Effect of Isoleucine and Added Valine on Performance, Nutrients Digestibility and Gut Microbiota Composition of Pigs Fed with Very Low Protein Diets
Abstract
1. Introduction
2. Results
2.1. Growth Performance
2.2. Thermal Radiation
2.3. Nutrients Digestibility
2.4. Dual-Energy X-ray Absorptiometry Analysis
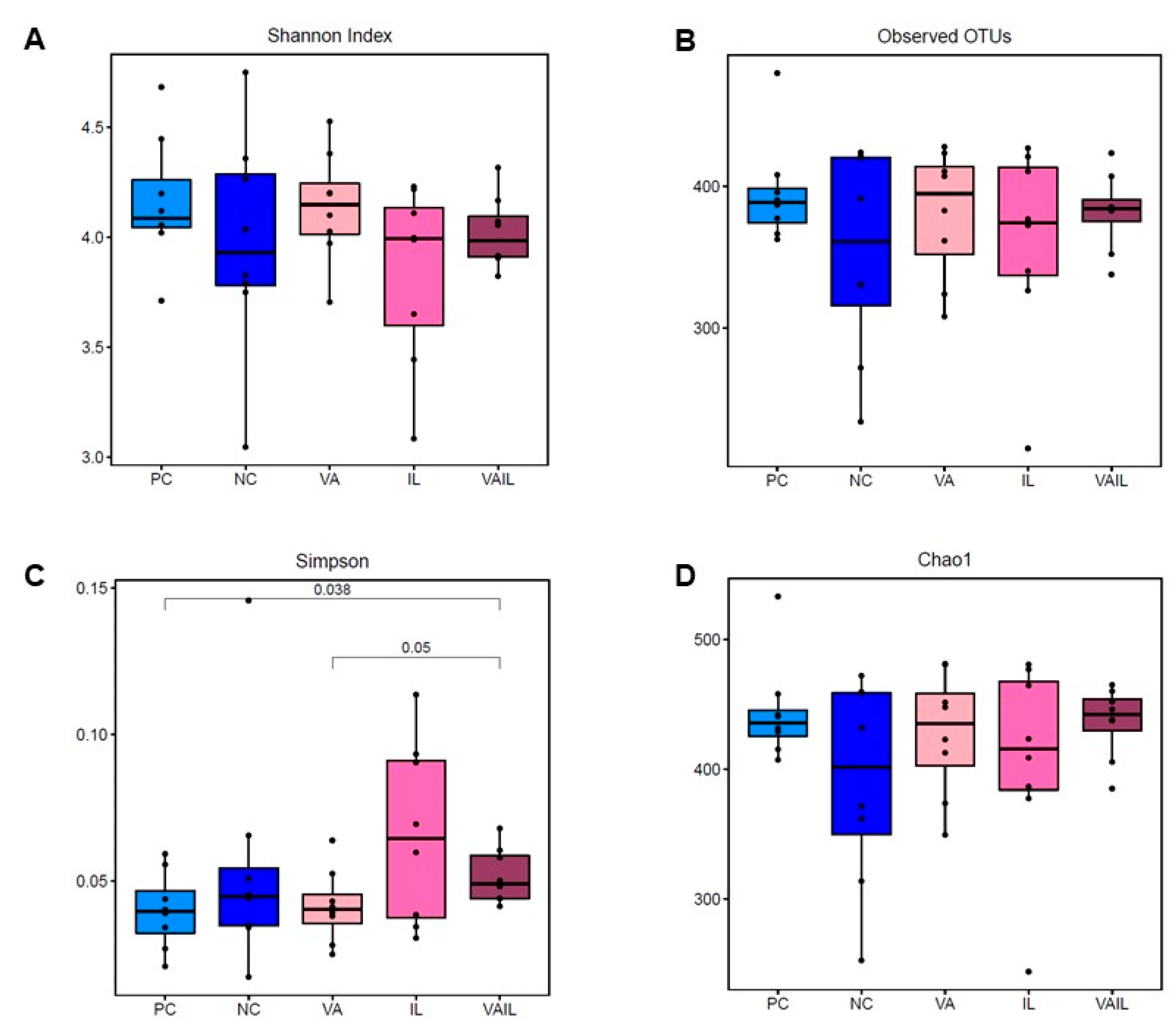
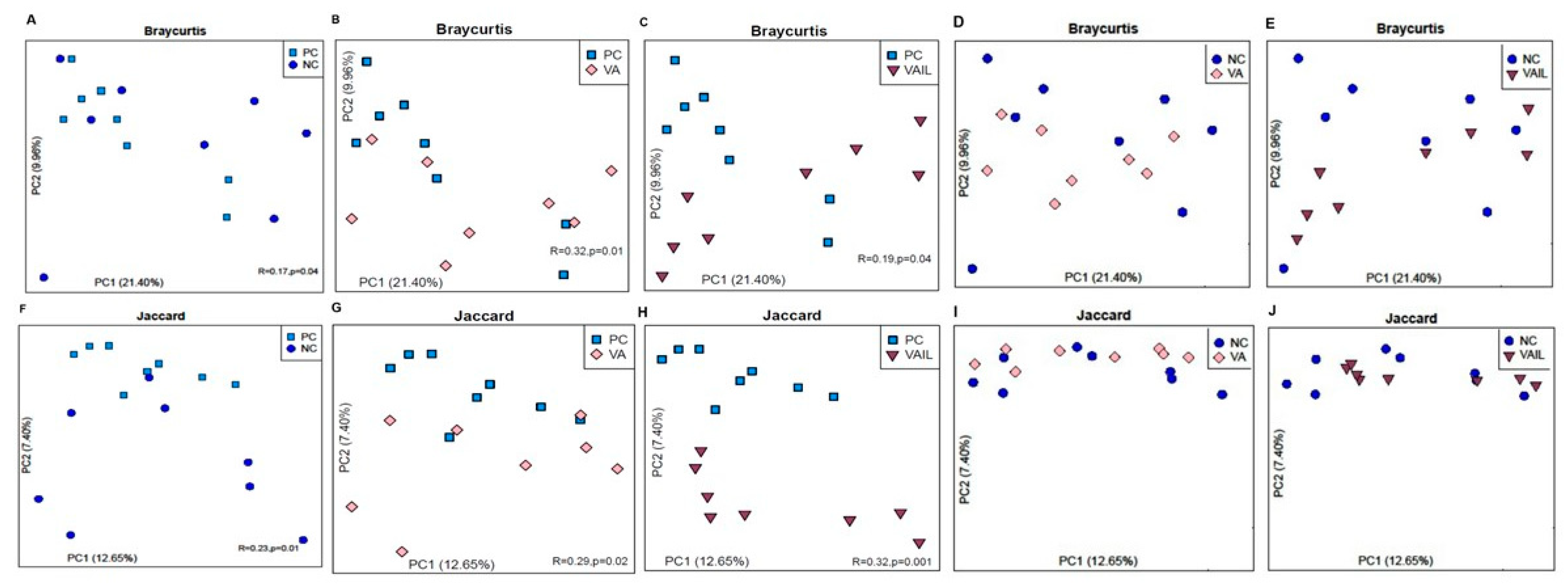
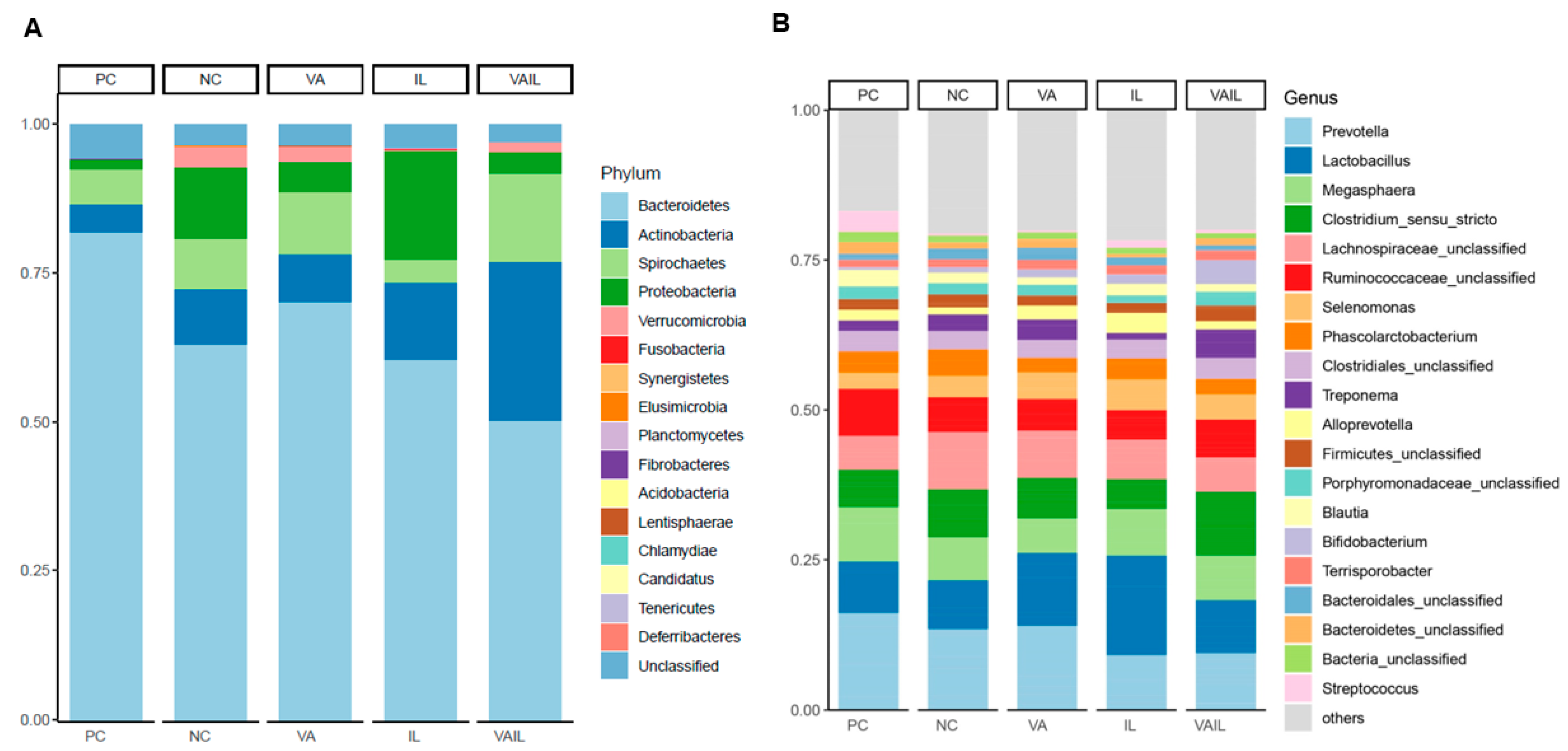
2.5. Colon Content Microbiota
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Diets and Experimental Design
4.3. Body Weight, Feed Intake, and Water Intake
4.4. Thermal Images
4.5. Feed and Fecal Samples Collection
4.6. Tissue Samples Collection
4.7. Diets and Fecal Samples Composition Analysis
4.8. Thermal Radiation Analysis
4.9. Apparent Fecal Digestibility
4.10. Dual-Energy X-ray Absorptiometry Analysis
4.11. Colon Contents DNA Isolation, Amplicon Sequencing, Sequence Data Analysis, and Taxonomic Classification
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Lordelo, M.; Gaspar, A.; Le Bellego, L.; Freire, J. Isoleucine and valine supplementation of a low-protein corn-wheat-soybean meal-based diet for piglets: Growth performance and nitrogen balance. J. Anim. Sci. 2008, 86, 2936–2941. [Google Scholar] [CrossRef]
- Opapeju, F.; Rademacher, M.; Blank, G.; Nyachoti, C. Effect of low-protein amino acid-supplemented diets on the growth performance, gut morphology, organ weights and digesta characteristics of weaned pigs. Animal 2008, 2, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Le Bellego, L.; van Milgen, J.; Noblet, J. Effect of high temperature and low-protein diets on the performance of growing-finishing pigs. J. Anim. Sci. 2002, 80, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Shriver, J.A.; Carter, S.D.; Sutton, A.L.; Richert, B.T.; Senne, B.W.; Pettey, L.A. Effects of adding fiber sources to reduced-crude protein, amino acid-supplemented diets on nitrogen excretion, growth performance, and carcass traits of finishing pigs. J. Anim. Sci. 2003, 81, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Madrid, J.; Martínez, S.; López, C.; Orengo, J.; López, M.J.; Hernández, F. Effects of low protein diets on growth performance, carcass traits and ammonia emission of barrows and gilts. Anim. Prod. Sci. 2012, 53, 146–153. [Google Scholar] [CrossRef]
- Figueroa, J.; Lewis, A.; Miller, P.S.; Fischer, R.; Gómez, R.; Diedrichsen, R. Nitrogen metabolism and growth performance of gilts fed standard corn-soybean meal diets or low-crude protein, amino acid-supplemented diets. J. Anim. Sci. 2002, 80, 2911–2919. [Google Scholar] [CrossRef]
- Deng, D.; Huang, R.L.; Li, T.J.; Wu, G.Y.; Xie, M.Y.; Tang, Z.R.; Kang, P.; Zhang, Y.M.; Fan, M.Z.; Kong, X.F.; et al. Nitrogen balance in barrows fed low-protein diets supplemented with essential amino acids. Livest. Sci. 2007, 109, 220–223. [Google Scholar] [CrossRef]
- He, L.; Wu, L.; Xu, Z.; Li, T.; Yao, K.; Cui, Z.; Yin, Y.; Wu, G. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 2016, 48, 21–30. [Google Scholar] [CrossRef]
- Portejoie, S.; Dourmad, J.Y.; Martinez, J.; Lebreton, Y. Effect of lowering dietary crude protein on nitrogen excretion, manure composition and ammonia emission from fattening pigs. Livest. Prod. Sci. 2004, 91, 45–55. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, Q.; Tan, B.; Guo, F.; Tang, M.; Li, T. Chronic feeding with protein-restricted diets affect ileal amino acid digestibility and the expression of nutrient-sensing, hormone secretion, gastrointestinal digestive enzyme, and nutrient transporter genes in young weaned pigs. Oncotarget 2018, 5, 1–15. Available online: https://www.oncotarget.com/article/24093/text/ (accessed on 10 January 2018). [CrossRef][Green Version]
- Spring, S.; Premathilake, H.; DeSilva, U.; Shili, C.; Carter, S.; Pezeshki, A. Low protein-high carbohydrate diets alter energy balance, gut microbiota composition and blood metabolomics profile in young pigs. Sci. Rep. 2020, 10, 3318. [Google Scholar] [CrossRef]
- Luo, Z.; Li, C.; Cheng, Y.; Hang, S.; Zhu, W. Effects of low dietary protein on the metabolites and microbial communities in the caecal digesta of piglets. Arch. Anim. Nutr. 2015, 69, 212–226. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; DeSilva, U.; Carter, S.; Pezeshki, A. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef]
- Habibi, M.; Shili, C.; Sutton, J.; Goodarzi, P.; Maylem, E.R.; Spicer, L.; Pezeshki, A. Branched-chain amino acids partially recover the reduced growth of pigs fed with protein-restricted diets through both central and peripheral factors. Anim Nutr. 2021, 7, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.; Lewis, A.; Miller, P.S.; Fischer, R.; Diedrichsen, R. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J. Anim. Sci. 2003, 81, 1529–1537. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effect of different dietary protein levels and amino acids supplementation patterns on growth performance, carcass characteristics and nitrogen excretion in growing-finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 75. [Google Scholar] [CrossRef]
- Gloaguen, M.; Le Floc’H, N.; Brossard, L.; Barea, R.; Primot, Y.; Corrent, E.; van Milgen, J. Response of piglets to the valine content in diet in combination with the supply of other branched-chain amino acids. Animal 2011, 5, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, M.; Le Floc’H, N.; Corrent, E.; Primot, Y.; van Milgen, J. Providing a diet deficient in valine but with excess leucine results in a rapid decrease in feed intake and modifies the postprandial plasma amino acid and α-keto acid concentrations in pigs. J. Anim. Sci. 2012, 90, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Wiltafsky, M.K.; Pfaffl, M.W.; Roth, F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010, 103, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.; Baker, D. Amino acid excesses for young pigs: Effects of excess methionine, tryptophan, threonine or leucine. J. Anim. Sci. 1987, 64, 1664–1671. [Google Scholar] [CrossRef]
- Habibi, M.; Goodarzi, P.; Shili, C.N.; Sutton, J.; Wileman, C.M.; Kim, D.M.; Lin, D.; Pezeshki, A. A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile. Int. J. Mol. Sci. 2022, 23, 3300. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Fernández, J.A. Isoleucine and valine supplementation of crude protein-reduced diets for pigs aged 5–8 weeks. Anim. Feed Sci. Technol. 2009, 154, 248–253. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Jia, H.; He, P.; Mao, X.; Qiao, S.; Zeng, X. Valine supplementation in a reduced protein diet regulates growth performance partially through modulation of plasma amino acids profile, metabolic responses, endocrine, and neural factors in piglets. J. Agric. Food Chem. 2018, 66, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, Y.H.; Kim, I.H. Effects of Bacillus licheniformis derived-protease supplementation, alone or in combination, with valine to low protein diet on growth performance and carcass quality grade in growing-finishing pigs. J. Appl. Anim. Res. 2021, 49, 180–184. [Google Scholar] [CrossRef]
- Ma, W.; Mao, P.; Fan, W.; Zhu, Y.; Guo, L. Valine and isoleucine supplementation improve performance and serum biochemical concentrations in growing gilts fed low-protein diets. Can. J. Anim. Sci. 2019, 99, 921–928. [Google Scholar] [CrossRef]
- Russell, L.E.; Kerr, B.J.; Easter, R.A. Limiting amino acids in an 11% crude protein corn-soybean meal diet for growing pigs. J. Anim. Sci. 1987, 65, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Mavromichalis, I.; Webel, D.M.; Emmert, J.L.; Moser, R.L.; Baker, D.H. Limiting order of amino acids in a low-protein corn-soybean meal-whey-based diet for nursery pigs. J. Anim. Sci. 1998, 76, 2833–2837. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, W.; Zeng, X.; Xie, C.; Thacker, P.; Htoo, J.; Qiao, S.Y. Estimation of the standardized ileal digestible valine to lysine ratio required for 25-to 120-kg pigs fed low crude protein diets supplemented with crystalline amino acids. J. Anim. Sci. 2015, 93, 4761–4773. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, A.; Bidner, T.; Payne, R.; Southern, L. Valine and isoleucine requirement of 20-to 45-kg pigs. J. Anim. Sci. 2012, 90, 2276–2284. [Google Scholar] [CrossRef]
- Powell, S.; Bidner, T.; Payne, R.; Southern, L. Growth performance of 20-to 50-kg pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J. Anim. Sci. 2011, 89, 3643–3650. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, S.; Zeng, X.; Liu, H.; Qiao, S. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian-Australas. J. Anim. Sci. 2015, 28, 1742. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.; Li, W.; Zhang, C.; Sun, K.; Ji, Y.; Wang, B.; Jiao, N.; He, B.; Wang, W.; et al. Dietary L-leucine supplementation enhances intestinal development in suckling piglets. Amino Acids 2015, 47, 1517–1525. [Google Scholar] [CrossRef]
- Duan, Y.; Tan, B.; Li, J.; Liao, P.; Huang, B.; Li, F.; Xiao, H.; Liu, Y.; Yin, Y. Optimal branched-chain amino acid ratio improves cell proliferation and protein metabolism of porcine enterocytesin in vivo and in vitro. Nutrition 2018, 54, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef]
- Zheng, L.; Wei, H.; Cheng, C.; Xiang, Q.; Pang, J.; Peng, J. Supplementation of branched-chain amino acids to a reduced-protein diet improves growth performance in piglets: Involvement of increased feed intake and direct muscle growth-promoting effect. Br. J. Nutr. 2016, 115, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Heng, J.; Song, H.; Shi, K.; Lin, X.; Chen, F.; Guan, W.; Zhang, S. Dietary branched-chain amino acids regulate food intake partly through intestinal and hypothalamic amino acid receptors in piglets. J. Agric. Food Chem. 2019, 67, 6809–6818. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Gao, J.; Htoo, J.K.; Chen, D. Regulation of intestinal health by branched-chain amino acids. Anim. Sci. J. 2018, 89, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Shili, C.N.; Sutton, J.; Goodarzi, P.; Pezeshki, A. Dietary branched-chain amino acids modulate the dynamics of calcium absorption and reabsorption in protein-restricted pigs. J. Anim. Sci. Biotechnol. 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.B.; Soto, J.A.; Stein, H.H. Effects on nitrogen balance and metabolism of branched-chain amino acids by growing pigs of supplementing isoleucine and valine to diets with adequate or excess concentrations of dietary leucine. J. Anim. Sci. 2020, 98, skaa346. [Google Scholar] [CrossRef]
- Yin, J.; Ma, J.; Li, Y.; Ma, X.; Chen, J.; Zhang, H.; Wu, X.; Li, F.; Liu, Z.; Li, T.; et al. Branched-chain amino acids, especially of leucine and valine, mediate the protein restricted response in a piglet model. Food Funct. 2020, 11, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Jeyapalan, A.S.; Orellana, R.A.; Wilson, F.A.; Nguyen, H.V.; Davis, T.A. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Murgas Torrazza, R.; Suryawan, A.; Gazzaneo, M.C.; Orellana, R.A.; Frank, J.W.; Nguyen, H.V.; Fiorotto, M.L.; El-Kadi, S.; Davis, T.A. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J. Nutr. 2010, 140, 2145–2152. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, K.; Liu, Z.; Gong, M.; Ruan, Z.; Deng, D.; Tan, B.; Liu, Z.; Wu, G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 2010, 39, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Branched-chain amino acids: Enzyme and substrate regulation. J. Nutr. 2006, 136, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Austic, R.E. The branched-chain amino acid antagonism in chicks. J. Nutr. 1978, 108, 1180–1191. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, L.; Zhu, J.; Chen, J.; Wang, Z.; Yue, Z.; Qiu, M.; Shan, A. Valine supplementation does not reduce lipid accumulation and improve insulin sensitivity in mice fed high-fat diet. ACS Omega 2020, 5, 30937–30945. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Guo, F. Impacts of essential amino acids on energy balance. Mol. Metab. 2022, 57, 101393. [Google Scholar] [CrossRef] [PubMed]
- Wiltafsky, M.K.; Schmidtlein, B.; Roth, F.X. Estimates of the optimum dietary ratio of standardized ileal digestible valine to lysine for eight to twenty-five kilograms of body weight pigs. J. Anim. Sci. 2009, 87, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Salmon, W.D. The significance of amino acid imbalance in nutrition. Am. J. Clin. Nutr. 1958, 6, 487–494. [Google Scholar] [CrossRef]
- Shili, C.N.; Broomhead, J.N.; Spring, S.C.; Lanahan, M.B.; Pezeshki, A. A novel corn-expressed phytase improves daily weight gain, protein efficiency ratio and nutrients digestibility and alters fecal microbiota in pigs fed with very low protein diets. Animals 2020, 10, 1926. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Kang, D.K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Borewicz, K.; White, B.A.; Singer, R.S.; Sreevatsan, S.; Tu, Z.J.; Isaacson, R.E. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet. Microbiol. 2011, 153, 124–133. [Google Scholar] [CrossRef]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a possible link between the intestinal microbiota and feed efficiency in pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann. N. Y. Acad. Sci. 2008, 1225, 280–288. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef]
- Koo, B.; Kim, J.W.; Nyachoti, C.M. Nutrient and energy digestibility, and microbial metabolites in weaned pigs fed diets containing Lactobacillus–fermented wheat. Anim. Feed Sci. Technol. 2018, 241, 27–37. [Google Scholar] [CrossRef]
- Marounek, M.; Fliegrova, K.; Bartos, S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl. Environ. Microbiol. 1989, 55, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Cai, G.; Yang, M.; Zeng, Z.; Ding, R.; Wang, X.; Zhuang, Z.; Zhou, S.; Li, S.; Yang, H.; et al. Exploring the fecal microbial composition and metagenomic functional capacities associated with feed efficiency in commercial DLY pigs. Front. Microbiol. 2019, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, M.; Fang, S.; Huang, X.; He, M.; Ke, S.; Gao, J.; Wu, J.; Zhou, Y.; Fu, H.; et al. Evaluating the profound effect of gut microbiome on host appetite in pigs. BMC Microbiol. 2018, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, R.; Sweeney, T.; Maher, S.; Ryan, M.T.; Thornton, K.; O’Doherty, J.V. Effects of reducing dietary crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Anim. Feed Sci. Technol. 2020, 270, 114693. [Google Scholar] [CrossRef]
- Christensen, H.; Bossé, J.; Angen, Ø.; Nørskov-Lauritsen, N.; Bisgaard, M. Immunological and molecular techniques used for determination of serotypes in Pasteurellaceae. In Book Immunological Methods in Microbiology, 1st ed.; Pavia, C.S., Gurtler, V., Eds.; Academic Press: London, UK, 2020; Volume 47, pp. 117–149. [Google Scholar] [CrossRef]
- Stetsko, T.I. Antibiotic resistance of bacteria of the family pasteurellaceae, pathogens of respiratory infections of cattle and pigs. Sci. Tech. Bull. State Sci. Res. Control Inst. Vet. Med. Prod. Fodd. Addit. Inst. Anim. Biol. 2021, 22, 197–208. [Google Scholar] [CrossRef]
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE 2017, 12, e0169851. [Google Scholar] [CrossRef] [PubMed]
- Allen-Vercoe, E.; Jobin, C. Fusobacterium and Enterobacteriaceae: Important players for CRC? Immunol. Lett. 2014, 162, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Abbondio, M.; Sau, R.; Tanca, A.; Pira, G.; Errigo, A.; Manetti, R.; Pes, G.M.; Dore, M.P.; Uzzau, S. Fecal microbiota signatures in celiac disease patients with poly-autoimmunity. Front. Cell. Infect. 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhou, J.; Su, Y.; Mao, K.; Wu, J.; Zhu, C.; He, L.; Cui, Y. Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2021, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.; Liu, T. Rational synthetic pathway refactoring of natural products biosynthesis in actinobacteria. Metab. Eng. 2017, 39, 228–236. [Google Scholar] [CrossRef]
- Zeyner, A.; Boldt, E. Effects of a probiotic Enterococcus faecium strain supplemented from birth to weaning on diarrhoea patterns and performance of piglets. J. Anim. Physiol. Anim. Nutr. 2006, 90, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bednorz, C.; Guenther, S.; Oelgeschläger, K.; Kinnemann, B.; Pieper, R.; Hartmann, S.; Tedin, K.; Semmler, T.; Neumann, K.; Schierack, P.; et al. Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli pathotypes that adhere to the gut mucosa. Appl. Environ. Microbiol. 2013, 79, 7896–7904. [Google Scholar] [CrossRef]
- Wang, Y.B.; Du, W.; Fu, A.K.; Zhang, X.P.; Huang, Y.; Lee, K.H.; Yu, K.; Li, W.F.; Li, Y.L. Intestinal microbiota and oral administration of Enterococcus faecium associated with the growth performance of new-born piglets. Benef. Microbes 2016, 7, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Strompfova, V.; Marciňáková, M.; Simonová, M.; Gancarčíková, S.; Jonecová, Z.; Sciranková, Ľ.; Koščová, J.; Buleca, V.; Čobanová, K.; Lauková, A. Enterococcus faecium EK13—An enterocin A-producing strain with probiotic character and its effect in piglets. Anaerobe 2006, 12, 242–248. [Google Scholar] [CrossRef]
- Seddon, B.; McHugh, R.C.; Schmitt, A. Brevibacillus brevis-a novel candidate biocontrol agent with broad-spectrum antifungal activity. In The BCPC Conference: Pests and Diseases, Proceedings of the International Conference Held at the Brighton Hilton Metropole Hotel, Brighton, UK, 13–16 November 2000; British Crop Protection Council: Farnham, UK, 2000; Volume 2, pp. 563–570. [Google Scholar]
- Che, J.; Ye, S.; Liu, B.; Deng, Y.; Chen, Q.; Ge, C.; Liu, G.; Wang, J. Effects of Brevibacillus brevis FJAT-1501-BPA on growth performance, faecal microflora, faecal enzyme activities and blood parameters of weaned piglets. Antonie Leeuwenhoek Int. J. Gen. 2016, 109, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Tu, W.; Lu, Y.; Cai, X. Pyrodextrin enhances intestinal function through changing the intestinal microbiota composition and metabolism in early weaned piglets. Appl. Microbiol. Biotechnol. 2020, 104, 4141–4154. [Google Scholar] [CrossRef]
- Goodarzi, P.; Habibi, M.; Roberts, K.; Sutton, J.; Shili, C.N.; Lin, D.; Pezeshki, A. Dietary tryptophan supplementation alters fat and glucose metabolism in a low-birthweight piglet model. Nutrients 2021, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and environmental microbiology. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, 141–145. [Google Scholar] [CrossRef] [PubMed]



| Measurements 1 | Diets 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | VA | IL | VAIL | |||
| Initial BW, kg | 6.76 | 6.69 | 6.89 | 6.68 | 6.68 | 0.12 | 0.98 |
| Final BW, kg | 23.92 | 17.98 a | 20.26 # | 18.21 | 20.19 ε,ω | 0.56 | 0.01 |
| ADG, kg/day | 0.50 | 0.32 a | 0.38 b | 0.33 | 0.39 d,ω | 0.02 | <0.01 |
| ADFI, kg/day | 0.78 | 0.62 a | 0.81 c | 0.68 | 0.78 ω | 0.02 | 0.04 |
| ADPI, kg/day | 0.15 | 0.08 a | 0.11 b,c | 0.09 | 0.10 d,ω | 0.01 | <0.01 |
| ADWI, L/day | 1.95 | 1.38 | 1.73 | 1.55 | 1.77 | 0.08 | 0.24 |
| G:F, kg/kg | 0.66 | 0.49 a | 0.47 b | 0.48 | 0.50 d | 0.02 | <0.01 |
| G:P, kg/kg | 3.47 | 3.66 | 3.62 | 3.73 | 3.74 | 0.09 | 0.91 |
| W:F, L/kg | 2.68 | 2.11 | 2.31 | 2.28 | 2.28 | 0.10 | 0.54 |
| Final body length, m | 0.65 | 0.61 | 0.65 | 0.61 | 0.63 | 0.01 | 0.08 |
| Final heart girth, m | 0.62 | 0.57 | 0.59 | 0.58 | 0.61 | 0.01 | 0.11 |
| Final wither height, m | 0.42 | 0.41 | 0.42 | 0.40 | 0.41 | 0.004 | 0.31 |
| Diets 1 | SEM 2 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| PC | NC | VA | IL | VAIL | |||
| BWG 1, kg | |||||||
| Week 1 | 1.48 | 1.09 a | 1.07 b | 1.32 | 1.09 | 0.06 | 0.08 |
| Week 2 | 3.23 | 2.00 a | 2.35 # | 1.82 | 2.19 ε | 0.12 | <0.01 |
| Week 3 | 4.11 | 2.44 a | 3.05 b,c | 2.24 | 2.54 d | 0.15 | <0.01 |
| Week 4 | 4.33 | 2.74 a | 3.05 b | 2.56 | 3.83 e | 0.16 | <0.01 |
| Week 5 | 4.85 | 3.03 a | 3.44 b | 3.27 | 3.89 ε,ω | 0.17 | 0.01 |
| MFI 1, kg | |||||||
| Week 1 | 0.44 | 0.40 | 0.42 | 0.35 | 0.42 | 0.02 | 0.54 |
| Week 2 | 0.65 | 0.55 | 0.63 | 0.48 | 0.59 | 0.03 | 0.21 |
| Week 3 | 0.83 | 0.63 * | 0.81 δ | 0.63 | 0.75 | 0.03 | 0.05 |
| Week 4 | 0.96 | 0.73 a | 0.96 c | 0.72 | 0.94 ω | 0.03 | <0.01 |
| Week 5 | 1.03 | 0.80 | 0.94 | 0.80 | 1.06 | 0.04 | 0.02 |
| CFI 1, kg | |||||||
| Week 1 | 3.09 | 2.78 | 2.96 | 2.48 | 2.96 | 0.12 | 0.56 |
| Week 2 | 4.56 | 3.86 | 4.40 | 3.34 | 4.15 | 0.21 | 0.20 |
| Week 3 | 5.78 | 4.41 * | 5.65 δ | 4.41 | 5.28 | 0.19 | 0.05 |
| Week 4 | 6.70 | 5.11 a | 6.74 c | 5.05 | 6.58 ω | 0.21 | <0.01 |
| Week 5 | 7.22 | 5.58 | 6.59 | 5.63 | 7.44 | 0.25 | 0.02 |
| CPI 1, kg | |||||||
| Week 1 | 0.62 | 0.38 a | 0.40 b | 0.39 | 0.37 d | 0.02 | <0.01 |
| Week 2 | 0.91 | 0.53 a | 0.65 #,δ | 0.51 | 0.66 | 0.04 | <0.01 |
| Week 3 | 1.08 | 0.58 a | 0.72 b,c | 0.55 | 0.64 d | 0.04 | <0.01 |
| Week 4 | 1.25 | 0.64 a | 0.86 b,c | 0.63 | 0.87 d,e | 0.05 | <0.01 |
| Week 5 | 1.35 | 0.74 a | 0.84 b | 0.70 | 0.98 d,e | 0.05 | <0.01 |
| G:F 1, kg/kg | |||||||
| Week 1 | 0.51 | 0.42 | 0.36 | 0.72 | 0.41 | 0.07 | 0.46 |
| Week 2 | 0.72 | 0.53 a | 0.55 b | 0.59 | 0.76 | 0.06 | 0.65 |
| Week 3 | 0.71 | 0.56 * | 0.55 b | 0.50 | 0.55 d | 0.02 | 0.01 |
| Week 4 | 0.65 | 0.54 a | 0.45 b,δ | 0.51 | 0.56 d | 0.02 | <0.01 |
| Week 5 | 0.69 | 0.53 a | 0.53 # | 0.60 | 0.56 d | 0.02 | 0.11 |
| G:P 1, kg/kg | |||||||
| Week 1 | 2.54 | 3.06 | 2.69 | 3.06 | 3.29 | 0.12 | 0.31 |
| Week 2 | 3.85 | 3.85 | 3.72 | 3.40 | 3.43 | 0.12 | 0.77 |
| Week 3 | 3.82 | 3.79 | 4.27 | 4.05 | 4.20 | 0.11 | 0.56 |
| Week 4 | 3.45 | 3.99 | 3.51 | 3.80 | 4.24 d | 0.09 | 0.04 |
| Week 5 | 3.70 | 3.71 | 4.13 | 4.55 | 4.41 | 0.13 | 0.13 |
| Measurements | Diets 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| PC | NC | VA | IL | VAIL | |||
| Fat, % | 12.70 | 13.80 | 16.35 # | 19.69 | 14.77 | 0.62 | <0.01 |
| Lean, % | 84.97 | 83.71 | 81.01 δ | 77.99 | 82.94 | 0.64 | <0.01 |
| BMC 1, g | 47.09 | 39.65 | 45.78 | 39.10 | 41.23 | 1.58 | 0.38 |
| BMD 1, g/cm2 | 0.31 | 0.29 | 0.31 | 0.29 | 0.30 | 0.01 | 0.61 |
| Diets 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | |||||||||
| Ingredients 2, % | PC | NC | VA | IL | VAIL | PC | NC | VA | IL | VAIL | |
| Corn, yellow dent | 36.98 | 46.35 | 67.62 | 67.49 | 67.50 | 67.35 | 66.20 | 84.04 | 83.93 | 83.94 | 83.82 |
| Soybean meal, 47.5% CP | 17.00 | 26.40 | 6.60 | 6.60 | 6.60 | 6.60 | 21.28 | 2.83 | 2.83 | 2.83 | 2.83 |
| Fish meal, menhaden | 6.00 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 |
| Whey, dried | 25.00 | 4.50 | 4.50 | 4.50 | 4.50 | 4.50 | ─ | ─ | ─ | ─ | ─ |
| Corn starch | ─ | 13.88 | 10.62 | 10.62 | 10.62 | 10.62 | 4.00 | 2.91 | 2.91 | 2.91 | 2.91 |
| Lactose | 7.00 | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ |
| Plasma spray-dried | 5.80 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 |
| Corn oil | 0.37 | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ |
| Dicalcium phosphate 18.5% | 0.85 | 1.35 | 1.73 | 1.73 | 1.73 | 1.73 | 1.15 | 1.55 | 1.55 | 1.55 | 1.55 |
| Limestone | 0.39 | 0.50 | 0.41 | 0.41 | 0.41 | 0.41 | 0.47 | 0.34 | 0.34 | 0.34 | 0.34 |
| Salt | 0.13 | 0.59 | 0.59 | 0.59 | 0.59 | 0.59 | 0.48 | 0.48 | 0.48 | 0.48 | 0.48 |
| Chromium oxide | ─ | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin premix | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Trace mineral premix | ─ | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Zinc oxide, 72% Zn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| L-Lysine, HCl | 0.26 | 0.35 | 0.85 | 0.85 | 0.85 | 0.85 | 0.36 | 0.83 | 0.83 | 0.83 | 0.83 |
| DL-methionine | 0.13 | 0.11 | 0.20 | 0.20 | 0.20 | 0.20 | 0.09 | 0.17 | 0.17 | 0.17 | 0.17 |
| L-threonine | 0.04 | 0.10 | 0.37 | 0.37 | 0.37 | 0.37 | 0.10 | 0.36 | 0.36 | 0.36 | 0.36 |
| L-tryptophan | ─ | 0.01 | 0.11 | 0.11 | 0.11 | 0.11 | 0.01 | 0.10 | 0.10 | 0.10 | 0.10 |
| L-isoleucine | ─ | ─ | ─ | ─ | 0.33 | 0.33 | ─ | ─ | ─ | 0.31 | 0.31 |
| L-valine | ─ | ─ | ─ | 0.48 | ─ | 0.48 | ─ | ─ | 0.44 | ─ | 0.44 |
| L-alanine | ─ | ─ | 0.54 | 0.19 | 0.33 | ─ | ─ | 0.53 | 0.20 | 0.32 | ─ |
| Calculated Chemical Composition3 | |||||||||||
| Dry matter, % | 90.38 | 90.36 | 90.14 | 90.05 | 90.16 | 90.18 | 88.93 | 88.97 | 88.98 | 88.98 | 88.99 |
| ME, Mcal/kg | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.33 | 3.34 | 3.34 | 3.34 | 3.34 |
| Crude protein, % | 21.97 | 20.30 | 14.00 | 14.00 | 14.00 | 14.00 | 18.93 | 12.93 | 12.93 | 12.93 | 12.93 |
| Crude fiber, % | 1.61 | 2.23 | 1.86 | 1.85 | 1.85 | 1.85 | 2.47 | 2.07 | 2.07 | 2.07 | 2.06 |
| Crude fat, % | 3.23 | 2.72 | 3.08 | 3.08 | 3.08 | 3.08 | 3.28 | 3.54 | 3.54 | 3.54 | 3.53 |
| Calcium, % | 0.85 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 |
| Total phosphorus, % | 0.70 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Available phosphorus, % | 0.62 | 0.46 | 0.51 | 0.51 | 0.51 | 0.51 | 0.39 | 0.44 | 0.44 | 0.44 | 0.44 |
| SID Lysine, % | 1.50 | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 |
| SID Threonine, % | 0.88 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 |
| SID Methionine, % | 0.43 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| SID Tryptophan, % | 0.25 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| SID Isoleucine, % | 0.78 | 0.74 | 0.41 | 0.41 | 0.74 | 0.74 | 0.67 | 0.36 | 0.36 | 0.67 | 0.67 |
| SID Valine, % | 0.96 | 0.86 | 0.53 | 1.01 | 0.53 | 1.01 | 0.79 | 0.48 | 0.92 | 0.48 | 0.92 |
| SID Leucine, % | 1.65 | 1.50 | 1.05 | 1.05 | 1.05 | 1.05 | 1.46 | 1.03 | 1.03 | 1.03 | 1.02 |
| SID Histidine, % | 0.51 | 0.47 | 0.30 | 0.30 | 0.30 | 0.30 | 0.45 | 0.28 | 0.28 | 0.28 | 0.28 |
| SID Arginine, % | 1.13 | 1.19 | 0.62 | 0.62 | 0.62 | 0.62 | 1.09 | 0.55 | 0.55 | 0.55 | 0.55 |
| SID Phenylalanine, % | 0.90 | 0.86 | 0.51 | 0.51 | 0.51 | 0.51 | 0.80 | 0.47 | 0.47 | 0.47 | 0.47 |
| SID Valine: SID Lysine | 0.64 | 0.64 | 0.39 | 0.75 | 0.39 | 0.75 | 0.64 | 0.39 | 0.75 | 0.39 | 0.75 |
| SID Isoleucine: SID Lysine | 0.52 | 0.55 | 0.30 | 0.30 | 0.55 | 0.55 | 0.54 | 0.29 | 0.29 | 0.54 | 0.54 |
| Diets 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | |||||||||
| Chemical Composition | PC | NC | VA | IL | VAIL | PC | NC | VA | IL | VAIL | |
| Dry matter, % | 90.60 | 88.10 | 87.60 | 86.80 | 87.70 | 87.00 | 87.30 | 86.80 | 86.60 | 86.90 | 86.50 |
| Crude protein, % | 23.00 | 20.00 | 13.70 | 13.50 | 14.40 | 13.50 | 18.70 | 13.20 | 12.80 | 12.40 | 13.20 |
| Crude fiber, % | 1.40 | 2.00 | 1.70 | 1.80 | 1.90 | 1.40 | 2.90 | 2.00 | 1.90 | 1.90 | 2.00 |
| Calcium, % | 1.06 | 0.92 | 0.66 | 0.83 | 0.88 | 0.78 | 0.78 | 0.64 | 0.66 | 0.71 | 0.67 |
| Phosphorus, % | 0.94 | 0.76 | 0.67 | 0.74 | 0.79 | 0.70 | 0.61 | 0.61 | 0.67 | 0.69 | 0.69 |
| Taurine 2, % | 0.20 | 0.19 | 0.28 | 0.19 | 0.20 | 0.20 | 0.26 | 0.28 | 0.21 | 0.21 | 0.20 |
| Hydroxyproline, % | 0.11 | 0.09 | 0.05 | 0.06 | 0.10 | 0.08 | 0.06 | 0.06 | 0.09 | 0.06 | 0.06 |
| Aspartic acid, % | 2.10 | 2.09 | 1.02 | 1.06 | 1.30 | 0.93 | 1.81 | 0.88 | 0.89 | 0.94 | 0.97 |
| Threonine, % | 1.06 | 0.93 | 0.85 | 0.75 | 0.92 | 0.80 | 0.86 | 0.75 | 0.72 | 0.94 | 0.98 |
| Serine, % | 0.95 | 0.91 | 0.51 | 0.51 | 0.59 | 0.47 | 0.81 | 0.47 | 0.47 | 0.49 | 0.50 |
| Glutamic acid, % | 3.45 | 3.57 | 1.94 | 2.03 | 2.41 | 1.85 | 3.31 | 1.80 | 1.79 | 1.86 | 1.89 |
| Proline, % | 1.17 | 1.11 | 0.73 | 0.76 | 0.81 | 0.72 | 1.05 | 0.72 | 0.76 | 0.76 | 0.77 |
| Lanthionine 2, % | 0.02 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 |
| Glycine, % | 0.95 | 0.88 | 0.53 | 0.52 | 0.63 | 0.49 | 0.83 | 0.51 | 0.52 | 0.53 | 0.51 |
| Alanine, % | 1.14 | 1.02 | 1.29 | 0.97 | 1.08 | 0.69 | 1.00 | 1.19 | 0.84 | 1.21 | 0.72 |
| Cysteine, % | 0.44 | 0.39 | 0.23 | 0.24 | 0.29 | 0.21 | 0.33 | 0.23 | 0.20 | 0.24 | 0.26 |
| Valine, % | 1.18 | 1.04 | 0.61 | 1.03 | 0.74 | 1.04 | 0.94 | 0.55 | 0.98 | 0.60 | 0.97 |
| Methionine, % | 0.47 | 0.44 | 0.36 | 0.35 | 0.44 | 0.39 | 0.39 | 0.37 | 0.29 | 0.34 | 0.38 |
| Isoleucine, % | 0.93 | 0.91 | 0.49 | 0.50 | 0.89 | 0.75 | 0.81 | 0.43 | 0.41 | 0.74 | 0.71 |
| Leucine, % | 1.89 | 1.73 | 1.12 | 1.17 | 1.29 | 1.12 | 1.63 | 1.09 | 1.10 | 1.12 | 1.14 |
| Tyrosine, % | 0.73 | 0.68 | 0.39 | 0.39 | 0.47 | 0.39 | 0.63 | 0.39 | 0.39 | 0.38 | 0.39 |
| Phenylalanine, % | 1.01 | 1.02 | 0.56 | 0.60 | 0.69 | 0.54 | 0.92 | 0.53 | 0.54 | 0.53 | 0.56 |
| Hydroxylysine, % | 0.06 | 0.04 | 0.00 | 0.03 | 0.04 | 0.03 | 0.00 | 0.00 | 0.04 | 0.02 | 0.04 |
| Ornithine 2, % | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Lysine, % | 1.69 | 1.69 | 1.39 | 1.32 | 1.56 | 1.31 | 1.40 | 1.29 | 1.37 | 1.40 | 1.29 |
| Histidine, % | 0.54 | 0.54 | 0.32 | 0.32 | 0.37 | 0.28 | 0.51 | 0.30 | 0.30 | 0.31 | 0.30 |
| Arginine, % | 1.19 | 1.29 | 0.65 | 0.65 | 0.81 | 0.56 | 1.16 | 0.58 | 0.59 | 0.61 | 0.60 |
| Tryptophan, % | 0.33 | 0.28 | 0.23 | 0.25 | 0.26 | 0.28 | 0.22 | 0.20 | 0.22 | 0.24 | 0.22 |
| Valine: Lysine | 0.70 | 0.62 | 0.44 | 0.78 | 0.47 | 0.79 | 0.67 | 0.43 | 0.72 | 0.43 | 0.75 |
| Isoleucine: Lysine | 0.55 | 0.54 | 0.35 | 0.38 | 0.57 | 0.57 | 0.58 | 0.33 | 0.30 | 0.53 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodarzi, P.; Wileman, C.M.; Habibi, M.; Walsh, K.; Sutton, J.; Shili, C.N.; Chai, J.; Zhao, J.; Pezeshki, A. Effect of Isoleucine and Added Valine on Performance, Nutrients Digestibility and Gut Microbiota Composition of Pigs Fed with Very Low Protein Diets. Int. J. Mol. Sci. 2022, 23, 14886. https://doi.org/10.3390/ijms232314886
Goodarzi P, Wileman CM, Habibi M, Walsh K, Sutton J, Shili CN, Chai J, Zhao J, Pezeshki A. Effect of Isoleucine and Added Valine on Performance, Nutrients Digestibility and Gut Microbiota Composition of Pigs Fed with Very Low Protein Diets. International Journal of Molecular Sciences. 2022; 23(23):14886. https://doi.org/10.3390/ijms232314886
Chicago/Turabian StyleGoodarzi, Parniyan, Caitlyn Marie Wileman, Mohammad Habibi, Katherine Walsh, Julia Sutton, Cedrick Ndhumba Shili, Jianmin Chai, Jiangchao Zhao, and Adel Pezeshki. 2022. "Effect of Isoleucine and Added Valine on Performance, Nutrients Digestibility and Gut Microbiota Composition of Pigs Fed with Very Low Protein Diets" International Journal of Molecular Sciences 23, no. 23: 14886. https://doi.org/10.3390/ijms232314886
APA StyleGoodarzi, P., Wileman, C. M., Habibi, M., Walsh, K., Sutton, J., Shili, C. N., Chai, J., Zhao, J., & Pezeshki, A. (2022). Effect of Isoleucine and Added Valine on Performance, Nutrients Digestibility and Gut Microbiota Composition of Pigs Fed with Very Low Protein Diets. International Journal of Molecular Sciences, 23(23), 14886. https://doi.org/10.3390/ijms232314886









