Trypanosoma cruzi-Derived Molecules Induce Anti-Tumour Protection by Favouring Both Innate and Adaptive Immune Responses
Abstract
:1. Introduction
2. Results
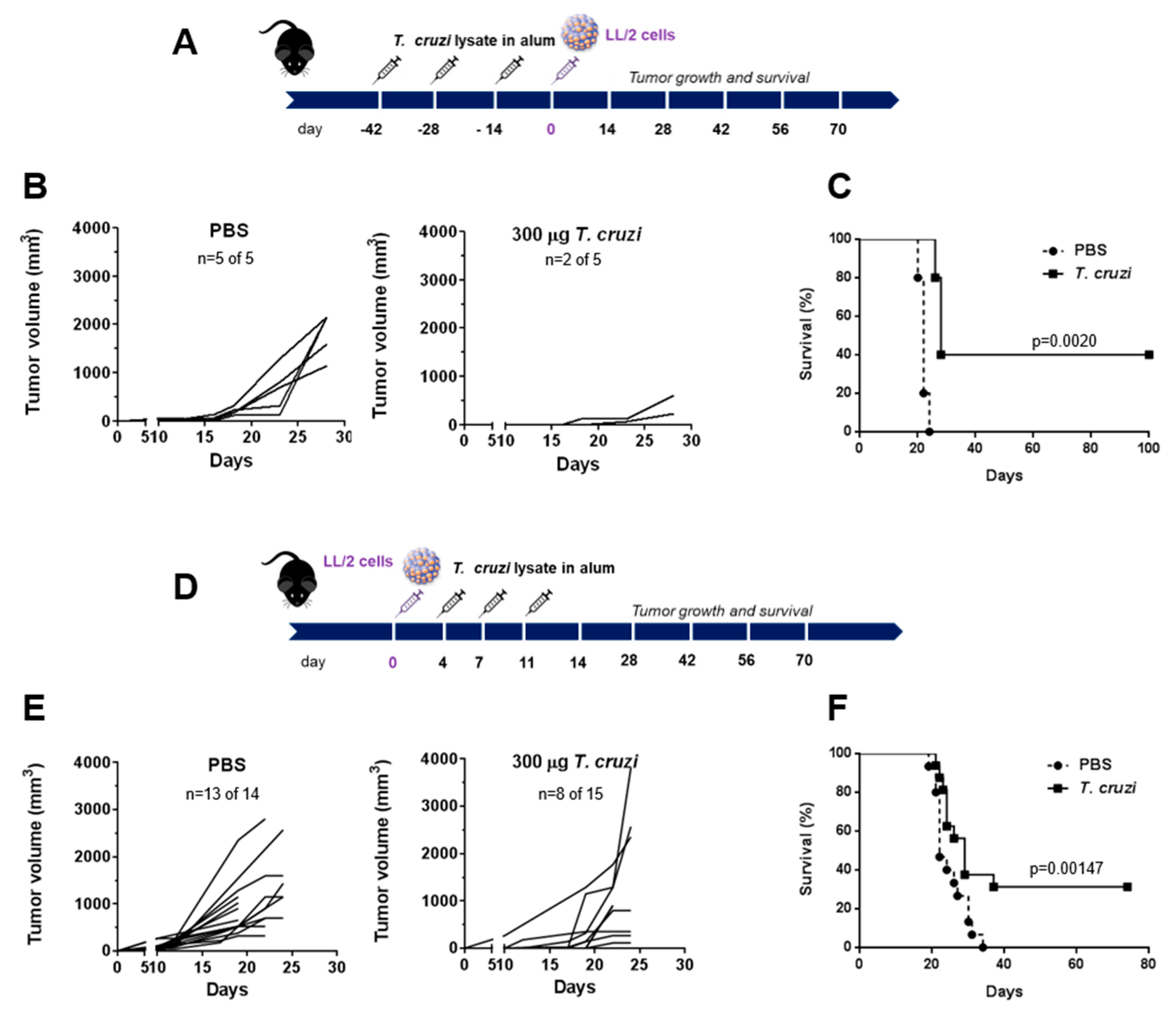
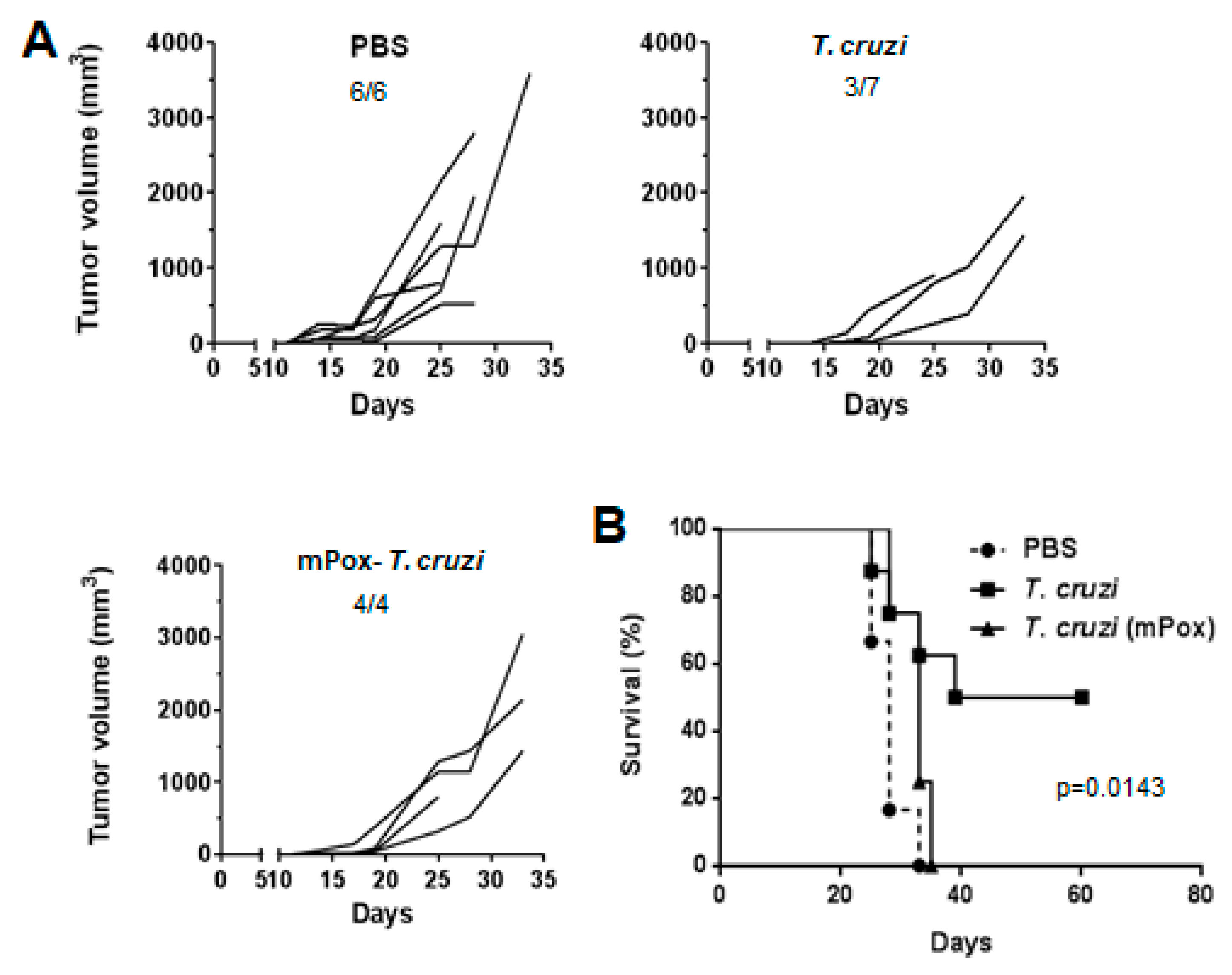
2.1. A Lysate from T. cruzi Epimastigotes Prevents Tumour Growth
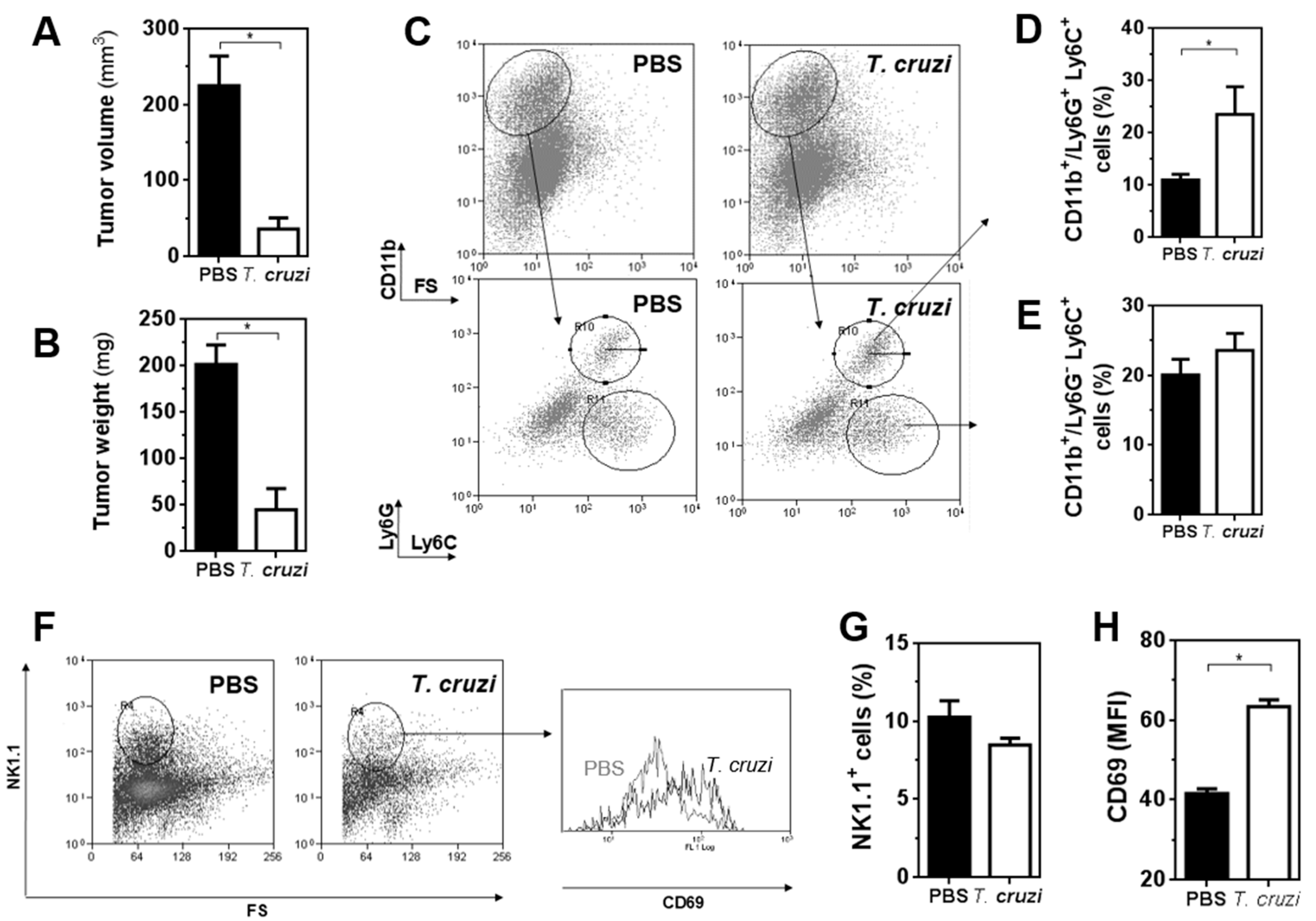
2.2. T. cruzi Epimastigote Lysate Administration Is Associated with Higher Activation of NK Cells in Tumour-Bearing Mice
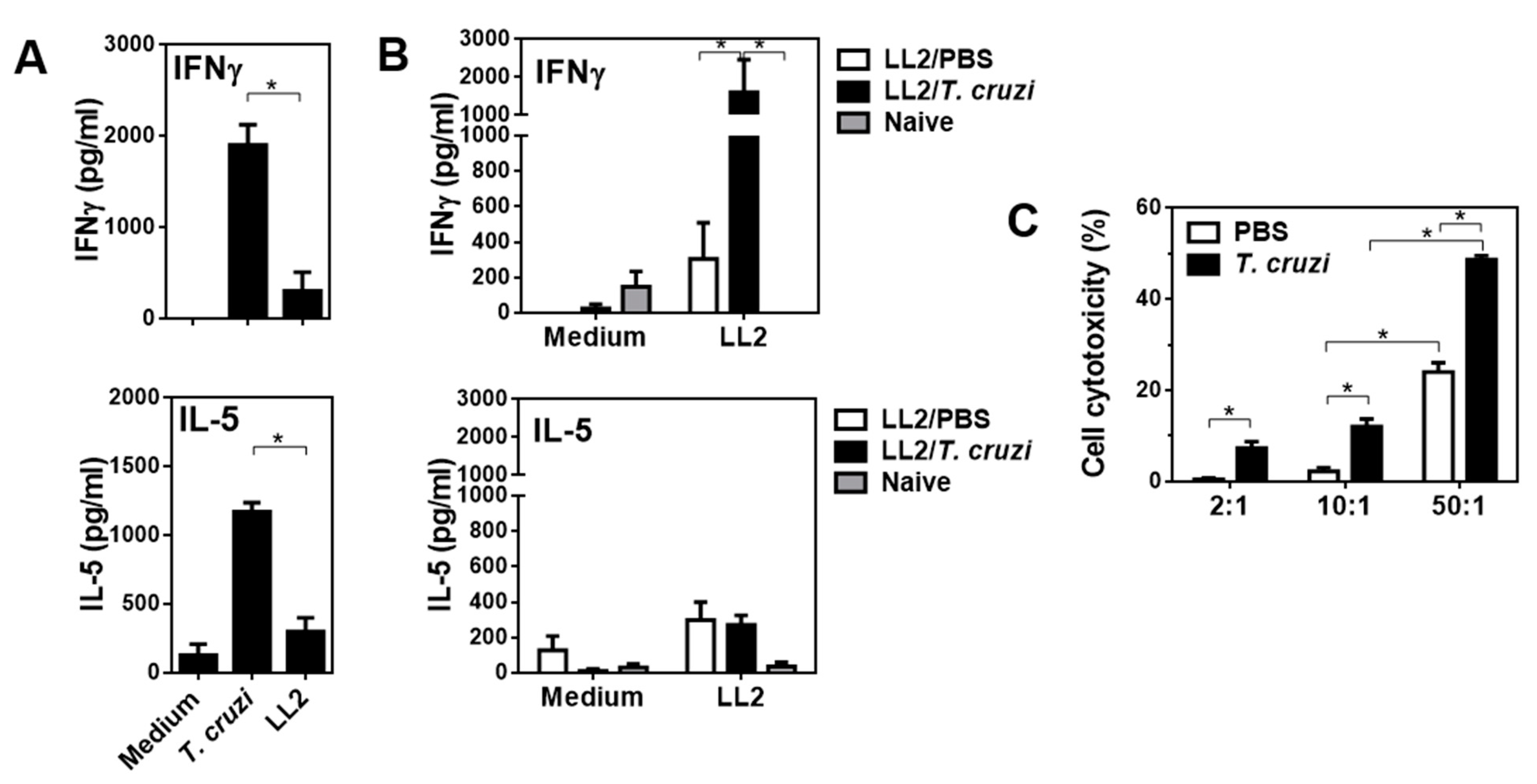
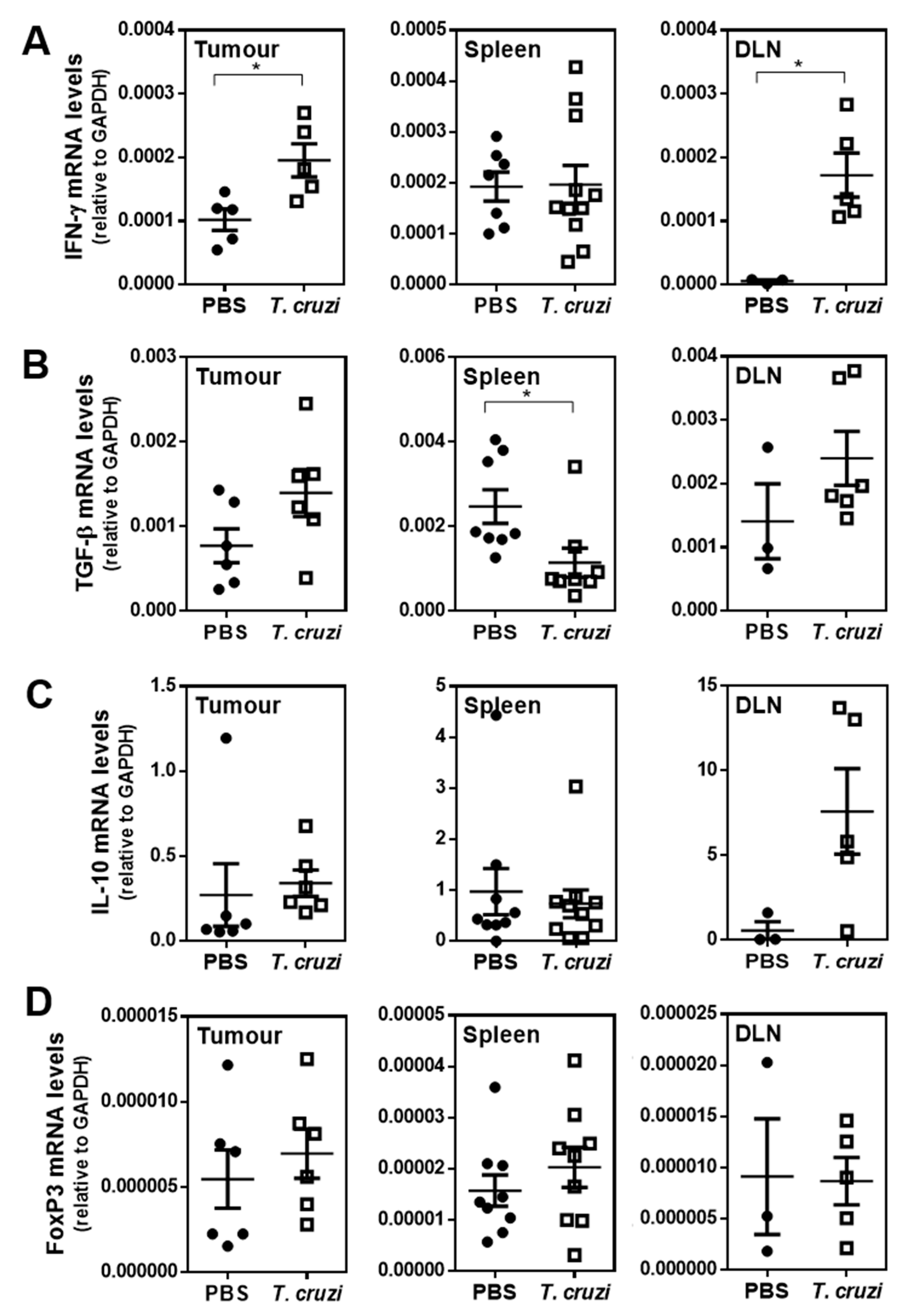
2.3. T. cruzi-Treated Mice Present a Th1 Immune Response Characterised by Increased Cytotoxicity
2.4. T. cruzi-Treatment Induces IgG Antibodies That Recognise LL/2 Tumour Cells
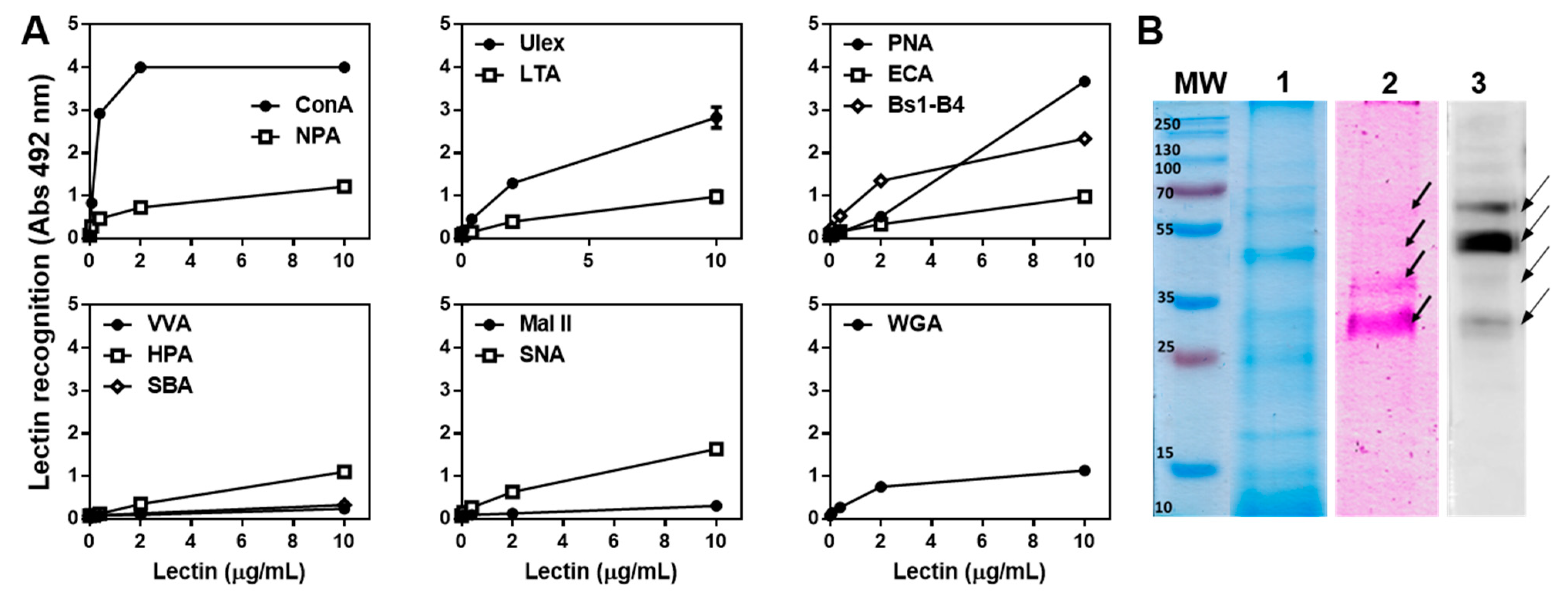
2.5. Glycans in T. cruzi Epimastigote Protein Lysate Mediate Tumour Protection
2.6. The T. cruzi Epimastigote Lysate Contains Mannose-Rich Glycan Structures
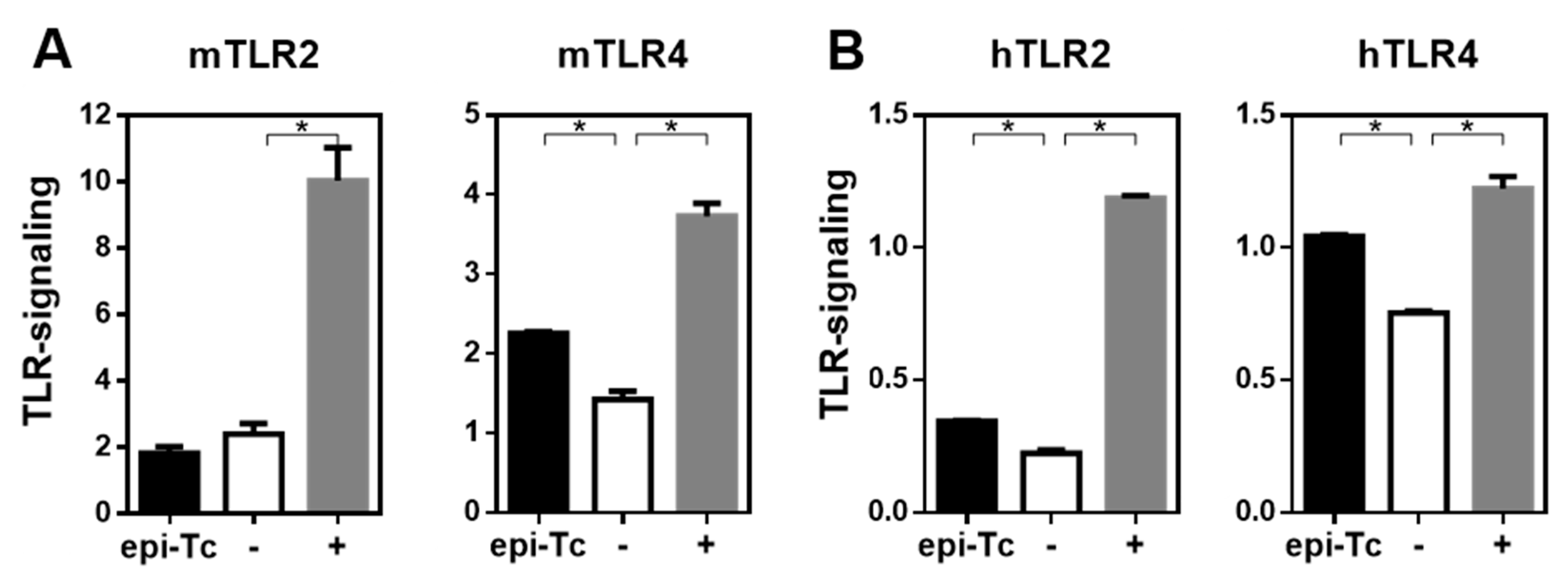
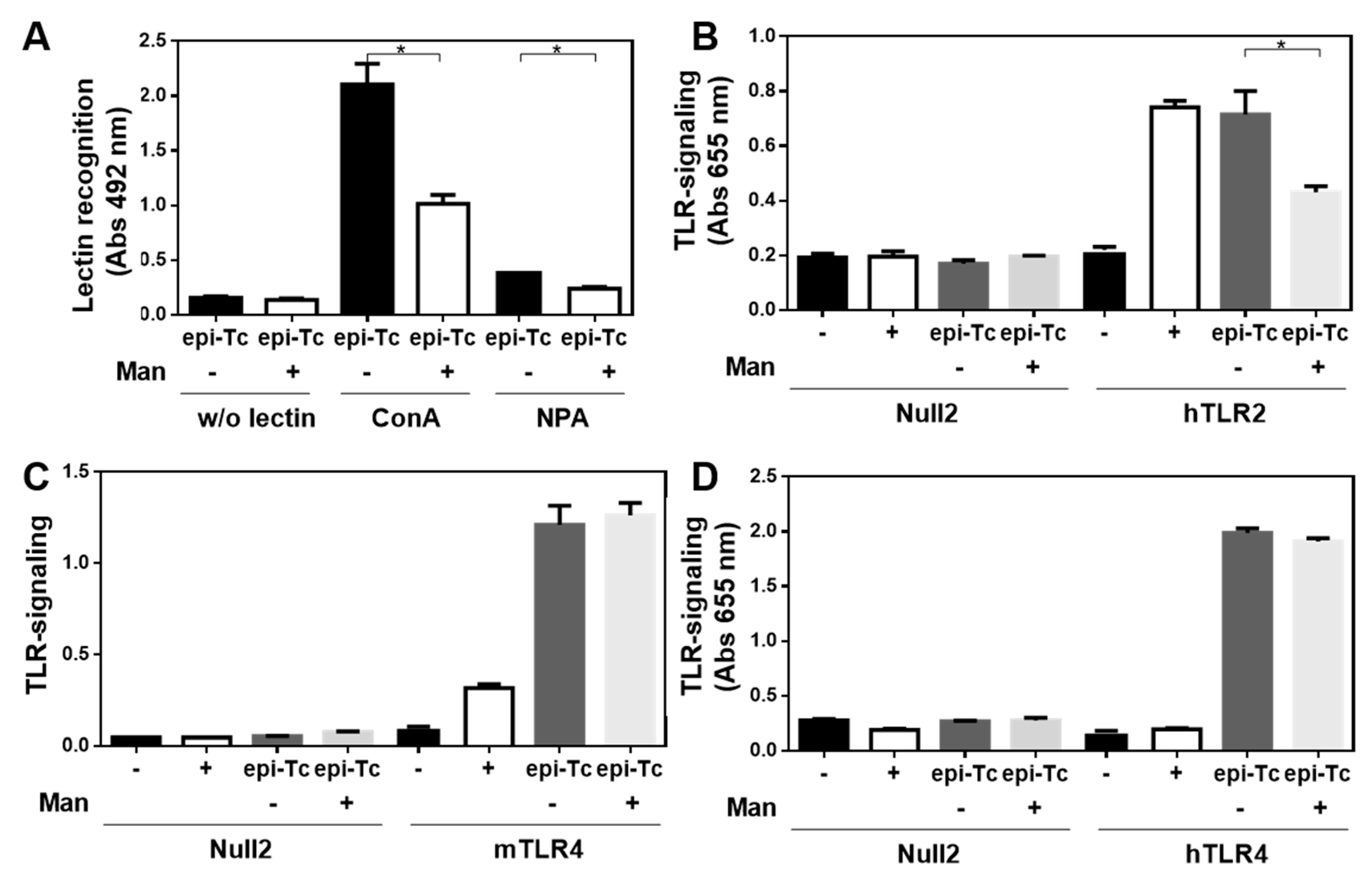
2.7. Mannose-Rich Parasite Glycans Induce TLR-Signalling
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Mice
4.3. Preparation of T. cruzi Lysate
4.4. Oxidation of T. cruzi Lysate
4.5. Evaluation of the Anti-Tumour Potential of the T. cruzi Lysate
4.6. Evaluation of Cellular Immune Response
4.7. Evaluation of Humoral Immune Response
4.8. Analyses of Immune Cells in Tumours by Flow Cytometry
4.9. Gene Expression by Quantitative RT-PCR
4.10. Lectin Recognition by Enzyme-Linked Lectin Assay (ELLA)
4.11. SDS-PAGE and Lectin Blot
4.12. TLR-Signalling Assays
4.13. Deglycosylation of T. cruzi Lysate with Immobilised α-Mannosidase
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flieswasser, T.; Van Loenhout, J.; Freire Boullosa, L.; Van den Eynde, A.; De Waele, J.; Van Audenaerde, J.; Lardon, F.; Smits, E.; Pauwels, P.; Jacobs, J. Clinically Relevant Chemotherapeutics Have the Ability to Induce Immunogenic Cell Death in Non-Small Cell Lung Cancer. Cells 2020, 9, 1474. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, Z.; Zhang, T.; Yang, L.; Xian, J.; Li, Y.; Li, W. Immunotherapy in non-small cell lung cancer: Rationale, recent advances and future perspectives. Precis. Clin. Med. 2021, 4, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; Haderi, A.; Roediger, A.; Yuan, M. Should we screen for lung cancer? A 10-country analysis identifying key decision-making factors. Health Policy 2022, 126, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Punekar, S.R.; Shum, E.; Grello, C.M.; Lau, S.C.; Velcheti, V. Immunotherapy in non-small cell lung cancer: Past, present, and future directions. Front. Oncol. 2022, 12, 877594. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Desrichard, A.; Snyder, A.; Chan, T.A. Cancer Neoantigens and Applications for Immunotherapy. Clin. Cancer Res. 2016, 22, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Raez, L.E.; Cassileth, P.A.; Schlesselman, J.J.; Sridhar, K.; Padmanabhan, S.; Fisher, E.Z.; Baldie, P.A.; Podack, E.R. Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 2800–2807. [Google Scholar] [CrossRef]
- Cho, J.H. Immunotherapy for Non-small-cell Lung Cancer: Current Status and Future Obstacles. Immune Netw. 2017, 17, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current state of immunotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 196–211. [Google Scholar] [CrossRef] [Green Version]
- Akgul, H.; Tez, M.; Unal, A.E.; Keskek, M.; Sayek, I.; Ozcelik, T. Echinococcus against cancer: Why not? Cancer 2003, 98, 1999–2000. [Google Scholar] [CrossRef]
- Garcia, S.B.; Aranha, A.L.; Garcia, F.R.; Basile, F.V.; Pinto, A.P.; de Oliveira, E.C.; Zucoloto, S. A retrospective study of histopathological findings in 894 cases of megacolon: What is the relationship between megacolon and colonic cancer? Rev. Inst. Med. Trop. Sao Paulo 2003, 45, 91–93. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, A.B.; Lemos, E.M.; Adad, S.J.; Correa-Oliveira, R.; Furness, J.B.; D’Avila Reis, D. Megacolon in Chagas disease: A study of inflammatory cells, enteric nerves, and glial cells. Hum. Pathol. 2007, 38, 1256–1264. [Google Scholar] [CrossRef]
- Berriel, E.; Freire, T.; Chiale, C.; Rodriguez, E.; Moron, G.; Fernandez-Grana, G.; Crispo, M.; Berois, N.; Osinaga, E. Human hydatid cyst fluid-induced therapeutic anti-cancer immune responses via NK1.1+ cell activation in mice. Cancer Immunol. Immunother. 2021, 70, 3617–3627. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Leite, M.S.; Miranda, J.A.; Andrade, A.L.; Garcia, S.B.; Luquetti, A.O.; Moreira, H. Chronic Trypanosoma cruzi infection associated with low incidence of 1,2-dimethylhydrazine-induced colon cancer in rats. Carcinogenesis 2001, 22, 737–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, C.A.; Yu, D.; Gee, M.; Ngo, C.V.; Sevignani, C.; Goldschmidt, M.; Golovkina, T.V.; Evans, S.; Lee, W.F.; Thomas-Tikhonenko, A. Cutting edge: Systemic inhibition of angiogenesis underlies resistance to tumors during acute toxoplasmosis. J. Immunol. 2001, 166, 5878–5881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, J.R.; Byrne, K.T.; Lizotte, P.H.; Toraya-Brown, S.; Scarlett, U.K.; Alexander, M.P.; Sheen, M.R.; Fox, B.A.; Bzik, D.J.; Bosenberg, M.; et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J. Immunol. 2013, 190, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, J.R.; Fox, B.A.; Sanders, K.L.; Lizotte, P.H.; Cubillos-Ruiz, J.R.; Scarlett, U.K.; Rutkowski, M.R.; Conejo-Garcia, J.R.; Fiering, S.; Bzik, D.J. Avirulent Toxoplasma gondii generates therapeutic antitumor immunity by reversing immunosuppression in the ovarian cancer microenvironment. Cancer Res. 2013, 73, 3842–3851. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; He, Z.; Qin, L.; Li, Q.; Shi, X.; Zhao, S.; Chen, L.; Zhong, N.; Chen, X. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS ONE 2011, 6, e24407. [Google Scholar] [CrossRef]
- Ubillos, L.; Freire, T.; Berriel, E.; Chiribao, M.L.; Chiale, C.; Festari, M.F.; Medeiros, A.; Mazal, D.; Rondan, M.; Bollati-Fogolin, M.; et al. Trypanosoma cruzi extracts elicit protective immune response against chemically induced colon and mammary cancers. Int. J. Cancer 2016, 138, 1719–1731. [Google Scholar] [CrossRef] [Green Version]
- Zenina, A.V.; Kravtsov, E.G.; Tsetsegsaikhan, B.; Yashina, N.V.; Dalin, M.V.; Karpenko, L.P.; Sheklakova, L.A.; Kallinikova, V. The study of immunological component in antitumor effect of Trypanosoma cruzi. Bull. Exp. Biol. Med. 2008, 145, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Klaver, E.J.; Kuijk, L.M.; Laan, L.C.; Kringel, H.; van Vliet, S.J.; Bouma, G.; Cummings, R.D.; Kraal, G.; van Die, I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol. 2013, 43, 191–200. [Google Scholar] [CrossRef]
- Rodriguez, E.; Noya, V.; Cervi, L.; Chiribao, M.L.; Brossard, N.; Chiale, C.; Carmona, C.; Giacomini, C.; Freire, T. Glycans from Fasciola hepatica Modulate the Host Immune Response and TLR-Induced Maturation of Dendritic Cells. PLoS Negl. Trop. Dis. 2015, 9, e0004234. [Google Scholar] [CrossRef]
- Buonocore, S.; Haddou, N.O.; Moore, F.; Florquin, S.; Paulart, F.; Heirman, C.; Thielemans, K.; Goldman, M.; Flamand, V. Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. J. Leukoc. Biol. 2008, 84, 713–720. [Google Scholar] [CrossRef]
- Garley, M.; Jablonska, E.; Dabrowska, D. NETs in cancer. Tumour Biol. 2016, 37, 14355–14361. [Google Scholar] [CrossRef]
- Jamieson, T.; Clarke, M.; Steele, C.W.; Samuel, M.S.; Neumann, J.; Jung, A.; Huels, D.; Olson, M.F.; Das, S.; Nibbs, R.J.; et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Investig. 2012, 122, 3127–3144. [Google Scholar] [CrossRef] [PubMed]
- Soo, A.; Maher, B.; McCarthy, J.; Nolke, L.; Wood, A.; Watson, R.W. Pre-operative determination of an individual’s neutrophil response: A potential predictor of early cardiac transplant cellular rejection. J. Heart Lung Transplant. 2009, 28, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Korbel, D.S.; Newman, K.C.; Almeida, C.R.; Davis, D.M.; Riley, E.M. Heterogeneous human NK cell responses to Plasmodium falciparum-infected erythrocytes. J. Immunol. 2005, 175, 7466–7473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lantier, L.; Poupee-Beauge, A.; di Tommaso, A.; Ducournau, C.; Epardaud, M.; Lakhrif, Z.; Germon, S.; Debierre-Grockiego, F.; Mevelec, M.N.; Battistoni, A.; et al. Neospora caninum: A new class of biopharmaceuticals in the therapeutic arsenal against cancer. J. Immunother. Cancer 2020, 8, e001242. [Google Scholar] [CrossRef]
- Sanabria, M.X.; Vargas-Inchaustegui, D.A.; Xin, L.; Soong, L. Role of natural killer cells in modulating dendritic cell responses to Leishmania amazonensis infection. Infect. Immun. 2008, 76, 5100–5109. [Google Scholar] [CrossRef] [Green Version]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef] [PubMed]
- Trelford, C.B.; Dagnino, L.; Di Guglielmo, G.M. Transforming growth factor-beta in tumour development. Front. Mol. Biosci. 2022, 9, 991612. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; McKenzie, I.F. Cellular mucins: Targets for immunotherapy. Crit. Rev. Immunol. 1994, 14, 293–309. [Google Scholar] [CrossRef] [PubMed]
- de Veer, M.J.; Curtis, J.M.; Baldwin, T.M.; DiDonato, J.A.; Sexton, A.; McConville, M.J.; Handman, E.; Schofield, L. MyD88 is essential for clearance of Leishmania major: Possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 2003, 33, 2822–2831. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Almeida, I.C.; Takeuchi, O.; Akira, S.; Valente, E.P.; Procopio, D.O.; Travassos, L.R.; Smith, J.A.; Golenbock, D.T.; Gazzinelli, R.T. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 2001, 167, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Debierre-Grockiego, F.; Azzouz, N.; Schmidt, J.; Dubremetz, J.F.; Geyer, H.; Geyer, R.; Weingart, R.; Schmidt, R.R.; Schwarz, R.T. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J. Biol. Chem. 2003, 278, 32987–32993. [Google Scholar] [CrossRef] [Green Version]
- Yarovinsky, F.; Zhang, D.; Andersen, J.F.; Bannenberg, G.L.; Serhan, C.N.; Hayden, M.S.; Hieny, S.; Sutterwala, F.S.; Flavell, R.A.; Ghosh, S.; et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 2005, 308, 1626–1629. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef]
- Hu, W.; Spaink, H.P. The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target. Biology 2022, 11, 246. [Google Scholar] [CrossRef]
- Gravina, H.D.; Antonelli, L.; Gazzinelli, R.T.; Ropert, C. Differential use of TLR2 and TLR9 in the regulation of immune responses during the infection with Trypanosoma cruzi. PLoS ONE 2013, 8, e63100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bott, E.; Carneiro, A.B.; Gimenez, G.; Lopez, M.G.; Lammel, E.M.; Atella, G.C.; Bozza, P.T.; Belaunzaran, M.L. Lipids from Trypanosoma cruzi Amastigotes of RA and K98 Strains Generate a Pro-inflammatory Response via TLR2/6. Front. Cell. Infect. Microbiol. 2018, 8, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, D.L.; Blumenstein, B.A.; Crawford, E.D.; Montie, J.E.; Scardino, P.; Grossman, H.B.; Stanisic, T.H.; Smith, J.A., Jr.; Sullivan, J.; Sarosdy, M.F.; et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N. Engl. J. Med. 1991, 325, 1205–1209. [Google Scholar] [CrossRef]
- Kidner, T.B.; Morton, D.L.; Lee, D.J.; Hoban, M.; Foshag, L.J.; Turner, R.R.; Faries, M.B. Combined intralesional Bacille Calmette-Guerin (BCG) and topical imiquimod for in-transit melanoma. J. Immunother. 2012, 35, 716–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, M.; Wang, H.; Zhang, M.; Tian, Y.; Wang, Y.; Li, B.; Yu, J.; Dou, J.; Xi, T.; Zhou, C. The anti-lung cancer activity of SEP is mediated by the activation and cytotoxicity of NK cells via TLR2/4 In Vivo. Biochem. Pharmacol. 2014, 89, 119–130. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin. Cancer Res. 2011, 17, 6742–6753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malyala, P.; Singh, M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 2008, 97, 2041–2044. [Google Scholar] [CrossRef]
- Rodriguez, E.; Francia, K.; Brossard, N.; Garcia Vallejo, J.J.; Kalay, H.; van Kooyk, Y.; Freire, T.; Giacomini, C. Immobilization of beta-galactosidase and alpha-mannosidase onto magnetic nanoparticles: A strategy for increasing the potentiality of valuable glycomic tools for glycosylation analysis and biological role determination of glycoconjugates. Enzyme Microb. Technol. 2018, 117, 45–55. [Google Scholar] [CrossRef]
- Puri, K.D.; Gopalakrishnan, B.; Surolia, A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4). FEBS Lett. 1992, 312, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, K.; Collet-Cassart, D.; Kobayashi, K.; Vaerman, J.P. Jacalin: Isolation, characterization, and influence of various factors on the Jacalin-IgA1 interaction studied by precipitin reaction and latex agglutination. Mol. Immunol. 1988, 25, 69–83. [Google Scholar] [CrossRef]
- Sanchez, J.F.; Lescar, J.; Chazalet, V.; Audfray, A.; Gagnon, J.; Alvarez, R.; Breton, C.; Imberty, A.; Mitchell, E.P. Biochemical and structural analysis of Helix pomatia agglutinin: A hexameric lectin with a novel fold. J. Biol. Chem. 2006, 281, 20171–20180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dam, T.K.; Gerken, T.A.; Cavada, B.S.; Nascimento, K.S.; Moura, T.R.; Brewer, C.F. Binding studies of α-GalNAc-specific lectins to the α-GalNAc (Tn-antigen) form of porcine submaxillary mucin and its smaller fragments. J. Biol. Chem. 2007, 282, 28256–28263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piller, V.; Piller, F.; Cartron, J.P. Comparison of the carbohydrate-binding specificities of seven N-acetyl-D-galactosamine-recognizing lectins. Eur. J. Biochem. 1990, 191, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.; Jarvis, D.L. Letter to the Glyco-Forum: Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 2011, 21, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, N.; Goldstein, I.J.; Broekaert, W.F.; Nsimba-Lubaki, M.; Peeters, B.; Peumans, W.J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac (alpha 2-6) Gal/GalNAc sequence. J. Biol. Chem. 1987, 262, 1596–1601. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, T.; Landeira, M.; Giacomini, C.; Festari, M.F.; Pittini, Á.; Cardozo, V.; Brosque, A.; Monin, L.; da Costa, V.; Faral-Tello, P.; et al. Trypanosoma cruzi-Derived Molecules Induce Anti-Tumour Protection by Favouring Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2022, 23, 15032. https://doi.org/10.3390/ijms232315032
Freire T, Landeira M, Giacomini C, Festari MF, Pittini Á, Cardozo V, Brosque A, Monin L, da Costa V, Faral-Tello P, et al. Trypanosoma cruzi-Derived Molecules Induce Anti-Tumour Protection by Favouring Both Innate and Adaptive Immune Responses. International Journal of Molecular Sciences. 2022; 23(23):15032. https://doi.org/10.3390/ijms232315032
Chicago/Turabian StyleFreire, Teresa, Mercedes Landeira, Cecilia Giacomini, María Florencia Festari, Álvaro Pittini, Viviana Cardozo, Alina Brosque, Leticia Monin, Valeria da Costa, Paula Faral-Tello, and et al. 2022. "Trypanosoma cruzi-Derived Molecules Induce Anti-Tumour Protection by Favouring Both Innate and Adaptive Immune Responses" International Journal of Molecular Sciences 23, no. 23: 15032. https://doi.org/10.3390/ijms232315032





