Abstract
Flavonoids are a category of plant-derived compounds which exhibit a large number of health-related effects. One of the most well-known and studied flavonoids is kaempferol, which can be found in a wide variety of herbs and plant families. Apart from their anticarcinogenic and anti-inflammatory effects, kaempferol and its associated compounds also exhibit antibacterial, antifungal, and antiprotozoal activities. The development of drugs and treatment schemes based on these compounds is becoming increasingly important in the face of emerging resistance of numerous pathogens as well as complex molecular interactions between various drug therapies. In addition, many of the kaempferol-containing plants are used in traditional systems all over the world for centuries to treat numerous conditions. Due to its variety of sources and associated compounds, some molecular mechanisms of kaempferol antimicrobial activity are well known while others are still under analysis. This paper thoroughly documents the vegetal and food sources of kaempferol as well as the most recent and significant studies regarding its antimicrobial applications.
1. Introduction
In general, natural substances have been a recent target of research for their numerous health benefits and also for their potential as the basis for new drugs [1,2,3]. The use of plants and herbs is documented by numerous authors both in Europe [4] and elsewhere [5,6,7,8]. The aim of such research is two-pronged, both to explore new opportunities for effective therapeutical agents, and also to elucidate the correlation between a decreased incidence of health problems and the consumption of certain food types. Regarding this last aim, it is the logical course of action, since certain diets are correlated with negative mortality and morbidity incidence rates [9,10,11,12,13]—in addition, based on the research of [14] specific diet choices after the diagnosis of cancer may improve survival rates.
The focus of this paper is kaempferol, (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one), a flavonoid with many promising health benefits found in a variety of plants. Kaempferol is named in honor of Engelbert Kaempfer, a German doctor, naturalist, and historian who lived during the 17th century and made a significant contribution to transporting medical knowledge from Japan to the West [15]. Kaempferol, as a chemical compound, was discovered in Camelia sinensis (tea tree) [16] and exhibits a host of different positive health-related effects.
In this review, we will present a thorough view of the studies which have aimed to ascertain the use of kaempferol against pathogens, namely protozoa, fungi and bacteria, describing the molecular mechanisms of action, where literature data is available. We will also explain the relative importance of the pathogens described to justify the importance of the studies on kaempferol as a novel basis for therapies and drug design. A number of these researches have focused on the extracts of plants that are included in traditional medical systems in different countries and regions. Accordingly, we will also describe the traditional use of kaempferol-containing plants and we will also present the most prominent plant species which contain kaempferol in regard to biosynthesis and availability of the substance.
2. Biosynthesis and Availability of Kaempferol
Kaempferol is a flavonoid; flavonoids are regarded as the largest group of secondary plant metabolites. They are polyphenolic compounds of low molecular weight and are used by plants to stimulate and regulate their growth and for defense purposes [17]. Flavonoids are divided into a number of groups based on their chemical composition, namely flavones, flavonols, flavanones, isoflavonoids, neoflavonoids, catechins (flavanols), anthocyanins and chalcones [18]. The antioxidant properties of polyphenols—flavonoids are such compounds—are already well known [19]; more than 104 types of flavonoids are estimated to exist [20,21]. Other proven effects of flavonoids include hepatoprotective [22,23,24], antimicrobial [25,26], renoprotective [27,28], antidiabetic [29,30], cardioprotective [31,32], anti-arthritic [33], neuroprotective [34,35,36,37], gastroprotective [38,39] and anti-mutagenic [40,41,42,43,44], among others [16].
Recently, there has been an increasing amount of research interest in the anti-carcinogenic potential of kaempferol [45,46], as a positive correlation between its consumption and reduced cancer incidence has been documented [47]; this is in addition to existing epidemiological studies linking increased flavonoid consumption with reduced cancer incidence [48,49]. The anti-inflammatory role of kaempferol has also been concisely presented by [50], while even its anti-adipogenic potential has come under investigation [51].
The basic structure of all flavonoids, regardless of their subclass, is a 15-carbon benopyranone or benzopyran in which the three-carbon bridge between the phenyl groups is commonly cyclized with oxygen forming a C6-C3-C6 flavan nucleus [19,52,53].
Kaempferol is specifically classified as a flavonol [54] and has the molecular formula C15H10O6 (Figure 1).
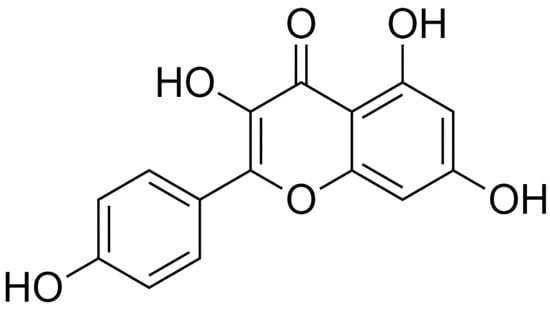
Figure 1.
The two-dimensional structural formula of kaempferol.
2.1. Biosynthetic Pathways of Kaempferol
Flavonoids are synthetized via the shikimic acid pathway [55], a process that occurs in the plants’ plastids [56,57,58]. More than 2000 compounds are known, with nearly 500 occurring in a free-aglycone state and the rest as O- or C-glycosides. Flavonols, in their free forms as aglycones, have lipophilic properties, yet most flavonols produced in plants are attached to a sugar moiety, the glycoside form, and are water-soluble [59]. The hydroxyl functional groups present in each flavonol are potential sites for linkage to saccharides as O-glycosides [60]. The saccharides most commonly attached to flavonols are monosaccharides such as glucose, rhamnose, galactose, arabinose, and xylose [32], and the disaccharide rutinose (glucose and rhamnose connected by a β-glycosidic bond) [61].
2.2. Bioavailability of Kaempferol
The pharmacokinetics of kaempferol has been studied in vitro and in vivo, both in rats and humans. Flavonols such as kaempferol are commonly ingested as glycosides. The types and attachments of saccharide impact bioavailability, and also bioactivity [62].
Glycosides are highly polar compounds, a property that greatly impacts their absorption, whereas the intermediate polarity of aglycones facilitates it. For some types of glycosides, previous hydrolysis to absorbable aglycones is needed, and others can be absorbed without hydrolysis [54].
Like other flavonoids, kaempferol is mainly absorbed in the small intestine. The lipophilicity of aglycone kaempferol facilitates its absorption by passive diffusion, but evidence suggests that it can also be absorbed by facilitated diffusion or active transport [63]. The nature of sugar linking will influence the compound uptake, as enterocytes have a preference for glucose, as membrane-bound beta-glucosidase breaks down the glucoside before absorption [64]. Kaempferol glucosides with many sugar units in their structure travel to the large intestine, where gut microbiota will remove terminal saccharides exposing the glucose, and then absorption by enterocytes occurs [65]. Studies have shown that kaempferol-O-glycosides decomposition can be extended to a breakdown process known as C-ring fission (C-ring is the central ring of the flavonoid structure) to form simple phenolic compounds such as 4-hydroxyphenylacetic acid, phloroglucinol and 4- methylphenol, which can either be absorbed or excreted in feces [66,67,68].
After absorption, conjugated forms of kaempferol, some phenolic compounds produced by the colon microflora, kaempferol, and some kaempferol glycosides can reach systemic circulation and tissues and are transported along with intestinal metabolites to the liver, where a part of them are metabolized (mainly compounds with poor hydro solubility). In the liver, and also in enterocytes, metabolism involves the phase I (oxidation and O-demethylation) and the phase II pathway (sulfation, glucuronidation, and methylation) followed by distribution to body tissues and urine excretion [69,70,71].
Pure kaempferol can be isolated from several plant species (Table 1), in the Divisions Pteridophyta, Coniferophyta, and Angiosperms of the Kingdom Plantae [72]. In addition, the glycosides of kaempferol can be identified in many plant families [73,74,75,76,77,78,79,80]—a detailed account of the various kaempferol chemical compounds and derivatives may be found in recent studies [54]. The kaempferol content of some common foodstuffs is provided in Table 2.

Table 1.
Plant species containing pure kaempferol [54,81,82,83].

Table 2.
Kaempferol content of some common foodstuffs (fresh unless otherwise specified).
3. Kaempferol as an Antibacterial Agent
The antibacterial properties of the secondary metabolites of plants have been in the foreground of research in the last two decades [189,190,191,192,193,194,195]. Such research is even more important considering the emergence of numerous resistant [196,197] and multi-drug resistant (MRD) bacteria [198]. Kaempferol-containing extracts and preparations, as well as pure kaempferol compounds, have been tested as possible antibacterials for quite some time [121,144,199].
The investigation into the action mechanisms behind the antibacterial activity of kaempferol has proven difficult due to the large variety within the family of kaempferol derivatives but also due to the diversity in morphology and functions between the numerous species of bacteria. However, some theories have been advanced and validated regarding the potential action mechanisms in specific bacteria. For instance, [200] have shown that a mixture of kaempferol 3-O-b-(200-acetyl) galactopyranoside and quercetin exerts antibacterial effects through cell membrane disruption, followed by activation of apoptosis and DNA fragmentation in M. luteus cells. Kaempferol was also the most effective tested flavonoid in damaging the cell membrane of Escherichia coli in a study by [201], where the findings were objectified by showing bacterial protein leakage into the extracellular environment. Moreover, kaempferol and quercetin interact with 3-oxyacyl-[acyl carrier protein] reductase (FabG) and enoyl-acyl carrier protein reductase therefore inhibiting the biosynthesis of fatty acids by Mycobacterium, Pseudomonas aeruginosa, and Vibrio cholerae thus hindering the function of the cell envelope as well as the impeding creation of bacterial biofilms [202,203,204]. Another important antibacterial mechanism was demonstrated for E. coli, where kaempferol was shown to be the most effective flavonoid in directly inhibiting the bacterial DNA gyrase [205]; similarly, kaempferol inhibited the DNA gyrase in methicillin-resistant Staphylococcus aureus [206]. Kaempferol was also able to inhibit DNA helicases, more specifically SAPriA in Staphylococcus aureus, as shown by [207].
Actions of kaempferol compounds against Porphyromonas gingivalis, Prevotella intermedia, and Cutibacterium acnes have been described by [144,208]. The research of [209] had already indicated the antibacterial effect of the extract of S. hymettia against Enterobacter cloacae, and also other bacteria, as will be presented below. The extract of Helichrysum compactum, which contained pure kaempferol and also kaempferol-3-O-glucoside, proved to have a degree of antibacterial activity [178]. It is also possible, that the extract from Nephelium lappaceum, which contains kaempferol compounds, has antimicrobial activity [210]. A local Malaysian herb, kacip Fatimah, i.e., the plant Labisa pumila Benth, which contains kaempferol, was found to have some antibacterial activity against Micrococcus luteus, Bacillus subtillis, Bacillus cereus, Staphylococcus aureus, Enterobacter aerogenes, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa, albeit at relatively low bacterial loads [211]. The extract of Uapaca heudelotti proved effective against S. pneumoniae [212], as well as against other pathogens. It is also important to note that while some kaempferol-containing extracts may not have significant antibacterial action on their own, they may potentiate the action of some antibiotics [213].
Subsequently, we will present the most important research on the antimicrobial activities of kaempferol against different bacterial genera (Table 3), which are human pathogens of particular interest.

Table 3.
Kaempferol compounds and their antibacterial activities based on current research.
3.1. Antibacterial Activity against Acinetobacter baumannii
This coccobacillus was once considered of low importance, from a medical standpoint, but has now emerged as a prominent healthcare unit-acquired and community-acquired infection. It frequently causes pulmonary infections and septicemia in immunocompromised patients [231]. Its antibiotic resistance and increased survival in harsh environments [232,233,234] further enhance its pathogenicity. At the moment, the results of the kaempferol-containing compounds against this pathogen are quite promising, and this can be important in the face of ever-increasing antibiotic resistance [96,215].
The compound kaempferol-3,7-O-α-l-dirhamnoside was found to be moderately inhibitory against A. baumannii [214]. A novel nanotechnology application involving a blend loaded with kaempferol nanocrystals showed very promising results against A. baumannii [215]; the research focused on treating infected wounds. Kaempferol-containing propolis extracts have also proved effective against A. baumannii in vitro [216]. The action of a further kaempferol-containing compound, the extract of Geranium ibericum subsp. jubatum, was also found to be almost as effective as some commercial antibiotics against this pathogen in vitro [132]. Earlier research [96] indicated that the kaempferol-containing extract of K. fedtschenkoi was effective against this pathogen.
3.2. Antibacterial Activity against Bacillus Spp.
In general, the bacteria of this genus are aerobic ([235]; and references therein), rod-shaped bacteria [236], which are spore-forming and resistant to disinfectants and harsh environmental conditions [236,237]. According to [238], only a handful of species from this genus are pathogenic; although current research has focused on the antibacterial actions of kaempferol against B. subtilis, which is non-pathogenic, the existing research experience can be potentially used in the future to find effective antimicrobial phytochemicals against the pathogenic bacillus species, namely B. anthracis and B. cereus [239,240].
An extract of taif’s rose (Rosa damascena Mill. var. trigintipetala) exhibited antimicrobial activity against B. subtilis as well as other microorganisms. The extract contained kaempferol amongst other compounds [218]. Earlier research by [214] indicated that the antibacterial action of kaempferol-3,7-O-α-l-dirhamnoside, on its own, was quite moderate. The fermented aerial part of Bupleurum chinense also contains kaempferol and exhibited promising antibacterial action against this bacterium [172]. The compound kaempferol-3-O-glucoside, which was isolated from the stem bark of Uapaca heudelotti, was also effective in that regard [212].
Based on the research of [167], the kaempferol compounds of the extract of Buddleja indica Lam. enable it to act as a local antiseptic, effective against B. subtilis. The novel research of [100] on the extract of Astragalus creticus, which, among other compounds, contains kaempferol and kaempferol-7-O-β-D-glucopyranose, proved their efficacy against this pathogen. Finally, the conjugation of kaempferol with silver nanoparticles proved effective against B. subtilis [217].
3.3. Antibacterial Activity against Escherichia coli
These bacteria are physiological colonizers of the gastrointestinal tract; the colonization begins typically shortly after birth. They typically do not cause disease in immunocompetent patients but will become pathogenetic, if they migrate to other locations or if their host becomes immunocompromised [241]. The most well-described E. coli pathogenic categories are the enteropathogenic E. coli (EPEC), the enterohaemorrhagic E. coli (EHEC), the enterotoxigenic E. coli (ETEC), the enteroaggregative E. coli (EAEC), the enteroinvasive E. coli (EIEC) and the diffusely adherent E. coli (DAEC) [242]. Commonly, E. coli infections are centered around the gastrointestinal and urogenital systems. Although most such infections can be easily treated, the emergence of multi-drug resistant (MDR) E. coli presents a novel therapeutical challenge [243].
The anti-microbial action of kaempferol-3,7-O-α-l-dirhamnoside was moderate against E. coli [214]. Success in that regard was also documented by [209] who used the extract of S. hymettia. The extract of B. chinense, which contained kaempferol-3-O-β-D-rutinoside and kaempferol proved effective against this bacterium [172]. The propolis extracts studied by [216] were also found to be effective against this pathogen. The combination of kaempferol with silver nanoparticles was also proven to be effective against E. coli [217]. The phytochemical extracts of [218,219] also proved effective against E. coli.
3.4. Antibacterial Activity against Klebsiella pneumoniae
Klebsiella pneumoniae represents an important human opportunistic pathogen and an emerging concern in clinical settings [244]. It accounts for virtually one-third of the total Gram-negative bacterial infections [245]. Klebsiella infections, especially in nosocomial settings, are rather severe [246]. The emergence of K. pneumoniae strains which are resistant to even last-line antibiotics [244,247] means that is not improbable, in the near future, that new compounds, whether natural or artificial, will be required to counter it. Interestingly, a strain of Klebsiella was found to even be resistant to chlorine treatment in water [248].
The anti-microbial action of kaempferol-3,7-O-α-l-dirhamnoside, was moderately effective against K. pneumoniae [214]. A similar anti-Klebsiella activity was also found by [172]. Earlier research by [220], on the extract of Argyreia speciosa, which was determined to contain kaempferol 7-O-methyl-3-sulphate, showed that it was inhibitory for K. pneumoniae growth. Similar successful antibacterial action was documented by [209], who used the extract of S. hymettia. The extract studied by [132] proved to also be effective against K. pneumoniae, as well as the extract studied by [219].
3.5. Antibacterial Activity against Mycobacterium Spp.
From the Mycobacterium genus, the most well-known and dangerous pathogen is Mycobacterium tuberculosis, which is the causative agent of tuberculosis, one of the oldest human diseases [249]. Although a vaccine against the disease exists, it is of varying efficiency [250] and has proven incapable of stopping the global epidemic [251]. While there exist antibiotics effective against tuberculosis during the last few years, the increase in antibiotic resistance of M. tuberculosis has led to the emergence of multi (MDR) [252], extensively (XDR), extremely (XXDR) and total (TDR) drug-resistant strains; these are estimated to kill about 75 · 106 people, in the next three decades [253]. Although resistance-conferring mutations may reduce the overall fitness of the bacteria, it has been suggested by [254,255,256] that the resistant bacteria may find ways to circumvent this limitation. Thus, it is evident that tuberculosis may again come to the foreground as a major disease, even in Western countries. M. bovis infects primarily cattle but can also spread to humans [257,258,259,260]; however, it is not of particular importance as a human pathogen [261]. Rather, its study is of interest in understanding the pathogenetic mechanism of M. tuberculosis [262].
Based on the research of [230], a leaf and hardwood extract from Vatairea macrocarpa, a plant used in Brazilian folk medicine, exhibited antibacterial action, in an in vivo model, in rat paws infected with M. bovis. The action of kaempferol-3-O-rhamnopyranoside was supplemented by that of other flavonoids in the extract. The extract was also found to have significant anti-inflammatory parameters.
The extract of Argyreia speciosa was found to have antibacterial properties against M. tuberculosis [220]. Another medicinal plant, Doliocarpus dentatus, proved to be effective in a rat model, as an antimycobacterial agent; the phenolic extract of its leaves contains kaempferol 3-O-α-L-rhamnopyranoside [229]. The extract of Pluchea indica, which contained kaempferol, was identified as a potent inhibitor of the M. tuberculosis CYP121 in a recent study by [180]. Finally, pure kaempferol from Bauhinia vahlii, was found, along with other flavonols, to be effective against M. tuberculosis [102].
3.6. Antibacterial Activity against Pseudomonas aeruginosa
This is a versatile opportunistic pathogen, from a metabolic point of view, which can cause both localized and systemic infections in humans, of varying degrees of severity [263]; recently, it has come to the foreground as a potent causative agent of nosocomial infections [264]. People already suffering from cystic fibrosis and COPD are at an increased risk of contracting P. aeruginosa, even outside of healthcare units [265,266,267]. It is of particular note that in cystic fibrosis patients, the bacterium may persist for decades [268]. Although some of the P. aeruginosa infections are relatively easily treated [269,270,271], other cases are still characterized by increased morbidity and mortality [267,272,273,274]. It has been observed that there is increasing resistance to antibiotics, of many P. aeruginosa strains, which is caused both by acquired and intrinsic mechanisms; this necessitates the development of new treatment avenues [275].
The research of [209], who tested the extract of S. hymettia, indicated that kaempferol-containing compounds were effective against P. aeruginosa. The extract of Bryophyllum pinnatum (Lank.) Oken also had some antibacterial activity against P. aeruginosa [221].
The extract prepared by [96] exhibited good antibacterial activity against P. aeruginosa. The extract of Bupleurum chinense, which has been already mentioned, proved effective against this pathogen [172]. The extract from Y. gigantea, which contains kaempferol-3-O-α-l-rhamnoside, was found to have an antimicrobial potential against this pathogen [219].
3.7. Antibacterial Activity against Salmonella Spp.
Salmonella is a common pathology in both developed and developing countries and represents a major public concern [276,277]; there are over 2600 recorded serotypes [278]. Salmonellae are foodborne pathogens, found mostly in poultry, eggs, and dairy products [279]. Recently, there has been an increase in the number of antibiotic-resistant strains; these are strains of increased virulence that are associated with increased mortality [280].
The extract of Uapaca heudelotti was effective, as an antimicrobial, against S. typhi [212]. Another effective antimicrobial against this pathogen is the extract of Bryophyllum pinnatum (Lank.) Oken [221]. The extract from Yucca gigantea also had an effect against S. typhimurium [219].
3.8. Antibacterial Activity against Staphylococcus Spp.
Staphylococcus aureus is a frequent human commensal and a common cause of various infections in humans. It can cause a wide variety of pathologies and associated symptoms, ranging from skin and soft tissue infections to infective endocarditis [281]; different staphylococcal strains are characterized by different aggressiveness properties [282]. The importance of S. aureus as a pathogen is further highlighted by the emergence of increasing antibiotic resistance [283,284]. The particular strain of Staphylococcus aureus which is resistant to methicillin is commonly referred to as MRSA (methicillin-resistant Staphylococcus aureus); it is a significant problem for health systems worldwide, both from a medical and a healthcare cost standpoint [285,286]. Its incidence rates present significant variations depending on the countries and healthcare unit location but are nevertheless quite significant [287,288]. The situation is aggravated even more since different types of MRSA have been identified, namely the healthcare-associated MRSA (HA-MRSA), the community-associated MRSA (CA-MRSA), and the livestock-acquired MRSA (LA-MRSA) [289]. A rather more benign species is S. epidermidis, a commensal which is not a frequent cause of disease, but it is of increasing importance in nosocomial settings; in healthcare unit settings, its infection rates are approximately commensurate with those of S. aureus [290].
The early research of [214] indicated that kaempferol-3,7-O-α-l-dirhamnoside was particularly effective against S. aureus. The research of [209], on the extract of Scabiosa hymettia, which contained two kaempferol-based flavonoids, corroborated the antibacterial action of kaempferol and its derivatives, against S. aureus; it was also active against S. epidermidis. The extract of M. scaber, a plant used in traditional West African medicine, also proved effective against S. aureus [223]. It must be noted that in this last case, when the compounds of the extract were tested separately, kaempferol-3-O-rutinoside exhibited a low antibacterial action suggesting that either the antibacterial effects were attributable to other compounds or that it has some sort of synergistic action with some of the other compounds found in the extract. A degree of antibacterial activity, against S. aureus, was exhibited by some of the extracts of Allium ursinum from Bulgaria [224]. The extract of Bryophyllum pinnatum (Lank.) Oken also exhibited interesting antibacterial properties against S. aureus [221].
The experiments of [222] determined that both kaempferol 3-O-α-L-(2″,4″-di-E-p-coumaroyl)-rhamnoside (C2) and kaempferol 3-O-α L-(2″-Z-p-coumaroyl-4″-E-p-coumaroyl)-rhamnoside (C3), exerted a strong antibacterial activity against different MRSA strains in vitro. These compounds were extracted from Laurus nobilis, and were virtually ineffective against Streptococcus pneumoniae, Pseudomonas aeruginosa, and Serratia marcescens. These same compounds were later found to have a synergistic effect with fluoroquinolones; namely, they increased the minimum inhibitory concentrations of these antibiotics. The same does not apply to hydrophobic quinolones [206].
Contemporary research [225], studying the anti-MRSA activities of the extract from Platanus occidentalis, determined that the numerous contained kaempferol compounds exhibited a satisfactory level of anti-MRSA activity. The research of [227], expounding upon the previous data, identified four isomers of kaempferol-3-O-α-L-(2”,3”-di-p-coumaroyl)-rhamnoside, from the same plant, which all exhibit anti-MRSA activity. It is possible that the main effect of such kaempferol-containing extracts is mostly attributable to the inhibition of the synthesis of the staphylococcal proteins, as determined by [228]. The earlier research of [226] also identified kaempferol-3-O-(2″,3″,4″-tri-O-galloyl)-α-l-rhamnopyranoside, along with other compounds, in the extract of Calliandra tergemina (L.) Benth. The anti-MRSA activity of the extract was verified experimentally.
The already mentioned research of [215] indicated that kaempferol, in the form of nanocrystals, was effective against multi-drug resistant (MDR) S. aureus. Two different propolis extracts, which contained kaempferol, also proved effective against S. aureus [216]. The earlier research of [96] also indicated the effectiveness of a kaempferol-containing extract, against S. aureus. Another extract, from the plant B. chinense also proved effective against S. aureus [172]; the results from the extract of Uapaca heudelotti against S. aureus were also positive [212]. The combination of kaempferol with Ag nanoparticles was also effective against this pathogen [217]. A successful result was also obtained by [219], who studied the effects of the extract of Y. gigantea; this extract was effective against S. epidermidis too.
3.9. Antibacterial Activity against Enterococci
In the last decades, enterococci have become a concern as nosocomial pathogens of note [291]; they have the potential to cause serious infections [292,293]. The most important pathogens of the genus are Enterococcus faecium and Enterococcus faecalis [294]. In particular, vancomycin-resistant enterococci (VRE) present a serious challenge because not only can they resist many antibiotics but they are quick to accrue further resistance [295].
Based on the research of [214], kaempferol-3,7-O-α-l-dirhamnoside was quite effective against Enterococcus faecalis. The extract of Laurus nobilis, of [222], was also effective against VRE. The team of [140] isolated kaempferol, amongst other compounds, from the plant Combretum erythrophyllum and found that it was effective, as an antibacterial, against E. faecalis.
3.10. Antibacterial Activity against Proteus Spp.
Perhaps the most important representative of the infectious species of the genus Proteus is P. mirabilis, which causes infections of the urinary tract, such as cystitis and pyelonephritis; many cases of asymptomatic bacteriuria have been also documented, predominantly in elderly patients and individuals having type 2 diabetes [296,297]. Such infections are also associated with urinary stone formation and even become life-threatening [298]. P. vulgaris has also been implicated in resistant healthcare unit-acquired infections [299]. Proteus infections can lead to catheter obstruction in catheterized patients [300] and the urinary stones created may act as a focal point for further bacterial infections [301]; indeed, catheterization is perhaps the dominant risk factor in Proteus infections [302,303]. The bacteria of this genus are associated with numerous determinants of antibiotic resistance [304,305] and there is even a number of MDR Proteus strains [306,307,308,309]; the prevalence of such strains was recently estimated to be quite high, at least in certain settings [310].
The already-mentioned extract of [218] was effective against P. vulgaris. Of all the microorganisms tested in this study, P. vulgaris proved to be the most susceptible. On the other hand, Proteus mirabilis proved quite resistant to kaempferol-3,7-O-α-l-dirhamnoside [214]. However, the extract studied by [132] was effective against this species, as well as the extract of Uapaca heudelotti [212]. This bacterial species proved also susceptible to the extract of Y. gigantea, which contains kaempferol-3-O-α-l-rhamnoside [219].
3.11. Antibacterial Activity against Vibrio cholerae
Cholera is most certainly an ancient disease of humans, although it has become a major health concern after the 19th century; it is a physiological inhabitant of aquatic ecosystems [311,312,313]. There is a number of pathogenic biotypes and there are several virulence factors [314]. The emergence of resistant strains of Vibrio cholerae has been documented in the past [315] and further resistance mechanisms continue to be observed [316].
Kaempferol and some of its derivatives were found to be effective against Vibrio cholerae, showing good antibacterial activity; in particular, kaempferol did not exhibit side effects such as toxicity to lymphocytes [140].
4. Antifungal Properties of Kaempferol
A very small number of fungi species are pathogenic to humans [317]; of these pathogenic fungi, some cause mild infections, while others, such as Candida spp. and Aspergillus spp., can even cause life-threatening, systemic infections [318]. Based on recent research, infections by Candida species, in hospital settings, represent an increasing health problem [319,320]. While fungi of this genus are generally benign, they can be the cause of oral candidiasis; in women, a significant percentage will suffer, sometime in their lives, from vaginal candidiasis [321,322]. Infections by Candida species are mostly determined by risk factors [323,324].
Likewise, Aspergillus fumigatus, while harmless to the immunocompetent host, will cause aspergillosis in immunocompromised patients; this represents an increasing concern with the number of such patients rising [325,326,327,328]. It is important to note that it is virtually impossible to evade exposure to this pathogen, as humans ingest hundreds of its conidia on a daily basis [329,330,331]. Another important aspect of A. fumigatus infections is that they may occur in the cavities left over in patients who have recovered from tuberculosis [332,333]; this is interesting, considering that kaempferol is known to be effective against M. tuberculosis, as already discussed. Therefore, for this specific case, a kaempferol-containing agent could both suppress the initial infection and act preventatively against possible aspergillosis.
Finally, Cryptococcus neoformans is one of the deadliest fungal pathogens [334] and, according to a recent survey kills thousands of infected patients each year [335]. Its importance as a disease of global interest was realized after the 1970s [336,337]. Several risk factors are associated with an increased risk of cryptococcosis infection [338]; for example, as happens with other pathogens, cryptococcosis is particularly dangerous for HIV/AIDS patients [339]. In general, despite the availability of antifungal drugs, there is an emerging resistance as the microorganisms adapt [340]; coupled with the known side effects of many antifungal drugs [341], the importance of the development of novel therapeutic strategies, based on natural compounds becomes all the more obvious.
Probably the first antifungal action of a kaempferol-containing compound was documented by [223], who tested the extract of Mitracarpus scaber, a plant used in traditional West African medicine. Both isolated kaempferol-3-O-[3-O-acetyl-6-O-(E)-p-coumaroyl]-b-d-glucopyranoside and kaempferol 3-O-b-D-kaempferol 3-O-b-D-glucopyranoside, from S. hymettia, were found to be active in vitro against C. albicans, C. glabrata and C. tropicalis [209]. As mentioned above, the kaempferol compound of the extract was not as effective when tested in isolated form. Moderate antifungal activity, against C. albicans was exhibited by some kaempferol-containing extracts from Allium ursinum [224]. The extract from Labisa pumila Benth, discussed before, also has a quite notable antifungal effect [211]. The extract from Bryophyllum pinnatum (Lank.) Oken exhibited interesting antifungal activity against C. albicans, C. parapsilosis and also Cryptococcus neoformans [221].
A comparatively weak inhibitory activity, at maximum concentration, was exhibited by kaempferol-3-O-(6”-galloyl)-β-D-glucopyranoside, isolated from Baseonema acuminatum [342]. Pure kaempferol also proved very effective against C. albicans both in vitro and in vivo in mice [343]. A possible effect against fungi of the Candida spp. was reported for the extract of Trachyspermum ammi, which contained kaempferol-(coumaroyl glucosyl)-rhamnoside [344]. The extract from Y. gigantea had a definite antifungal effect [219]. In the recent research of [345], it was determined, based on binding mechanisms, that kaempferol, at least when contained in an extract, may have a significant fungicidal effect in cases of vaginal candidiasis.
The antifungal activity of kaempferol was also proven by the research of [132]. The extract prepared by [218], which also contained many other phytochemicals, had antifungal activity against Candida albicans and Aspergillus fumigatus. Moreover, significant fungistatic activity was exhibited by the bark extract of Spondias mombin [213]. A summary of the research on the antifungal properties of kaempferol mentioned in the text is presented in Table 4.

Table 4.
Kaempferol-containing extracts and compounds with a verified antifungal potential.
5. Antiprotozoal Properties of Kaempferol
Plant extracts containing kaempferol have shown antiprotozoal activity based on a number of researches against some of the most common protozoal pathogens (Table 5). In this section, we will review the most prominent research and list the plants identified as possible sources of cure and prevention (Table 6).

Table 5.
Protozoal diseases discussed in the text and their causative agents.

Table 6.
Antiprotozoal activity of kaempferol compounds based on current research.
5.1. Antiprotozoal Action against Entamoeba histolytica and Giardia lamblia
Probably the first description of an antiprotozoal activity of a kaempferol-containing extract was made by [136] who studied the extract of Helianthemum glomeratum against Entamoeba histolytica in vitro, with successful results. Shortly after, kaempferol was also isolated from the roots of Cuphea pinetorum; it too was effective against E. histolytica and Giardia lamblia [356,357] in vitro. The importance of kaempferol in the antiprotozoal activity of these extracts was verified by [141]. The antiprotozoal activity of H. glomeratum, and the importance of kaempferol, were also examined by [358]. These plants are used in Mayan traditional medicine. The same promising results against E. histolytica were obtained when using the extract of Morinda morindoides [160]. The potency of kaempferol against E. histolytica was also demonstrated by [85]. Against both E. histolytica and G. lamblia, kaempferol-3,7-dimethylether was shown to have a degree of antiprotozoal activity in vitro [363]. This antiprotozoal activity of kaempferol against E. histolytica is important in the wider context of the activity of numerous flavonoids against this parasite [367]; such natural compounds may enable the development of new drugs against resistant parasites.
5.2. Antiprotozoal Action against Trypanosoma Spp.
This disease, also known as the sleeping sickness, is caused by Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense. It is a disease endemic to African countries [368]. It is mainly transmitted by flies of the genus Glossina, although transmission by other blood-sucking insects has also been documented [369,370]; it has even been proposed that due to the different possibilities of transmission, there may be outbreaks of this disease in non-endemic areas [371]. The treatment of this disease is based on a few drugs, which can be divided into two groups, the blood–brain barrier-crossing drugs (melarsoprol, eflornithine, nifurtimox) and the non-blood–brain barrier-crossing drugs (pentamidine, suramin) [372]. While currently there is a decrease in human African trypanosomiasis cases [373]. At the moment, resistance to treatment is not a massive issue for this particular disease, although resistant cases have been clinically reported [374]; furthermore, effective treatment options are required for the final stages of the disease [374].
Kaempferol-7-methylether was one of the compounds identified in the extract of Alomia myriadenia which was very effective against Trypanosoma cruzi in vitro [359]. On the other hand, the kaempferol-containing extract of Conyza filaginoides, was not found to be effective against Trypanosoma spp. and Giardia spp. [375]. Possibly, kaempferol is also important in the antiprotozoal activity exhibited by the bark extract of Cayratia trifolia Linn [98]. Contrary to that, the results of [376] were disappointing in that regard. Another research, focusing on kaempferol-3-O-methylether-5-O-β-D-glucoside and kaempferol-8-hydroxy-3,7-O-dimethylether-5-O-β-D-glucoside, from the extract of the plant Zanthoxylum pistaciifolium Griseb. found that they had no significant activity against either T. cruzi or T. brucei [377]. The compound 4′-methoxykaempferol, isolated from the extract of temperate propolis, proved to be quite effective against T. brucei [365]. The extract of Lotus corniculatus L. was found to be effective against Trypanosoma spp. [366].
5.3. Antiprotozoal Action against Plasmidium Spp.
Malaria is a well-known disease since ancient times and is caused by the amoeboid intracellular parasite Plasmodium; five of the 172 Plasmodium species are infectious to humans (P. malariae, P.falciparum, P.vivax, P.ovale, P.knowlesi); others are rarely infectious [378,379,380]. Regardless, their morphology and biology are quite similar [381]. The transmission of malaria is performed through its vectors, the female mosquitoes of the genus Anopheles [382]; subsequently, the parasite will infect first the hepatocytes and then the erythrocytes [383]. Currently, the most widespread therapy against malaria is the use of artemisinin and artemisinin-based combination therapy (ACT) [384]. In addition, there is an emerging resistance to antimalarial drugs, which threatens future efforts to eliminate the disease [385]. While endemic malaria is a major health concern, it may even be a health hazard in non-endemic countries [386].
Interestingly, 8-(1;1)-DMA-kaempferide, a flavonoid very similar to kaempferol [387], was found to have an antiprotozoal potential against Plasmidium falciparum [361]. Based on the research of [179], the extract of Eupatorium perfoliatum L. exhibited an in vitro antiprotozoal activity against P. falciparum; the extract contained kaempferol but the dimeric guaianolide was shown to be the most important part of the antiprotozoal activity. Some kaempferol metabolites proved to be effective against the malaria parasite when isolated in vitro [362]. The inability of kaempferol to influence negatively the formation of hemozoin, lead [388] to suggest that the in vitro antiplasmodial activity of kaempferol must not be related to any heme-binding activity pathway. The aforementioned study of [377], found two kaempferol glycosides to be ineffective against P. falciparum. Finally, the extract of Lotus corniculatus L., which contains pure kaempferol alongside some other kaempferol compounds has antiprotozoal activity against Plasmodium spp. [366].
5.4. Antiprotozoal Action against Leishmania Spp.
Leishmaniasis is a tropical and subtropical disease, mainly transmitted to humans through the sand flies of the genuses Phlebotomus and Lutzomyia [389]. The disease is extremely dangerous and presents a variety of symptoms; occasionally it can be fatal [390]. There exist over 20 species of the Leishmania parasite which can infect humans; leishmaniasis is a zoonosis and can be divided into visceral, cutaneous, and mucocutaneous [391]. The traditional treatment for leishmaniasis is based on antimonials, against which there is, however, increasing resistance [392]; antimonials also have frequent and rather severe side effects [393,394].
Compounds from the extract of K. pinatta were found to have antileishmanial activity [360]. In an in vitro assay, kaempferol-3,7-di-O-methylether was found to be able to induce cell death in Leishmania amazonensis [364]. When isolated from temperate propolis, 4′,7-dimethoxykaempferol was found to be quite effective against L. amazonensis in vitro [365].
6. Kaempferol-Containing Plants in Traditional Medical Systems
As mentioned elsewhere in this paper, a number of plants that contain kaempferol compounds are included in many traditional medical systems, all around the world. It is interesting to note that their traditional applications frequently correspond with their current effects under research. In this section, we will group the most important such plants, and their applications mentioned in this paper (Table 7), and then briefly examine the importance of some kaempferol-containing plants in the context of traditional Chinese medicine.

Table 7.
Correlation between ethnobotanical and described uses of certain kaempferol-containing plants.
Kaempferol-Containing Plants in the Context of Traditional Chinese Medicine
Herbal medicine is still regarded as an integral part of Traditional Chinese Medicine [437], and continues to be relevant in all parts of the world. As has been already proven, the study of these ancient practices can lead to novel therapies and drug discovery [438].
The use of the flower of the clove, known as dingxiang, is indicated to counteract the invasion of cold, and also to warm the kidneys; associated clinical signs include vomiting, hiccup, diarrhea, impotence, and leg weakness [439].
Bupleurum chinense, also known as radix bupleuri, or chaixu, is used in a variety of herbal formulas, which are associated with harmonizing lesser yang-stage disorders; some formulas, are also used against malaria. Other formulas, containing B. chinense are used to release exterior wind and heat [440]. To be more precise, radix bupleuri is derived from the roots of B. chinense [441]. Modern phytochemical research indicates that it has a wide range of pharmacological effects [442,443,444,445,446,447].
Geranium is also used in Traditional Chinese Medicine, both in anti-inflammatory and anti-microorganism applications [448]; current research has verified its anti-inflammatory potential [449].
Astragalus creticus is a plant that is endemic both to Greece and China [450]; it is used, either alone or in herbal formulas to warm the meridians and dispel cold [440]. In warming the meridians, it is ideal for rectifying the deficiency of the lung, spleen, and stomach meridians [439]. In Western Medicine terminology, it is used in cases of body weakness, as a diuretic, against digestive system disorders, or simply as a food supplement [451]. This is one of the most widespread plant genuses, and it has numerous ethnobotanical applications [452,453,454,455,456,457,458]. It would be interesting to compare the similarities between the applications of these plants in different medical systems.
Lastly, propolis, which is a bee product containing plant elements, not a plant per se, is an integral compound of many medicinal systems. It is used in traditional Chinese medicine, for its anti-inflammatory properties [459] and also has an anti-diabetic potential [460].
7. Discussion and Conclusions
In recent years, the field of phytochemistry has been rapidly developing with the aim of developing new drugs based on plant-derived compounds. At the same time, the field of ethnopharmacology studies the use of traditional medicinal plants of different regions and their possible applications in modern medical and pharmacological practice. Such approaches are integrated into the innovative practices which constitute the driving force behind the development of new therapeutical approaches [461].
As discussed in this paper, plants that contain kaempferol and its associated compounds have been tested for a number of effects, from anticarcinogenic to antibacterial, antifungal, and antiprotozoal. Indeed, the identification of natural compounds with anticarcinogenic potential has been a mainstay of medical research in the last decades [462]. Applications of such products have been proposed by [463] and the related new perspectives in drug discovery of many such natural agents have been summarized by [464].
Regarding the focus of this paper, in light of the promising effects of kaempferol compounds in the field of clinical microbiology, it can be said with a degree of certainty that it represents a novel potential for drug design. This is all the more important given the emerging resistance of many pathogens to traditional drugs. We may further postulate that given the wide range of kaempferol effects, drugs that may combat more than one condition may be developed; for example, using kaempferol as the basic agent, infections in cancer patients may be treated, combatting the pathogen and the cancer cells at the same time. This is a subject for future research.
Finally, as presented in the last part of the paper, kaempferol-containing plants are found in the traditional medicinal systems of almost every region; this attests to the efficacy of such treatments. In the particular case of traditional Chinese medicine, more often than not, such plants are used in conjunction with other plants and herbs, in herbal formulas. We propose, from a future research perspective, that these formulas should be tested, initially in vitro, to ascertain the relative efficacy of their components, and whether the kaempferol compounds of the ingredients can exert their actions on their own or in tandem with some of the other contained compounds.
Author Contributions
Conceptualization, A.P., K.P., C.S. and C.C.; resources, I.A.B., E.M.P., D.C.P. and A.C.; writing—original draft preparation, A.P., K.P., I.A.B., E.M.P., D.C.P., A.C., R.S.C., C.S., C.C. and D.O.C.; writing—review and editing, A.P., K.P., A.C., C.S., C.C. and D.O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Seidel, V. Plant-Derived Chemicals: A Source of Inspiration for New Drugs. Plants 2020, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Petran, M.; Dragos, D.; Gilca, M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s–1970s). J. Ethnobiol. Ethnomedicine 2020, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium majus–an integrative review: Traditional knowledge versus modern findings. Complement. Med. Res. 2010, 17, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, B.; Verma, P.; Bhalla, M.; Gilca, M. Phyto-pharmacotherapeutics of Cyperus rotundus Linn.(Motha): An overview. Indian J. Nat. Prod. Res. 2012, 3, 467–476. [Google Scholar]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384s–392s. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, P.; De Masi, L.; Carafa, V.; Rigano, D.; Scisciola, L.; Iside, C.; Grassi, R.; Molinari, A.M.; Aversano, R.; Nebbioso, A.; et al. Anticancer activities of anthocyanin extract from genotyped Solanum tuberosum L. “Vitelotte”. J. Funct. Foods 2015, 19, 584–593. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Krauss, R.M. Diet, lipids, and cardiovascular disease. Curr. Opin. Lipidol. 2016, 27, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Lange, K.M.; Makulska-Gertruda, E.; Nakamura, Y.; Reissmann, A.; Kanaya, S.; Hauser, J. Ketogenic diets and Alzheimer’s disease. Food Sci. Hum. Wellness 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Playdon, M.C.; Nagle, C.M.; Ibiebele, T.I.; Ferrucci, L.M.; Protani, M.M.; Carter, J.; Hyde, S.E.; Neesham, D.; Nicklin, J.L.; Mayne, S.T.; et al. Pre-diagnosis diet and survival after a diagnosis of ovarian cancer. Br. J. Cancer 2017, 116, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K. On the Dissemination of Acupuncture to Europe. JournalNX 2020, 6, 201–209. [Google Scholar]
- Farombi, E.O.; Akinmoladun, A.C.; Owumi, S.E. Anti-Cancer Foods: Flavonoids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 224–236. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Farombi, E.O.; Shrotriya, S.; Surh, Y.-J. Kolaviron inhibits dimethyl nitrosamine-induced liver injury by suppressing COX-2 and iNOS expression via NF-κB and AP-1. Life Sci. 2009, 84, 149–155. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Amobonye, A.E.; Komolafe, K.; Akinmoladun, A.C. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J. Biol. Sci. 2014, 21, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Flores, L.F.; Casas-Grajales, S.; Hernández-Aquino, E.; Vargas-Pozada, E.E.; Muriel, P. Chapter 47—Antioxidant, Antiinflammatory, and Antifibrotic Properties of Quercetin in the Liver. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 653–674. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorganic Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Athira, K.V.; Madhana, R.M.; Lahkar, M. Flavonoids, the emerging dietary supplement against cisplatin-induced nephrotoxicity. Chem. -Biol. Interact. 2016, 248, 18–20. [Google Scholar] [CrossRef]
- Cai, H.-D.; Su, S.-L.; Qian, D.-W.; Guo, S.; Tao, W.-W.; Cong, X.D.; Tang, R.; Duan, J.-A. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol. 2017, 206, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, M.K.; Veerapur, V.; Nayak, Y.; Mudgal, P.P.; Mathew, G. Chapter 13—Antidiabetic, Antihyperlipidemic and Antioxidant Effects of the Flavonoids. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 143–161. [Google Scholar] [CrossRef]
- Keshari, A.K.; Kumar, G.; Kushwaha, P.S.; Bhardwaj, M.; Kumar, P.; Rawat, A.; Kumar, D.; Prakash, A.; Ghosh, B.; Saha, S. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J. Ethnopharmacol. 2016, 181, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, M.T.; Crown, O.O.; Akinmoladun, A.C.; Akindahunsi, A.A. Rutin and quercetin show greater efficacy than nifedipin in ameliorating hemodynamic, redox, and metabolite imbalances in sodium chloride-induced hypertensive rats. Hum. Exp. Toxicol. 2014, 33, 602–608. [Google Scholar] [CrossRef]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.G.; Oommen, B. Chapter 1—Antioxidant Flavonoids for Arthritis Treatment: Human and Animal Models. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 1–16. [Google Scholar] [CrossRef]
- Choudhary, N.; Bijjem, K.R.V.; Kalia, A.N. Antiepileptic potential of flavonoids fraction from the leaves of Anisomeles malabarica. J. Ethnopharmacol. 2011, 135, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Falode, J.A.; Akinmoladun, A.C.; Olaleye, M.T.; Akindahunsi, A.A. Sausage tree (Kigelia africana) flavonoid extract is neuroprotective in AlCl(3)-induced experimental Alzheimer’s disease. Pathophysiology 2017, 24, 251–259. [Google Scholar] [CrossRef]
- Preethi Pallavi, M.C.; Sampath Kumar, H.M. Chapter 8—Nutraceuticals in Prophylaxis and Therapy of Neurodegenerative Diseases. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–376. [Google Scholar] [CrossRef]
- De Lira Mota, K.S.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, Â.; Monteiro Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with Gastroprotective Activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef]
- Antonisamy, P.; Subash-Babu, P.; Albert-Baskar, A.; Alshatwi, A.A.; Aravinthan, A.; Ignacimuthu, S.; Choi, K.C.; Lee, S.C.; Kim, J.-H. Experimental study on gastroprotective efficacy and mechanisms of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. in different experimental models. J. Funct. Foods 2016, 25, 302–313. [Google Scholar] [CrossRef]
- Nwankwo, J.O.; Tahnteng, J.G.; Emerole, G.O. Inhibition of aflatoxin B1 genotoxicity in human liver-derived HepG2 cells by kolaviron biflavonoids and molecular mechanisms of action. Eur. J. Cancer Prev. 2000, 9, 351–361. [Google Scholar] [CrossRef]
- Snijman, P.W.; Swanevelder, S.; Joubert, E.; Green, I.R.; Gelderblom, W.C.A. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): Some dose–response effects on mutagen activation–flavonoid interactions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 631, 111–123. [Google Scholar] [CrossRef]
- Carvalho-Silva, L.B.D.; Dionísio, A.P.; Pereira, A.C.D.S.; Wurlitzer, N.J.; Brito, E.S.D.; Bataglion, G.A.; Brasil, I.M.; Eberlin, M.N.; Liu, R.H. Antiproliferative, antimutagenic and antioxidant activities of a Brazilian tropical fruit juice. LWT—Food Sci. Technol. 2014, 59, 1319–1324. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Leal Specian, A.F.; Ribeiro, D.L.; Tuttis, K.; Vilegas, W.; Martínez-López, W.; Dokkedal, A.L.; Saldanha, L.L.; de Syllos Cólus, I.M.; Varanda, E.A. Antimutagenicity and induction of antioxidant defense by flavonoid rich extract of Myrcia bella Cambess. in normal and tumor gastric cells. J. Ethnopharmacol. 2015, 176, 345–355. [Google Scholar] [CrossRef]
- Zarev, Y.; Foubert, K.; Lucia de Almeida, V.; Anthonissen, R.; Elgorashi, E.; Apers, S.; Ionkova, I.; Verschaeve, L.; Pieters, L. Antigenotoxic prenylated flavonoids from stem bark of Erythrina latissima. Phytochemistry 2017, 141, 140–146. [Google Scholar] [CrossRef]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-Pot Synthesis of Hyperoside by a Three-Enzyme Cascade Using a UDP-Galactose Regeneration System. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Sacerdote, C.; Ricceri, F.; Weiderpass, E.; Roswall, N.; Buckland, G.; St-Jules, D.E.; Overvad, K.; Kyrø, C.; Fagherazzi, G.; et al. Flavonoid and lignan intake in relation to bladder cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Cancer 2014, 111, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: Incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Park, U.H.; Hwang, J.T.; Youn, H.; Kim, E.J.; Um, S.J. Kaempferol antagonizes adipogenesis by repressing histone H3K4 methylation at PPARγ target genes. Biochem. Biophys. Res. Commun. 2022, 617, 48–54. [Google Scholar] [CrossRef]
- Beltz, L.A.; Bayer, D.K.; Moss, A.L.; Simet, I.M. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med. Chem. 2006, 6, 389–406. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Feliciano, A.S. Flavonoids: From Structure to Health Issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef] [PubMed]
- Mousdale, D.M.; Coggins, J.R. Subcellular localization of the common shikimate-pathway enzymes in Pisum sativum L. Planta 1985, 163, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Zamir, L.O.; Jensen, R.A. Chloroplasts of higher plants synthesize L-phenylalanine via L-arogenate. Proc. Natl. Acad. Sci. USA 1986, 83, 7231–7235. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Bode, R. Chorismate mutase isoforms from seeds and seedlings of Papaver somniferum. Phytochemistry 1992, 31, 2983–2987. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Fortunato, R.H.; Spotorno, V.G. Analysis of flavonoid glycosides with potential medicinal properties on Bauhinia uruguayensis and Bauhinia forficata subspecies pruinosa. Nat. Prod. Res. 2019, 33, 2574–2578. [Google Scholar] [CrossRef]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef]
- Viskupicova, J.; Ondrejovič, M.; Sturdik, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Kall, M.; Justesen, U.; Schou, A.; Dragsted, L.O. Human absorption and excretion of flavonoids after broccoli consumption. Cancer Lett. 1997, 114, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Dietary flavonoids as antioxidants. Forum. Nutr. 2009, 61, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc. 1998, 73, 203–266. [Google Scholar] [CrossRef]
- Markham, K.R.; Geiger, H.; Jaggy, H. Kaempferol-3-O-glucosyl(1-2)rhamnoside from Ginkgo biloba and a reappraisal of other gluco(1-2, 1-3 and 1-4)rhamnoside structures. Phytochemistry 1992, 31, 1009–1011. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, F.; Wang, J.; Li, Y.; Zhuang, S. Coumaroyl flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry 2001, 58, 1251–1256. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.J. Selective responses of three Ginkgo biloba leaf-derived constituents on human intestinal bacteria. J. Agric. Food Chem. 2002, 50, 1840–1844. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M. Flavonoids from the genus Taxus. Z. Naturforsch C J. Biosci. 2004, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, L.L.; Weemhoff, J.L.; Bedir, E.; Khan, I.A.; Harmatz, J.S.; Goldman, P.; Greenblatt, D.J. Inhibition of human cytochromes P450 by components of Ginkgo biloba. J. Pharm. Pharmacol. 2004, 56, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.D.; Jeong, D.G.; Hwang, Y.H.; Ryu, J.M.; Kim, J. Inhibitors of Osteoclast Differentiation from Cephalotaxus koreana. J. Nat. Prod. 2007, 70, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Nam, J.I.; Kim, S.H.; Kim, J.H.; Yoon, J.H.; Kim, K.S. Kaempferol and quercetin, essential ingredients in Ginkgo biloba extract, inhibit interleukin-1beta-induced MUC5AC gene expression in human airway epithelial cells. Phytother. Res. 2009, 23, 1708–1712. [Google Scholar] [CrossRef]
- Group, T.A.P.; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- I, P. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Christenhusz, M.; Reveal, J.; Farjon, A.; Gardner, M.; Mill, R.; Chase, M. A new classification and linear sequence of extant gymnosperms. Nov. Magnolia Press Phytotaxa 2010, 19, 55–70. [Google Scholar] [CrossRef]
- Arot Manguro, L.O.; Ugi, I.; Hermann, R.; Lemmen, P. Flavonol and drimane-type sesquiterpene glycosides of Warburgia stuhlmannii leaves. Phytochemistry 2003, 63, 497–502. [Google Scholar] [CrossRef]
- Calzada, F.; Correa-Basurto, J.; Barbosa, E.; Mendez-Luna, D.; Yepez-Mulia, L. Antiprotozoal Constituents from Annona cherimola Miller, a Plant Used in Mexican Traditional Medicine for the Treatment of Diarrhea and Dysentery. Pharmacogn. Mag. 2017, 13, 148–152. [Google Scholar] [CrossRef]
- Rodríguez Galdón, B.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Flavonoids in onion cultivars (Allium cepa L.). J. Food Sci. 2008, 73, C599–C605. [Google Scholar] [CrossRef] [PubMed]
- Keyhanian, S.; Stahl-Biskup, E. Phenolic constituents in dried flowers of aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. 2007, 73, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Vachálková, A.; Eisenreichová, E.; Haladová, M.; Mucaji, P.; Józová, B.; Novotný, L. Potential carcinogenic and inhibitory activity of compounds isolated from Lilium candidum L. Neoplasma 2000, 47, 313–318. [Google Scholar] [PubMed]
- Francis, J.A.; Rumbeiha, W.; Nair, M.G. Constituents in Easter lily flowers with medicinal activity. Life Sci. 2004, 76, 671–683. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Zhang, P.; Li, Z.L.; Wang, Y. Antiinflammatory constituents from the roots of Smilax bockii warb. Arch. Pharmacal Res. 2005, 28, 395–399. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Zhou, J.-R.; Hon, P.-M.; Li, S.-L.; Zhou, Y.; Li, L.-L.; Ye, W.-C.; Xu, H.-X.; Shaw, P.-C.; But, P.P.-H. Lignans from Dysosma versipellis with Inhibitory Effects on Prostate Cancer Cell Lines. J. Nat. Prod. 2007, 70, 283–286. [Google Scholar] [CrossRef]
- Marín, C.; Boutaleb-Charki, S.; Díaz, J.G.; Huertas, O.; Rosales, M.J.; Pérez-Cordon, G.; Guitierrez-Sánchez, R.; Sánchez-Moreno, M. Antileishmaniasis activity of flavonoids from Consolida oliveriana. J. Nat. Prod. 2009, 72, 1069–1074. [Google Scholar] [CrossRef]
- Je Ma, C.; Jung, W.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Calpain inhibitory flavonoids isolated from Orostachys japonicus. J. Enzym. Inhib. Med. Chem. 2009, 24, 676–679. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorganic Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Song, E.K.; Kim, J.H.; Kim, J.S.; Cho, H.; Nan, J.X.; Sohn, D.H.; Ko, G.I.; Oh, H.; Kim, Y.C. Hepatoprotective phenolic constituents of Rhodiola sachalinensis on tacrine-induced cytotoxicity in Hep G2 cells. Phytother. Res. 2003, 17, 563–565. [Google Scholar] [CrossRef]
- Richwagen, N.; Lyles, J.T.; Dale, B.L.F.; Quave, C.L. Antibacterial Activity of Kalanchoe mortagei and K. fedtschenkoi Against ESKAPE Pathogens. Front. Pharmacol. 2019, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kim, H.J.; Jin, C.; Lee, Y.S. Antioxidant caffeic acid derivatives from leaves of Parthenocissus tricuspidata. Arch. Pharmacal Res. 2004, 27, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, S.; Gupta, J.; Arya, R.; Gupta, A. A review on chemical and biological properties of Cayratia trifolia Linn. (Vitaceae). Pharmacogn. Rev. 2011, 5, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhang, Y.; Yang, Z.; Cheng, Q.; Hu, L. Triterpene saponins from Gynostemma cardiospermum. J. Nat. Prod. 2006, 69, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.A.; Chaudhry, B.A.; Uzair, M.; Imran, M.; Haneef, M.; Ashfaq, K. Biological and phytochemical investigations of crude extracts of Astragalus creticus. Pak. J. Pharm. Sci. 2021, 34, 403–409. [Google Scholar] [PubMed]
- Mazimba, O.; Majinda, R.R.; Modibedi, C.; Masesane, I.B.; Cencič, A.; Chingwaru, W. Tylosema esculentum extractives and their bioactivity. Bioorganic Med. Chem. 2011, 19, 5225–5230. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ketha, A.; Hieu, H.V.; Tatipamula, V.B. In vitro antimycobacterial studies of flavonols from Bauhinia vahlii Wight and Arn. 3 Biotech 2021, 11, 128. [Google Scholar] [CrossRef]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. Vitr. 2008, 22, 1965–1970. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Jimenez, P.C.; Wilke, D.V.; Leal, L.K.; Cunha, G.M.; Silveira, E.R.; Canuto, K.M.; Viana, G.S.; Moraes, M.E.; de Moraes, M.O.; et al. Antiproliferative effects of several compounds isolated from Amburana cearensis A. C. Smith. Z. Naturforsch C J. Biosci. 2003, 58, 675–680. [Google Scholar] [CrossRef]
- Terreaux, C.; Wang, Q.; Ioset, J.R.; Ndjoko, K.; Grimminger, W.; Hostettmann, K. Complete LC/MS analysis of a Tinnevelli senna pod extract and subsequent isolation and identification of two new benzophenone glucosides. Planta Med. 2002, 68, 349–354. [Google Scholar] [CrossRef]
- Nsonde Ntandou, G.F.; Banzouzi, J.T.; Mbatchi, B.; Elion-Itou, R.D.; Etou-Ossibi, A.W.; Ramos, S.; Benoit-Vical, F.; Abena, A.A.; Ouamba, J.M. Analgesic and anti-inflammatory effects of Cassia siamea Lam. stem bark extracts. J. Ethnopharmacol. 2010, 127, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Calvo, T.R.; Cardoso, C.R.P.; da Silva Moura, A.C.; dos Santos, L.C.; Colus, I.M.S.; Vilegas, W.; Varanda, E.A. Mutagenic Activity of Indigofera truxillensis and I. suffruticosa Aerial Parts. Evid. -Based Complement. Altern. Med. 2011, 2011, 323276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhan, W.Q.; Liu, X.; Jiang, S.X. Antioxidant activities of extracts and flavonoid compounds from Oxytropis falcate Bunge. Nat. Prod. Res. 2008, 22, 1650–1656. [Google Scholar] [CrossRef]
- Ali, A.A.; Mohamed, M.H.; Kamel, M.S.; Fouad, M.A.; Spring, O. Studies on Securigera securidacea (L.) Deg. et Dörfl. (Fabaceae) seeds, an antidiabetic Egyptian folk medicine. Pharmazie 1998, 53, 710–715. [Google Scholar]
- Xiang, W.; Li, R.T.; Mao, Y.L.; Zhang, H.J.; Li, S.H.; Song, Q.S.; Sun, H.D. Four new prenylated isoflavonoids in Tadehagi triquetrum. J. Agric. Food Chem. 2005, 53, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M. Chemical constituents from the seeds of Trifolium alexandrinum. Nat. Prod. Res. 2008, 22, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.K.; Sharma, K.; Sharma, N.; Sharma, A.; Singh, H.P.; Sinha, A.K. Microwave-assisted efficient extraction of different parts of Hippophae rhamnoides for the comparative evaluation of antioxidant activity and quantification of its phenolic constituents by reverse-phase high-performance liquid chromatography (RP-HPLC). J. Agric. Food Chem. 2008, 56, 374–379. [Google Scholar] [CrossRef]
- Wei, B.L.; Lu, C.M.; Tsao, L.T.; Wang, J.P.; Lin, C.N. In vitro anti-inflammatory effects of quercetin 3-O-methyl ether and other constituents from Rhamnus species. Planta Med. 2001, 67, 745–747. [Google Scholar] [CrossRef]
- Goel, R.K.; Pandey, V.B.; Dwivedi, S.P.; Rao, Y.V. Antiinflammatory and antiulcer effects of kaempferol, a flavone, isolated from Rhamnus procumbens. Indian J. Exp. Biol. 1988, 26, 121–124. [Google Scholar]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory activity of flavonoids from Prunus davidiana and other flavonoids on total ROS and hydroxyl radical generation. Arch. Pharmacal Res. 2003, 26, 809–815. [Google Scholar] [CrossRef]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa L. leaves extracts and their radical scavenging activity. Z. Naturforsch C J. Biosci. 2007, 62, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Piacente, S.; Pizza, C.; Burke, A.; Khan, A.I.; Hay, A.J. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem. Biophys. Res. Commun. 1996, 229, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Suntornsuk, L.; Anurukvorakun, O. Precision improvement for the analysis of flavonoids in selected Thai plants by capillary zone electrophoresis. Electrophoresis 2005, 26, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Giannasi, D.E. Flavonoids and other chemical constituents of fossil miocene zelkova (ulmaceae). Science 1977, 196, 877–878. [Google Scholar] [CrossRef]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Rocha, L.; Marston, A.; Potterat, O.; Kaplan, M.A.C.; Stoeckli-Evans, H.; Hostettmann, K. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense. Phytochemistry 1995, 40, 1447–1452. [Google Scholar] [CrossRef]
- Odabas, M.S.; Camas, N.; Cirak, C.; Radusiene, J.; Janulis, V.; Ivanauskas, L. The quantitative effects of temperature and light intensity on phenolics accumulation in St. John’s wort (Hypericum perforatum). Nat. Prod. Commun. 2010, 5, 535–540. [Google Scholar] [CrossRef]
- Nguemeving, J.R.; Azebaze, A.G.B.; Kuete, V.; Eric Carly, N.N.; Beng, V.P.; Meyer, M.; Blond, A.; Bodo, B.; Nkengfack, A.E. Laurentixanthones A and B, antimicrobial xanthones from Vismia laurentii. Phytochemistry 2006, 67, 1341–1346. [Google Scholar] [CrossRef]
- Pattamadilok, D.; Suttisri, R. Seco-Terpenoids and Other Constituents from Elateriospermum tapos. J. Nat. Prod. 2008, 71, 292–294. [Google Scholar] [CrossRef]
- Oksüz, S.; Gürek, F.; Lin, L.Z.; Gil, R.R.; Pezzuto, J.M.; Cordell, G.A. Aleppicatines A and B from Euphorbia aleppica. Phytochemistry 1996, 42, 473–478. [Google Scholar] [CrossRef]
- Sousa, M.; Ousingsawat, J.; Seitz, R.; Puntheeranurak, S.; Regalado, A.; Schmidt, A.; Grego, T.; Jansakul, C.; Amaral, M.D.; Schreiber, R.; et al. An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: A potential treatment for cystic fibrosis. Mol. Pharmacol. 2007, 71, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 275–279. [Google Scholar] [PubMed]
- Penna, C.; Marino, S.; Vivot, E.; Cruañes, M.C.; Muñoz, J.D.D.; Cruañes, J.; Ferraro, G.; Gutkind, G.; Martino, V. Antimicrobial activity of Argentine plants used in the treatment of infectious diseases. Isolation of active compounds from Sebastiania brasiliensis. J. Ethnopharmacol. 2001, 77, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.S.; Song, K.S.; Seong, Y.H.; Bae, K. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharmacal Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Zhou, W.; Feng, M.; Zhou, P. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol. Pharm. Bull. 2008, 31, 743–747. [Google Scholar] [CrossRef]
- Gayosso-De-Lucio, J.A.; Torres-Valencia, J.M.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P. Ellagitannins from Geranium potentillaefolium and G. bellum. Nat. Prod. Commun. 2010, 5, 1934578X1000500407. [Google Scholar] [CrossRef]
- Şeker, M.E.; Ay, E.; Aktaş Karaçelik, A.; Hüseyinoǧlu, R.; Efe, D. First determination of some phenolic compounds and antimicrobial activities of Geranium ibericum subsp. jubatum: A plant endemic to Turkey. Turk. J. Chem. 2021, 45, 60–70. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Newman, M.; Greenham, J.; Eagles, J. Chrysin and other leaf exudate flavonoids in the genus Pelargonium. Phytochemistry 1997, 46, 1349–1353. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Imsic, M.; Jones, R.; Trenerry, V.C.; Tomkins, B. Characterization of flavonol conjugates in immature leaves of pak choi [Brassica rapa L. Ssp. chinensis L. (Hanelt.)] by HPLC-DAD and LC-MS/MS. J. Agric. Food Chem. 2006, 54, 4855–4860. [Google Scholar] [CrossRef]
- Bennett, R.N.; Rosa, E.A.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Calzada, F.; Lopéz, R.; Meckes, M.; Cedillo-Rivera, R. Flavonoids of the Aerial Parts of Helianthemum Glomeratum. Int. J. Pharmacogn. 1995, 33, 351–352. [Google Scholar] [CrossRef]
- Papiez, M.; Gancarczyk, M.; Bilińska, B. The compounds from the hollyhock extract (Althaea rosea Cav. var. nigra) affect the aromatization in rat testicular cells in vivo and in vitro. Folia Histochem. Et Cytobiol. 2002, 40, 353–359. [Google Scholar]
- Yang, H.; Protiva, P.; Cui, B.; Ma, C.; Baggett, S.; Hequet, V.; Mori, S.; Weinstein, I.B.; Kennelly, E.J. New Bioactive Polyphenols from Theobroma g randiflorum (“Cupuaçu”). J. Nat. Prod. 2003, 66, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.; Wolfman, C.; Levi de Stein, M.; Wasowski, C.; Peña, C.; Medina, J.H.; Paladini, A.C. Isolation of pharmacologically active benzodiazepine receptor ligands from Tilia tomentosa (Tiliaceae). J. Ethnopharmacol. 1994, 44, 47–53. [Google Scholar] [CrossRef]
- Martini, N.D.; Katerere, D.R.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef]
- Calzada, F. Additional antiprotozoal constituents from Cuphea pinetorum, a plant used in Mayan traditional medicine to treat diarrhoea. Phytother. Res. 2005, 19, 725–727. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker Constituents of the Natural Antioxidant Eucalyptus Leaf Extract for the Evaluation of Food Additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065. [Google Scholar] [CrossRef]
- Chen, K.C.; Chuang, C.M.; Lin, L.Y.; Chiu, W.T.; Wang, H.E.; Hsieh, C.L.; Tsai, T.; Peng, R.Y. The polyphenolics in the aqueous extract of Psidium guajava kinetically reveal an inhibition model on LDL glycation. Pharm. Biol. 2010, 48, 23–31. [Google Scholar] [CrossRef][Green Version]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- van Elswijk, D.A.; Schobel, U.P.; Lansky, E.P.; Irth, H.; van der Greef, J. Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry 2004, 65, 233–241. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kim, J.H.; Hong, M.H.; Seog, H.M.; Oh, S.H.; Lee, P.J.; Kim, G.J.; Kim, H.M.; Um, J.Y.; Ko, S.-G. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-κB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2007, 110, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shoer, M.; Ma, G.E.; Li, X.H.; Koonchanok, N.M.; Geahlen, R.L.; Chang, C.J. Flavonoids from Koelreuteria henryi and other sources as protein-tyrosine kinase inhibitors. J. Nat. Prod. 1993, 56, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Deng, Z.W.; Lei, H.M.; Fu, H.Z.; Li, J. Polyphenolic compounds from the leaves of Koelreuteria paniculata Laxm. J. Asian Nat. Prod. Res. 2002, 4, 287–295. [Google Scholar] [CrossRef]
- Arriaga, A.C.; de Mesquita, A.C.; Pouliquen, Y.B.; de Lima, R.A.; Cavalcante, S.H.; de Carvalho, M.G.; de Siqueira, J.A.; Alegrio, L.V.; Braz-Filho, R. Chemical constituents of Simarouba versicolor. An. Da Acad. Bras. De Ciências 2002, 74, 415–424. [Google Scholar] [CrossRef]
- Salvador, M.J.; Ferreira, E.O.; Mertens-Talcott, S.U.; De Castro, W.V.; Butterweck, V.; Derendorf, H.; Dias, D.A. Isolation and HPLC quantitative analysis of antioxidant flavonoids from Alternanthera tenella Colla. Z. Für Nat. C 2006, 61, 19–25. [Google Scholar] [CrossRef]
- Aung, H.H.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Ahmed, A.A.; Pare, P.W.; Mabry, T.J. Phenolic constituents from the leaves of the carnivorous plant Nepenthes gracilis. Fitoterapia 2002, 73, 445–447. [Google Scholar] [CrossRef]
- Kataoka, M.; Hirata, K.; Kunikata, T.; Ushio, S.; Iwaki, K.; Ohashi, K.; Ikeda, M.; Kurimoto, M. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J. Gastroenterol. 2001, 36, 5–9. [Google Scholar] [CrossRef]
- Parveen, Z.; Deng, Y.; Saeed, M.K.; Dai, R.; Ahamad, W.; Yu, Y.H. Antiinflammatory and analgesic activities of Thesium chinense Turcz extracts and its major flavonoids, kaempferol and kaempferol-3-O-glucoside. Yakugaku Zasshi. J. Pharm. Soc. Jpn. 2007, 127, 1275–1279. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Said, A.; Tundis, R.; Hawas, U.W.; Rashed, K.; Menichini, F.; Frega, N.G.; Menichini, F. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum. Nutr. 2009, 64, 264–270. [Google Scholar] [CrossRef]
- Crublet, M.-L.; Long, C.; Sévenet, T.; Hadi, H.A.; Lavaud, C. Acylated flavonol glycosides from leaves of Planchonia grandis. Phytochemistry 2003, 64, 589–594. [Google Scholar] [CrossRef]
- Sumino, M.; Sekine, T.; Ruangrungsi, N.; Igarashi, K.; Ikegami, F. Ardisiphenols and other antioxidant principles from the fruits of Ardisia colorata. Chem. Pharm. Bull. 2002, 50, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.M.; Svoboda, G.H.; Schiff, P.L., Jr.; Slatkin, D.J.; Knapp, J.E. Chemical constituents of Echites hirsuta (Apocynaceae). J. Pharm. Sci. 1979, 68, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Palu, K.; West, B.J.; Su, C.X.; Zhou, B.N.; Jensen, J.C. Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. J. Nat. Prod. 2007, 70, 859–862. [Google Scholar] [CrossRef]
- Cimanga, R.K.; Kambu, K.; Tona, L.; Hermans, N.; Apers, S.; Totté, J.; Pieters, L.; Vlietinck, A.J. Cytotoxicity and in vitro susceptibility of Entamoeba histolytica to Morinda morindoides leaf extracts and its isolated constituents. J. Ethnopharmacol. 2006, 107, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Majinda, R.R.; Motswaledi, M.; Waigh, R.D.; Waterman, P.G. Phenolic and antibacterial constituents of Vahlia capensis. Planta Med. 1997, 63, 268–270. [Google Scholar] [CrossRef]
- Ye, M.; Yan, Y.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Tu-Si-Zi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1469–1484. [Google Scholar] [CrossRef]
- Umehara, K.; Nemoto, K.; Ohkubo, T.; Miyase, T.; Degawa, M.; Noguchi, H. Isolation of a new 15-membered macrocyclic glycolipid lactone, Cuscutic Resinoside a from the seeds of Cuscuta chinensis: A stimulator of breast cancer cell proliferation. Planta Med. 2004, 70, 299–304. [Google Scholar] [CrossRef]
- Huang, H.-C.; Syu, K.-Y.; Lin, J.-K. Chemical Composition of Solanum nigrum Linn Extract and Induction of Autophagy by Leaf Water Extract and Its Major Flavonoids in AU565 Breast Cancer Cells. J. Agric. Food Chem. 2010, 58, 8699–8708. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kang, M.W.; Roh, J.H.; Choi, S.U.; Zee, O.P. Cytotoxic phenolic compounds from Chionanthus retusus. Arch. Pharmacal Res. 2009, 32, 1681–1687. [Google Scholar] [CrossRef]
- de Laurentis, N.; Stefanizzi, L.; Milillo, M.A.; Tantillo, G. Flavonoids from leaves of Olea europaea L. cultivars. Ann. Pharm. Françaises 1998, 56, 268–273. [Google Scholar]
- Youssef, F.S.; Altyar, A.E.; Omar, A.M.; Ashour, M.L. Phytoconstituents, In Vitro Anti-Infective Activity of Buddleja indica Lam., and In Silico Evaluation of its SARS-CoV-2 Inhibitory Potential. Front. Pharmacol. 2021, 12, 619373. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, A.; Karioti, A.; Gousiadou, C.; Lax Vivancos, V.; Kyriazopoulos, P.; Golegou, S.; Skaltsa, H. Depsides and Other Polar Constituents from Origanum dictamnus L. and Their in Vitro Antimicrobial Activity in Clinical Strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and phenolic compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Yassa, N.; Mozaffarian, V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak. J. Pharm. Sci. 2010, 23, 300–304. [Google Scholar] [PubMed]
- Pistelli, L.; Noccioli, C.; Giachi, I.; Dimitrova, B.; Gevrenova, R.; Morelli, I.; Potenza, D. Lupane-triterpenes from Bupleurum flavum. Nat. Prod. Res. 2005, 19, 783–788. [Google Scholar] [CrossRef]
- Guo, S.; Guo, R.; Xia, Y.; Dong, T.; Wang, H.; Zhan, H. Quantitative analysis of fermented aerial part of Bupleurum chinense and prediction of their antimicrobial activity. Zhongguo Zhong Yao Za Zhi 2020, 45, 4238–4245. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ishikawa, H.; Sanchez, Y.; Ogura, T.; Kubo, Y.; Kubo, I. Antioxidative constituents in Heterotheca inuloides. Bioorganic Med. Chem. 1997, 5, 865–871. [Google Scholar] [CrossRef]
- Hidalgo Báez, D.; de los Ríos, C.; Crescente, O.; Caserta, A. Antibacterial and chemical evaluation of Chromolaena moritziana. J. Ethnopharmacol. 1998, 59, 203–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Shi, Y.-P. Separation and Determination of Flavonoids in Ixeridium gracile by Capillary Electrophoresis. J. Chromatogr. Sci. 2007, 45, 600–604. [Google Scholar] [CrossRef]
- Kim, D.K. Antioxidative components from the aerial parts of Lactuca scariola L. Arch. Pharmacal Res. 2001, 24, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Z.; Choi, S.U.; Lee, K.R. Phytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch. Pharmacal Res. 2004, 27, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Süzgeç, S.; Meriçli, A.H.; Houghton, P.J.; Cubukçu, B. Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia 2005, 76, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Hensel, A.; Costa, F.B.; Brun, R.; Kaiser, M.; Schmidt, T.J. An unusual dimeric guaianolide with antiprotozoal activity and further sesquiterpene lactones from Eupatoriumperfoliatum. Phytochemistry 2011, 72, 635–644. [Google Scholar] [CrossRef]
- Prasasty, V.D.; Cindana, S.; Ivan, F.X.; Zahroh, H.; Sinaga, E. Structure-based discovery of novel inhibitors of Mycobacterium tuberculosis CYP121 from Indonesian natural products. Comput. Biol. Chem. 2020, 85, 107205. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef]
- Li, M.M.; Wang, K.; Pan, Z.H.; Chen, X.Q.; Peng, L.Y.; Li, Y.; Cheng, X.; Zhao, Q.S. Two new sesquiterpene glucosides from Dennstaedtia scabra (Wall.) Moore. Chem. Pharm. Bull. 2009, 57, 1123–1125. [Google Scholar] [CrossRef]
- Lin, Y.L.; Shen, C.C.; Huang, Y.J.; Chang, Y.Y. Homoflavonoids from Ophioglossum petiolatum. J. Nat. Prod. 2005, 68, 381–384. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; Beltsville Human Nutrition Research Center, Agricultural Research Service: Beltsville, MD, USA, 2011. [Google Scholar]
- Fieschi, M.; Codignola, A.; Luppi Mosca, A.M. Mutagenic Flavonol Aglycones in Infusions and in Fresh and Pickled Vegetables. J. Food Sci. 1989, 54, 1492–1495. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Herrmann, K. Flavonols and flavones in food plants: A review†. Int. J. Food Sci. Technol. 1976, 11, 433–448. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive Secondary Metabolites from Bacillus subtilis: A Comprehensive Review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Makuwa, S.C.; Serepa-Dlamini, M.H. The Antibacterial Activity of Crude Extracts of Secondary Metabolites from Bacterial Endophytes Associated with Dicoma anomala. Int. J. Microbiol. 2021, 2021, 8812043. [Google Scholar] [CrossRef]
- Aminah, N.S.; Laili, E.R.; Rafi, M.; Rochman, A.; Insanu, M.; Tun, K.N.W. Secondary metabolite compounds from Sida genus and their bioactivity. Heliyon 2021, 7, e06682. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid. -Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef]
- Popescu, G.A.; Șerban, R.; Iosif, I.; Codiță, I.; Dorobăț, O.; Tălăpan, D.; Buzea, M.; Szekely, E.; Dorneanu, O.; Bota, K.; et al. Antimicrobial resistance of germs isolated from invasive infections—Romania 2012. BMC Infect. Dis. 2013, 13, O16. [Google Scholar] [CrossRef]
- Rafila, A.; Tălăpan, D.; Dorobat, O.; Popescu, G.; Piţigoi, D.; Florea, D.; Buicu, F. Emergence of Carbapenemase-producing Enterobacteriaceae, a Public Health Threat: A Romanian Infectious Disease Hospital Based Study. Rev. Romana De Med. De Lab. 2015, 23, 295–301. [Google Scholar] [CrossRef]
- Tălăpan, D.; Rafila, A. Five-Year Survey of Asymptomatic Colonization with Multidrug-Resistant Organisms in a Romanian Tertiary Care Hospital. Infect. Drug Resist. 2022, 15, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Orjala, J.; Sticher, O.; Rali, T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J. Nat. Prod. 1999, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Luo, X.-G.; Si, C.-L.; Wang, N.; Zhou, H.; He, J.-F.; Zhang, T.-C. Antibacterial active compounds from Hypericum ascyron L. induce bacterial cell death through apoptosis pathway. Eur. J. Med. Chem. 2015, 96, 436–444. [Google Scholar] [CrossRef]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial mechanism of flavonoids against Escherichia coli ATCC 25922 by model membrane study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Cronan, J.E.; Thomas, J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 2009, 459, 395–433. [Google Scholar] [CrossRef] [PubMed]
- Rowlett, V.W.; Mallampalli, V.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of Membrane Phospholipid Alterations in Escherichia coli on Cellular Function and Bacterial Stress Adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef]
- Al-Nour, M.Y.; Ibrahim, M.M.; Elsaman, T. Ellagic Acid, Kaempferol, and Quercetin from Acacia nilotica: Promising Combined Drug With Multiple Mechanisms of Action. Curr. Pharmacol. Rep. 2019, 5, 255–280. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure-activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Liu, M.H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic effect of kaempferol glycosides purified from Laurus nobilis and fluoroquinolones on methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, C.C.; Chen, C.C.; Yang, K.J.; Huang, C.Y. Inhibition of Staphylococcus aureus PriA Helicase by Flavonol Kaempferol. Protein. J. 2015, 34, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Kim, I.H.; Seo, J.J. In vitro activity of kaempferol isolated from the Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. J. Microbiol. 2007, 45, 473–477. [Google Scholar] [PubMed]
- Christopoulou, C.; Graikou, K.; Chinou, I. Chemosystematic Value of Chemical Constituents from Scabiosa hymettia (Dipsacaceae). Chem. Biodivers. 2008, 5, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; de Luna, R.D.; Cruz, W.C.; Rideout, J.A. Monoterpene Lactones from the Seeds of Nephelium lappaceum. J. Nat. Prod. 2005, 68, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.Z.; Ahmad, S. Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of Labisa pumila Benth. Molecules 2011, 16, 4438–4450. [Google Scholar] [CrossRef] [PubMed]
- Achika, J.I.; Ayo, R.G.; Oyewale, A.O.; Habila, J.D. Flavonoids with antibacterial and antioxidant potentials from the stem bark of Uapaca heudelotti. Heliyon 2020, 6, e03381. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.A.; da Cruz, R.P.; Dos Santos, A.T.L.; Almeida-Bezerra, J.W.; Machado, A.J.T.; Dos Santos, J.F.S.; Rocha, J.E.; Boligon, A.A.; Bezerra, C.F.; de Freitas, T.S.; et al. HPLC-DAD analysis and antimicrobial activities of Spondias mombin L. (Anacardiaceae). 3 Biotech 2022, 12, 61. [Google Scholar] [CrossRef]
- Özçelik, B.; Orhan, I.; Toker, G. Antiviral and Antimicrobial Assessment of Some Selected Flavonoids. Z. Für Nat. C 2006, 61, 632–638. [Google Scholar] [CrossRef]
- Rofeal, M.; El-Malek, F.A.; Qi, X. In vitro assessment of green polyhydroxybutyrate/chitosan blend loaded with kaempferol nanocrystals as a potential dressing for infected wounds. Nanotechnology 2021, 32, 37. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Tlak Gajger, I.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous Polyethylene Glycol as a Safer Alternative to Ethanolic Propolis Extracts with Comparable Antioxidant and Antimicrobial Activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef]
- Kannanoor, M.; Lakshmi, B.A.; Kim, S. Synthesis of silver nanoparticles conjugated with kaempferol and hydrocortisone and an evaluation of their antibacterial effects. 3 Biotech 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Galal, T.M.; Al-Yasi, H.M.; Fawzy, M.A.; Abdelkader, T.G.; Hamza, R.Z.; Eid, E.M.; Ali, E.F. Evaluation of the Phytochemical and Pharmacological Potential of Taif’s Rose (Rosa damascena Mill var. trigintipetala) for Possible Recycling of Pruning Wastes. Life 2022, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.M.; El-Sherbeni, S.A.; El-Kadem, A.H.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Negm, W.A. Elucidation of the Metabolite Profile of Yucca gigantea and Assessment of Its Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2022, 27, 1329. [Google Scholar] [CrossRef] [PubMed]
- Habbu, P.V.; Mahadevan, K.M.; Shastry, R.A.; Manjunatha, H. Antimicrobial activity of flavanoid sulphates and other fractions of Argyreia speciosa (Burm.f) Boj. Indian J. Exp. Biol. 2009, 47, 121–128. [Google Scholar]
- Tatsimo, S.J.; Tamokou Jde, D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef]
- Otsuka, N.; Liu, M.H.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Anti-methicillin resistant Staphylococcus aureus (MRSA) compounds isolated from Laurus nobilis. Biol. Pharm. Bull. 2008, 31, 1794–1797. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Sanogo, R.; Marino, A.; Aquino, R.; D’Angelo, V.; Germanò, M.P.; De Pasquale, R.; Pizza, C. Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett. Appl. Microbiol. 2000, 30, 105–108. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mansoor, A.A.; Gross, A.; Ashfaq, M.K.; Jacob, M.; Khan, S.I.; Hamann, M.T. Methicillin-resistant Staphylococcus aureus (MRSA)-active metabolites from Platanus occidentalis (American Sycamore). J. Nat. Prod. 2009, 72, 2141–2144. [Google Scholar] [CrossRef]
- Chan, E.W.; Gray, A.I.; Igoli, J.O.; Lee, S.M.; Goh, J.K. Galloylated flavonol rhamnosides from the leaves of Calliandra tergemina with antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochemistry 2014, 107, 148–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Valeriote, F.; Swartz, K.; Chen, B.; Hamann, M.T.; Rodenburg, D.L.; McChesney, J.D.; Shaw, J. HPLC Plasma Assay of a Novel Anti-MRSA Compound, Kaempferol-3-O-Alpha-L-(2”,3”-di-p-coumaroyl)rhamnoside, from Sycamore Leaves. Nat. Prod. Commun. 2015, 10, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, N.J.; Stemmer, P.M.; Media, J.; Swartz, K.; Wang, X.; Aube, N.; Hamann, M.T.; Valeriote, F.; Shaw, J. The anti-MRSA compound 3-O-alpha-L-(2″,3″-di-p-coumaroyl)rhamnoside (KCR) inhibits protein synthesis in Staphylococcus aureus. J. Proteom. 2020, 210, 103539. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.B.; Leitão, M.M.; Kassuya, R.M.; Macorini, L.F.; Moreira, F.M.F.; Cardoso, C.A.L.; Coelho, R.G.; Pott, A.; Gelfuso, G.M.; Croda, J.; et al. Anti-inflammatory, antimycobacterial and genotoxic evaluation of Doliocarpus dentatus. J. Ethnopharmacol. 2017, 204, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.D.; Silva-Filho, S.E.; Santos Radai, J.A.; Arena, A.C.; Fraga, T.L.; Lima Cardoso, C.A.; Croda, J.; Leite Kassuya, C.A. Anti-inflammatory properties of ethanolic extract from Vatairea macrocarpa leaves. J. Ethnopharmacol. 2021, 278, 114308. [Google Scholar] [CrossRef]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Hashemi, F.B.; Pourakbari, B.; Aziemzadeh, M.; Bahador, A. Antimicrobial Resistance of Acinetobacter baumannii to Imipenem in Iran: A Systematic Review and Meta-Analysis. Open Microbiol J 2016, 10, 32–42. [Google Scholar] [CrossRef]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe-Barbu, I.; Barbu, I.C.; Popa, L.I.; Pîrcălăbioru, G.G.; Popa, M.; Măruțescu, L.; Niță-Lazar, M.; Banciu, A.; Stoica, C.; Gheorghe, Ș.; et al. Temporo-spatial variations in resistance determinants and clonality of Acinetobacter baumannii and Pseudomonas aeruginosa strains from Romanian hospitals and wastewaters. Antimicrob. Resist. Infect. Control 2022, 11, 115. [Google Scholar] [CrossRef]
- Gu, H.-J.; Sun, Q.-L.; Luo, J.-C.; Zhang, J.; Sun, L. A First Study of the Virulence Potential of a Bacillus subtilis Isolate From Deep-Sea Hydrothermal Vent. Front. Cell Infect. Microbiol. 2019, 9, 183. [Google Scholar] [CrossRef]
- Cote, C.K.; Heffron, J.D.; Bozue, J.A.; Welkos, S.L. Chapter 102—Bacillus anthracis and Other Bacillus Species. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1789–1844. [Google Scholar] [CrossRef]
- Brown, K.L. Control of bacterial spores. Br. Med. Bull. 2000, 56, 158–171. [Google Scholar] [CrossRef]
- Spencer, R.C. Bacillus anthracis. J. Clin. Pathol. 2003, 56, 182–187. [Google Scholar] [CrossRef]
- Hoffmaster, A.R.; Hill, K.K.; Gee, J.E.; Marston, C.K.; De, B.K.; Popovic, T.; Sue, D.; Wilkins, P.P.; Avashia, S.B.; Drumgoole, R.; et al. Characterization of Bacillus cereus isolates associated with fatal pneumonias: Strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J. Clin. Microbiol. 2006, 44, 3352–3360. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Sanchis, V. The Pore-Forming Haemolysins of Bacillus Cereus: A Review. Toxins 2013, 5, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Pormohammad, A.; Pouriran, R.; Azimi, H.; Goudarzi, M. Prevalence of integron classes in Gram-negative clinical isolated bacteria in Iran: A systematic review and meta-analysis. Iran. J. Basic Med. Sci. 2019, 22, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Surleac, M.; Barbu, I.; Paraschiv, S.; Popa, L.; Gheorghe, I.; Marutescu, L.; Popa, M.; Avram, I.; Tălăpan, D.; Nita-Lazar, M.; et al. Whole genome sequencing snapshot of multi-drug resistant Klebsiella pneumoniae strains from hospitals and receiving wastewater treatment plants in Southern Romania. PLoS ONE 2020, 15, e0228079. [Google Scholar] [CrossRef]
- Popa, L.; Gheorghe, I.; Barbu, I.; Surleac, M.; Paraschiv, S.; Marutescu, L.; Popa, M.; Gradisteanu Pircalabioru, G.; Tălăpan, D.; Nita-Lazar, M.; et al. Multidrug Resistant Klebsiella pneumoniae ST101 Clone Survival Chain From Inpatients to Hospital Effluent After Chlorine Treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Scriba, T.J.; Netea, M.G.; Ginsberg, A.M. Key recent advances in TB vaccine development and understanding of protective immune responses against Mycobacterium tuberculosis. Semin. Immunol. 2020, 50, 101431. [Google Scholar] [CrossRef]
- Popescu, G.G.; Arghir, O.C.; Fildan, A.P.; Spanu, V.; Cambrea, S.C.; Rafila, A.; Buicu, F.C. Antibiotic resistance of Mycobacterium tuberculosis; mechanism and specific therapeutic response. Farmacia 2020, 68, 197–205. [Google Scholar] [CrossRef]
- Allué-Guardia, A.; García, J.I.; Torrelles, J.B. Evolution of Drug-Resistant Mycobacterium tuberculosis Strains and Their Adaptation to the Human Lung Environment. Front. Microbiol. 2021, 12, 612675. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G. Antibiotic resistance: Counting the cost. Curr. Biol. 1996, 6, 1219–1221. [Google Scholar] [CrossRef][Green Version]
- Böttger, E.C.; Springer, B.; Pletschette, M.; Sander, P. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nat. Med. 1998, 4, 1343–1344. [Google Scholar] [CrossRef]
- Andersson, D.I.; Levin, B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999, 2, 489–493. [Google Scholar] [CrossRef]
- Fanning, A.; Edwards, S. Mycobacterium bovis infection in human beings in contact with elk (Cervus elaphus) in Alberta, Canada. Lancet 1991, 338, 1253–1255. [Google Scholar] [CrossRef]
- Cooke, M.M.; Gear, A.J.; Naidoo, A.; Collins, D.M. Accidental Mycobacterium bovis infection in a veterinarian. New Zealand Vet. J. 2002, 50, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.; Bartlett, P.; Frawley, B.; O’Brien, D.; Miller, C.; Boulton, M. Mycobacterium bovis (bovine TB) exposure as a recreational risk for hunters: Results of a Michigan Hunter Survey, 2001. Int. J. Tuberc. Lung Dis. 2003, 7, 1001–1009. [Google Scholar] [PubMed]
- Wilkins, M.J.; Meyerson, J.; Bartlett, P.C.; Spieldenner, S.L.; Berry, D.E.; Mosher, L.B.; Kaneene, J.B.; Robinson-Dunn, B.; Stobierski, M.G.; Boulton, M.L. Human Mycobacterium bovis infection and bovine tuberculosis outbreak, Michigan, 1994–2007. Emerg. Infect. Dis. 2008, 14, 657. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R.; Gortázar, C.; Corner, L.A.L. Mycobacterium bovis: A Model Pathogen at the Interface of Livestock, Wildlife, and Humans. Vet. Med. Int. 2012, 2012, 236205. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, J.M.; Contreras-Martinez, L.M.; Livny, J.; Smith, D.; McDonough, K.A.; Belfort, M. Multiple small RNAs identified in Mycobacterium bovis BCG are also expressed in Mycobacterium tuberculosis and Mycobacterium smegmatis. Nucleic Acids Res. 2010, 38, 4067–4078. [Google Scholar] [CrossRef]
- Klockgether, J.; Tümmler, B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Res 2017, 6, 1261. [Google Scholar] [CrossRef]
- Buhl, M.; Peter, S.; Willmann, M. Prevalence and risk factors associated with colonization and infection of extensively drug-resistant Pseudomonas aeruginosa: A systematic review. Expert Rev. Anti Infect. Ther. 2015, 13, 1159–1170. [Google Scholar] [CrossRef]
- Murphy, T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009, 15, 138–142. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, C.F.; Silva, A.R.; Burth, P.; Rocco, P.R.; Castro-Faria, M.V.; Castro-Faria-Neto, H.C. Possible mechanisms of Pseudomonas aeruginosa-associated lung disease. Int. J. Med. Microbiol. 2016, 306, 20–28. [Google Scholar] [CrossRef]
- Talwalkar, J.S.; Murray, T.S. The Approach to Pseudomonas aeruginosa in Cystic Fibrosis. Clin. Chest Med. 2016, 37, 69–81. [Google Scholar] [CrossRef]
- Winstanley, C.; O’Brien, S.; Brockhurst, M.A. Pseudomonas aeruginosa Evolutionary Adaptation and Diversification in Cystic Fibrosis Chronic Lung Infections. Trends Microbiol. 2016, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D. Pseudomonas aeruginosa infection and inflammation during contact lens wear: A review. Optom. Vis. Sci. 2007, 84, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Fournier, A.; Voirol, P.; Krähenbühl, M.; Bonnemain, C.L.; Fournier, C.; Pantet, O.; Pagani, J.L.; Revelly, J.P.; Dupuis-Lozeron, E.; Sadeghipour, F.; et al. Antibiotic consumption to detect epidemics of Pseudomonas aeruginosa in a burn centre: A paradigm shift in the epidemiological surveillance of Pseudomonas aeruginosa nosocomial infections. Burns 2016, 42, 564–570. [Google Scholar] [CrossRef]
- Döring, G.; Parameswaran, I.G.; Murphy, T.F. Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease. FEMS Microbiol. Rev. 2011, 35, 124–146. [Google Scholar] [CrossRef] [PubMed]
- Langan, K.M.; Kotsimbos, T.; Peleg, A.Y. Managing Pseudomonas aeruginosa respiratory infections in cystic fibrosis. Curr. Opin. Infect. Dis. 2015, 28, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.L.; Ferrer, M.; Marti, J.D.; Comaru, T.; Torres, A. Ventilator-associated pneumonia. Semin. Respir. Crit. Care Med. 2014, 35, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar]
- Popa, G.; Popa, M.I. Salmonella spp. Infection—A continuous threat worldwide. GERMS 2021, 11, 87–96. [Google Scholar] [CrossRef]
- Allerberger, F.; Liesegang, A.; Grif, K.; Khaschabi, D.; Prager, R.; Danzl, J.; Höck, F.; Ottl, J.; Dierich, M.P.; Berghold, C.; et al. Occurrence of Salmonella enterica serovar Dublin in Austria. Wien Med. Wochenschr. 2003, 153, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Chiu, C.H.; Wu, T.L.; Su, L.H.; Chu, C.; Chia, J.H.; Kuo, A.J.; Chien, M.S.; Lin, T.Y. The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype choleraesuis. N. Engl. J. Med. 2002, 346, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Sandulescu, O.; Bleotu, C.; Matei, L.; Streinu-Cercel, A.; Oprea, M.; Dragulescu, E.; Chifiriuc, M.; Rafila, A.; Pirici, D.; Tălăpan, D.; et al. Comparative evaluation of aggressiveness traits in staphylococcal strains from severe infections versus nasopharyngeal carriage. Microb. Pathog. 2016, 102, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Davies, J.K.; Johnson, P.D.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef]
- Durai, R.; Ng, P.C.; Hoque, H. Methicillin-resistant Staphylococcus aureus: An update. Aorn J. 2010, 91, 599–606. [Google Scholar] [CrossRef]
- Johnson, A.P. Methicillin-resistant Staphylococcus aureus: The European landscape. J. Antimicrob. Chemother. 2011, 66 (Suppl. 4), iv43–iv48. [Google Scholar] [CrossRef]
- Dulon, M.; Haamann, F.; Peters, C.; Schablon, A.; Nienhaus, A. MRSA prevalence in European healthcare settings: A review. BMC Infect. Dis. 2011, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Grema, H.A.; Geidam, Y.A.; Gadzama, G.B.; Ameh, J.A.; Suleiman, A. Methicillin resistant Staphylococcus aureus (MRSA): A review. Adv. Anim. Vet. Sci. 2015, 3, 79–98. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, D.R.; Culver, D.H.; Gaynes, R.P. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 1991, 91, 72s–75s. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D. Enterococci. Biologic and epidemiologic characteristics and in vitro susceptibility. Arch. Intern. Med. 1982, 142, 2006–2009. [Google Scholar] [CrossRef]
- Maki, D.G.; Agger, W.A. Enterococcal Bacteremia: Clinical Features, the Risk of Endocarditis, and Management. Medicine 1988, 67, 248. [Google Scholar] [CrossRef]
- Noskin, G.A.; Peterson, L.R.; Warren, J.R. Enterococcus faecium and Enterococcus faecalis bacteremia: Acquisition and outcome. Clin. Infect. Dis. 1995, 20, 296–301. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-Resistant Enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.; Daniil, I.; Sotiropoulos, A.; Balampani, E.; Kokolaki, A.; Bousboulas, S.; Konstantopoulou, S.; Skliros, E.; Petropoulou, D.; Pappas, S. Prevalence of asymptomatic bacteriuria in type 2 diabetic subjects with and without microalbuminuria. BMC Res. Notes 2010, 3, 169. [Google Scholar] [CrossRef]
- Matthews, S.J.; Lancaster, J.W. Urinary tract infections in the elderly population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and Urinary Tract Infections. Microbiol. Spectr. 2015, 3, 3–5. [Google Scholar] [CrossRef]
- Charkhian, H.; Bodaqlouie, A.; Soleimannezhadbari, E.; Lotfollahi, L.; Shaykh-Baygloo, N.; Hosseinzadeh, R.; Yousefi, N.; Khodayar, M. Comparing the Bacteriostatic Effects of Different Metal Nanoparticles Against Proteus vulgaris. Curr. Microbiol. 2020, 77, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Warren, J.W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 1987, 25, 2216–2217. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Warren, J.W. Urinary Tract Infections: Molecular Pathogenesis and Clinical Management; ASM Press: Washington, DC, USA, 1996; pp. 3–27. [Google Scholar]
- Jacobsen, S.M.; Shirtliff, M.E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2011, 2, 460–465. [Google Scholar] [CrossRef]
- Yuan, F.; Huang, Z.; Yang, T.; Wang, G.; Li, P.; Yang, B.; Li, J. Pathogenesis of Proteus mirabilis in Catheter-Associated Urinary Tract Infections. Urol. Int. 2021, 105, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.M.; Collis, C.M. Antibiotic resistance in gram-negative bacteria: The role of gene cassettes and integrons. Drug Resist. Updat. 1998, 1, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Trecarichi, E.M.; Fiori, B.; Losito, A.R.; D’Inzeo, T.; Campana, L.; Ruggeri, A.; Di Meco, E.; Liberto, E.; Fadda, G.; et al. Multidrug-resistant Proteus mirabilis bloodstream infections: Risk factors and outcomes. Antimicrob. Agents Chemother. 2012, 56, 3224–3231. [Google Scholar] [CrossRef]
- Pagani, L.; Migliavacca, R.; Pallecchi, L.; Matti, C.; Giacobone, E.; Amicosante, G.; Romero, E.; Rossolini, G.M. Emerging extended-spectrum beta-lactamases in Proteus mirabilis. J. Clin. Microbiol. 2002, 40, 1549–1552. [Google Scholar] [CrossRef]
- Endimiani, A.; Luzzaro, F.; Brigante, G.; Perilli, M.; Lombardi, G.; Amicosante, G.; Rossolini, G.M.; Toniolo, A. Proteus mirabilis bloodstream infections: Risk factors and treatment outcome related to the expression of extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 2005, 49, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Nahum, K.; Saidel-Odes, L.; Riesenberg, K.; Schlaeffer, F.; Borer, A. Urinary tract infections caused by multi-drug resistant Proteus mirabilis: Risk factors and clinical outcomes. Infection 2010, 38, 41–46. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Literacka, E.; Zioga, A.; Giani, T.; Baraniak, A.; Fiett, J.; Sadowy, E.; Tassios, P.T.; Rossolini, G.M.; Gniadkowski, M.; et al. Evolution and spread of a multidrug-resistant Proteus mirabilis clone with chromosomal AmpC-type cephalosporinases in Europe. Antimicrob. Agents Chemother. 2011, 55, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Vaez, H.; Kalarestaghi, H.; Sahebkar, A.; Khademi, F. Prevalence of antibiotic resistance of Proteus species in urinary tract infections in Iran: A systematic review and meta-analysis. Gene Rep. 2022, 27, 101632. [Google Scholar] [CrossRef]
- Colwell, R.R.; Kaper, J.; Joseph, S.W. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: Occurrence and distribution in Chesapeake Bay. Science 1977, 198, 394–396. [Google Scholar]
- Garay, E.; Arnau, A.; Amaro, C. Incidence of Vibrio cholerae and related vibrios in a coastal lagoon and seawater influenced by lake discharges along an annual cycle. Appl. Environ. Microbiol. 1985, 50, 426–430. [Google Scholar] [CrossRef]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Reidl, J.; Klose, K.E. Vibrio cholerae and cholera: Out of the water and into the host. FEMS Microbiol. Rev. 2002, 26, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.I.; Huq, I.; Alim, A.R.M.A.; Yunus, M. Emergence of Multiply Antibiotic-Resistant Vibrio cholerae in Bangladesh. J. Infect. Dis. 1980, 142, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic resistance in Vibrio cholerae: Understanding the ecology of resistance genes and mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; De Louvois, J. Candida vaginitis. Postgrad. Med. J. 1979, 55, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr. History and new insights into host defense against vaginal candidiasis. Trends Microbiol. 2004, 12, 220–227. [Google Scholar] [CrossRef]
- Cohen, J.; Denning, D.W.; Viviani, M.A. Epidemiology of invasive aspergillosis in European cancer centres. EORTC Invasive Fungal Infections Cooperative Group. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 392–393. [Google Scholar] [CrossRef]
- Rogers, T. Epidemiology and control of nosocomial fungal infections. Curr. Opin. Infect. Dis. 1995, 8, 287–290. [Google Scholar] [CrossRef]
- Ruchlemer, R.; Yinnon, A.M.; Hershko, C. Changes in the natural history of invasive pulmonary aspergillosis in neutropenic leukemic patients. Isr. J. Med. Sci. 1996, 32, 1089–1092. [Google Scholar]
- Meidani, M.; Naeini, A.E.; Rostami, M.; Sherkat, R.; Tayeri, K. Immunocompromised patients: Review of the most common infections happened in 446 hospitalized patients. J. Res. Med. Sci. 2014, 19, S71–S73. [Google Scholar]
- Goodley, J.M.; Clayton, Y.M.; Hay, R.J. Environmental sampling for aspergilli during building construction on a hospital site. J. Hosp. Infect. 1994, 26, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chazalet, V.; Debeaupuis, J.P.; Sarfati, J.; Lortholary, J.; Ribaud, P.; Shah, P.; Cornet, M.; Vu Thien, H.; Gluckman, E.; Brücker, G.; et al. Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J. Clin. Microbiol. 1998, 36, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Hospenthal, D.R.; Kwon-Chung, K.J.; Bennett, J.E. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: Lack of correlation. Med. Mycol. 1998, 36, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Bennett, J.E. Medical mycology. Rev. Do Inst. De Med. Trop. De São Paulo 1992, 34, 504. [Google Scholar] [CrossRef]
- Pennington, J. Respiratory Infections: Diagnosis and Management; Raven Press: New York, NY, USA, 1983. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Knoke, M.; Schwesinger, G. One hundred years ago: The history of cryptococcosis in Greifswald. Medical mycology in the nineteenth century. Mycoses 1994, 37, 229–233. [Google Scholar] [CrossRef]
- Casadevall, A.; Perfect, J.R. Cryptococcus Neoformans; Citeseer: Princeton, NJ, USA, 1998; Volume 595. [Google Scholar]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Badicut, I.; Paraschiv, S.; Borcan, A.; Tălăpan, D.; Dorobat, O. Risk factors in HIV/AIDS patients with cryptococcal meningitis. BMC Infect. Dis. 2014, 14, P29. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.; Braca, A.; De Tommasi, N.; Norscia, I.; Morelli, I.; Battinelli, L.; Mazzanti, G. Phenolic compounds from Baseonema acuminatum leaves: Isolation and antimicrobial activity. Planta Med. 2004, 70, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, M.; Dimitrova, P.; Patkar, S.; Saso, L.; Ivanovska, N. Inhibition of Candida albicans extracellular enzyme activity by selected natural substances and their application in Candida infection. Can. J. Microbiol. 2008, 54, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Kundu, A. Macroporous resin-assisted enrichment, characterizations, antioxidant and anticandidal potential of phytochemicals from Trachyspermum ammi. J. Food Biochem. 2022, 46, e13847. [Google Scholar] [CrossRef]
- Pushkala, V.P.; Sulekha, S.M.P.; Mathukumar, S.; Ragavi, B.; Sowmiya, U. Molecular Docking Analysis of Siddha Formulation Parangipattai Chooranam Against Vaginal Candidiasis. Appl. Biochem. Biotechnol. 2022, 194, 1039–1050. [Google Scholar] [CrossRef]
- Slack, A. Parasitic causes of prolonged diarrhoea in travellers—Diagnosis and management. Aust. Fam. Physician 2012, 41, 782–786. [Google Scholar]
- El-Dib, N.A. Entamoeba histolytica: An Overview. Curr. Trop. Med. Rep. 2017, 4, 11–20. [Google Scholar] [CrossRef]
- Wolfe, M.S. Giardiasis. Clin. Microbiol. Rev. 1992, 5, 93–100. [Google Scholar] [CrossRef]
- Minetti, C.; Chalmers, R.M.; Beeching, N.J.; Probert, C.; Lamden, K. Giardiasis. BMJ 2016, 355, i5369. [Google Scholar] [CrossRef]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Greenwood, B. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am. J. Trop. Med. Hyg. 2004, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Autino, B.; Noris, A.; Russo, R.; Castelli, F. Epidemiology of malaria in endemic areas. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012060. [Google Scholar] [CrossRef] [PubMed]
- Abdo, M.G.; Elamin, W.M.; Khalil, E.A.; Mukhtar, M.M. Antimony-resistant Leishmania donovaniin eastern Sudan: Incidence and in vitro correlation. East Mediterr. Health J. 2003, 9, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Elsayed, G.A.; Buskas, T.; Boons, G.-J. Synthesis of Proteophosphoglycans of Leishmania major and Leishmania mexicana. J. Org. Chem. 2005, 70, 1691–1697. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Calzada, F.; Alanis, A.D.; Meckes, M.; Tapia-Contreras, A.; Cedillo-Rivera, R. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to some medicinal plants used by the people of Southern Mexico. Phytother. Res. 1998, 12, 70–72. [Google Scholar] [CrossRef]
- Calzada, F.; Meckes, M.; Cedillo-Rivera, R. Antiamoebic and antigiardial activity of plant flavonoids. Planta Med. 1999, 65, 78–80. [Google Scholar] [CrossRef]
- Meckes, M.; Calzada, F.; Tapia-Contreras, A.; Cedillo-Rivera, R. Antiprotozoal properties of Helianthemum glomeratum. Phytother. Res. 1999, 13, 102–105. [Google Scholar] [CrossRef]
- Scio, E.; Ribeiro, A.; Alves, T.M.; Romanha, A.J.; Dias de Souza Filho, J.; Cordell, G.A.; Zani, C.L. Diterpenes from Alomia myriadenia (Asteraceae) with cytotoxic and trypanocidal activity. Phytochemistry 2003, 64, 1125–1131. [Google Scholar] [CrossRef]
- Muzitano, M.F.; Tinoco, L.W.; Guette, C.; Kaiser, C.R.; Rossi-Bergmann, B.; Costa, S.S. The antileishmanial activity assessment of unusual flavonoids from Kalanchoe pinnata. Phytochemistry 2006, 67, 2071–2077. [Google Scholar] [CrossRef]
- de Monbrison, F.; Maitrejean, M.; Latour, C.; Bugnazet, F.; Peyron, F.; Barron, D.; Picot, S. In vitro antimalarial activity of flavonoid derivatives dehydrosilybin and 8-(1;1)-DMA-kaempferide. Acta Trop. 2006, 97, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Risinger, A.L.; Nair, S.; Peng, J.; Anderson, T.J.; Du, L.; Powell, D.R.; Mooberry, S.L.; Cichewicz, R.H. Identification of Compounds with Efficacy against Malaria Parasites from Common North American Plants. J. Nat. Prod. 2016, 79, 490–498. [Google Scholar] [CrossRef]
- Pérez-González, M.Z.; Gutiérrez-Rebolledo, G.A.; Yépez-Mulia, L.; Rojas-Tomé, I.S.; Luna-Herrera, J.; Jiménez-Arellanes, M.A. Antiprotozoal, antimycobacterial, and anti-inflammatory evaluation of Cnidoscolus chayamansa (Mc Vaugh) extract and the isolated compounds. Biomed Pharmacother. 2017, 89, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.V.; Queiroz, A.C.; Silva, J.F.M.; Silva, A.E.; Silva, J.K.S.; Silva, G.R.; Silva, E.C.O.; Souza, S.T.; Fonseca, E.J.S.; Camara, C.A.; et al. Flavonoids induce cell death in Leishmania amazonensis: In vitro characterization by flow cytometry and Raman spectroscopy. Analyst 2019, 144, 5232–5244. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Ebiloma, G.U.; Williams, R.; Alfayez, I.A.; Natto, M.J.; Alenezi, S.; Siheri, W.; AlQarni, M.; Igoli, J.O.; Fearnley, J.; et al. Activity of Compounds from Temperate Propolis against Trypanosoma brucei and Leishmania mexicana. Molecules 2021, 26, 3912. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.M.; Hammoda, H.M.; Radwan, M.M.; El-Gazzar, N.S.; Wanas, A.S.; ElSohly, M.A.; El-Demellawy, M.A.; Abdel-Rahman, N.M.; Sallam, S.M. Phytochemical and pharmacological appraisal of the aerial parts of Lotus corniculatus L. growing in Egypt. Nat. Prod. Res. 2021, 35, 5914–5917. [Google Scholar] [CrossRef]
- Martínez-Castillo, M.; Pacheco-Yepez, J.; Flores-Huerta, N.; Guzmán-Téllez, P.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Campos-Rodríguez, R.; Shibayama, M. Flavonoids as a Natural Treatment Against Entamoeba histolytica. Front. Cell Infect. Microbiol. 2018, 8, 209. [Google Scholar] [CrossRef]
- Simarro, P.P.; Cecchi, G.; Paone, M.; Franco, J.R.; Diarra, A.; Ruiz, J.A.; Fèvre, E.M.; Courtin, F.; Mattioli, R.C.; Jannin, J.G. The Atlas of human African trypanosomiasis: A contribution to global mapping of neglected tropical diseases. Int. J. Health Geogr. 2010, 9, 57. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dia, M.L. Mechanical transmission of Trypanosoma congolense in cattle by the African tabanid Atylotus agrestis. Exp. Parasitol. 2003, 105, 226–231. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dia, M.L. Trypanosoma vivax: Mechanical transmission in cattle by one of the most common African tabanids, Atylotus agrestis. Exp. Parasitol. 2003, 103, 35–43. [Google Scholar] [CrossRef]
- Büscher, P.; Bart, J.M.; Boelaert, M.; Bucheton, B.; Cecchi, G.; Chitnis, N.; Courtin, D.; Figueiredo, L.M.; Franco, J.R.; Grébaut, P.; et al. Do Cryptic Reservoirs Threaten Gambiense-Sleeping Sickness Elimination? Trends Parasitol. 2018, 34, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Bouteille, B.; Oukem, O.; Bisser, S.; Dumas, M. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 2003, 17, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Qian, Z.Y.; Hide, G.; Lai, D.H.; Lun, Z.R.; Wu, Z.D. Human African trypanosomiasis: The current situation in endemic regions and the risks for non-endemic regions from imported cases. Parasitology 2020, 147, 922–931. [Google Scholar] [CrossRef]
- Baker, N.; de Koning, H.P.; Mäser, P.; Horn, D. Drug resistance in African trypanosomiasis: The melarsoprol and pentamidine story. Trends Parasitol. 2013, 29, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal activity of the constituents of Conyza filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Kozykeyeva, R.A.; Datkhayev, U.M.; Srivedavyasasri, R.; Ajayi, T.O.; Patsayev, A.K.; Kozykeyeva, R.A.; Ross, S.A. Isolation of Chemical Compounds and Essential Oil from Agrimonia asiatica Juz. and Their Antimicrobial and Antiplasmodial Activities. Sci. World J. 2020, 2020, 7821310. [Google Scholar] [CrossRef] [PubMed]
- Heredia Díaz, Y.; Tuenter, E.; Garcia-Díaz, J.; Ochoa Pacheco, A.; Cos, P.; Pieters, L.; Escalona Arranz, J.C. Novel flavonol-3-O-methylethers from Zanthoxylum pistaciifolium Griseb. (Rutaceae). Nat. Prod. Res. 2021, 36, 4869–4878. [Google Scholar] [CrossRef]
- Antinori, S.; Galimberti, L.; Milazzo, L.; Corbellino, M. Biology of human malaria plasmodia including Plasmodium knowlesi. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012013. [Google Scholar] [CrossRef]
- Singh, B.; Daneshvar, C. Human infections and detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 2013, 26, 165–184. [Google Scholar] [CrossRef]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Vuk, I.; Rajic, Z.; Zorc, B. Malaria and antimalarial drugs. Farm. Glas. 2008, 64, 51. [Google Scholar]
- Josling, G.A.; Llinás, M. Sexual development in Plasmodium parasites: Knowing when it’s time to commit. Nat. Rev. Microbiol. 2015, 13, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef]
- Castelli, F.; Tomasoni, L.R.; Matteelli, A. Advances in the treatment of malaria. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012064. [Google Scholar] [CrossRef] [PubMed]
- Menard, D.; Dondorp, A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef]
- Lipan, L.; Alexandru, M.; Dedu, A.; Viaşu, A.; Piţigoi, D.; Rafila, A. Current state of the epidemiology of malaria in Romania. Bacteriol. Virusol. Parazitol. Epidemiol. 2011, 56, 25–38. [Google Scholar] [PubMed]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. In Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 579–605. [Google Scholar] [CrossRef]
- Ortiz, S.; Vásquez-Ocmín, P.G.; Cojean, S.; Bouzidi, C.; Michel, S.; Figadère, B.; Grougnet, R.; Boutefnouchet, S.; Maciuk, A. Correlation study on methoxylation pattern of flavonoids and their heme-targeted antiplasmodial activity. Bioorg. Chem. 2020, 104, 104243. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Res 2017, 6, 750. [Google Scholar] [CrossRef]
- Andrade-Narváez, F.J.; Vargas-González, A.; Canto-Lara, S.B.; Damián-Centeno, A.G. Clinical picture of cutaneous leishmaniases due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Mem. Inst. Oswaldo Cruz. 2001, 96, 163–167. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.A.; Merlotto, M.R.; Ramos, P.M.; Marques, M.E.A. American tegumentary leishmaniasis: Severe side effects of pentavalent antimonial in a patient with chronic renal failure. An. Bras. De Dermatol. 2019, 94, 355–357. [Google Scholar] [CrossRef]
- Vasarri, M.; Barletta, E.; Vinci, S.; Ramazzotti, M.; Francesconi, A.; Manetti, F.; Degl’Innocenti, D. Annona cherimola Miller fruit as a promising candidate against diabetic complications: An in vitro study and preliminary clinical results. Foods 2020, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Solís, J.; Calzada, F.; Barbosa, E.; Valdés, M. Antihyperglycemic and antilipidemic properties of a tea infusion of the leaves from Annona cherimola miller on streptozocin-induced type 2 diabetic mice. Molecules 2021, 26, 2408. [Google Scholar] [CrossRef] [PubMed]
- Galani, V.J.; Patel, B.G.; Patel, N.B. Argyreia speciosa (Linn. f.) sweet: A comprehensive review. Pharmacogn. Rev. 2010, 4, 172–178. [Google Scholar] [CrossRef]
- Joseph, A.; Mathew, S.; Skaria, B.P.; Sheeja, E. Medicinal uses and biological activities of Argyreia speciosa sweet (Hawaiian baby woodrose)-an overview. Indian J. Nat. Prod. Resour. 2011, 2, 286–291. [Google Scholar]
- Ullah, M.; Khan, M.U.; Mahmood, A.; Malik, R.N.; Hussain, M.; Wazir, S.M.; Daud, M.; Shinwari, Z.K. An ethnobotanical survey of indigenous medicinal plants in Wana district south Waziristan agency, Pakistan. J. Ethnopharmacol. 2013, 150, 918–924. [Google Scholar] [CrossRef]
- Mali, R.G.; Mahajan, S.G.; Mehta, A.A. Phcog Rev.: Plant review Rakta Kanchan (Bauhinia variegata): Chemistry traditional and medicinal uses—A review. Pharmacogn. Rev. 2007, 1, 314–319. [Google Scholar]
- Dhale, D. Phytochemical screening and antimicrobial activity of Bauhinia variegata Linn. J. Ecobiotechnol. 2011, 3, 4–7. [Google Scholar]
- Akinpelu, D.A. Antimicrobial activity of Bryophyllum pinnatum leaves. Fitoterapia 2000, 71, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A. Bryophyllum pinnatum (Lam.) Kurz.: Phytochemical and pharmacological profile: A review. Pharmacogn. Rev. 2009, 3, 364. [Google Scholar]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Singab, A.N.B.; Wink, M. Metabolic Profiling of Buddleia indica Leaves using LC/MS and Evidence of their Antioxidant and Hepatoprotective Activity Using Different In Vitro and In Vivo Experimental Models. Antioxidants 2019, 8, 412. [Google Scholar] [CrossRef]
- Yao, R.Y.; Zou, Y.F.; Chen, X.F. Traditional Use, Pharmacology, Toxicology, and Quality Control of Species in Genus Bupleurum L. Chin. Herb. Med. 2013, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Li, Q.; Sun, S.; Wang, Y.; Lin, R.; Zhang, Y.; Dai, J.; Zheng, N. The Medicinal Plant Pair Bupleurum chinense-Scutellaria baicalensis—Metabolomics and Metallomics Analysis in a Model for Alcoholic Liver Injury. Front. Pharmacol. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Angelica, B.C.; Maria, R.A.V.A.; Gabino, A.M.G.; Patricia, S.S.M.; Rafael, P.P. The traditional medicinal and food uses of four plants in Oaxaca, Mexico. J. Med. Plants Res. 2011, 5, 3404–3411. [Google Scholar]
- Barbosa, E.; Calzada, F.; Campos, R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J. Ethnopharmacol. 2007, 109, 552–554. [Google Scholar] [CrossRef]
- Aponte, J.C.; Vaisberg, A.J.; Rojas, R.; Caviedes, L.; Lewis, W.H.; Lamas, G.; Sarasara, C.; Gilman, R.H.; Hammond, G.B. Isolation of Cytotoxic Metabolites from Targeted Peruvian Amazonian Medicinal Plants. J. Nat. Prod. 2008, 71, 102–105. [Google Scholar] [CrossRef]
- Tetik, F.; Civelek, S.; Cakilcioglu, U. Traditional uses of some medicinal plants in Malatya (Turkey). J. Ethnopharmacol. 2013, 146, 331–346. [Google Scholar] [CrossRef]
- Meckes, M.; Torres, J.; Calzada, F.; Rivera, J.; Camorlinga, M.; Lemus, H.; Rodríguez, G. Antibacterial Properties of Helianthemum glomeratum, a Plant Used in Maya Traditional Medicine to Treat Diarrhoea. Phytother. Res. 1997, 11, 128–131. [Google Scholar] [CrossRef]
- Yang, X.; Summerhurst, D.K.; Koval, S.F.; Ficker, C.; Smith, M.L.; Bernards, M.A. Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation. Phytother. Res. 2001, 15, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, J.; Yuan, W.; Li, M.; Yang, L.; Sun, Y.; Wang, X.; Zhao, Y. New triterpene saponins from flowers of Impatiens balsamina L. and their anti-hepatic fibrosis activity. J. Funct. Foods 2017, 33, 188–193. [Google Scholar] [CrossRef]
- Costa, S.S.; Muzitano, M.F.; Camargo, L.M.M.; Coutinho, M.A.S. Therapeutic Potential of Kalanchoe Species: Flavonoids and other Secondary Metabolites. Nat. Prod. Commun. 2008, 3, 1934578X0800301236. [Google Scholar] [CrossRef]
- Abdullah, N.; Hosseinpour Chermahini, D.S.; Chua, L.S.; Sarmidi, M. Labisia pumila: A review on its traditional, phytochemical and biological uses. World Appl. Sci. J. 2013, 27, 1297–1306. [Google Scholar] [CrossRef]
- Zakaria, A.A.; Noor, M.H.M.; Ahmad, H.; Hassim, H.A.; Mazlan, M.; Latip, M.Q.A. A Review on Therapeutic Effects of Labisia pumila on Female Reproductive Diseases. Biomed Res. Int. 2021, 2021, 9928199. [Google Scholar] [CrossRef] [PubMed]
- Zmeeva, O.; Kolomiets, N.; Abramets, N.; Bondarchuk, R. Lotus corniculatus L. is a perspective species of the genus Lotus L. 2017, 5–14. [Google Scholar]
- Gürağaç Dereli, F.T.; Khan, H.; Sobarzo-Sánchez, E.; Akkol, E.K. Antidepressant Potential of Lotus corniculatus L. subsp. corniculatus: An Ethnobotany Based Approach. Molecules 2020, 25, 1299. [Google Scholar] [CrossRef]
- Germano, M.; Sanogo, R.; Costa, C.; Fulco, R.; D’angelo, V.; Torre, E.; Viscomi, M.; De Pasquale, R. Hepatoprotective properties in the rat of Mitracarpus scaber (Rubiaceae). J. Pharm. Pharmacol. 1999, 51, 729–734. [Google Scholar] [CrossRef]
- Meite, S.; N’guessan, J.; Bahi, C.; Yapi, H.; Djaman, A.; Guina, F.G. Antidiarrheal activity of the ethyl acetate extract of Morinda morindoides in rats. Trop. J. Pharm. Res. 2009, 8, 201–207. [Google Scholar] [CrossRef]
- Mohammed, A.; Tam, D.N.H.; Vu, T.L.H.; Tieu, T.M.; Elfaituri, M.K.; Trinh, N.N.; Sagheir, E.A.; Tran, L.; Loc, T.T.H.; Low, S.K.; et al. Morinda morindoides: A systematic review of its therapeutic activities. South Afr. J. Bot. 2020, 131, 93–103. [Google Scholar] [CrossRef]
- Suriyaphan, O. Nutrition, health benefits and applications of Pluchea indica (L.) Less leaves. Mahidol Univ. J. Pharm. Sci. 2014, 41, 1–10. [Google Scholar]
- Vongsak, B.; Kongkiatpaiboon, S.; Jaisamut, S.; Konsap, K. Comparison of active constituents, antioxidant capacity, and α-glucosidase inhibition in Pluchea indica leaf extracts at different maturity stages. Food Biosci. 2018, 25, 68–73. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid Based Complement Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, K.; Kovacheva, N.; Vosman, B.; Zhang, L.; Rajapakse, S.; Atanassov, A.; Atanassov, I. Microsatellite analysis of Rosa damascena Mill. accessions reveals genetic similarity between genotypes used for rose oil production and old Damask rose varieties. Theor. Appl. Genet. 2005, 111, 804–809. [Google Scholar] [CrossRef]
- Dalfardi, B.; Heydari, M.; Golzari, S.E.J.; Mahmoudi Nezhad, G.S.; Hashempur, M.H. Al-Baghdadi’s description of venous blood circulation. Int. J. Cardiol. 2014, 174, 209–210. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa damascena as holy ancient herb with novel applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef]
- Nayebi, N.; Khalili, N.; Kamalinejad, M.; Emtiazy, M. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complement. Ther. Med. 2017, 34, 129–140. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, M.; Akoumianaki, A.; Bertsouklis, K.F. Propagation of Scabiosa hymettia (Boiss. & Spruner) by stem cuttings. Acta Hortic. 2019, 1242, 763–766. [Google Scholar]
- Siqueira, S.; dos Santos Falcão-Silva, V.; de Fátima Agra, M.; Dariva, C.; de Siqueira-Júnior, J.P.; Fonseca, M.J.V. Biological activities of Solanum paludosum Moric. extracts obtained by maceration and supercritical fluid extraction. J. Supercrit. Fluids 2011, 58, 391–397. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Ngbolua, K.; Tshibangu, D.; Mpiana, P.; Mihigo, S.; Mavakala, B.; Ashande, M.; Muanyishay, L. Anti-sickling and antibacterial activities of Some Extracts from Gardenia ternifolia subsp. jovis-tonantis (Welw.) Verdc.(Rubiaceae) and Uapaca heudelotii Baill.(Phyllanthaceae). Infection 2015, 4, 6. [Google Scholar] [CrossRef]
- Asante-Kwatia, E.; Gyimah, L.; Mensah, A.Y.; Sarpong, K.; Obeng, A.K. Authentication and quality control of Uapaca heudelotii Baill.—An investigation of pharmacognostic, phytochemical and physicochemical properties of its leaves and stem bark. Plant Sci. Today 2022, 9, 477–485. [Google Scholar] [CrossRef]
- Baviloni, P.D.; dos Santos, M.P.; Aiko, G.M.; de Lima Reis, S.R.; Latorraca, M.Q.; da Silva, V.C.; Dall’Oglio, E.L.; de Sousa Júnior, P.T.; Lopes, C.F.; Baviera, A.M.; et al. Mechanism of anti-hyperglycemic action of Vatairea macrocarpa (Leguminosae): Investigation in peripheral tissues. J. Ethnopharmacol. 2010, 131, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Yucca: A medicinally significant genus with manifold therapeutic attributes. Nat. Prod. Bioprospecting 2012, 2, 231–234. [Google Scholar] [CrossRef]
- Andrews, C.M.; Wyne, K.; Svenson, J.E. The use of traditional and complementary medicine for diabetes in rural Guatemala. J. Health Care Poor Underserved 2018, 29, 1188–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Woerdenbag, H.J. Traditional Chinese herbal medicine. Pharm. World Sci. 1995, 17, 103–112. [Google Scholar] [CrossRef]
- Gu, S.; Pei, J. Innovating Chinese Herbal Medicine: From Traditional Health Practice to Scientific Drug Discovery. Front. Pharmacol. 2017, 8, 381. [Google Scholar] [CrossRef]
- Gongwang, L. Chinese Herbal Medicine; Hua Xia Publishing House: Beijing, China, 1999. [Google Scholar]
- Bensky, D.; Barolet, R. Chinese Herbal Medicine—Formulas & Strategies; Eastland Press Inc.: Seattle, WA, USA, 1990. [Google Scholar]
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology. Biomed Res. Int. 2017, 2017, 7597596. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chang, W.L.; Lee, S.C.; Liu, Y.G.; Lin, H.C.; Chen, C.J.; Yen, C.Y.; Yu, D.S.; Lin, S.Z.; Harn, H.J. Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. 2003, 73, 2383–2394. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Liu, L.T.; Shieh, D.E.; Lin, C.C. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003, 69, 705–709. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, W.; Cao, S.; Jiang, X.; Yin, M.; Tang, W. Preparation of bupleurum nasal spray and evaluation on its safety and efficacy. Chem. Pharm. Bull. 2006, 54, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Y.; Di, H.Y.; Li, H.; Cheng, X.Q.; Zhang, Y.Y.; Chen, D.F. Bupleurum chinense DC polysaccharides attenuates lipopolysaccharide-induced acute lung injury in mice. Phytomedicine 2012, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Sun, H.M.; Xing, J.; Qin, X.M.; Du, G.H. Chemical and biological comparison of raw and vinegar-baked Radix Bupleuri. J. Ethnopharmacol. 2015, 165, 20–28. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, J.; Liu, J.; Li, Y.; Zhou, Y.; Mao, T.; Li, Z.; Qin, X.; Jin, S. Geranium wilfordii maxim.: A review of its traditional uses, phytochemistry, pharmacology, quality control and toxicology. J. Ethnopharmacol. 2022, 285, 114907. [Google Scholar] [CrossRef]
- Huang, M.; Yao, P.W.; Chang, M.D.; Ng, S.K.; Yu, C.H.; Zhang, Y.F.; Wen, M.L.; Yang, X.Y.; Lai, Y.K. Identification of anti-inflammatory fractions of Geranium wilfordii using tumor necrosis factor-alpha as a drug target on Herbochip®—An array-based high throughput screening platform. BMC Complement. Altern. Med. 2015, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Tilikidis, A. Chinese Herbal Therapy—Herbs and Formulas; Akadimia of Ancient Greek and Traditional Chinese Medicine Athens: Athens, Greece, 2017. (In Greek) [Google Scholar]
- Amiri, M.S.; Joharchi, M.R.; Nadaf, M.; Nasseh, Y. Ethnobotanical knowledge of Astragalus spp.: The world’s largest genus of vascular plants. Avicenna J. Phytomed. 2020, 10, 128–142. [Google Scholar]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J. Ethnopharmacol. 2002, 82, 131–145. [Google Scholar] [CrossRef]
- Chaudhary, L.B.; Rana, T.S.; Anand, K.K. Current status of the systematics of Astragalus L.(Fabaceae) with special reference to the Himalayan species in India. Taiwania 2008, 53, 338–355. [Google Scholar]
- Kumar, G.P.; Gupta, S.; Murugan, M.P.; Bala Singh, S. Ethnobotanical studies of Nubra Valley-A cold arid zone of Himalaya. Ethnobot. Leafl. 2009, 2009, 9. [Google Scholar]
- Ghasemi Pirbalouti, A.; Momeni, M.; Bahmani, M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan Districts, Ilam Province, Iran. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 368–385. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.F.; Martin, K.; Travis, L. Prehistoric plant use at beaver creek rock shelter, Southwestern Montana, USA. Ethnobot. Res. Appl. 2014, 12, 355–384. [Google Scholar] [CrossRef][Green Version]
- Chermat, S.; Gharzouli, R. Ethnobotanical study of medicinal flora in the North East of Algeria-An empirical knowledge in Djebel Zdimm (Setif). J. Mater. Sci. Eng. 2015, 5, 50–59. [Google Scholar]
- Lakhdari, W.; Dehliz, A.; Acheuk, F.; Mlik, R.; Hammi, H.; DOUMANDJI-MITICHE, B.; Gheriani, S.; Berrekbia, M.; Guermit, K.; Chergui, S. Ethnobotanical study of some plants used in traditional medicine in the region of Oued Righ (Algerian Sahara). J. Med. Plants 2016, 4, 204–211. [Google Scholar]
- Sun, L.; Liao, L.; Wang, B. Potential Antinociceptive Effects of Chinese Propolis and Identification on Its Active Compounds. J. Immunol. Res. 2018, 2018, 5429543. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, M.; Shou, Q.; Li, Y.; Hu, F. Biological activities of chinese propolis and brazilian propolis on streptozotocin-induced type 1 diabetes mellitus in rats. Evid. -Based Complement. Altern. Med. 2011, 2011, 468529. [Google Scholar] [CrossRef]
- Periferakis, A.; Bolocan, A.; Ion, D. A Review of Innovation in Medicine. Technol. Innov. Life Sci. 2022, 1, 42–48. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Scheau, C.; Mihai, L.; Bădărău, I.; Caruntu, C. Emerging applications of some important natural compounds in the field of oncology. Farmacia 2020, 68, 984–991. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).