Breast Cancer Vaccine Containing a Novel Toll-like Receptor 7 Agonist and an Aluminum Adjuvant Exerts Antitumor Effects
Abstract
:1. Introduction
2. Results
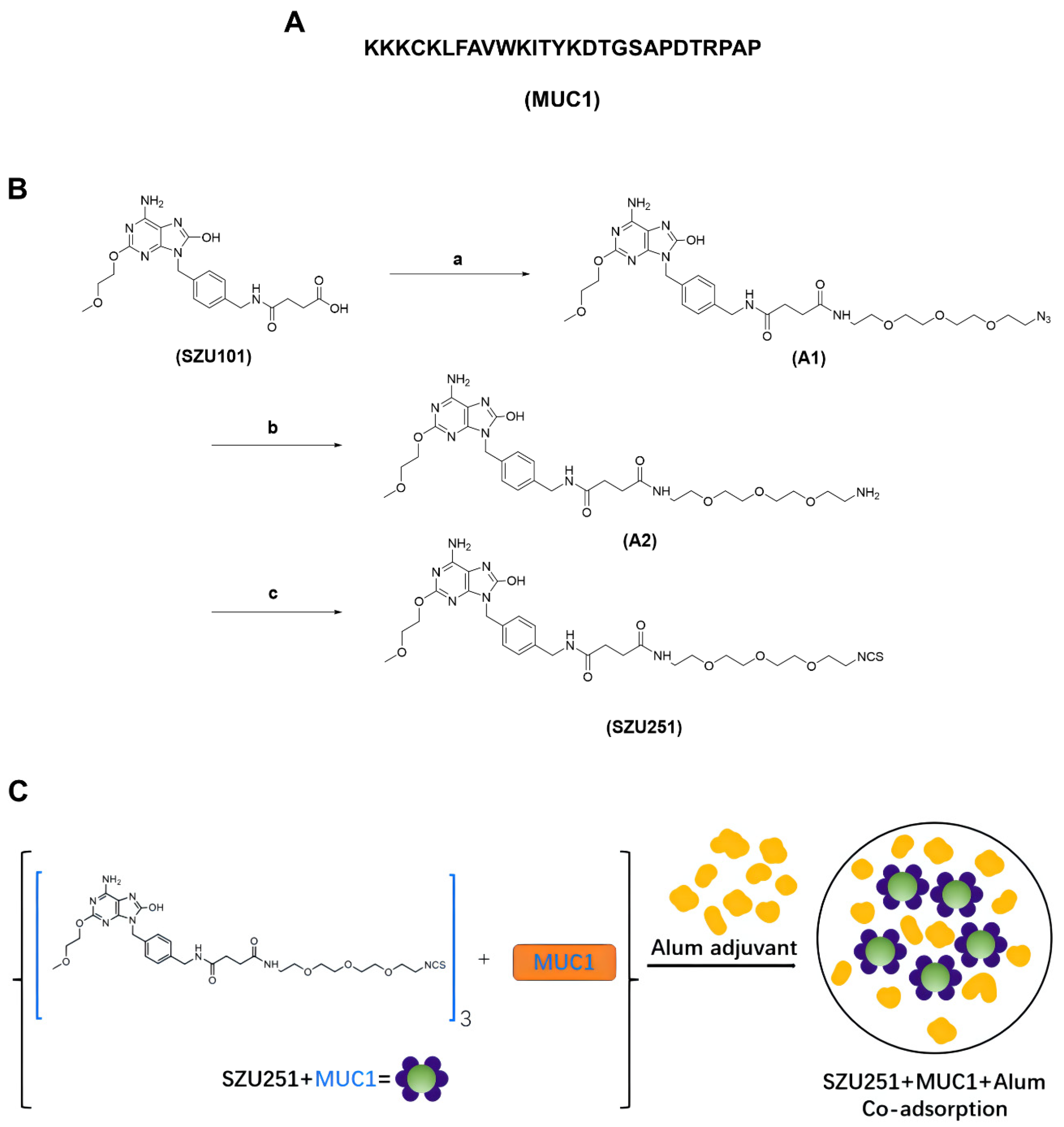
2.1. Chemical Synthesis of the Vaccine Candidates
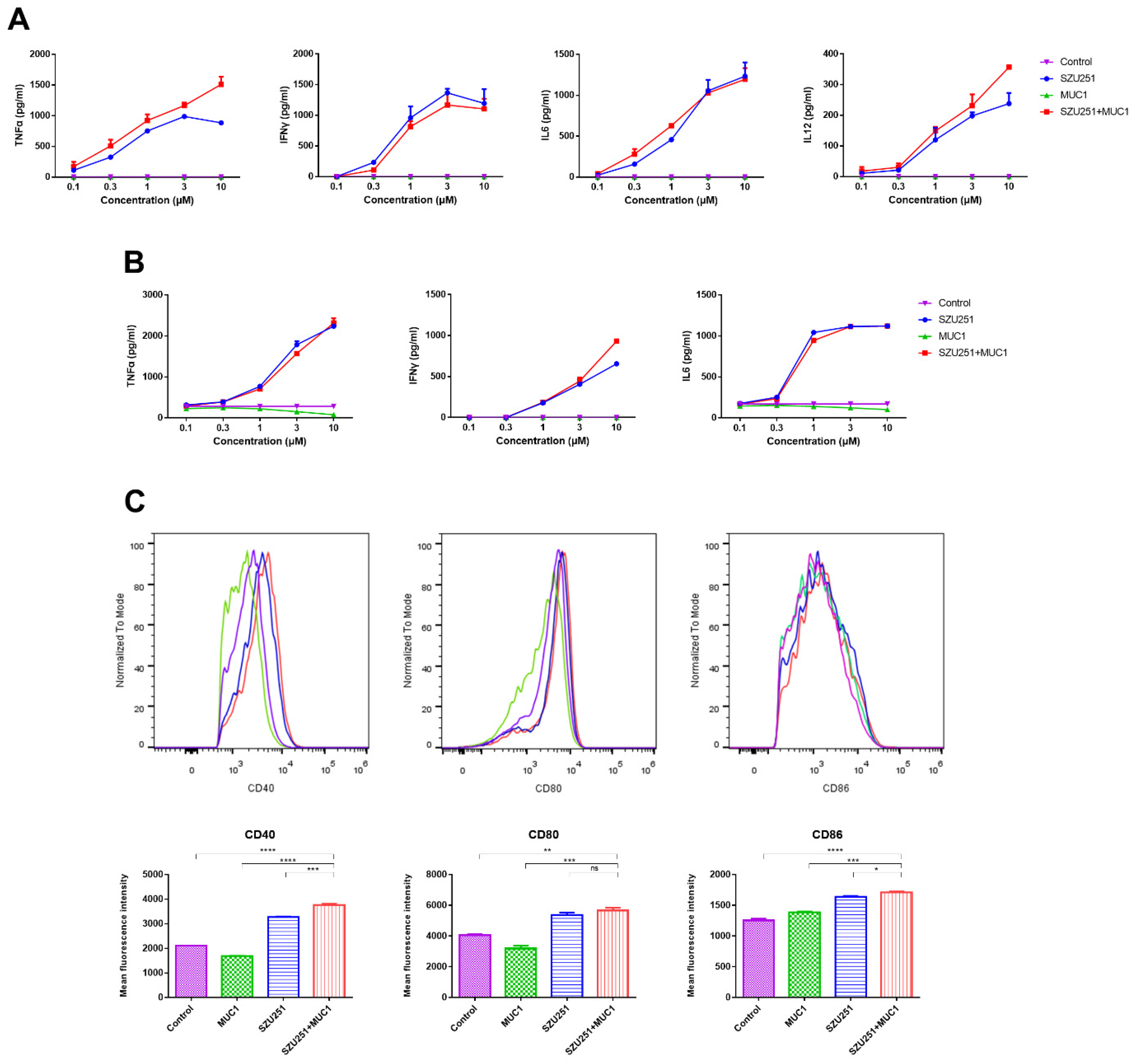
2.2. In Vitro Immunostimulatory Responses of the Vaccine Candidates
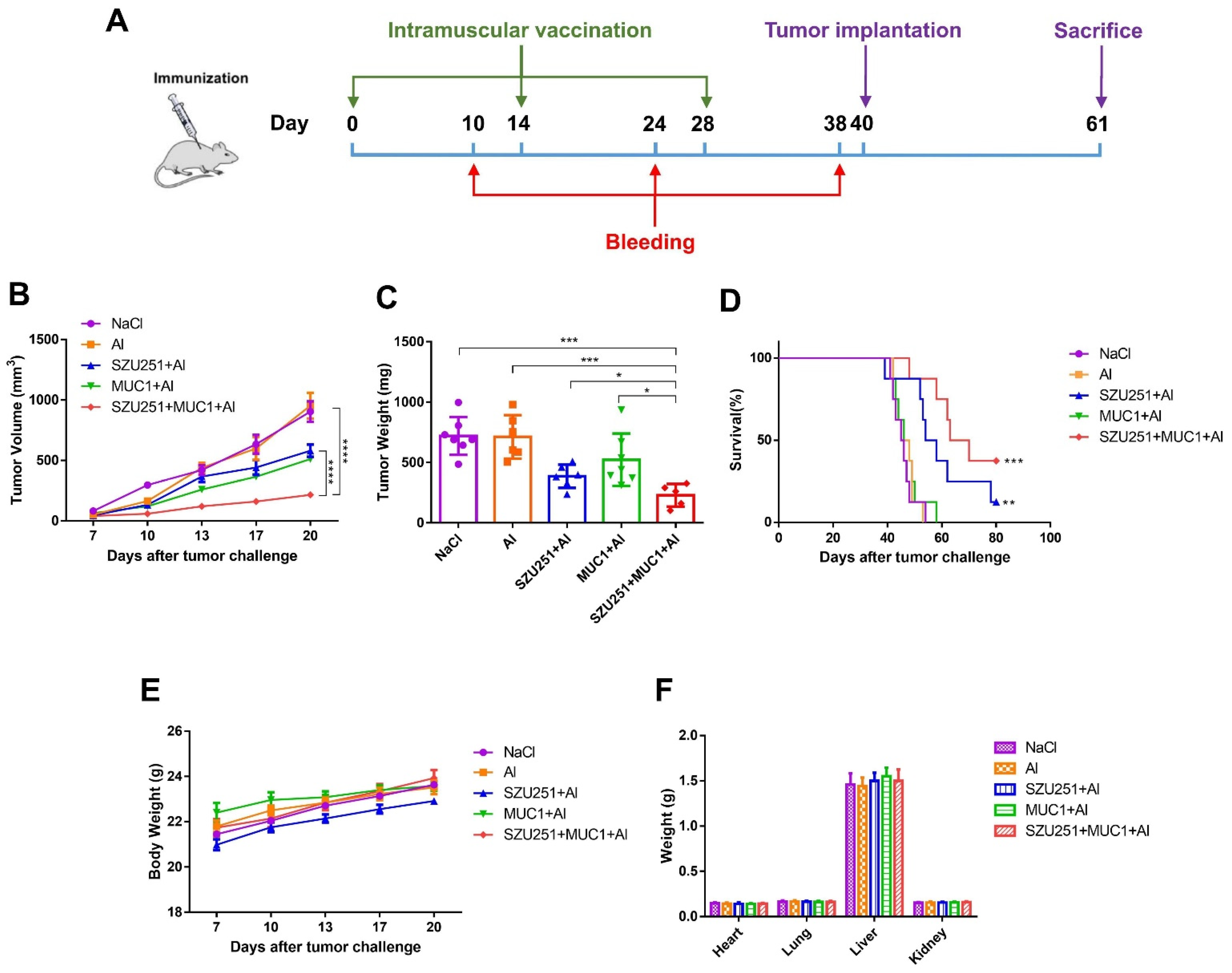
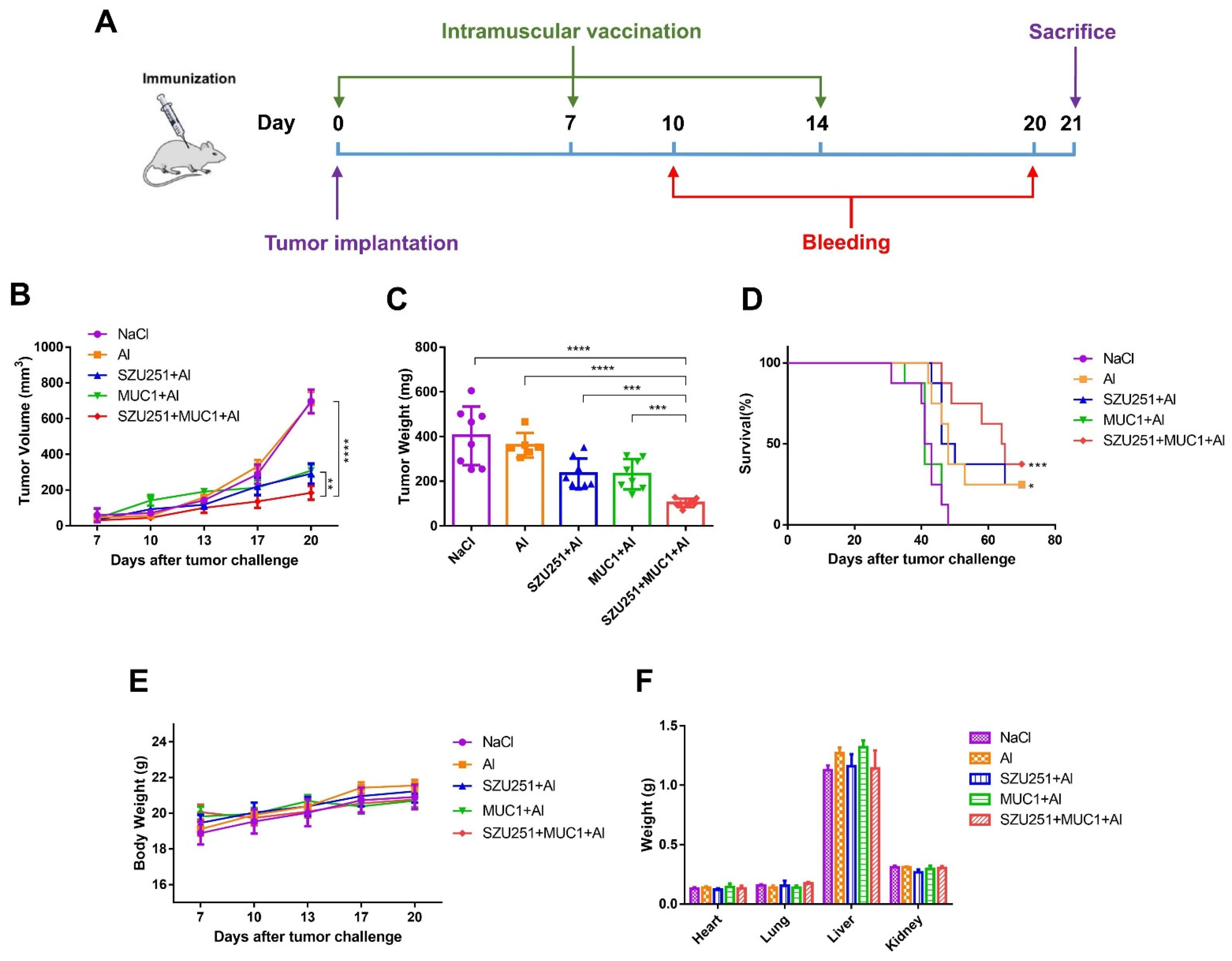
2.3. SZU251 + MUC1 + Al Inhibited 4T1 Mouse Breast Tumor Growth in the Prophylactic Schedule
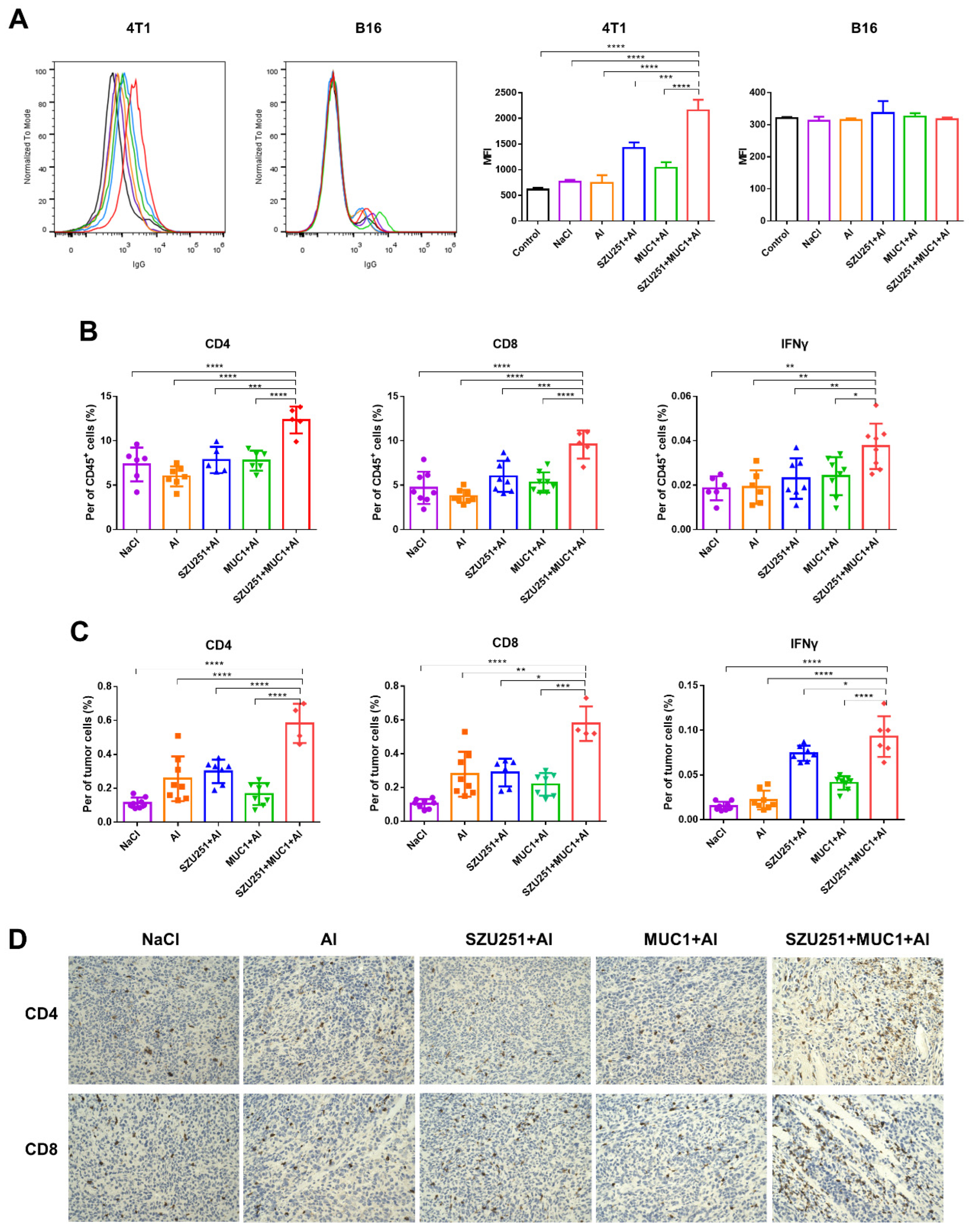
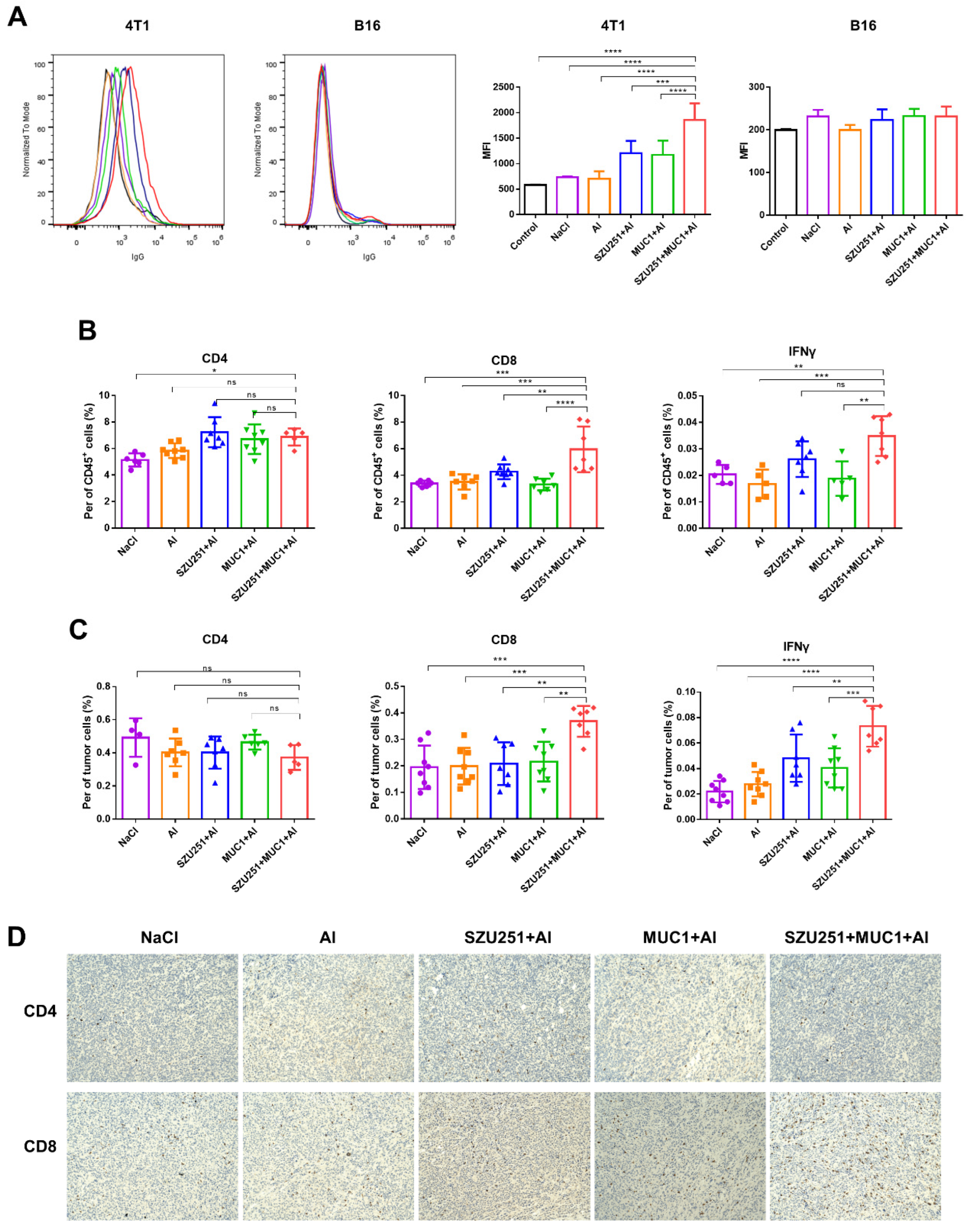
2.4. SZU251 + MUC1 + Al Induced Tumor-Specific Immune Responses in the Prophylactic Schedule
2.5. SZU251 + MUC1 + Al Inhibited 4T1 Mouse Breast Tumor Growth in the Therapeutic Schedule
2.6. SZU251 + MUC1 + Al Induced Tumor-Specific Immune Responses in the Therapeutic Schedule
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Mice and Cell Lines
4.3. HEK-Blue Assay
4.4. BMDCs Preparation
4.5. BMDCs Maturation
4.6. Spleen Lymphocytes Preparation
4.7. Analysis of Cytokine Levels by ELISA
4.8. Immunization of Mice
4.9. Analysis of Antibody Titers and Subtypes by ELISA
4.10. Analysis of Antigen Recognition Ability
4.11. Evaluation of Antitumor Effects in Vaccinated Mice
4.12. Detection of T Lymphocytes
4.13. Immunohistochemistry (IHC) Staining
4.14. Toxicity Studies
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokhtari, Y.; Pourbagheri-Sigaroodi, A.; Zafari, P.; Bagheri, N.; Ghaffari, S.H.; Bashash, D. Toll-like receptors (TLRs): An old family of immune receptors with a new face in cancer pathogenesis. J. Cell. Mol. Med. 2021, 25, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Petes, C.; Odoardi, N.; Gee, K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front. Immunol. 2017, 8, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, R.; Jiao, A.; Zhang, B. Targeting toll-like receptors on T cells as a therapeutic strategy against tumors. Int. Immunopharmacol. 2022, 107, 108708. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Que, H.; Chen, L.; Lan, T.; Hong, W.; He, C.; Yang, J.; Wei, Y.; Wei, X. Lymph-Node-Targeted Cholesterolized TLR7 Agonist Liposomes Provoke a Safe and Durable Antitumor Response. Nano Lett. 2021, 21, 7960–7969. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.L.; Kheirolomoom, A.; Fite, B.Z.; Wu, B.; Lau, K.; Baikoghli, M.; Raie, M.N.; Tumbale, S.K.; Foiret, J.; et al. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2021, 330, 1080–1094. [Google Scholar] [CrossRef]
- Narayanan, J.S.S.; Ray, P.; Hayashi, T.; Whisenant, T.C.; Vicente, D.; Carson, D.A.; Miller, A.M.; Schoenberger, S.P.; White, R.R. Irreversible Electroporation Combined with Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol. Res. 2019, 7, 1714–1726. [Google Scholar] [CrossRef]
- Wang, X.; Yu, B.; Cao, B.; Zhou, J.; Deng, Y.; Wang, Z.; Jin, G. A chemical conjugation of JQ-1 and a TLR7 agonist induces tumoricidal effects in a murine model of melanoma via enhanced immunomodulation. Int. J. Cancer 2021, 148, 437–447. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Diao, Y.; Gao, N.; Wan, Y.; Zhong, J.; Zheng, H.; Wang, Z.; Jin, G. Gastric cancer vaccines synthesized using a TLR7 agonist and their synergistic antitumor effects with 5-fluorouracil. J. Transl. Med. 2018, 16, 120. [Google Scholar] [CrossRef] [Green Version]
- Beatson, R.E.; Taylor-Papadimitriou, J.; Burchell, J.M. MUC1 immunotherapy. Immunotherapy 2010, 2, 305–327. [Google Scholar] [CrossRef]
- Rashidijahanabad, Z.; Huang, X. Recent advances in tumor associated carbohydrate antigen based chimeric antigen receptor T cells and bispecific antibodies for anti-cancer immunotherapy. Semin. Immunol. 2020, 47, 101390. [Google Scholar] [CrossRef]
- Stergiou, N.; Urschbach, M.; Gabba, A.; Schmitt, E.; Kunz, H.; Besenius, P. The Development of Vaccines from Synthetic Tumor-Associated Mucin Glycopeptides and their Glycosylation-Dependent Immune Response. Chem. Rec. 2021, 21, 3313–3331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.H.; Li, Y.T.; Zhang, R.Y.; Liu, Y.L.; You, Z.W.; Bian, M.M.; Wen, Y.; Wang, J.; Du, J.J.; Guo, J. Alum Adjuvant and Built-in TLR7 Agonist Synergistically Enhance Anti-MUC1 Immune Responses for Cancer Vaccine. Front. Immunol. 2022, 13, 857779. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.J. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. USA 2012, 109, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tang, L.; Gao, N.; Diao, Y.; Zhong, J.; Deng, Y.; Wang, Z.; Jin, G.; Wang, X. Synthetic MUC1 breast cancer vaccine containing a Toll-like receptor 7 agonist exerts antitumor effects. Oncol. Lett. 2020, 20, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-associated O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Quoix, E.; Lena, H.; Losonczy, G.; Forget, F.; Chouaid, C.; Papai, Z.; Gervais, R.; Ottensmeier, C.; Szczesna, A.; Kazarnowicz, A.; et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): Results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016, 17, 212–223. [Google Scholar] [CrossRef]
- Guckel, B.; Rentzsch, C.; Nastke, M.D.; Marme, A.; Gruber, I.; Stevanovic, S.; Kayser, S.; Wallwiener, D. Pre-existing T-cell immunity against mucin-1 in breast cancer patients and healthy volunteers. J. Cancer Res. Clin. Oncol. 2006, 132, 265–274. [Google Scholar] [CrossRef]
- Fremd, C.; Stefanovic, S.; Beckhove, P.; Pritsch, M.; Lim, H.; Wallwiener, M.; Heil, J.; Golatta, M.; Rom, J.; Sohn, C.; et al. Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. Oncoimmunology 2016, 5, e1057387. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef]
- Beesu, M.; Salyer, A.C.; Brush, M.J.; Trautman, K.L.; Hill, J.K.; David, S.A. Identification of High-Potency Human TLR8 and Dual TLR7/TLR8 Agonists in Pyrimidine-2,4-diamines. J. Med. Chem. 2017, 60, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, S.W.; Vandenberghe, P.; de Boer, M.; Ceuppens, J.L. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol. Rev. 1996, 153, 47–83. [Google Scholar] [CrossRef] [PubMed]
- Guy, B. The perfect mix: Recent progress in adjuvant research. Nat. Rev. Microbiol. 2007, 5, 505–517. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Akilesh, H.M.; Buechler, M.B.; Duggan, J.M.; Hahn, W.O.; Matta, B.; Sun, X.; Gessay, G.; Whalen, E.; Mason, M.; Presnell, S.R.; et al. Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes. Science 2019, 363, eaao5213. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Egawa, S.; Cai, Q.; Kalinska, A.; Bykovskaya, S.N.; Lotze, M.T.; Kapsenberg, M.L.; Storkus, W.J.; Kalinski, P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: Helper role of CD8+ T cells in the development of T helper type 1 responses. J. Exp. Med. 2002, 195, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Zaragoza, M.; Hernández-Ávila, R.; Viedma-Rodríguez, R.; Arenas-Aranda, D.; Ostoa-Saloma, P. Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (Review). Oncol. Rep. 2015, 34, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Sato-Kaneko, F.; Yao, S.; Ahmadi, A.; Zhang, S.S.; Hosoya, T.; Kaneda, M.M.; Varner, J.A.; Pu, M.; Messer, K.S.; Guiducci, C.; et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017, 2, e93397. [Google Scholar] [CrossRef]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef] [PubMed]
| Group | NaCl | Al | SZU251 + Al | MUC1 + Al | SZU251 + MUC1 + Al |
|---|---|---|---|---|---|
| WBC (109/L) | 30.05 ± 4.96 | 28.70 ± 9.49 | 27.68 ± 7.59 | 18.63 ± 5.74 | 13.85 ± 3.24 |
| RBC (1012/L) | 11.17 ± 0.56 | 11.64 ± 0.95 | 11.74 ± 0.69 | 11.07 ± 0.71 | 11.40 ± 0.49 |
| HGB (g/L) | 175.20 ± 7.73 | 186.50 ± 9.33 | 181.25 ± 5.56 | 171.20 ± 10.69 | 169.00 ± 8.69 |
| HCT (%) | 55.40 ± 2.42 | 57.45 ± 4.62 | 55.24 ± 3.23 | 55.36 ± 3.23 | 54.12 ± 2.91 |
| MCV (fL) | 49.66 ± 0.47 | 49.40 ± 0.86 | 47.11 ± 0.98 | 50.08 ± 1.00 | 47.52 ± 1.08 |
| PLT (109/L) | 1022.67 ± 168.50 | 1045.50 ± 258.30 | 957.50 ± 208.63 | 795.50 ± 138.85 | 809.00 ± 43.70 |
| Antibody | Titer | |||||
|---|---|---|---|---|---|---|
| NaCl | Al | SZU251 + Al | MUC1 + Al | SZU251 + MUC1 + Al | ||
| IgG | Day 10 | 0 | 0 | 0 | 1000 | 16,000 |
| Day 24 | 0 | 0 | 0 | 32,000 | 1,024,000 | |
| Day 38 | 0 | 0 | 0 | 256,000 | 2,048,000 | |
| IgG1 | Day 10 | 0 | 0 | 0 | 0 | 0 |
| Day 24 | 0 | 0 | 0 | 8000 | 64,000 | |
| Day 38 | 0 | 0 | 0 | 8000 | 128,000 | |
| IgG2a | Day 10 | 0 | 0 | 0 | 0 | 32,000 |
| Day 24 | 0 | 0 | 0 | 16,000 | 2,048,000 | |
| Day 38 | 0 | 0 | 0 | 16,000 | 2,048,000 | |
| IgM | Day 10 | 0 | 0 | 0 | 0 | 16,000 |
| Day 24 | 0 | 0 | 0 | 4000 | 128,000 | |
| Day 38 | 0 | 0 | 0 | 16,000 | 128,000 | |
| Group | NaCl | Al | SZU251 + Al | MUC1 + Al | SZU251 + MUC1 + Al |
|---|---|---|---|---|---|
| WBC (109/L) | 13.33 ± 0.78 | 9.68 ± 5.12 | 15.48 ± 5.31 | 12.60 ± 2.52 | 15.30 ± 3.48 |
| RBC (1012/L) | 9.85 ± 0.40 | 10.00 ± 0.94 | 9.57 ± 0.33 | 8.70 ± 0.46 | 9.34 ± 0.81 |
| HGB (g/L) | 144.80 ± 4.15 | 142.00 ± 7.00 | 144.40 ± 7.54 | 134.00 ± 5.29 | 140.20 ± 9.40 |
| HCT (%) | 44.83 ± 1.35 | 45.93 ± 3.95 | 43.86 ± 1.95 | 40.56 ± 1.70 | 43.34 ± 3.67 |
| MCV (fL) | 46.92 ± 0.47 | 46.03 ± 0.76 | 45.77 ± 0.21 | 46.98 ± 0.89 | 45.87 ± 0.35 |
| PLT (109/L) | 739.40 ± 128.80 | 633.80 ± 288.80 | 1056.00 ± 214.99 | 933.40 ± 188.03 | 956.75 ± 231.80 |
| Antibody | Titer | |||||
|---|---|---|---|---|---|---|
| NaCl | Al | SZU251 + Al | MUC1 + Al | SZU251 + MUC1 + Al | ||
| IgG | Day 10 | 0 | 0 | 0 | 32,000 | 64,000 |
| Day 20 | 0 | 0 | 0 | 128,000 | 1,024,000 | |
| IgG1 | Day 10 | 0 | 0 | 0 | 0 | 0 |
| Day 20 | 0 | 0 | 0 | 32,000 | 32,000 | |
| IgG2a | Day 10 | 0 | 0 | 0 | 32,000 | 64,000 |
| Day 20 | 0 | 0 | 0 | 32,000 | 1,024,000 | |
| IgM | Day 10 | 0 | 0 | 0 | 8000 | 128,000 |
| Day 20 | 0 | 0 | 0 | 32,000 | 256,000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, Y.; Zhou, J.; Wang, J.; Jin, G.; Wang, X. Breast Cancer Vaccine Containing a Novel Toll-like Receptor 7 Agonist and an Aluminum Adjuvant Exerts Antitumor Effects. Int. J. Mol. Sci. 2022, 23, 15130. https://doi.org/10.3390/ijms232315130
Zhang S, Liu Y, Zhou J, Wang J, Jin G, Wang X. Breast Cancer Vaccine Containing a Novel Toll-like Receptor 7 Agonist and an Aluminum Adjuvant Exerts Antitumor Effects. International Journal of Molecular Sciences. 2022; 23(23):15130. https://doi.org/10.3390/ijms232315130
Chicago/Turabian StyleZhang, Shuquan, Yu Liu, Ji Zhou, Jiaxin Wang, Guangyi Jin, and Xiaodong Wang. 2022. "Breast Cancer Vaccine Containing a Novel Toll-like Receptor 7 Agonist and an Aluminum Adjuvant Exerts Antitumor Effects" International Journal of Molecular Sciences 23, no. 23: 15130. https://doi.org/10.3390/ijms232315130
APA StyleZhang, S., Liu, Y., Zhou, J., Wang, J., Jin, G., & Wang, X. (2022). Breast Cancer Vaccine Containing a Novel Toll-like Receptor 7 Agonist and an Aluminum Adjuvant Exerts Antitumor Effects. International Journal of Molecular Sciences, 23(23), 15130. https://doi.org/10.3390/ijms232315130





