Abstract
Cells have the ability to communicate with their immediate and distant neighbors through the release of extracellular vesicles (EVs). EVs facilitate intercellular signaling through the packaging of specific cargo in all type of cells, and perturbations of EV biogenesis, sorting, release and uptake is the basis of a number of disorders. In this review, we summarize recent advances of the complex roles of the sphingolipid ceramide and lysosomes in the journey of EV biogenesis to uptake.
1. Introduction
Extracellular vesicles (EVs) are secretory components that are well known as biologic communicatory systems that contain multiple signaling molecules, including RNAs, proteins, and lipids. Based on the size and origin of biogenesis, EVs are classified into three groups: exosomes, microvesicles (MVs) and apoptotic bodies [1]. During multivesicular body (MVB) formation, depending on nature of the cargo that is packaged into the early endosome, intraluminal vesicles (ILVs) can end up for targeted cargo degradation or be released as exosomes from the cell [2]. Both mechanisms are regulated by endosomal sorting complex required for transport (ESCRT)-dependent and -independent mechanisms, and these vesicles are in a crosstalk with lysosomes, autophagosomes, Golgi apparatus, mitochondria and other organelles [3,4]. Lysosomes are the main recipients of ILVs trapped inside MVBs. These organelles can degrade the inner content of the multivesiclar body or release exosomes during lysosomal exocytosis [5]. Ceramides have a key role in EV biogenesis. Ceramides are a diverse family of structural and bioactive lipid molecules that are essential for cell function [6]. As ceramides are the building blocks of bilayers membrane in all vesicles, many researchers have investigated the role of ceramides in EVs biogenesis. In this review we will focus on the role of ceramides in EV biogenesis, release and their effects in recipient cells.
2. Ceramides
2.1. Ceramide Structure
Ceramides are the principal sphingolipid metabolites composed of a Fatty Acid (FA) linked via an amide bond to a long-chain aliphatic amino alcohol or sphingoid base (sphingosine, phytosphingosine, dihydrosphingosine, or 6-hydroxysphingosine). Ceramides are a diverse family of structures that vary on FA chain length (medium chain, C12 to C14; long chain, C16 to C18; very-long chain, C20 to C24; ultra-long chain, ≥C26) and saturation, as well as by the length of the sphingoid base and the modification such as the presence of hydroxyl group on sphingoid backbone [7]. To date, more than 1500 structurally different ceramides and additional ceramide subclasses have been identified and many more are currently discovered due to continuous advances in mass spectrometry approaches and protocols [8,9].
2.2. Biogenesis of Ceramides: Role of Endoplasmic Reticulum, Golgi and Lysosome
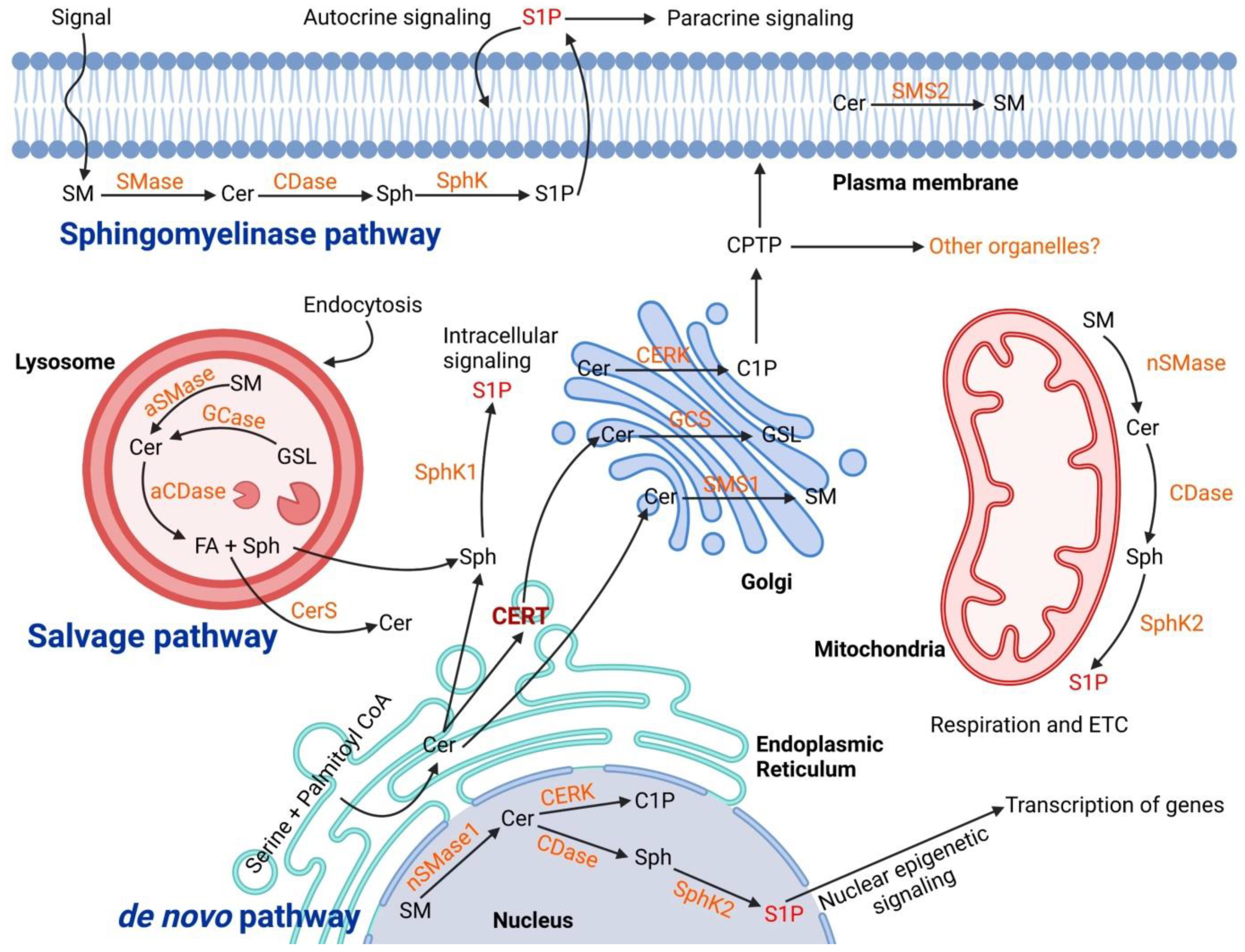
Sphingolipid metabolism is extremely complex and is regulated by the activity of more than 40 enzymes [10]. Ceramide is the core of sphingolipid metabolism and can be produced or utilized through several pathways. There are three main synthesis pathways of ceramide: the de novo pathway, the sphingomyelinase (SMase) pathway (or catabolic pathway), and the salvage pathway (Figure 1).
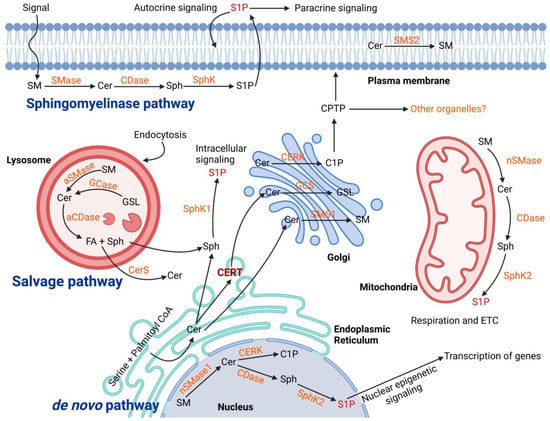
Figure 1.
Biogenesis of ceramide in cellular organelles and the complex biochemical pathways in downstream. The ceramide main synthesis routes are De novo in the Endoplasmic Reticulum, Salvage pathway in Lysosome, and Sphingomyelinase both in lysosome and plasma membrane. The transfer of ceramides to the Golgi is mediated through ceramide transfer protein (CERT) within vesicles. In the Golgi, different classes of sphingolipid are synthesized and transported to the cellular sites of action. In the plasma membrane and lysosomes, the main catabolic pathways of sphingolipids are “sphingomyelinase (SMase)” to produce ceramides. The produced ceramides go under further metabolism to generate other sphingolipid species such as sphingosine (Sph), sphingosine-1 phosphate (S1P) and also sphingomyelin (SM). Abbreviation: Acid Ceramidase (aCDase), Ceramidase (CDase), Ceramide Kinase (CERK), Ceramide Synthase (CerS), Glucocerebrosidase (GCase), Glucosylceramide Synthase (GCS), Glycosphingolipid (GSL), Sphingomyelin Synthase (SMS).
The de novo pathway originates in the endoplasmic reticulum by the condensation of serine and palmitoyl CoA, catalyzed by serine palmitoyltransferase (SPT), to produce 3-ketodihydrosphingosine that is then converted by the 3-ketodihydrosphingosine reductase to dihydrosphingosine (sphinganine). Sphinganine is then acylated to dihydroceramide by ceramide synthase, which is later desaturated by dihydroceramide desaturase to form ceramides [11]. Ceramides can be delivered from ER to the Golgi apparatus either by a vesicular route (vesicular transport) or nonvesicular transport by specific translocation via the action of ceramide transfer protein (CERT) [12]. Ceramide transported to the Golgi complex is further modified by the addition of several head groups to form different classes of complex sphingolipids such as sphingomyelin (ceramide from vesicular transport) and glycosphingolipids (ceramide from nonvesicular transport or CERT) [10]. In the Golgi compartment, ceramides that serve as the substrate for sphingomyelins flip across membrane leaflets whereas the synthesis of glucosylceramide from ceramide happens on the cytosolic side of the Golgi and then glucosylceramides flip for the synthesis of higher glycosphingolipids [13]. However, the machinery involved in this flipping is not yet well defined. Sphingomyelin (major product of ceramide conversion) is obtained by the activity of sphingomyelin synthase enzymes SMS1 and SMS2 [14]. SMS1 is a Golgi enzyme and SMS2 is present in the plasma membrane. The lipids are then transferred to the plasma membrane (the major cellular reservoir) via the vesicular transport pathway or lipid-transfer proteins.
The SMase pathway is a sphingolipid catabolic pathway that produces phosphocholine and ceramide at the plasma membrane of cells or in lysosomes by the activity of SMase [15]. Constitutive catabolism occurs in the late endosomes and lysosomes where ceramides can be additionally metabolized by acid ceramidases to generate sphingosine (Sph) and free FA by the enzymatic activity of ceramidases [16]. FAs and sphingosine can then leave the lysosome and Sph can be phosphorylated by Sph kinases (SphK) to form sphingosine-1 phosphate (S1P) [17]. Ceramide in the lysosomal compartment can also be produced from the breakdown of globoside by hexosaminidase from sulfatide that loses a sulfate group by arylsulfatase A and from ganglisoides GM1 that loses one unit of galactose. Ceramides, FAs and sugar (obtained by the catabolism of glycosphingolipids) are transferred from the lysosomes through specific membrane proteins and reach once again the Golgi apparatus where they are used in the biosynthetic pathway [18]. Several reports have revealed the role of lysosomes for sphingolipid metabolism and its importance for cell physiology [17]. In particular, many researchers have focused their studies on the metabolism of ceramide in lysosome and sphingolipid storage disorders such as Sandhoff disease, Fabry disease, Gaucher disease, Krabbe disease, Niemann Pick disease, Farber disease and preeclampsia [19,20].
Ceramides can also be generated by “salvage pathway or recycling pathway” in the late endosomes and lysosomes through recycling of sphingosine. In the salvage pathway, the long-chain sphingoid base sphingosine produced from the metabolism of complex sphingolipids is converted to ceramide through the action of a number of enzymes including SMases, cerebrosidases, ceramidases, and ceramide synthases [13].
2.3. Physiopathological Function of Ceramides
Sphingolipids are versatile molecules that have vital roles as structural components of cell membranes and as signal transduction molecules involved in regulation of many of the most critical cellular events, including growth, differentiation, programmed cell death and tumorigenesis. Ceramides are fundamental precursors of complex sphingolipids and components of cell membranes. Ceramides, in particular, long chain and very-long chain ones are the major lipid class present in the human stratum corneum barrier, the remotest layer of the epidermis that safeguards underlying tissue from mechanical stress, dryness, illness, and chemicals [21]. Ceramides are also the main component of a particular group of lipid membrane microdomains termed “ceramide-enriched membrane domains” that are involved in the induction and magnification of receptor and stress-mediated signaling in the majority of cell types [22]. More fundamentally, ceramides are also important bioactive lipids involved in cellular regulatory circuits. Indeed, the heterogeneity of ceramides, as well as the flexibility of sphingolipid metabolism, suggests a variety of possible mechanisms to regulate cell fate and functions. Several recent pieces of evidence have indicated that ceramides are critical mediators of various cell death pathways, including apoptosis, autophagy, necrosis, and necroptosis [10,20,23,24].
Altered levels of ceramides trigger signaling pathways leading to cell death in various cell types. For instance, elevation of ceramide levels in the lung result in endothelial cell death, which is linked to the development of emphysema-like disease in murine models [25]. Rotstein et al. showed that ceramides would provoke apoptosis and inflammation in endothelial and retinal pigmented epithelium cells, which bring to several retinopathies [26]. Similarly, treatment of fetal rat lung epithelial cells with C16 ceramide induces autophagy and apoptosis in a temporal pattern [27]. Recent research has also indicated that ceramide can selectively regulate cell fate differently in the placenta trophoblast layers. High levels of ceramide bring excessive autophagy that leads to Type II cell-death in syncytiotrophoblast layer [20,23]. Instead, in the cytotrophoblast layer the sphingolipid accumulation can activate the homeostatic process of mitophagy or caspase-independent type III programmed cell death [28].
Altered ceramide metabolism has been documented in several types of cancers [29] and several studies suggest that there are diverse and controversial functions of endogenously generated ceramides in malignant cells. For example, C16 ceramide has been implicated in cancer cell proliferation, whereas C18 ceramide mediates cell death [30]. Due to its importance in tumorigenesis, some of the metabolic enzymes of ceramide synthesis are the targets of current chemotherapy drugs [31].
Ceramides can regulate a variety of pathways that promote inflammation. Ceramide inhibits the Akt pathway by activation of PP2A or PKCζ, thereby blocking insulin signaling which leads to insulin resistance and decreased cell survival [32,33]. Moreover, ceramides can influence innate immune responses regulating autophagy, promote the processing and secretion of the pro-inflammatory cytokines IL-1β and IL-18, and induce apoptosis [34]. Furthermore, numerous age-related, neurological, and neuroinflammatory diseases are shown to be regulated by intracellular levels of ceramide [35].
Several pieces of evidence have indicated that ceramide levels are important for lung development to maintain lung cell homeostasis and correct host response to airway microbial infections [36]. Furthermore, the lipids play a pivotal role in pulmonary fibrosis, chronic obstructive pulmonary disease (COPD), and asthma [37]. Ceramide accumulation in blood vessels and heart is present in subjects with cardiovascular disease (such as hypertension and heart failure) [38] and plasma and placentae from a pregnancy complicated by preeclampsia [20].
3. Extracellular Vesicles (EVs)
3.1. EV Biogenesis and Cargo Loading
EVs represent a heterogenous population of vesicles that are classified into three groups typically based on their size and biogenesis: exosomes (30–200 nm), MVs (100–1000 nm) and apoptotic bodies (>1000 nm) [1]. Here, we use EV as generic term for all secreted vesicles from cells, since most research described in the literature has employed heterogeneous populations of EVs and often failed to fully characterize the isolated EVs [1,39]. Although several molecular families and organelles have been shown to have important roles in the EV biogenesis (e.g., Rab, ESCRT, ceramide, syntenin, syndecans, tetraspanins, etc.), trafficking (e.g., Rab, actin, etc.), and fusion of MVBs with the plasma membrane (e.g., SNAREs), the focus of this review is on sphingolipid ceramide as one of the lipids critical for EV formation as well as lysosomes as the main organelle for the biogenesis, cargo loading and release of EVs.
Even though the most distinguishable hallmark of EV biogenesis is the involvement of the ESCRT pathway, EVs can be generated through an ESCRT-independent pathway. Packaging of endocytosed parts of plasma membrane, receptors, encapsulated ubiquitinated proteins, and accumulation of those in the form of ILVs ensures regulation of cell signaling and transition of the early endosome into the late endosome, also known as MVB (Figure 2). This mechanism is regulated by the classical ESCRT-dependent mechanism, which is also involved in EV release. Damaged protein cargo coming from the plasma membrane and Golgi apparatus is packaged into the MVB inside ILVs. The typical fate of the MVB is its fusion with the lysosome [40,41]. The ESCRT pathway is involved in many processes, including cellular abscission, viral budding, and even daughter cell separation during cell division in eukaryotic cells [40,42,43]. Of these processes, one that stands out is the EV release through the ESCRT pathway, and is predominantly associated with packaging and recycling ubiquitylated proteins [41,44]. However, SMase, tetraspanins and other supporting proteins are involved in EV biogenesis that are not restricted to ESCRT only EV biogenesis [6,45]. Furthermore, ceramide is considered the main building block of EVs and can be involved in both ESCRT-dependent and -independent pathways. The ceramides involved in EV biogenesis pathways are less strictly ESCRT linked and mostly associated with direct membrane budding [3,6,46]. Both pathways can be observed in metazoa (which we will use as an example), yeast and archaea, but the nature of the cargo is not the same [42,45,47].
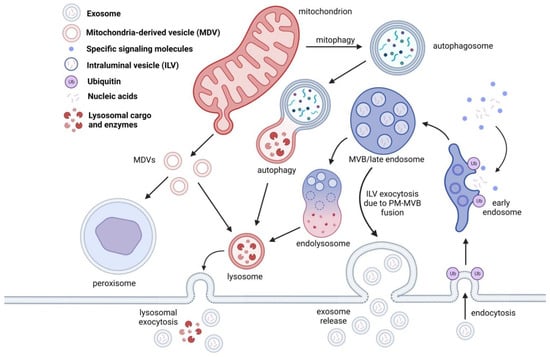
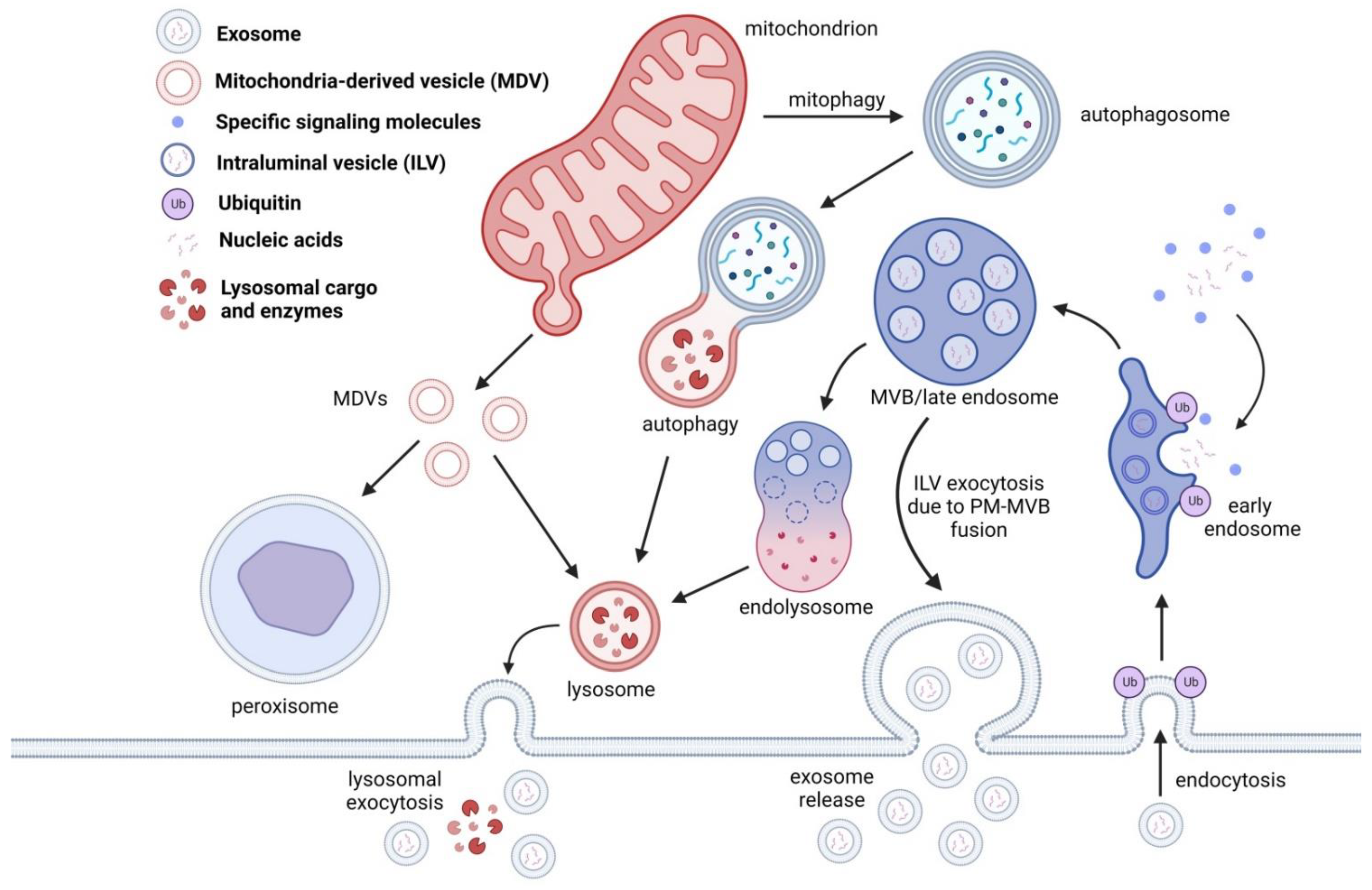
Figure 2.
EVs are generated through the endosomal pathway. Once the early endosome is formed, ILVs are packaged with proteins destined for degradation, nucleic acids, and other signaling molecules. Once the cargo is tagged for degradation, an endolysosome is formed and will proceed to lysosomal degradation. In some cases, the ILVs are released during MVB-PM fusion and are consequently released as exosomes. In some cases, lysosomal cargo containing ILVs can also release exosomes through lysosomal exocytosis. Lysosomes are also involved in protein degradation through autophagy, mitophagy and mitochondria derived vesicles (MDVs). MDVs, unlike MVBs, are targeted for peroxisomal cargo degradation.
3.1.1. ESCRT-Independent Mechanism
MVB biogenesis and EV formation can occur without the presence of ESCRTs in a ubiquitin-independent manner. Despite simultaneously silencing all four ESCRTs, EV generation can still occur [3]. One of the many key regulators of ESCRT-independent EV pathway is Ras-associated binding 31 (Rab31) (Figure 3). Once Rab31 is phosphorylated by epidermal growth factor receptor (EGFR), it will let flotillin proteins traffick EGFR inside the MVB for packaging into ILVs in an ESCRT-independent manner [46]. Flotillins will engage in lipid rafts to drive the inclusion of EGFR into MVBs in order to form ILVs in an ESCRT-independent manner. Rab31 recruits TBC1D2B (a GTPase activating protein) in order to inactivate Rab7 [46], a small GTPase key regulator of transport to late endocytic compartments such as late endosomes and lysosomes [48]. Once Rab7 is inactive, lysosomal degradation of MVBs is interrupted and enables the release of ILVs from the cell [46]. Rab31 is thus essential increased EV release, as it can recruit TBC1D2 to inactivate Rab7. This will prevent MVB-lysosome fusion and will result in ILV release [46]. Another study has shown that sphingholipid ceramide can be enriched in EVs that are generated in an ESCRT-independent manner [6]. However, the release of such EVs in an ESCRT-independent manner was significantly reduced upon inhibiting neutral sphingomyelinase (nSMase), an enzyme that generates ceramides from sphingomyelin [6]. Several proteins including tetraspanins Tspan8, CD9, Rab proteins, flotillin-1 and other classical EV biomarkers are shared between ESCRT-dependent and -independent pathways [3,46,49]. Some of the most prominent hallmarks that distinguish between the two pathways is the packaging of RNA and the use of ceramides as building blocks for EV generation [47]. A greater proportion of lipids in EV formation can represent another hallmark of the ESCRT-independent EV biogenesis pathway [6]. The cargo was secreted into a distinct subdomain of the endosomal membrane that required ceramides in ESCRT-independent manner. The application of a nSMase2 inhibitor such as GW4868 reduces the number of secreted EVs due to the lack of core building blocks for the vesicles, including the proteolipid protein [6,50]. While nSMase2 converts sphingomyelin into ceramide, the main building block of EVs [6], ceramide blockage is responsible only for a fraction of EV release [3,6,51]. Furthermore, EV release can be both ceramide and nSMase2 independent [52,53]. Interestingly, the lipidome of the EVs can distinguish drastically from larger vesicles, such as MVs. MVs will have a lipidome more of a plasma membrane, whereas EVs are more rigid, as they contain lipid rafts, phospholipases A2, C, D. In addition they are enriched with common EV biomarkers heat shock proteins-70 and -90 [54,55].
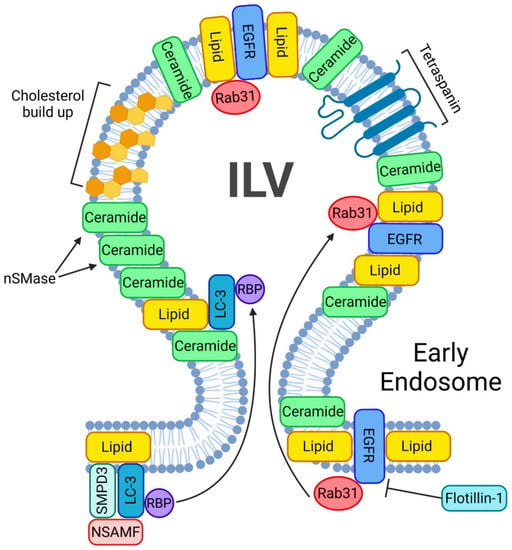
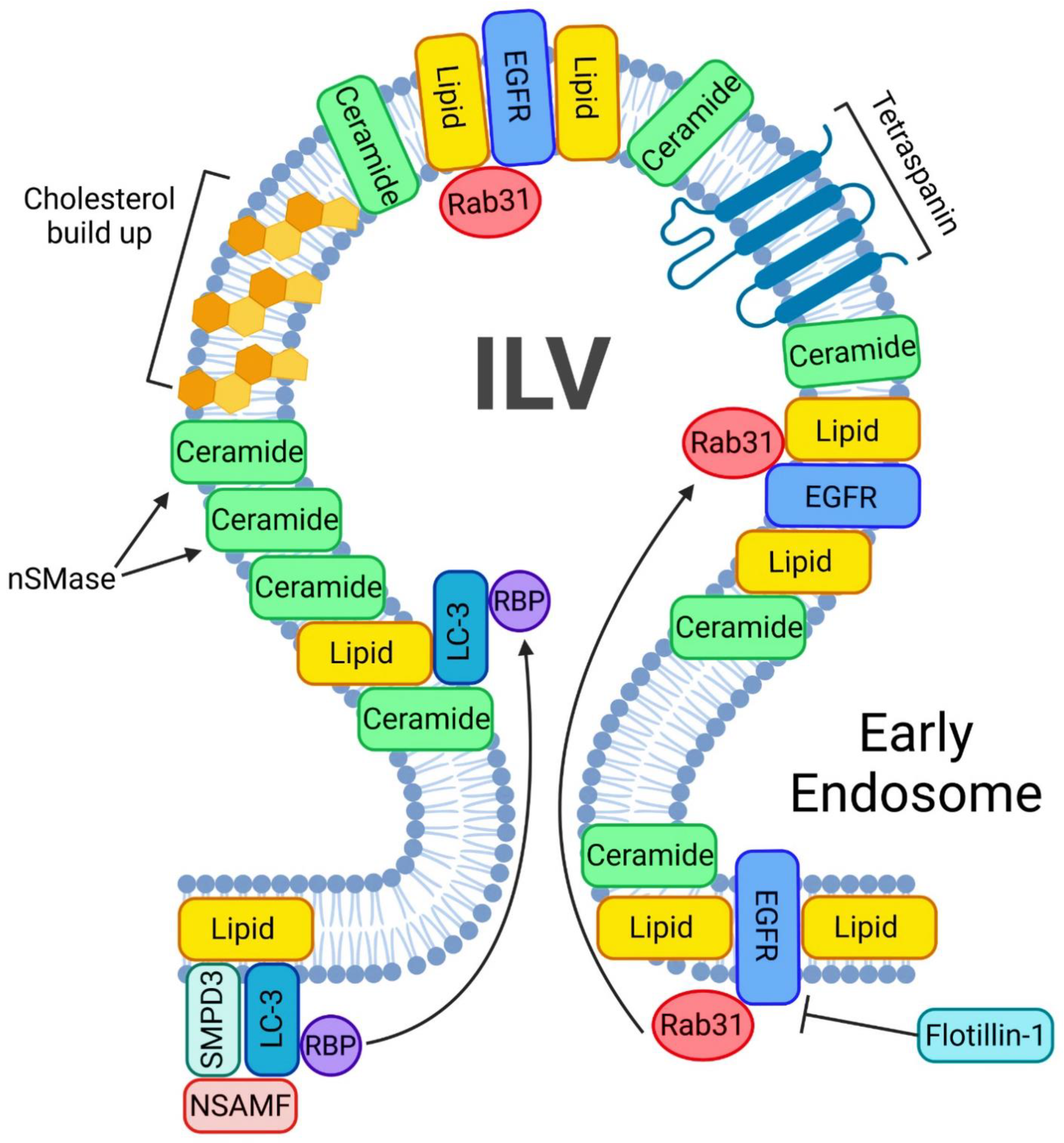
Figure 3.
ESCRT-independent pathway of exosome biogenesis and release. A crucial step in starting the ESCRT-independent exosome biogenesis is the phosphorylation of Rab31. A phosphorylated Rab31 will allow flotillins to deliver the EGFRs into the ILV. nSMases will help accumulate ceramides inside the ILVs. In addition, such ILVs will be enriched in ceramides and cholesterol. Another mechanism of lipid buildup inside the ILV involves LC3, which also incorporates RNA binding sites and small non-coding RNAs.
LC3 is a central protein in the autophagy pathway that can selectively recruit cargo to the autophagosome via interacting with cargo receptors. This protein can selectively incorporate RNA binding proteins (RBP) and non-coding RNA into extracellular vesicles in an ESCRT-independent manner. Such LC3-dependent extracellular vesicle loading starts with LC3 positioning on the outside of the MVB. Once on the MVB, three proteins—SMPD3, NSMAF and RBP—will surround LC3 in order for LC3 to undergo intraluminal budding. This mechanism will drive ceramide production at the MVB membrane to facilitate EV biogenesis [56].
3.1.2. ESCRT-Dependent Mechanism
The ESCRT-dependent EV biogenesis is an evolutionary conserved pathway, mainly regulated by Ubiquitin (Ub) which mediates degradation of a wide variety of membrane proteins in lysosomes by sorting them into the ILVs of MVBs [4]. There are 4 ESCRT complexes, ESCRT-0, -I, -II, and -III, that are accompanied by accessory proteins such as the Vps4-Vta1 ATPase in complex with Alix. Alix is involved in ILV generation and packaging into the MVB, cargo packaging into ILVs, and ILV/exosome release from the cell (Figure 4). Each stage decides the fate of the ILVs, whether they will be released as EVs or undergo lysosomal protein degradation [51]. ESCRT-0 is absent in some protists and plants, and is not necessary for initial MVB formation. The main role of this pathway is to safely deliver utilize the ubiquitinated protein cargo (Ub-cargo) for degradation [44,57]. The early endosomal protein Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) and the signal transducing adaptor molecules (STAMs) each contain a Ub-interacting motif. Hrs and STAMs form a complex that leads to transport of early endosomes. Ub-cargo will cluster along the endosomal membrane in order to be part of the ESCRT-0, a Vps27-Hse1 heterodimer complex in yeast, or its mammalian analogue Hrs/STAM1 [58]. Once Ub-cargo is localized along the early endosome, the heterodimer Hrs-STAM1/2 will complex along Eps15 and the Ub-cargo to form the ESCRT-0 complex [57]. Subsequently, after the ESCRT-0 complex is generated a vesicular envelope containing clathrin will be formed [59]. Such localization of clathrin, STAM2, Hrs and Eps15 is controlled by MVB forming protein Vps4 [59]. Vps4 presence is important for tetraspanin vesicle formation [60].
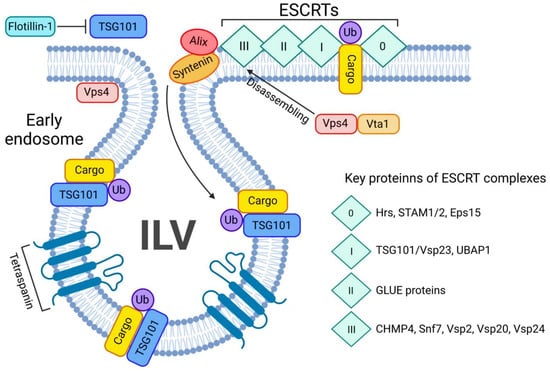
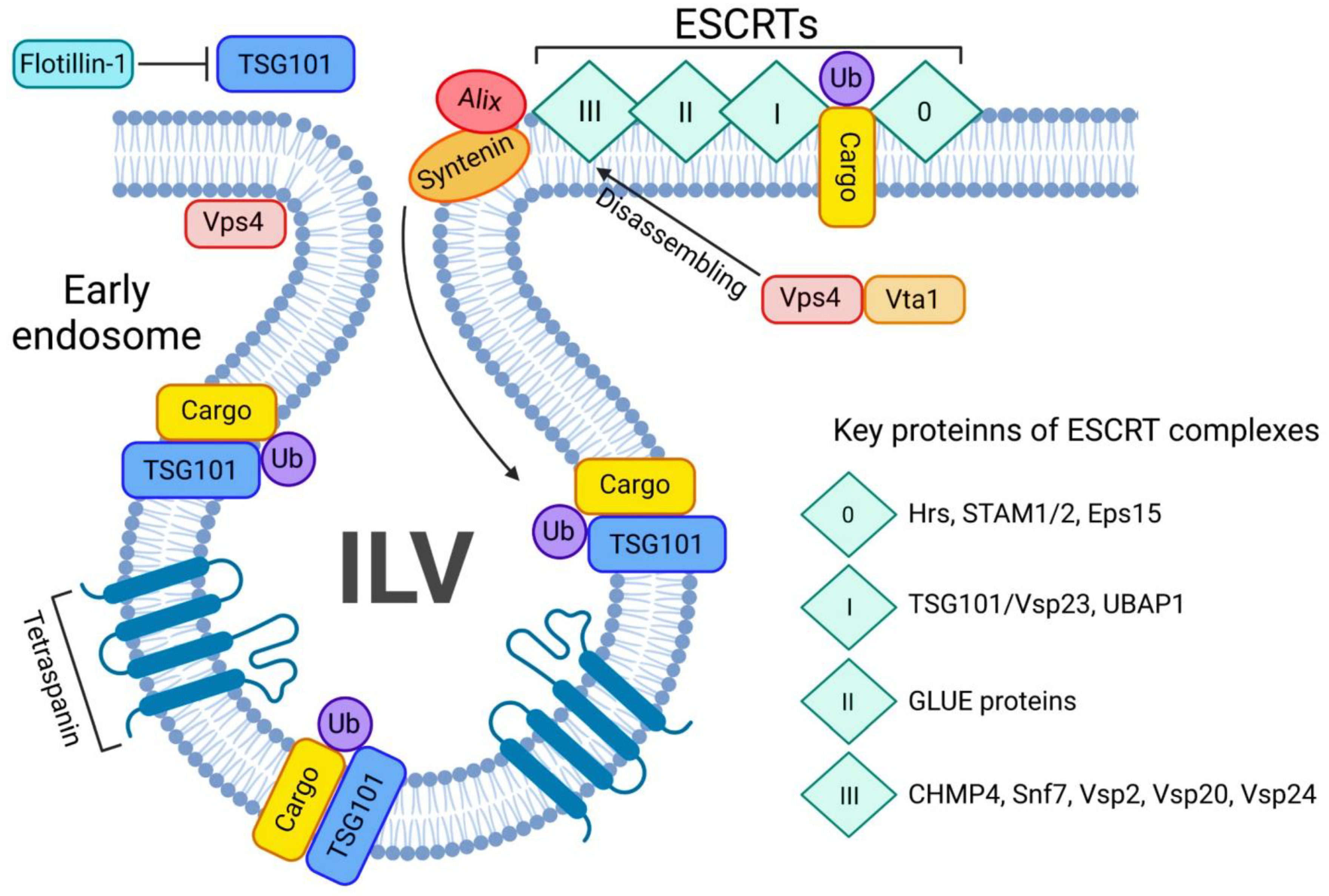
Figure 4.
ESCRT-dependent pathway of exosome biogenesis and release. The ESCRT-dependent exosome biogenesis starts with assembling all 4 ESCRT complexes on the ubiquitin-bound cargo (Ub-cargo). The process is tightly regulated by key proteins such as Alix and syntenin. Once ESCRTs bind the Ub-cargo, TSG101 navigates the cargo inside the ILV and the Vsp4/Vta1 complex will disassemble the ESCRT 0-III complex. Tetraspanins will favour the budding of the ILV.
The whole transition of unwanted cargo from its internalization by the cell until exosome release or lysosomal degradation by the ESCRT-machinery, several transition steps are involved during each ESCRT-complex formation, The transition of ESCRT-0 to ESCRT-I is tightly regulated by Flotillin-1, as this protein regulates TSG-101 activity, cargo recognition and sorting during the transition [61,62]. TSG-101, a regulator of vesicular trafficking, binds to Ub-cargo proteins, as well as is a key player in sorting this cargo into the MVB [63]. Hrs/Vps27 will bind directly to the amino terminus of the TSG101/Vps23 subunit of ESCRT-I to further promote endosomal formation. The ESCRT-I subunits are essential for MVB generation and its stabilization [61,64]. A proline rich motif of Vps23 directs ESCRT-I towards the midbody during membrane scission once it meets ESCRT-III. TSG-101 will guide the ESCRT-I complex and favor its interaction with ESCRT-0, Ub-cargo [64], and the GRAM-like Ub-binding in Eap45 (GLUE) protein from ESCRT-II will bridge via NZF with Ub-associated protein 1 (UBAP1), a component of ESCRT-I complex [65], while GLUE and UBAP1 both bind to different Ub-cargo. Without each of the previous ESCRT steps, the budding of membrane into lumen will not occur and subsequently would block ESCRT-III formation and ILV scission [61,64]. Due to the presence of Vps23, ESCRT-III regulates all previous ESCRT complexes. As a transient complex, ESCRT-III is the most important ESCRT-complex, as it is the final step where ILVs are released as exosomes or the cargo is shuttled to the lysosome for degradation [44,66]. Upon anchoring of Vps20 to the membrane surface, Alix directly binds to CHMP4 [67]. Subsequently, after spiralization and convexity of the polymerized CHMP4 is formed, Vps24 and Vps2 subsequently pinches off the vesicle [51,68,69]. Alix, along with Syntenin and Syndecans, two other key regulators of endocytosis and release of exosomes, thus regulate EV biogenesis [63]. This is a stage where the Ub-cargo can deubiquitinated by an Ub hydrolase Doa4, which is also recruited by Alix, to bind to Snf7 on ESCRT-III, and leads to restoration of the MVB to potentially enable EV release instead of shuttling cargo to the lysosome [70,71]. Later the ESCRT-III complex will be dissembled by the Vps4/Vta1 complex, recycled and ready for the next cycle [72]. It worth mentioning that tetraspanins, such as the classical exosomal biomarkers CD63, CD81 and CD82, enable vesicle budding by creating membrane curvatures and favoring cargo sorting into vesicles [42,59,73]. While EV biogenesis is often described as ESCRT-dependent or -independent, these two pathways might not be wholly independent [74]. Exosomal release could be a synergistic co-release by several pathways, which could even ensure a backup mechanism for the unique and more prominent ones.
3.2. Mitophagy and Mitochondria-Lysosome Cargo Shuttling Route
As the “cell’s engine”, mitochondria can undergo fusion and fission in order to maintain quality control and prevent unnecessary inflammation [75]. Mitochondrial fusion is viewed as a last resort to save the organelle, a process where damaged mitochondria are fusing with healthy mitochondria to minimize waste and ensure mitochondrial quality control [76,77]. However, once the organelle is beyond repair, mitochondrial autophagy, termed as mitophagy will remove damaged mitochondria. The process of selective degradation of mitochondria by autophagy involves lysosomal degradation of mitochondrial fragments. Key regulators of mitophagy, such as Parkin and PINK1, are involved in preventing diseases such as neurodegenerative disorders [78]. In addition, mitochondrial cargo can also be shuttled from the mitochondrion inside mitochondria-derived vesicles (MDVs) [79,80]. Stunningly, MDVs are of similar size, shape, and cargo content as exosomes. Mostly, ubiquitinated or sumoylated cargo inside the MDVs is shuttled to lysosomes or peroxisomes for degradation [81,82] in a similar manner as the ILV-bearing MVBs. Such similarities point towards similar key role of recycling damaged proteins through generating ILVs and MDVs, as vesicles that encapsulate and deliver unwanted cargo to the lysosome [83,84]. Another shared feature is the lysosomal degradation of MDV and MVB cargo is due to SNARE protein family [85,86].
4. Ceramides in EV Biogenesis
4.1. Ceramides in EVs Formation
Lipid metabolism and in particular the sphingolipid ceramide is involved in EV formation [6,87]. Ceramide production at the level of the plasma membrane and endosomes regulates EV biogenesis [6,87] (Figure 5). Menck et al. have demonstrated that inhibition of nSMase in human and mouse cells prevents exosome release and significantly increases the release of MVs from the plasma membrane [87]. nSMase and the product ceramide are also involved in the ESCRT-independent pathway for the formation of EVs. Indeed, the internalization of EVs into late endosomes can be triggered by ceramide. Because of their singular cone-shaped structure, ceramides initiate spontaneous membrane invagination that allows for ILV formation into the MVBs and for the maintenance of vesicle shape and structure. In a study conducted by Trajkovic et al., the authors demonstrated that the exosomes were ceramide-enriched and that inhibiting nSMase inhibits exosome release [6]. Moreover, several data indicate that EV miRNA cargo is segregated into distinct subdomains on the endosomal membrane and that transfer of miRNA into the lumen of the endosome required the presence of ceramide [6,88] as well as that proteolipid protein sorting into EVs derived by an oligodendrocyte cell line (myelinating cells of the central nervous system) [6].
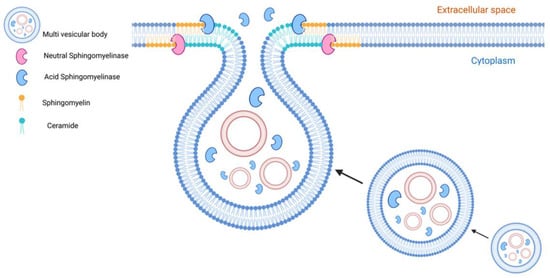
Figure 5.
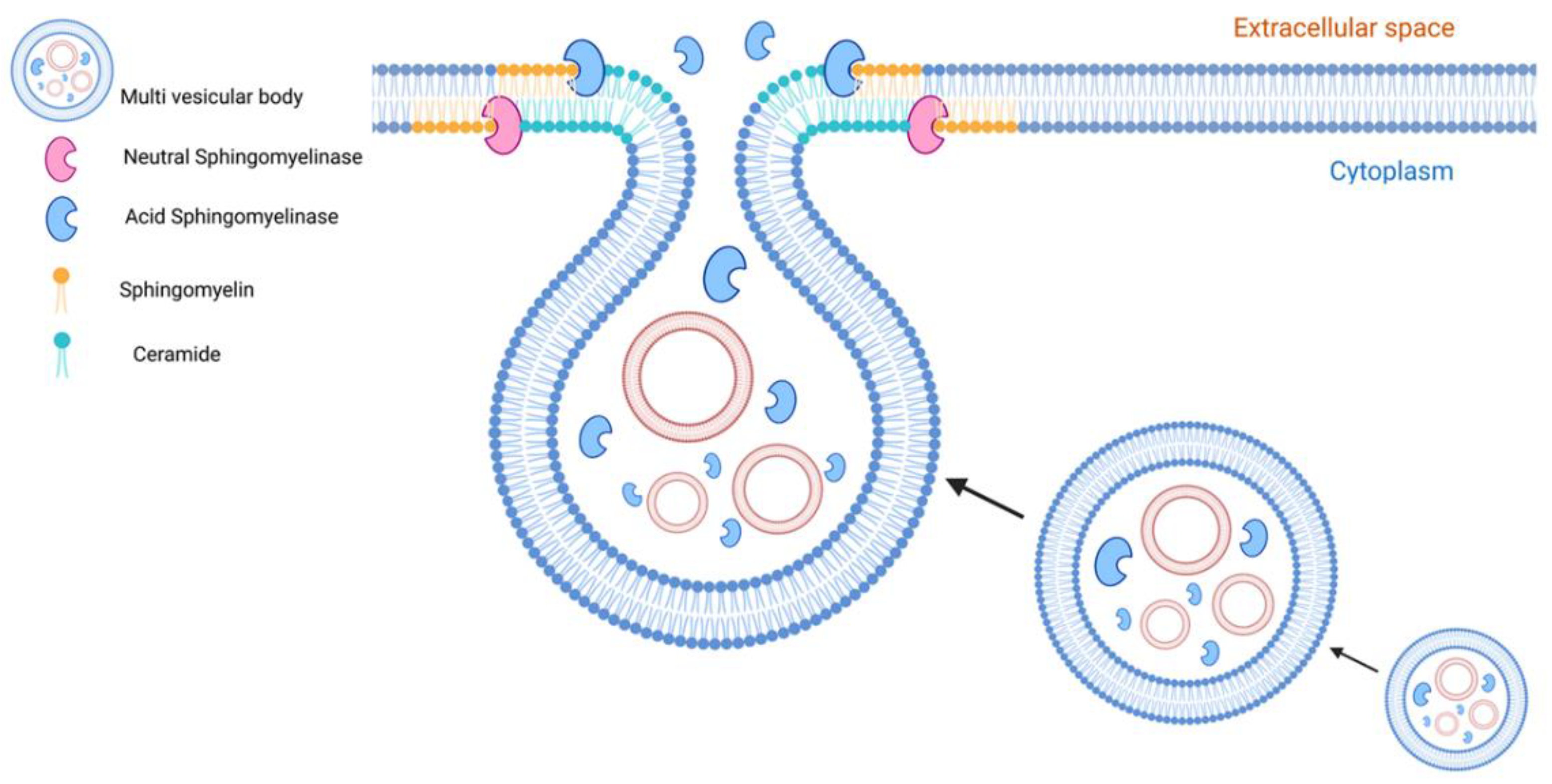
Role of sphingomyelinases and ceramides in EV formation. Sphingomyelinases are active at the level of endosomes and cell membranes whereas their natural products, ceramides, are involved in the biogenesis and secretion of EVs. Neutral sphingomyelinase (nSMase) and ceramides participate in the endosomal formation of exosomes in an ESCRT-independent pathway. Acid sphingomyelinases (aSMase) targeted directly or indirectly via lysosomal exocytosis to the cell membrane contribute to creating ceramide-enriched microdomains that are involved in microvesicle and exosome formation and secretion.
The acid sphingomyelinase (aSMase) is also involved in EV formation and release. The acidic enzyme can move onto the cell surface via direct translocation or lysosomal exocytosis in response to stimuli such as CD95 or Pseudomonas aeruginosa infection as well as in wounded cells [89,90,91]. Bianco and colleagues demonstrated that aSMase activity triggers MVs release from glial cells, a process which requires p38 MAPK activation [92]. SMase has been shown to be associated with membrane lipid raft microdomains and its activity results in localized ceramide build-up, thereby creating ceramide-enriched microdomains and inducing membrane curvature [20,28]. In trophoblast cells, alteration of membrane curvature facilitates the budding from the inner plasma membrane to form early endosomes that, when cholesterol-rich, promotes endosome transport for secretion via the MVB pathway for the secretion of EVs [91]. Interestingly, other enzymes of the sphingolipid metabolism such as SMS1 and SMS2 could finely regulate EV formation. The inhibition or silencing of these two enzymes, respectively, lead to the accumulation of ceramide in the trans-Golgi and plasma membrane increasing the release of EVs by microglia cells [93].
4.2. Ceramides in EV Secretion
Ceramide has been shown to promote EV production and release in vitro and in vivo. Treatment of several cell lines with exogenous ceramide leads to an increase in the release of EVs [91,94,95]. However, the role of ceramide in EV release is cell dependent and it has been reproduced in several but not all cell lines [2]. Secretion of exosomal miRNAs in HEK293 cells depends on a ceramide-triggered secretory mechanism; on the other hand, inhibition of de novo ceramide formation and nSMase2 did not inhibit the secretion of EVs in PC-3 cells [53,96]. In a recent study, Matsui et al. demonstrated that different EVs are secreted from the apical and basolateral sides of epithelial cells by two independent mechanisms. The apical release of EVs is dependent on the ALIX–Syntenin1–Syndecan1 machinery and the basolateral release of EVs is facilitated by sphingomyelinase-dependent ceramide production [97]. Using mouse model of Alzheimer’s disease (AD), Yuyama et al. showed that treatment and oral administration of plant ceramides promotes the release of neuron-derived EVs with the ability to clear Amyloid-β (Aβ) in neuronal cultures and in mice [98]. Ceramide-dependent upregulation of EVs release may effectively release neuronal EVs and prevent AD pathology [99], suggesting ceramides as a potential therapeutic candidate for AD via secretion of Aβ-enriched neuron-derived EVs.
4.3. Ceramide-Enriched EVs
Lipids are important constituents of EVs. Interestingly, the lipid composition of EVs is quite peculiar and is not the same as the donor cells, as EVs are enriched in phosphatidylserine, sphingolipids and cholesterol. A high content of sphingolipids and cholesterol are characteristics of lipid rafts microdomains. Indeed, EVs share these common characteristics of membrane microdomains, particularly lipid bilayer membrane for protection and stability of encapsulated material against detergents [100].
Several pieces of evidence demonstrated that EVs can directly transport lipids from donor cells to recipient cells, altering various cellular processes such as metabolism, release of secretory proteins, and activation of immune cells [101,102]. As previously mentioned, ceramide has a pivotal role in regulating the fate and the activities of cells. For instance, astrocytes treated with Aβ results in the release of EVs enriched with ceramide and pro-apoptosis prostate apoptosis response-4 (PAR-4) [103]. These ceramide-enriched EVs induce apoptosis in recipient astrocytes, which may contribute to the development of AD [103]. Recent data has also indicated increased in serum EVs carrying very long-chain C24:1 C24:1 ceramide in aging and can induce senescence of bone-derived mesenchymal stem cells [104].
The organization of ceramides in the membrane of ceramide-enriched EVs is not fully understood. Elsherbini and colleagues described a distinctive population of EVs that is highly enriched with the sphingolipid ceramide [105]. They proposed that ceramide in the EV membrane was organized in ceramide-rich platforms (CRPs), which are circulating lipid raft microdomain that mediate interaction of ceramide with ceramide-associated proteins (CAPs) [105]. Several stimuli can trigger the release of ceramide-enriched EVs. Treatment with the cytokines (TNF-α and IFN-γ) in an oligodendroglioma cell line induced the formation and release of ceramide (C16-, C24-, and C24:1-Cer species and dihydroCer species) enriched EVs [106]. Treatment of embryonic hippocampal cells with vitamin D3 augments ceramide content in EVs [107]. To date, we do not have an accurate molecular description of the formation of ceramide-enriched EVs.
The levels of ceramide in EVs are associated with various disease conditions [108]. Ermini and co-workers demonstrated that in early-onset preeclampsia syncytiotrophoblast release ceramide-enriched EVs active L-SMPD1 into the maternal circulation [91]. The SMPD1 and ceramide in the EVs promote activation of endothelial cells and impair endothelial tubule formation [91]. Human hepatocytes have also been shown to release ceramide-enriched pro-inflammatory EVs that are involved in macrophage-mediated hepatic inflammation, one of the characteristics of nonalcoholic steatohepatitis [109]. Elsherbini and colleagues demonstrated that serum from a transgenic mouse model of familial AD and serum from AD patients contain ceramide-enriched EVs that are associated with Aβ [105].
5. Lysosomes and EVs
Currently there are two main mechanisms involved in lysosomal degradation of ILVs—through its biogenesis inside the MVB or via the autophagy pathway [110,111]. After several cycles of membrane fusion events, an endocytic vesicle will fuse with the early endosome that will later mature into MVB. Once the unwanted cargo starts entering the host cell through endocytosis via the plasma membrane (PM), an early endosome is formed. This cargo will accumulate by entering the early endosome through inwards budding and formation of ILVs. By going through several rounds of ILV formation, the early endosome will grow in size and becomes a late endosome, also known as MVB [112,113]. At this point, the mature MVB is able to fuse with a lysosome. In the majority of cases, this fusion develops an endolysosome whereby the lysosomal lumenal hydrolases initiate a chain of catabolic reactions in order to recycle the protein content of the MVB. In some cases, ILVs inside the MVB are trafficked to the PM and released as exosomes, whereas the majority of MVBs will fuse with lysosomes to form an endolysosome [2].
5.1. Lysosomal Endocytosis (LE) and EV Release
Once the cargo is packaged into the ILV, it is considered for elimination due to its unwanted cargo. Although lysosomes are considered as terminal organelles that systematically degrade and recycle cargo, some of the cargo can escape through fusion and release of the lysosomal content through the PM [114,115]. Once the MVB fuses with the lysosome, the processing of the MVB cargo does not appear start immediately, as there are reports that ILVs can be released during a process called lysosomal exocytosis (LE). LE is involved in maintaining cell homeostasis. The release of LE derived vesicles is necessary to prevent disorders, such as lysosomal storage disorders, fibrosis, neurodegenerative diseases and neoplastic transformation of the cell. In these LE-derived vesicles, intact ILVs are exosomes that are loaded with potentially harmful ubiquitinated cargo [5,110,116]. Organelles, such as MVB and autophagosome, can shift from fusion with the lysosome to fusion with the PM for extracellular release of its content. Interestingly, autophagosomes can also fuse with the late endosomes (also known as MVB) and produce amphisomes which later fuse with the lysosome or fuse with the PM for release of the contents. The MVB-autophagosome-lysosome crosstalk is highly dynamic, as it can shift the cargo from degradation to exocytosis release, and vice versa [110]. One such protein involved in the MVB-autophagosome-lysosome axis is S1P, a pro-survival and anti-apoptotic factor that opposes ceramide pro-apoptotic activity [117,118,119]. The S1P receptor, called G protein (Gi)-coupled sphingosine 1-phosphate (S1P) receptor, is involved in MVB maturation as well as cargo sorting into the ILVs [119]. Several autophagy genes, such as Atg5 and the Atg12–13 complex, regulate the release of exosomes. Such decrease or increase in exosome release can be due to drug-stimulated pH increase and further LE [120]. Furthermore, the interaction of Alix, an ESCRT component, with Atg12-Atg3 is critical during early stages of autophagy [121,122].
LE is a ubiquitous Ca2+-dependent mechanism involved in PM repair [123], neuron protection and prevention of neurodegenerative diseases [110,124], immune response and antigen presentation [125,126] and intracellular Ca2+ induced release of exosomes [127,128]. A unique role of LE is to regulate ATP levels due to its potential toxicity in excess amounts [129,130]. More interestingly, LE is closely linked with PM repair in order to restore the natural barrier and selective permeability of the cell. Lysosomes located near the PM damage site will rush to relocate to restore PM integrity [131]. Once the cell is ruptured, a massive Ca2+ influx activates a synaptotagmin VII (SynVII)-dependent mechanism of PM repair. During this process, lysosomal proteins VAMP7, C2B and C2A will assist SynVII in disassembling and restructuring the lysosome in order to integrate it into the damaged area of the PM. The restructuring process also involves the interaction with the PM surface-protein SNAP-23 and Syntaxin 4 [132,133]. Once a complex of Syntaxin 4/VAMP7/SNAP-23 is formed (also known as the SNARE-complex), the lysosome will dock with the PM [134]. Such docking requires increased Ca2+ levels, which subsequently will trigger interaction between SynVII and SNARE-complex [135,136]. A patch protein complex, in the presence of Ca2+ and the lysosomal calcium channel mycolipin-1 (TRPML-1), will ensure a smooth transition of the lysosomal content with the PM [111,137,138]. Furthermore, acid ceramides are critical for TRPML-1 channel-mediated Ca2+ release, which controls lysosome-MVB fusion and exosome release in podocytes [111]. Lysosomal Ca2+ release through TRPML-1 activates calcineurin, which will bind and de-phosphorylate the transcription factor EB (TFEB). De-phosphorylation of TFEB will promote its nuclear translocation, which is linked with LE and autophagy, as well as proper lysosomal cargo clearance and fusion between the lysosome and PM [139,140]. A delay in ILV degradation inside the lysosome can later result in exosome release during LE [141], which directly links exosome release with TRPML-1-mediated LE [142]. Furthermore, a delay in tumor ILV lysosomal degradation can induce cell migration via activating the MAPK pathway [143]. All this highlight the role and importance of lysosome and LE in exosome release, uptake and degradation.
The processes regulating lysosome function and exosome release are tightly linked. Lysosomal alkalized pH is linked with impaired lysosomal function and an increase in exosome release [144,145]. An increased release of exosomes is associated with several lysosomal storage diseases (LSDs). There is evidence that lysosomal dysfunction by stored material can be the cause of ILV release from MVB via PM-MVB fusion in order to ensure cellular homeostasis [146]. A major viral oncoprotein latent membrane protein 1 (LMP1) can activate mammalian target of rapamycin complex 1 (mTORC1) in order to suppress host autophagy and favor cellular growth and proliferation. Interestingly, LMP1 and mTORC1 activation also opposes exosome release [147,148,149]. Inhibition of the mTORC1 component mTOR1 induces Ca2+ release through TRPML-1. This Ca2+ release is regulated by transmembrane BAX inhibitor motif containing 6 (TMBIM6), a Ca2+ channel-like protein. This increase in lysosomal Ca2+ activates PP3/calcineurin and trigger TFEB translocation into the nucleus, leading to autophagy and lysosomal biogenesis [150]. The classical exosome biomarker CD63, one of the key factors in exosome production and endosomal cargo sorting, coordinates endosomal packaging and autophagy. A reduction in CD63 is associated with development of autophagosomes. This means that the balance shifts from ILV-lysosome cargo degradation towards MVB-PM fusion and CD63+ exosome release [147,151]. However, it is still unclear if the exosomes escaped before or after the MVB is fused with the lysosome.
LE is important for PM remodeling and reorganization. LE is particularly vital in phagocytic cells, such as macrophages when they rapidly reassemble actin under stress situations. Macrophages can internalize large amounts of lipids [152,153,154]. Actin reorganization is driving the whole process and contributes in filopodia, lamellipodia and other processes of membrane ruffling. Such phagocytosis necessitates considerable intracellular membrane relocation and LE is the central process for reorganization and integration of the membrane [154,155]. Another study found that LE is associated with increased exosome release [141]. LE is negatively regulated by neuraminidase 1 (NEU1), a sialidase mutated in the glycoprotein storage disease sialidosis. The complete deletion of NFU1 triggers LE and increased exosome release. These exosomes carry ubiquitous cargo, such as misfolded transforming growth factors, and could cause pathogenesis. These exosomes, once taken up by normal fibroblasts, convert them into myofibroblasts with altered proliferative and migratory properties [141]. In addition, phagocytic macrophages are sensitive to lysosomal dysfunction and consequently use exosome release to get rid of unwanted cargo, which can downstream regulate immune responses or contribute to disease progression [156,157]. In summary, there is clear evidence that the lysosome has a central role in exosome release under stress and LE conditions. Furthermore, such LE-regulated release of exosomes can contribute to initiation or progression of certain diseases.
5.2. Lysosome and EV Uptake
EVs are found inside the lysosome of recipient cells as soon as 1 h after uptake [158,159]. The mode of uptake in phagocytic cells, especially macrophages, is likely through pinocytosis and phagocytosis [160,161,162,163]. Smaller and larger particles (~100 nM and >500 nm, respectively) are internalized through phagocytosis [164]. As an example, leukemia cell-derived exosomes can be taken via DNM2-dependent phagocytosis and co-localize with phagolysosome biomarkers, which indicates that foreign exosomes are likely to be recycled and eliminated [165,166]. During pinocytosis, smaller particles (<100 nm; micropinocytosis) or larger particles (up to 5000 nm; micropinocytosis) are taken up [167,168]. Pinocytosis of these vesicles can be facilitated through the cooperation of caveolin and/or clathrin [169,170], proteins that are vital for endosomal budding and packaging. The blocking of clathrin-mediated endocytosis inhibits EV uptake [161,171]. Similarly, knockdown of calveolin impaired the uptake of EVs [159]. Macropinocytosis, on the other hand, allows for a larger number of EVs to be taken up [162]. Macropinocytosis is a non-selective uptake, and like any pinocytosis will likely involve degradation of the EV cargo once it’s inside the cell through the proteasome or the lysosome [172,173,174].
5.3. Lysosomal Storage Diseases (LSDs)
LSDs are a group of about 70 rare genetic conditions that cause a toxic buildup of substrates that damages cells and organs due to defective functioning of the lysosome. LSDs are more common during pregnancy or soon after birth, with adult LSD cases less severe than newborns. To date, there is no cure for the various LSDs, with current treatment lessening the damage. LSD state occurs once undigested protein cargo is accumulated inside the lysosome-endosome system due genetic mutations or pharmaceutical treatment that led to deficiency of a specific enzyme required for digestion of glycoproteins or lipids [175]. Such lysosomal dysfunction is the cause of the rise of several rare diseases, depending on the route and type of altered protein cargo that is accumulated inside the cell. The most frequent disorders are associated with the mitochondria-lysosome axis. Intracellularly, lysosomal Ca2+ is released by TRPML1, and enters transfers to mitochondria once the mitochondria-lysosome contact is established. Loss of TRPML1 alters the mitochondria-lysosome axis and results in the LSD mucolipidosis type IV [176]. TRPML1 activation is critical for TFEB-mediated LE and lysosomal cargo clearance, which makes TRPML1 a promising target for treatment of various LSDs [139]. Other disorders, such as neurodegenerative Parkinson’s disease, ones, occur due to improper autophagic cascade and excessive overwhelming of lysosomal potential [177,178]. Strikingly, LSDs are linked with unusual and altered exosome release and uptake, and TRMPL1 and other key players of the LE represent promising targets for therapeutic strategies [142]. Owing to its importance in endosomal transport, the targeting of the cytoskeletal system that carry exosomes to the perinuclear region for further fusion with the lysosome is another promising strategy [171].
5.4. Lysosomal Dysfunction and EVs
Lysosomes are acidic organelles that degrade and recycle cargo mainly via autophagy or endocytosis, respectively. They also play important role on regulation of autophagy, mitophagy, and other protein-degradation processes [79,179]. Several diseases can arise due to lysosomal dysfunction, as exemplified by the neurodegenerative disorder [180]. In other diseases, such as AD and Parkinson’s, toxic proteins are aggregated inside EVs that once released, contribute to inflammation and disease progression [181,182,183]. Presence of key proteins, such as DJ-1, Parkin or PINK1 are all involved in lysosomal dysfunction, mitophagy, and neurodegenerative diseases [184,185]. Parkin, a protein involved in Parkinson’s disease, is vital for modulating endosomal organization and function of the endosomal-lysosomal pathway [186]. Mitophagy is highly dependent on PINK1 and Parkin, two key players in Parkinson’s disease, which involvement is regulated by exosomes. The exosome-mediated PINK1/Parkin-dependent mitophagy is important for intercellular communication in impaired neurodegeneration-involved cells [184]. Exosomes can carry several biomarkers associated with diseases. For example, several exosomal biomarkers are associated with AD, particularly neurotoxic amyloid-beta (Aβ), lactoferrin, Tau, Acetylcholine esterase (AChE), and amyloid precursor protein (APP). These exosomal proteins, particularly increased levels Aβ and Tau, further propagate disease progression of AD [187,188]. Similarly, in Parkinson’s disease, DJ-1, alpha-synuclein (a-syn), AChE and Tau, represent key exosomal biomarkers. Mostly detected in the plasma, DJ-1 and a-syn are prominent in exosomes of PD patients [189]. Such packaging of a-syn can be promoted by Secretory Carrier Membrane Protein 5 (SCAMP5), a protein regulated by TFEB. SCAMP5 inhibits autophagy flux by blocking the fusion of autophagosomes with lysosomes [190,191]. This exosome release has been shown to be Ca2+ -dependent [192]. Interestingly, a similar exosome mechanism can be observed in Huntington’s disease, which involves mutated huntigtonin (mHtt)-regulated decrease secretion of exosomes. The progression of Huntington’s disease may be inhibited through the administration of astrocyte-derived exosomes, which reduced the density of mHtt aggregates. In general, the study found that mHtt reduces the expression of αB-crystallin in astrocytes and decreases exosome secretion [193]. During Amyotrophic Lateral Sclerosis (ALS), neurons release exosomes and initiate motor neuron death. ALS-exosomes carried TDP43 and TSG-101, whereas cells had mutated TDP-43, as well as several mutations of the autophagy pathway. Exosomes carrying TDP43 can be the potential cause of TDP43 aggregation and TDP-43 proteinopathy and are linked to autophagy inhibition [194]. In all cases, autophagy, mitophagy and lysosomal activity are key factors that prevent the MVB rupture and distribution of exosome for ALS initiation and progression [188,195]. Exosomes containing these proteins can potentially be used as early disease biomarkers, such as for detection of cancer [196,197]. Furthermore, lysosomal exocytosis during cellular neurodegeration will enable release and potential detection of exosomes carrying biomarkers [124].
5.5. Cancer, Exosomes and Lysosomal Dysfunction
Exosomes carry specific cargo that can contribute to carcinogenesis. Several types of molecules, such as miRNA, are associated with tumor arise and progression, and are packaged into exosomes [1]. Since lysosomes are involved in numerous cellular processes, such as programmed cell death, antigen presentation, cell adhesion/migration and exosome release, there is a tight connection of this organelle with neoplastic transformation of cells. Lysosomes were shown to help cancer cells to survive stress due to lack of nutrients via autophagy [198]. Via adjusting the intracellular pH, lysosomes can also regulate degradation of CTLA-4, a key player in cancer cell evasion of T-cell specific response [199]. Acidic environment will affect cancer cell activity. However, via lysosomal exocytosis cathepsins are released and will promote cancer metastasis [200]. In cancer cells pH shifts will affect the ESCRT system. The MVB will move towards the positive end of the microtubule, and avoid lysosomal degradation in favor of fusing with the PM for exosome release, whereas alkaline pH reduced exosome secretion [201]. These exosome will carry tumor-associated miRNA with metastatic potential [202]. Under tumor conditions, the lack of nutrients will result in starvation for the rest of the normal cells linked with autophagy and endosomal pathway disorders [122]. Once extracellular content, including exosomes, are endocytosed, phagocytosed or pinocytosed, the content is delivered to the lysosome for breakdown and nutrient generation. In cancer cells, the nutrient microenvironment is depleted, and will require sacrifice in the form of healthy cell components [203]. All this links lysosomal dysfunction and exosome release with neoplastic transformation and metastasis.
6. Conclusions Remarks
EVs are a unique class of cell-to-cell communication whereby cells secrete a variety of macromolecules to distant cells. There is a growing appreciation of the precise role of EVs for cellular homeostasis and defects in EV biogenesis, cargo sorting, release, and uptake is the underlying cause of many disorders. In this review, we have highlighted the role of ceramides and lysosomes in the biology underpinning EV-based communication. While we are gaining deep insights of our knowledge of EVs, much remains unknown about the regulatory role of lysosomes and ceramides in physiological and pathological conditions, including the underappreciated heterogenous and diverse nature of vesicles. Nevertheless, we are at the point where we can exploit these pathways for therapeutic use of various vesicle associated disorders.
Author Contributions
Conceptualization, R.H., A.H., L.E., S.H., S.T.B. and B.Y.; writing, R.H., A.H., L.E., S.H., S.T.B. and B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cancer Research Society [935202, S.T.B.] and Canadian Institutes of Heath Research [426713, S.T.B.].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Aβ | Amyloid-beta |
| AChE | Acetylcholine esterase |
| AD | Alzheimer disease |
| AMPK | AMP-activated protein kinase |
| APP | Amyloid precursor protein |
| aSMase | Aacid sphingomyelinase |
| CAP | Ceramide-associated protein |
| CERT | Cramide transfer protein |
| CHMP4 | Charged multivesicular body protein 4 |
| COPD | Chronic obstructive pulmonary disease |
| CRP | Ceramide-rich platform |
| EGFR | Epidermal growth factor receptor |
| ESCRT | Endosomal sorting complex required for transport |
| ILVs | Intraluminal vesicles |
| LE | Lysosomal exocytosis |
| LMP1 | Latent membrane protein 1 |
| LSD | Lysosomal storage disease |
| MDV | Mitochondria-derived vesicle |
| mHtt | Mutated huntigtonin |
| mTORC1 | Mammalian target of rapamycin complec 1 |
| MVs | Microvesicles |
| MVB | Multivesicular body |
| nSMase2 | Neutral sphinghomyelinase 2 |
| PAR-4 | Prostate apoptosis response 4 |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PI | Phosphatidylinositol |
| PM | Plasma membrane |
| PS | Phosphatidylserine |
| Rab | Ras-associated binding |
| S1P | Sphingosine 1-phosphate |
| SCAMP5 | Secretory Carrier Membrane Protein 5 |
| SMase | Sphingomyelinase |
| SMS1 | Sphingomyelin synthase 1 |
| SMS2 | Sphingomyelin synthase 2 |
| SNARE | SNAP receptor |
| Sph | Sphingosine |
| SphK | Sph kinases |
| SPT | Serine palmitoyltransferase |
| SynVII | Synaptotagmin VII |
| TFEB | Transcription factor EB |
| TRPML1 | Transient Receptor Potential Channel Mucolipin-1 |
| Ub-cargo | Ubiquitinated protein cargo |
References
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. CMLS 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.I.; Cashikar, A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Tancini, B.; Buratta, S.; Delo, F.; Sagini, K.; Chiaradia, E.; Pellegrino, R.M.; Emiliani, C.; Urbanelli, L. Lysosomal Exocytosis: The Extracellular Role of an Intracellular Organelle. Membranes 2020, 10, 406. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Al Sazzad, M.A.; Yasuda, T.; Murata, M.; Slotte, J.P. The Long-Chain Sphingoid Base of Ceramides Determines Their Propensity for Lateral Segregation. Biophys. J. 2017, 112, 976–983. [Google Scholar] [CrossRef]
- Alonso, A.; Goni, F.M. The Physical Properties of Ceramides in Membranes. Annu. Rev. Biophys. 2018, 47, 633–654. [Google Scholar] [CrossRef]
- Suzuki, M.; Ohno, Y.; Kihara, A. Whole picture of human stratum corneum ceramides, including the chain-length diversity of long-chain bases. J. Lipid Res. 2022, 63, 100235. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Menaldino, D.S.; Bushnev, A.; Sun, A.; Liotta, D.C.; Symolon, H.; Desai, K.; Dillehay, D.L.; Peng, Q.; Wang, E.; Allegood, J.; et al. Sphingoid bases and de novo ceramide synthesis: Enzymes involved, pharmacology and mechanisms of action. Pharmacol. Res. 2003, 47, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K. Intracellular trafficking of ceramide by ceramide transfer protein. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Huitema, K.; van den Dikkenberg, J.; Brouwers, J.F.; Holthuis, J.C. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004, 23, 33–44. [Google Scholar] [CrossRef]
- Marchesini, N.; Hannun, Y.A. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Kolter, T.; Sandhoff, K. Principles of lysosomal membrane digestion: Stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005, 21, 81–103. [Google Scholar] [CrossRef]
- Taha, T.A.; Hannun, Y.A.; Obeid, L.M. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 2006, 39, 113–131. [Google Scholar] [CrossRef]
- Ryckman, A.E.; Brockhausen, I.; Walia, J.S. Metabolism of Glycosphingolipids and Their Role in the Pathophysiology of Lysosomal Storage Disorders. Int. J. Mol. Sci. 2020, 21, 6881. [Google Scholar] [CrossRef]
- Paciotti, S.; Albi, E.; Parnetti, L.; Beccari, T. Lysosomal Ceramide Metabolism Disorders: Implications in Parkinson’s Disease. J. Clin. Med. 2020, 9, 594. [Google Scholar] [CrossRef]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef]
- Moore, D.J.; Rawlings, A.V. The chemistry, function and (patho)physiology of stratum corneum barrier ceramides. Int. J. Cosmet. Sci. 2017, 39, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Becker, K.A.; Gulbins, E. Ceramide-enriched membrane domains--structure and function. Biochim. Biophys. Acta 2009, 1788, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.J.; Alahari, S.; Tagliaferro, A.; Post, M.; Caniggia, I. Augmented trophoblast cell death in preeclampsia can proceed via ceramide-mediated necroptosis. Cell Death Dis. 2017, 8, e2590. [Google Scholar] [CrossRef] [PubMed]
- Young, M.M.; Kester, M.; Wang, H.G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef]
- Petrache, I.; Petrusca, D.N.; Bowler, R.P.; Kamocki, K. Involvement of ceramide in cell death responses in the pulmonary circulation. Proc. Am. Thorac. Soc. 2011, 8, 492–496. [Google Scholar] [CrossRef]
- Rotstein, N.P.; Miranda, G.E.; Abrahan, C.E.; German, O.L. Regulating survival and development in the retina: Key roles for simple sphingolipids. J. Lipid Res. 2010, 51, 1247–1262. [Google Scholar] [CrossRef]
- Yeganeh, B.; Lee, J.; Bilodeau, C.; Lok, I.; Ermini, L.; Ackerley, C.; Caniggia, I.; Tibboel, J.; Kroon, A.; Post, M. Acid Sphingomyelinase Inhibition Attenuates Cell Death in Mechanically Ventilated Newborn Rat Lung. Am. J. Respir. Crit. Care Med. 2019, 199, 760–772. [Google Scholar] [CrossRef]
- Ausman, J.; Abbade, J.; Ermini, L.; Farrell, A.; Tagliaferro, A.; Post, M.; Caniggia, I. Ceramide-induced BOK promotes mitochondrial fission in preeclampsia. Cell Death Dis. 2018, 9, 298. [Google Scholar] [CrossRef]
- Barth, B.M.; Cabot, M.C.; Kester, M. Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med. Chem. 2011, 11, 911–919. [Google Scholar] [CrossRef]
- Saddoughi, S.A.; Ogretmen, B. Diverse functions of ceramide in cancer cell death and proliferation. Adv. Cancer Res. 2013, 117, 37–58. [Google Scholar] [CrossRef]
- Govindarajah, N.; Clifford, R.; Bowden, D.; Sutton, P.A.; Parsons, J.L.; Vimalachandran, D. Sphingolipids and acid ceramidase as therapeutic targets in cancer therapy. Crit. Rev. Oncol. Hematol. 2019, 138, 104–111. [Google Scholar] [CrossRef]
- Fox, T.E.; Houck, K.L.; O’Neill, S.M.; Nagarajan, M.; Stover, T.C.; Pomianowski, P.T.; Unal, O.; Yun, J.K.; Naides, S.J.; Kester, M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J. Biol. Chem. 2007, 282, 12450–12457. [Google Scholar] [CrossRef]
- Canals, D.; Roddy, P.; Hannun, Y.A. Protein phosphatase 1alpha mediates ceramide-induced ERM protein dephosphorylation: A novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. J. Biol. Chem. 2012, 287, 10145–10155. [Google Scholar] [CrossRef]
- Henry, B.; Ziobro, R.; Becker, K.A.; Kolesnick, R.; Gulbins, E. Acid sphingomyelinase. In Handbook of Experimental Pharmacology; Springer: Vienna, Austria, 2013; pp. 77–88. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Munoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [CrossRef]
- Petrache, I.; Berdyshev, E.V. Ceramide Signaling and Metabolism in Pathophysiological States of the Lung. Annu. Rev. Physiol. 2016, 78, 463–480. [Google Scholar] [CrossRef]
- Yang, Y.; Uhlig, S. The role of sphingolipids in respiratory disease. Ther. Adv. Respir. Dis. 2011, 5, 325–344. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Witwer, K.W.; Goberdhan, D.C.; O’Driscoll, L.; Thery, C.; Welsh, J.A.; Blenkiron, C.; Buzas, E.I.; Di Vizio, D.; Erdbrugger, U.; Falcon-Perez, J.M.; et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef]
- Stoorvogel, W. Resolving sorting mechanisms into exosomes. Cell Res. 2015, 25, 531–532. [Google Scholar] [CrossRef]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef]
- Van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Anderson, M.R.; Kashanchi, F.; Jacobson, S. Exosomes in Viral Disease. Neurotherapeutics 2016, 13, 535–546. [Google Scholar] [CrossRef]
- Mosesso, N.; Nagel, M.K.; Isono, E. Ubiquitin recognition in endocytic trafficking—With or without ESCRT-0. J. Cell Sci. 2019, 132, jcs232868. [Google Scholar] [CrossRef]
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef]
- Elsherbini, A.; Bieberich, E. Ceramide and Exosomes: A Novel Target in Cancer Biology and Therapy. Adv. Cancer Res. 2018, 140, 121–154. [Google Scholar] [CrossRef]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zoller, M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta 2015, 1852, 2362–2371. [Google Scholar] [CrossRef]
- Babst, M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr. Opin. Cell Biol. 2011, 23, 452–457. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Phuyal, S.; Hessvik, N.P.; Skotland, T.; Sandvig, K.; Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014, 281, 2214–2227. [Google Scholar] [CrossRef]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef]
- Leidal, A.M.; Debnath, J. LC3-dependent extracellular vesicle loading and secretion (LDELS). Autophagy 2020, 16, 1162–1163. [Google Scholar] [CrossRef]
- Mayers, J.R.; Fyfe, I.; Schuh, A.L.; Chapman, E.R.; Edwardson, J.M.; Audhya, A. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J. Biol. Chem. 2011, 286, 9636–9645. [Google Scholar] [CrossRef]
- Bilodeau, P.S.; Winistorfer, S.C.; Kearney, W.R.; Robertson, A.D.; Piper, R.C. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J. Cell Biol. 2003, 163, 237–243. [Google Scholar] [CrossRef]
- Bache, K.G.; Raiborg, C.; Mehlum, A.; Stenmark, H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 2003, 278, 12513–12521. [Google Scholar] [CrossRef]
- Jackson, C.E.; Scruggs, B.S.; Schaffer, J.E.; Hanson, P.I. Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophys. J. 2017, 113, 1342–1352. [Google Scholar] [CrossRef]
- Kaul, Z.; Mookherjee, D.; Das, S.; Chatterjee, D.; Chakrabarti, S.; Chakrabarti, O. Loss of tumor susceptibility gene 101 (TSG101) perturbs endoplasmic reticulum structure and function. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118741. [Google Scholar] [CrossRef]
- Meister, M.; Banfer, S.; Gartner, U.; Koskimies, J.; Amaddii, M.; Jacob, R.; Tikkanen, R. Regulation of cargo transfer between ESCRT-0 and ESCRT-I complexes by flotillin-1 during endosomal sorting of ubiquitinated cargo. Oncogenesis 2017, 6, e344. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Bishop, N.; Woodman, P. TSG101/mammalian VPS23 and mammalian VPS28 interact directly and are recruited to VPS4-induced endosomes. J. Biol. Chem. 2001, 276, 11735–11742. [Google Scholar] [CrossRef]
- Stefani, F.; Zhang, L.; Taylor, S.; Donovan, J.; Rollinson, S.; Doyotte, A.; Brownhill, K.; Bennion, J.; Pickering-Brown, S.; Woodman, P. UBAP1 is a component of an endosome-specific ESCRT-I complex that is essential for MVB sorting. Curr. Biol. CB 2011, 21, 1245–1250. [Google Scholar] [CrossRef]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219, e201904113. [Google Scholar] [CrossRef]
- McCullough, J.; Fisher, R.D.; Whitby, F.G.; Sundquist, W.I.; Hill, C.P. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 7687–7691. [Google Scholar] [CrossRef]
- Huber, S.T.; Mostafavi, S.; Mortensen, S.A.; Sachse, C. Structure and assembly of ESCRT-III helical Vps24 filaments. Sci. Adv. 2020, 6, eaba4897. [Google Scholar] [CrossRef]
- Pfitzner, A.K.; Mercier, V.; Jiang, X.; Moser von Filseck, J.; Baum, B.; Saric, A.; Roux, A. An ESCRT-III Polymerization Sequence Drives Membrane Deformation and Fission. Cell 2020, 182, 1140–1155.e18. [Google Scholar] [CrossRef]
- Buysse, D.; Pfitzner, A.K.; West, M.; Roux, A.; Odorizzi, G. The ubiquitin hydrolase Doa4 directly binds Snf7 to inhibit recruitment of ESCRT-III remodeling factors in S. cerevisiae. J. Cell Sci. 2020, 133, jcs241455. [Google Scholar] [CrossRef]
- Luhtala, N.; Odorizzi, G. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J. Cell Biol. 2004, 166, 717–729. [Google Scholar] [CrossRef]
- Yang, D.; Hurley, J.H. Structural role of the Vps4-Vta1 interface in ESCRT-III recycling. Structure 2010, 18, 976–984. [Google Scholar] [CrossRef]
- Sala-Valdes, M.; Ursa, A.; Charrin, S.; Rubinstein, E.; Hemler, M.E.; Sanchez-Madrid, F.; Yanez-Mo, M. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 2006, 281, 19665–19675. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Horbay, R.; Bilyy, R. Mitochondrial dynamics during cell cycling. Apoptosis 2016, 21, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Molecular machinery of mitochondrial fusion and fission. J. Biol. Chem. 2008, 283, 13501–13505. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Roberts, R.F.; Tang, M.Y.; Fon, E.A.; Durcan, T.M. Defending the mitochondria: The pathways of mitophagy and mitochondrial-derived vesicles. Int. J. Biochem. Cell Biol. 2016, 79, 427–436. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef]
- Sugiura, A.; Mattie, S.; Prudent, J.; McBride, H.M. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 2017, 542, 251–254. [Google Scholar] [CrossRef]
- Yonashiro, R.; Ishido, S.; Kyo, S.; Fukuda, T.; Goto, E.; Matsuki, Y.; Ohmura-Hoshino, M.; Sada, K.; Hotta, H.; Yamamura, H.; et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006, 25, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalo, O.; Fernandez-Delgado, I.; Sanchez-Madrid, F. Post-translational add-ons mark the path in exosomal protein sorting. Cell. Mol. Life Sci. CMLS 2018, 75, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.A.; Phillips, E.O.; Collier, C.L.; Jb Robinson, A.; Routledge, D.; Wood, R.E.; Assar, E.A.; Tumbarello, D.A. Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020, 39, e102539. [Google Scholar] [CrossRef]
- Hyenne, V.; Labouesse, M.; Goetz, J.G. The Small GTPase Ral orchestrates MVB biogenesis and exosome secretion. Small GTPases 2018, 9, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, G. A mitochondrial-derived vesicle HOPS to endolysosomes using Syntaxin-17. J. Cell Biol. 2016, 214, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Sonmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef] [PubMed]
- Janas, T.; Janas, M.M.; Sapon, K.; Janas, T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015, 589, 1391–1398. [Google Scholar] [CrossRef]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef]
- Tam, C.; Idone, V.; Devlin, C.; Fernandes, M.C.; Flannery, A.; He, X.; Schuchman, E.; Tabas, I.; Andrews, N.W. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 2010, 189, 1027–1038. [Google Scholar] [CrossRef]
- Ermini, L.; Farrell, A.; Alahari, S.; Ausman, J.; Park, C.; Sallais, J.; Melland-Smith, M.; Porter, T.; Edson, M.; Nevo, O.; et al. Ceramide-Induced Lysosomal Biogenesis and Exocytosis in Early-Onset Preeclampsia Promotes Exosomal Release of SMPD1 Causing Endothelial Dysfunction. Front. Cell Dev. Biol. 2021, 9, 652651. [Google Scholar] [CrossRef]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Sun, H.; Mikami, D.; Mioka, T.; Mukai, K.; Igarashi, Y. Lysosomal-associated transmembrane protein 4B regulates ceramide-induced exosome release. FASEB J. 2020, 34, 16022–16033. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, J.; Zhao, R.; Li, Y.; Peer, J.; Braun, A.L.; Zhao, L.; Wang, Y.; Tong, Z.; Huang, Y.; et al. Glutaminase 1 regulates the release of extracellular vesicles during neuroinflammation through key metabolic intermediate alpha-ketoglutarate. J. Neuroinflamm. 2018, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Matsui, T.; Osaki, F.; Hiragi, S.; Sakamaki, Y.; Fukuda, M. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 2021, 22, e51475. [Google Scholar] [CrossRef]
- Yuyama, K.; Takahashi, K.; Usuki, S.; Mikami, D.; Sun, H.; Hanamatsu, H.; Furukawa, J.; Mukai, K.; Igarashi, Y. Plant sphingolipids promote extracellular vesicle release and alleviate amyloid-beta pathologies in a mouse model of Alzheimer’s disease. Sci. Rep. 2019, 9, 16827. [Google Scholar] [CrossRef]
- Murai, Y.; Honda, T.; Yuyama, K.; Mikami, D.; Eguchi, K.; Ukawa, Y.; Usuki, S.; Igarashi, Y.; Monde, K. Evaluation of Plant Ceramide Species-Induced Exosome Release from Neuronal Cells and Exosome Loading Using Deuterium Chemistry. Int. J. Mol. Sci. 2022, 23, 10751. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.N.; Chen, J.; Zhang, C.J.; Xie, X.J.; Liao, D.F.; Qin, L. The crosstalk: Exosomes and lipid metabolism. Cell Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; de Jong, O.G.; Schiffelers, R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev. 2021, 173, 252–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): Potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef] [PubMed]
- Khayrullin, A.; Krishnan, P.; Martinez-Nater, L.; Mendhe, B.; Fulzele, S.; Liu, Y.; Mattison, J.A.; Hamrick, M.W. Very Long-Chain C24:1 Ceramide Is Increased in Serum Extracellular Vesicles with Aging and Can Induce Senescence in Bone-Derived Mesenchymal Stem Cells. Cells 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.; Qin, H.; Zhu, Z.; Tripathi, P.; Wang, G.; Crivelli, S.M.; Spassieva, S.D.; Bieberich, E. Extracellular Vesicles Containing Ceramide-Rich Platforms: “Mobile Raft” Isolation and Analysis. Methods Mol. Biol. 2021, 2187, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Szulc, Z.M.; Kurowska, E.; Hogan, E.L.; Bielawski, J.; Bielawska, A.; Bhat, N.R. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. J. Lipid Res. 2016, 57, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Cataldi, S.; Arcuri, C.; Mirarchi, A.; Lazzarini, A.; Garcia-Gil, M.; Beccari, T.; Curcio, F.; Albi, E. Vitamin D3 Enriches Ceramide Content in Exosomes Released by Embryonic Hippocampal Cells. Int. J. Mol. Sci. 2021, 22, 9287. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Giovagnoni, C.; Zhu, Z.; Tripathi, P.; Elsherbini, A.; Quadri, Z.; Pu, J.; Zhang, L.; Ferko, B.; Berkes, D.; et al. Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12233. [Google Scholar] [CrossRef] [PubMed]
- Kakazu, E.; Mauer, A.S.; Yin, M.; Malhi, H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J. Lipid Res. 2016, 57, 233–245. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Li, G.; Huang, D.; Hong, J.; Bhat, O.M.; Yuan, X.; Li, P.L. Control of lysosomal TRPML1 channel activity and exosome release by acid ceramidase in mouse podocytes. Am. J. Physiol. Cell Physiol. 2019, 317, C481–C491. [Google Scholar] [CrossRef]
- van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Katzmann, D.J. Biogenesis and function of multivesicular bodies. Annu. Rev. Cell Dev. Biol. 2007, 23, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.A.; Samyn, D.R.; Mattie, S.; Brett, C.L. Distinct features of multivesicular body-lysosome fusion revealed by a new cell-free content-mixing assay. Traffic 2018, 19, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; White-Gilbertson, S.; van de Vlekkert, D.; Janke, L.; Moshiach, S.; Campos, Y.; Finkelstein, D.; Gomero, E.; Mosca, R.; Qiu, X.; et al. Regulated lysosomal exocytosis mediates cancer progression. Sci. Adv. 2015, 1, e1500603. [Google Scholar] [CrossRef]
- Gleason, A.M.; Woo, E.G.; McKinney, C.; Sidransky, E. The Role of Exosomes in Lysosomal Storage Disorders. Biomolecules 2021, 11, 576. [Google Scholar] [CrossRef]
- Dadsena, S.; Bockelmann, S.; Mina, J.G.M.; Hassan, D.G.; Korneev, S.; Razzera, G.; Jahn, H.; Niekamp, P.; Muller, D.; Schneider, M.; et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 2019, 10, 1832. [Google Scholar] [CrossRef]
- Feuerborn, R.; Becker, S.; Poti, F.; Nagel, P.; Brodde, M.; Schmidt, H.; Christoffersen, C.; Ceglarek, U.; Burkhardt, R.; Nofer, J.R. High density lipoprotein (HDL)-associated sphingosine 1-phosphate (S1P) inhibits macrophage apoptosis by stimulating STAT3 activity and survivin expression. Atherosclerosis 2017, 257, 29–37. [Google Scholar] [CrossRef]
- Kajimoto, T.; Okada, T.; Miya, S.; Zhang, L.; Nakamura, S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 2013, 4, 2712. [Google Scholar] [CrossRef]
- Han, M.; Hu, J.; Lu, P.; Cao, H.; Yu, C.; Li, X.; Qian, X.; Yang, X.; Yang, Y.; Han, N.; et al. Exosome-transmitted miR-567 reverses trastuzumab resistance by inhibiting ATG5 in breast cancer. Cell Death Dis. 2020, 11, 43. [Google Scholar] [CrossRef]
- Murrow, L.; Debnath, J. Atg12-Atg3 Coordinates Basal Autophagy, Endolysosomal Trafficking, and Exosome Release. Mol. Cell. Oncol. 2018, 5, e1039191. [Google Scholar] [CrossRef]
- Murrow, L.; Malhotra, R.; Debnath, J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 2015, 17, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, J.V.; Gerasimenko, O.V.; Petersen, O.H. Membrane repair: Ca(2+)-elicited lysosomal exocytosis. Curr. Biol. CB 2001, 11, R971–R974. [Google Scholar] [CrossRef] [PubMed]
- Tsunemi, T.; Perez-Rosello, T.; Ishiguro, Y.; Yoroisaka, A.; Jeon, S.; Hamada, K.; Rammonhan, M.; Wong, Y.C.; Xie, Z.; Akamatsu, W.; et al. Increased Lysosomal Exocytosis Induced by Lysosomal Ca(2+) Channel Agonists Protects Human Dopaminergic Neurons from alpha-Synuclein Toxicity. J. Neurosci. 2019, 39, 5760–5772. [Google Scholar] [CrossRef] [PubMed]
- Tardif, V.; Riquelme, S.A.; Remy, S.; Carreno, L.J.; Cortes, C.M.; Simon, T.; Hill, M.; Louvet, C.; Riedel, C.A.; Blancou, P.; et al. Carbon monoxide decreases endosome-lysosome fusion and inhibits soluble antigen presentation by dendritic cells to T cells. Eur. J. Immunol. 2013, 43, 2832–2844. [Google Scholar] [CrossRef] [PubMed]
- Trombone, A.P.; Silva, C.L.; Lima, K.M.; Oliver, C.; Jamur, M.C.; Prescott, A.R.; Coelho-Castelo, A.A. Endocytosis of DNA-Hsp65 alters the pH of the late endosome/lysosome and interferes with antigen presentation. PLoS ONE 2007, 2, e923. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef]
- Savina, A.; Furlan, M.; Vidal, M.; Colombo, M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef]
- Rodriguez, A.; Webster, P.; Ortego, J.; Andrews, N.W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997, 137, 93–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Zhou, W.; Song, A.; Xu, T.; Luo, Q.; Wang, W.; Gu, X.S.; Duan, S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 2007, 9, 945–953. [Google Scholar] [CrossRef]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef]
- Andrews, N.W.; Chakrabarti, S. There’s more to life than neurotransmission: The regulation of exocytosis by synaptotagmin VII. Trends Cell Biol. 2005, 15, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.K.; Huynh, C.; Proux-Gillardeaux, V.; Galli, T.; Andrews, N.W. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 2004, 279, 20471–20479. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.W. Membrane repair and immunological danger. EMBO Rep. 2005, 6, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.C.; Weber, T.; Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 2004, 304, 435–438. [Google Scholar] [CrossRef]
- Weber, J.P.; Toft-Bertelsen, T.L.; Mohrmann, R.; Delgado-Martinez, I.; Sorensen, J.B. Synaptotagmin-7 is an asynchronous calcium sensor for synaptic transmission in neurons expressing SNAP-23. PLoS ONE 2014, 9, e114033. [Google Scholar] [CrossRef]
- Andrews, N.W.; Almeida, P.E.; Corrotte, M. Damage control: Cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014, 24, 734–742. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Q.; Yang, M.; Zhang, X.; Yu, L.; Lawas, M.; Li, X.; Bryant-Genevier, M.; Southall, N.T.; Marugan, J.; et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl. Acad. Sci. USA 2015, 112, E1373–E1381. [Google Scholar] [CrossRef]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Sbano, L.; Bonora, M.; Marchi, S.; Baldassari, F.; Medina, D.L.; Ballabio, A.; Giorgi, C.; Pinton, P. TFEB-mediated increase in peripheral lysosomes regulates store-operated calcium entry. Sci. Rep. 2017, 7, 40797. [Google Scholar] [CrossRef]
- van de Vlekkert, D.; Demmers, J.; Nguyen, X.X.; Campos, Y.; Machado, E.; Annunziata, I.; Hu, H.; Gomero, E.; Qiu, X.; Bongiovanni, A.; et al. Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Sci. Adv. 2019, 5, eaav3270. [Google Scholar] [CrossRef]
- Kim, M.S.; Muallem, S.; Kim, S.H.; Kwon, K.B.; Kim, M.S. Exosomal release through TRPML1-mediated lysosomal exocytosis is required for adipogenesis. Biochem. Biophys. Res. Commun. 2019, 510, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Watanabe, N.; Watanabe, M.; Sakamoto, M.; Sato, A.; Fujisaki, M.; Kubota, S.; Monzen, S.; Maruyama, A.; Nanashima, N.; et al. Exosomes derived from SW480 colorectal cancer cells promote cell migration in HepG2 hepatocellular cancer cells via the mitogen-activated protein kinase pathway. Int. J. Oncol. 2016, 48, 305–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alvarez-Erviti, L.; Seow, Y.; Schapira, A.H.; Gardiner, C.; Sargent, I.L.; Wood, M.J.; Cooper, J.M. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 2011, 42, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Hamdane, M.; Loyens, A.; Gele, P.; Drobeck, H.; Begard, S.; Galas, M.C.; Delacourte, A.; Beauvillain, J.C.; Buee, L.; et al. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 2007, 282, 18197–18205. [Google Scholar] [CrossRef] [PubMed]
- Tancini, B.; Buratta, S.; Sagini, K.; Costanzi, E.; Delo, F.; Urbanelli, L.; Emiliani, C. Insight into the Role of Extracellular Vesicles in Lysosomal Storage Disorders. Genes 2019, 10, 510. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Cheerathodi, M.R.; Nkosi, D.; York, S.B.; Meckes, D.G., Jr. Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Xia, C.; Xu, W.; Ai, X.; Zhu, Y.; Geng, P.; Niu, Y.; Zhu, H.; Zhou, W.; Huang, H.; Shi, X. Autophagy and Exosome Coordinately Enhance Macrophage M1 Polarization and Recruitment in Influenza A Virus Infection. Front. Immunol. 2022, 13, 722053. [Google Scholar] [CrossRef]
- Zou, W.; Lai, M.; Zhang, Y.; Zheng, L.; Xing, Z.; Li, T.; Zou, Z.; Song, Q.; Zhao, X.; Xia, L.; et al. Exosome Release Is Regulated by mTORC1. Adv. Sci. 2019, 6, 1801313. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, G.H.; Bhattarai, K.R.; Lee, M.S.; Back, S.H.; Kim, H.R.; Chae, H.J. TMBIM6 (transmembrane BAX inhibitor motif containing 6) enhances autophagy through regulation of lysosomal calcium. Autophagy 2021, 17, 761–778. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Barrio, M.M.; Moutel, S.; Bover, L.; Weck, M.; Brossart, P.; Teillaud, J.L.; Mordoh, J. CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood 2004, 104, 1183–1190. [Google Scholar] [CrossRef]
- Haka, A.S.; Barbosa-Lorenzi, V.C.; Lee, H.J.; Falcone, D.J.; Hudis, C.A.; Dannenberg, A.J.; Maxfield, F.R. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J. Lipid Res. 2016, 57, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Treede, I.; Braun, A.; Sparla, R.; Kuhnel, M.; Giese, T.; Turner, J.R.; Anes, E.; Kulaksiz, H.; Fullekrug, J.; Stremmel, W.; et al. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 2007, 282, 27155–27164. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Ridley, A.J. Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J. Immunol. 2000, 164, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Czibener, C.; Sherer, N.M.; Becker, S.M.; Pypaert, M.; Hui, E.; Chapman, E.R.; Mothes, W.; Andrews, N.W. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J. Cell Biol. 2006, 174, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Yang, C.L.; Ge, M.R.; Liu, Y.; Zhang, P.; Li, H.; Li, X.L.; Li, T.; Liu, Y.D.; Dou, Y.C.; et al. M1 Macrophage Derived Exosomes Aggravate Experimental Autoimmune Neuritis via Modulating Th1 Response. Front. Immunol. 2020, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, C.; Mai, C.; Hu, X.; Cheng, N.; Chen, W.; Peng, D.; Wang, L.; Ji, Z.; Xie, Y. The Biogenesis, Biological Functions, and Applications of Macrophage-Derived Exosomes. Front. Mol. Biosci. 2021, 8, 715461. [Google Scholar] [CrossRef]
- Koumangoye, R.B.; Sakwe, A.M.; Goodwin, J.S.; Patel, T.; Ochieng, J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE 2011, 6, e24234. [Google Scholar] [CrossRef]
- Yao, Z.; Qiao, Y.; Li, X.; Chen, J.; Ding, J.; Bai, L.; Shen, F.; Shi, B.; Liu, J.; Peng, L.; et al. Exosomes Exploit the Virus Entry Machinery and Pathway To Transmit Alpha Interferon-Induced Antiviral Activity. J. Virol. 2018, 92, e01578-18. [Google Scholar] [CrossRef]
- Chiba, M.; Kubota, S.; Sato, K.; Monzen, S. Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Sci. Rep. 2018, 8, 11972. [Google Scholar] [CrossRef]
- Holder, B.; Jones, T.; Sancho Shimizu, V.; Rice, T.F.; Donaldson, B.; Bouqueau, M.; Forbes, K.; Kampmann, B. Macrophage Exosomes Induce Placental Inflammatory Cytokines: A Novel Mode of Maternal-Placental Messaging. Traffic 2016, 17, 168–178. [Google Scholar] [CrossRef]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef] [PubMed]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Mrowczynski, O.D.; Madhankumar, A.B.; Sundstrom, J.M.; Zhao, Y.; Kawasawa, Y.I.; Slagle-Webb, B.; Mau, C.; Payne, R.A.; Rizk, E.B.; Zacharia, B.E.; et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 2018, 9, 36083–36101. [Google Scholar] [CrossRef]
- Oshima, M.; Seki, T.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H. Reciprocal Regulation of Chaperone-Mediated Autophagy/Microautophagy and Exosome Release. Biol. Pharm. Bull. 2019, 42, 1394–1401. [Google Scholar] [CrossRef]
- Qin, L.Y.; Liu, S.; Wang, C.R.; Wang, J.; Yue, X.; Yu, C. A brief review on the mechanism and function of macropinocytosis. Sheng Li Ke Xue Jin Zhan [Prog. Physiol.] 2006, 37, 41–44. [Google Scholar]
- Sullivan, A.L.; Grasso, J.A.; Weintraub, L.R. Micropinocytosis of transferrin by developing red cells: An electron-microscopic study utilizing ferritin-conjugated transferrin and ferritin-conjugated antibodies to transferrin. Blood 1976, 47, 133–143. [Google Scholar] [CrossRef]
- Ariotti, N.; Wu, Y.; Okano, S.; Gambin, Y.; Follett, J.; Rae, J.; Ferguson, C.; Teasdale, R.D.; Alexandrov, K.; Meunier, F.A.; et al. An inverted CAV1 (caveolin 1) topology defines novel autophagy-dependent exosome secretion from prostate cancer cells. Autophagy 2021, 17, 2200–2216. [Google Scholar] [CrossRef]
- Mellman, I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996, 12, 575–625. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef]
- Colin, M.; Delporte, C.; Janky, R.; Lechon, A.S.; Renard, G.; Van Antwerpen, P.; Maltese, W.A.; Mathieu, V. Dysregulation of Macropinocytosis Processes in Glioblastomas May Be Exploited to Increase Intracellular Anti-Cancer Drug Levels: The Example of Temozolomide. Cancers 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Arafiles, J.V.V.; Kawaguchi, Y.; Nakase, I.; Hirose, H.; Futaki, S. Stearylated Macropinocytosis-Inducing Peptides Facilitating the Cellular Uptake of Small Extracellular Vesicles. Bioconjugate Chem. 2022, 33, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Kobayashi, N.B.; Takatani-Nakase, T.; Yoshida, T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 2015, 5, 10300. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of lysosomal storage disorders. JAMA 1999, 281, 249–254. [Google Scholar] [CrossRef]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial Ca(2+) dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef]
- Manzoli, R.; Badenetti, L.; Rubin, M.; Moro, E. Lysosomal Function and Axon Guidance: Is There a Meaningful Liaison? Biomolecules 2021, 11, 191. [Google Scholar] [CrossRef]
- Robak, L.A.; Jansen, I.E.; van Rooij, J.; Uitterlinden, A.G.; Kraaij, R.; Jankovic, J.; International Parkinson’s Disease Genomics, C.; Heutink, P.; Shulman, J.M. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain A J. Neurol. 2017, 140, 3191–3203. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Reviews. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Cao, X.Y.; Lu, J.M.; Zhao, Z.Q.; Li, M.C.; Lu, T.; An, X.S.; Xue, L.J. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci. Lett. 2017, 644, 94–99. [Google Scholar] [CrossRef]
- Currim, F.; Singh, J.; Shinde, A.; Gohel, D.; Roy, M.; Singh, K.; Shukla, S.; Mane, M.; Vasiyani, H.; Singh, R. Exosome Release Is Modulated by the Mitochondrial-Lysosomal Crosstalk in Parkinson’s Disease Stress Conditions. Mol. Neurobiol. 2021, 58, 1819–1833. [Google Scholar] [CrossRef]
- Si, X.L.; Fang, Y.J.; Li, L.F.; Gu, L.Y.; Yin, X.Z.; Jun, T.; Yan, Y.P.; Pu, J.L.; Zhang, B.R. From inflammasome to Parkinson’s disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson’s disease? Exp. Neurol. 2021, 336, 113525. [Google Scholar] [CrossRef]
- Fei, D.; Xia, Y.; Zhai, Q.; Wang, Y.; Zhou, F.; Zhao, W.; He, X.; Wang, Q.; Jin, Y.; Li, B. Exosomes Regulate Interclonal Communication on Osteogenic Differentiation Among Heterogeneous Osteogenic Single-Cell Clones Through PINK1/Parkin-Mediated Mitophagy. Front. Cell Dev. Biol. 2021, 9, 687258. [Google Scholar] [CrossRef]
- Ho, D.H.; Yi, S.; Seo, H.; Son, I.; Seol, W. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. BioMed Res. Int. 2014, 2014, 704678. [Google Scholar] [CrossRef]
- Song, P.; Trajkovic, K.; Tsunemi, T.; Krainc, D. Parkin Modulates Endosomal Organization and Function of the Endo-Lysosomal Pathway. J. Neurosci. 2016, 36, 2425–2437. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Zhang, X.; Ye, Q.Y.; Wang, Y.Z. Increased DJ-1 and alpha-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 438. [Google Scholar] [CrossRef]
- Papadopoulos, V.E.; Nikolopoulou, G.; Antoniadou, I.; Karachaliou, A.; Arianoglou, G.; Emmanouilidou, E.; Sardi, S.P.; Stefanis, L.; Vekrellis, K. Modulation of beta-glucocerebrosidase increases alpha-synuclein secretion and exosome release in mouse models of Parkinson’s disease. Hum. Mol. Genet. 2018, 27, 1696–1710. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, M.; Bao, P.; Xu, W.; Xu, J. Secretory carrier membrane protein 5 is an autophagy inhibitor that promotes the secretion of alpha-synuclein via exosome. PLoS ONE 2017, 12, e0180892. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, T.; Li, X.J.; Li, S. Mutant Huntingtin Inhibits alphaB-Crystallin Expression and Impairs Exosome Secretion from Astrocytes. J. Neurosci. 2017, 37, 9550–9563. [Google Scholar] [CrossRef]
- Iguchi, Y.; Eid, L.; Parent, M.; Soucy, G.; Bareil, C.; Riku, Y.; Kawai, K.; Takagi, S.; Yoshida, M.; Katsuno, M.; et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain J. Neurol. 2016, 139 Pt 12, 3187–3201. [Google Scholar] [CrossRef]
- Wang, J.K.T.; Langfelder, P.; Horvath, S.; Palazzolo, M.J. Exosomes and Homeostatic Synaptic Plasticity Are Linked to Each other and to Huntington’s, Parkinson’s, and Other Neurodegenerative Diseases by Database-Enabled Analyses of Comprehensively Curated Datasets. Front. Neurosci. 2017, 11, 149. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Liu, M.; Tang, F.; Zhang, P.; Ai, C.; Fields, J.K.; Sundberg, E.J.; Latinovic, O.S.; Devenport, M.; et al. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 2019, 29, 609–627. [Google Scholar] [CrossRef]
- Mijanovic, O.; Brankovic, A.; Panin, A.N.; Savchuk, S.; Timashev, P.; Ulasov, I.; Lesniak, M.S. Cathepsin B: A sellsword of cancer progression. Cancer Lett. 2019, 449, 207–214. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Tan, S.; Xia, L.; Yi, P.; Han, Y.; Tang, L.; Pan, Q.; Tian, Y.; Rao, S.; Oyang, L.; Liang, J.; et al. Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. CR 2020, 39, 67. [Google Scholar] [CrossRef]
- Mosesson, Y.; Mills, G.B.; Yarden, Y. Derailed endocytosis: An emerging feature of cancer. Nat. Rev. Cancer 2008, 8, 835–850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).