The Molecular Gut-Brain Axis in Early Brain Development
Abstract
:1. Introduction
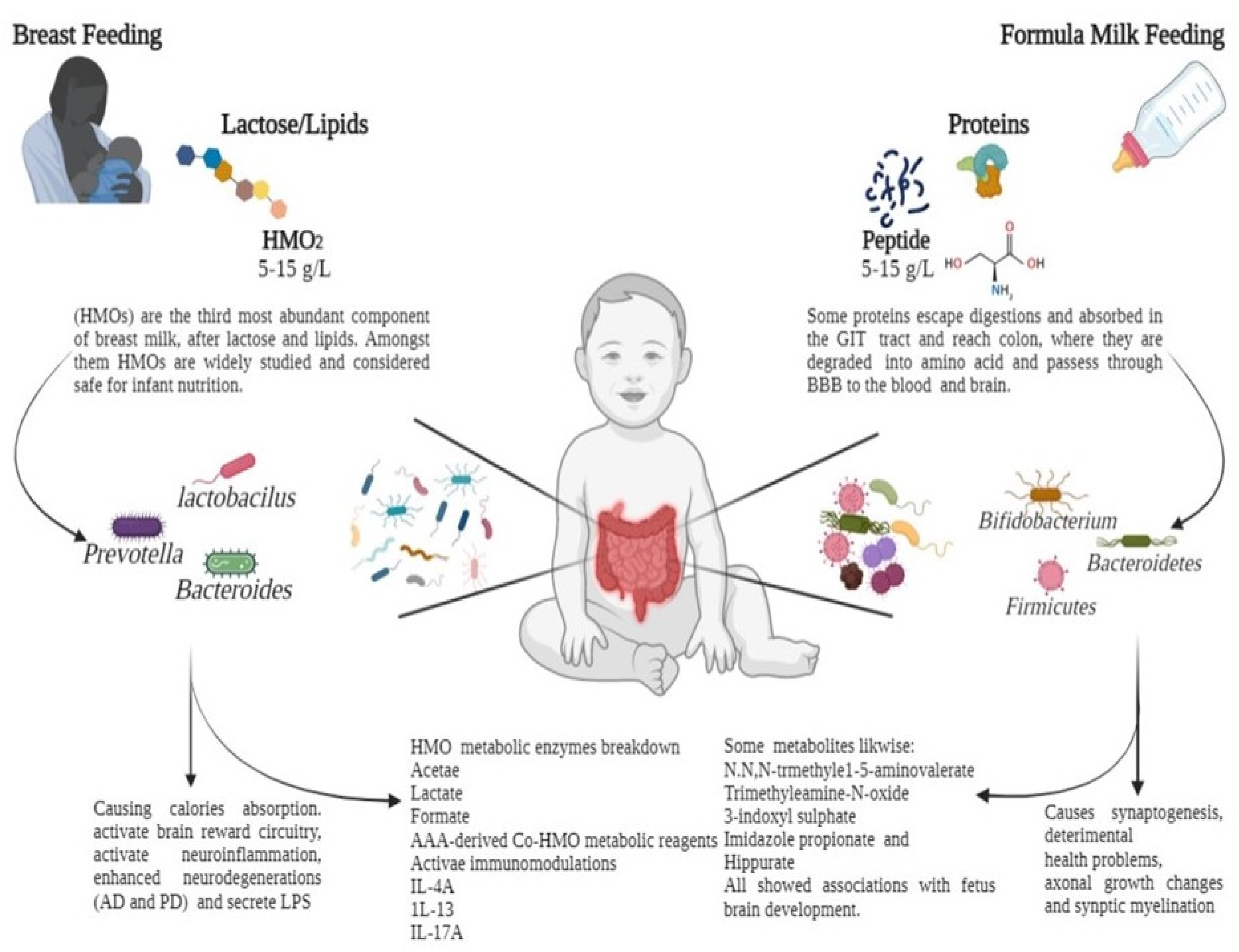
2. Gut Microbiota and Metabolic Factors
3. The Roles of the VN in the GBA and Brain Development
4. The Molecular Mechanisms of Gut Microbiota on Brain Development
4.1. The Dynamic Gut Microbiota and Brain Development
4.2. The Dynamic Gut Microbiota and Brain Structure Developments
4.3. The Gut Microbiota-Derived Neurotransmitters in Brain Development
4.4. The Effects of Gut Microbiota on Epigenetic Modifications and Brain Development
4.5. The Pathways of Gut Microbiota in Brain Development
5. The Role of the Molecular GBA in Postnatal Brain Development and Mental Disorders
6. The Role of the GBA in the Intergenerational Effects of Brain Development
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamashiro, K.; Kurita, N.; Urabe, T.; Hattori, N. Role of the Gut Microbiota in Stroke Pathogenesis and Potential Therapeutic Implications. Ann. Nutr. Metab. 2021, 77, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Chilloux, J.; Neves, A.L.; Boulangé, C.L.; Dumas, M.E. The microbial-mammalian metabolic axis: A critical symbiotic relationship. Curr. Opin. Clin. Nutr. Metab. Care. 2016, 19, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Caricilli, A.M.; Saad, M.J.A. The Role of Gut Microbiota on Insulin Resistance. Nutrients 2013, 5, 829–851. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Sandoval-Cabrera, A.; Romero-Figueroa, M.D.S. Modification of Gut Microbiota in Inflammatory Arthritis: Highlights and Future Challenges. Curr. Rheumatol. Rep. 2021, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The Role of Gut Microbiota in an Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef]
- Naureen, Z.; Farooq, S.; Zahoor, T.; Gilani, S.A. Effect of Probiotics on Gut Microbiota and Brain Interactions in the Context of Neurodegenerative and Neurodevelopmental Disorders, in Microbiome-Gut-Brain Axis; Springer: Singapore, 2022; pp. 383–399. [Google Scholar]
- Stopińska, K.; Radziwoń-Zaleska, M.; Domitrz, I. The Microbiota-Gut-Brain Axis as a Key to Neuropsychiatric Disorders: A Mini Review. J. Clin. Med. 2021, 10, 4640. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Nohara, M.; Yasuoka, A.; Kamei, A.; Shinozaki, F.; Kondo, K.; Inoue, R.; Kondo, T.; Abe, K. Mouse Model of Weak Depression Exhibiting Suppressed cAMP Signaling in the Amygdala, Lower Lipid Catabolism in Liver, and Correlated Gut Microbiota. Front. Behav. Neurosci. 2022, 16, 841450. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Liu, Y.; Wang, S.; Ma, J.; Yang, H. Distribution characteristics of intestinal microbiota during pregnancy and postpartum in healthy women. J. Matern. Neonatal Med. 2021, 35, 2915–2922. [Google Scholar] [CrossRef]
- Enav, H.; Bäckhed, F.; Ley, R.E. The developing infant gut microbiome: A strain-level view. Cell Host Microbe 2022, 30, 627–638. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, H.K. Host and Microbiome Interplay Shapes the Vaginal Microenvironment. Front. Immunol. 2022, 13, 919728. [Google Scholar] [CrossRef] [PubMed]
- Socha-Banasiak, A.; Pawłowska, M.; Czkwianianc, E.; Pierzynowska, K. From Intrauterine to Extrauterine Life—The Role of Endogenous and Exogenous Factors in the Regulation of the Intestinal Microbiota Community and Gut Maturation in Early Life. Front. Nutr. 2021, 8, 696966. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, J.; Zhang, F.; Hong, J.-S. Norepinephrine depleting toxin DSP-4 and LPS alter gut microbiota and induce neurotoxicity in α-synuclein mutant mice. Sci. Rep. 2020, 10, 15054. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Kumar, S.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2021, 38, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N -oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pinky, P.D.; Steinke, I.; Bloemer, J.; Ramesh, S.; Kariharan, T.; Rella, R.T.; Bhattacharya, S.; Dhanasekaran, M.; Suppiramaniam, V.; et al. Gut Metabolite TMAO Induces Synaptic Plasticity Deficits by Promoting Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2020, 13, 138. [Google Scholar] [CrossRef]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2020, 43, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar]
- Feng, X.; Li, Y.; Oppong, M.B.; Qiu, F. Insights into the intestinal bacterial metabolism of flavonoids and the bioactivities of their microbe-derived ring cleavage metabolites. Drug Metab. Rev. 2018, 50, 343–356. [Google Scholar] [CrossRef]
- Gasperotti, M.; Passamonti, S.; Tramer, F.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Fate of microbial metabolites of dietary polyphenols in rats: Is the brain their target destination? ACS Chem. Neurosci. 2015, 6, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLOS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef] [PubMed]
- DiviDiviccaro, S.; FitzGerald, J.A.; Cioffi, L.; Falvo, E.; Crispie, F.; Cotter, P.D.; O’Mahony, S.M.; Giatti, S.; Caruso, D.; Melcangi, R.C. Gut Steroids and Microbiota: Effect of Gonadectomy and Sex. Biomolecules 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Teratani, T. Role of the Vagus Nerve in the Gut-Brain Axis: Development and Maintenance of Gut Regulatory T Cells via the Liver-Brain-Gut Vago-Vagal Reflex. Brain Nerve 2022, 74, 971–977. [Google Scholar] [PubMed]
- Washburn, B.S.; Jiang, J.C.; Cummings, S.L.; Dixon, K.; Gietzen, D.W. Anorectic responses to dietary amino acid imbalance: Effects of vagotomy and tropisetron. Am. J. Physiol. Integr. Comp. Physiol. 1994, 266 Pt 2, R1922–R1927. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- AuAustelle, C.W.; Ba, G.H.O.; Thompson, S.; Gruber, E.; Kahn, A.; Manett, A.J.; Short, B.; Badran, B.W.; Bs, S.T.; Bs, E.G.; et al. A Comprehensive Review of Vagus Nerve Stimulation for Depression. Neuromodulation: Technol. Neural Interface 2021, 25, 309–315. [Google Scholar] [CrossRef]
- HummHummel, G.L.; Austin, K.; Cunningham-Hollinger, H.C. Comparing the maternal-fetal microbiome of humans and cattle: A translational assessment of the reproductive, placental, and fetal gut microbiomes. Biol. Reprod. 2022, 107, 371–381. [Google Scholar] [CrossRef]
- Celentano, C.; Matarrelli, B.; Pavone, G.; Vitacolonna, E.; Mattei, P.A.; Berghella, V.; Liberati, M. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: A randomized controlled trial. J. Matern. Neonatal Med. 2018, 33, 743–751. [Google Scholar] [CrossRef]
- Manta-Vogli, P.D.; Schulpis, K.H.; Dotsikas, Y.; Yannis, L. The significant role of amino acids during pregnancy: Nutritional support. J. Matern. Neonatal Med. 2018, 33, 334–340. [Google Scholar] [CrossRef]
- Li, P.; Tang, T.; Chang, X.; Fan, X.; Chen, X.; Wang, R.; Fan, C.; Qi, K. Abnormality in Maternal Dietary Calcium Intake During Pregnancy and Lactation Promotes Body Weight Gain by Affecting the Gut Microbiota in Mouse Offspring. Mol. Nutr. Food Res. 2018, 63, e1800399. [Google Scholar] [CrossRef]
- JanJandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Saraf, V.S.; Sheikh, S.A.; Ahmad, A.; Gillevet, P.M.; Bokhari, H.; Javed, S. Vaginal microbiome: Normalcy vs dysbiosis. Arch. Microbiol. 2021, 203, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Hu, M.; Hu, T.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J.; Waligora-Dupriet, A.-J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- NeNeuman, H.; Koren, O. The Pregnancy Microbiome. Nestlé Nutr. Inst. Workshop Ser. 2017, 88, 1–10. [Google Scholar]
- Greenhalgh, K.; Meyer, K.M.; Aagaard, K.M.; Wilmes, P. The human gut microbiome in health: Establishment and resilience of microbiota over a lifetime. Environ. Microbiol. 2016, 18, 2103–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirulli, F.; Musillo, C.; Berry, A. Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring. Neuroscience 2020, 447, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; Diéguez, E.; Campoy, C. Early nutrition and gut microbiome: Interrelationship between bacterial metabolism, immune system, brain structure, and neurodevelopment. Am. J. Physiol. Metab. 2019, 317, E617–E630. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Wang, Q.; O’Sullivan, O.; Greene-Diniz, R.; Cole, J.R.; Ross, R.; O’Toole, P.W. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 2010, 38, e200. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microbes Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Dougherty, M.W.; Kudin, O.; Mühlbauer, M.; Neu, J.; Gharaibeh, R.Z.; Jobin, C. Gut microbiota maturation during early human life induces enterocyte proliferation via microbial metabolites. BMC Microbiol. 2020, 20, 205. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, Z.; Liu, L.; Zhang, R.; Zhang, X.; Peng, C.; Geng, Y.; Zhou, F.; Han, Y.; Hou, X. Characteristics of gut microbiota of term small gestational age infants within 1 week and their relationship with neurodevelopment at 6 months. Front. Microbiol. 2022, 13, 912968. [Google Scholar] [CrossRef] [PubMed]
- ThThion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 2017, 172, 500–516.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luqman, A.; Nega, M.; Nguyen, M.T.; Ebner, P.; Götz, F. SadA-Expressing Staphylococci in the Human Gut Show Increased Cell Adherence and Internalization. Cell Rep. 2018, 22, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Roshchina, V.V. New Trends and Perspectives in the Evolution of Neurotransmitters in Microbial, Plant, and Animal Cells. Adv. Exp. Med. Biol. 2016, 874, 25–77. [Google Scholar]
- SarSarkar, C.; Basu, B.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. The immunoregulatory role of dopamine: An update. Brain, Behav. Immun. 2010, 24, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Färber, K.; Pannasch, U.; Kettenmann, H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell. Neurosci. 2005, 29, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Liu, L.-Z. Catecholamines inhibit microglial nitric oxide production. Brain Res. Bull. 2000, 52, 525–530. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [Green Version]
- Tsavkelova, E.A.; Botvinko, I.V.; Kudrin, V.S.; Oleskin, A.V. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl. Biochem. 2000, 372, 115–117. [Google Scholar]
- Zafra, F.; Lindholm, D.; Castrén, E.; Hartikka, J.; Thoenen, H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J. Neurosci. 1992, 12, 4793–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, J.; Zeppenfeld, D.; McConnell, E.; Pena, S.; Nedergaard, M. Norepinephrine: A Neuromodulator That Boosts the Function of Multiple Cell Types to Optimize CNS Performance. Neurochem. Res. 2012, 37, 2496–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Takanaga, H.; Ohtsuki, S.; Hosoya, K.I.; Terasaki, T. GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood-brain barrier. J. Cereb. Blood Flow Metab. 2001, 21, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sarraf, H. Transport of 14C-γ-aminobutyric acid into brain, cerebrospinal fluid and choroid plexus in neonatal and adult rats. Dev. Brain Res. 2002, 139, 121–129. [Google Scholar] [CrossRef]
- Shyamaladevi, N.; Jayakumar, A.R.; Sujatha, R.; Paul, V.; Subramanian, E.H. Evidence that nitric oxide production increases gamma-amino butyric acid permeability of blood-brain barrier. Brain Res. Bull. 2002, 57, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bjurstöm, H.; Wang, J.; Ericsson, I.; Bengtsson, M.; Liu, Y.; Mendu, S.K.; Issazadeh-Navikas, S.; Birnir, B. GABA, a natural immunomodulator of T lymphocytes. J. Neuroimmunol. 2008, 205, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krantis, A. GABA in the Mammalian Enteric Nervous System. News Physiol. Sci. Int. J. Physiol. Prod. jointly by Int. Union Physiol. Sci. Am. Physiol. Soc. 2000, 15, 284–290. [Google Scholar] [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrensel, A.; Ceylan, M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci 2015, 13, 239–244. [Google Scholar] [CrossRef]
- Abbott, N.J. Inflammatory Mediators and Modulation of Blood–Brain Barrier Permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- Sternberg, E.M.; Wedner, H.J.; Leung, M.K.; Parker, C.W. Effect of serotonin (5-HT) and other monoamines on murine macrophages: Modulation of interferon-gamma induced phagocytosis. J. Immunol. 1987, 138, 4360–4365. [Google Scholar] [PubMed]
- Kubera, M.; Maes, M.; Kenis, G.; Kim, Y.-K.; Lasoń, W. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor α and interleukin-6. Psychiatry Res. 2005, 134, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.-T.; Kuan, Y.-H.; Wang, J.; Hen, R.; Gershon, M.D. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J. Neurosci. 2009, 29, 9683–9699. [Google Scholar] [CrossRef] [Green Version]
- McVey Neufeld, K.A.; Perez-Burgos, A.; Mao, Y.K.; Bienenstock, J.; Kunze, W.A. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol. Motil. 2015, 27, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-H.; Li, J.; Dong, F.-Y.; Yang, J.-Y.; Liu, D.-J.; Yang, X.-M.; Wang, Y.-H.; Yang, M.-W.; Fu, X.-L.; Zhang, X.-X.; et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017, 153, 277–291.e19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romero, M.A.; Cota, I.; Casadesús, J. DNA methylation in bacteria: From the methyl group to the methylome. Curr. Opin. Microbiol. 2015, 25, 9–16. [Google Scholar] [CrossRef]
- Wiseman, A.K.; Tiedemann, R.L.; Fan, H.; Shen, H.; Madaj, Z.; McCabe, M.T.; Pappalardi, M.B.; Jones, P.A. Chromosome-specific retention of cancer-associated DNA hypermethylation following pharmacological inhibition of DNMT1. Commun. Biol. 2022, 5, 528. [Google Scholar] [CrossRef]
- Omar, N.N.; Mosbah, R.A.; Sarawi, W.S.; Rashed, M.M.; Badr, A.M. Rifaximin Protects against Malathion-Induced Rat Testicular Toxicity: A Possible Clue on Modulating Gut Microbiome and Inhibition of Oxidative Stress by Mitophagy. Molecules 2022, 27, 4069. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, T.K.; Goel, H.; Goyal, K.; Pandey, A.K.; Benjamin, M.; Khan, F.; Pandey, P.; Mittan, S.; Iqbal, D.; Alsaweed, M.; et al. Elucidations of Molecular Mechanism and Mechanistic Effects of Environmental Toxicants in Neurological Disorders. CNS Neurol. Disord.—Drug Targets 2023, 22, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.G.; Houseman, E.A.; Green, B.B.; Lesseur, C.; Armstrong, D.A.; Lester, B.; Marsit, C.J. Regions of variable DNA methylation in human placenta associated with newborn neurobehavior. Epigenetics 2016, 11, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, P.W.; Burt, A.; Karagas, M.; Jackson, B.P.; Punshon, T.; Lester, B.; Marsit, C.J. Association between placental toxic metal exposure and NICU Network Neurobehavioral Scales (NNNS) profiles in the Rhode Island Child Health Study (RICHS). Environ. Res. 2021, 204 Pt A, 111939. [Google Scholar] [CrossRef]
- Oakley, R.H.; Whirledge, S.D.; Petrillo, M.G.; Riddick, N.V.; Xu, X.; Moy, S.S.; Cidlowski, J.A. Combinatorial actions of glucocorticoid and mineralocorticoid stress hormone receptors are required for preventing neurodegeneration of the mouse hippocampus. Neurobiol. Stress 2021, 15, 100369. [Google Scholar] [CrossRef] [PubMed]
- Stroud, L.R.; Papandonatos, G.D.; Salisbury, A.L.; Phipps, M.G.; Huestis, M.A.; Niaura, R.; Padbury, J.F.; Marsit, C.J.; Lester, B.M. Epigenetic Regulation of Placental NR3C1, Mechanism Underlying Prenatal Programming of Infant Neurobehavior by Maternal Smoking? Child Dev. 2016, 87, 49–60. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Ren, D.; Zhao, A.; Cheng, Y.; Liu, Y.; Zhao, Y.; Yang, X. Eurotium cristatum reduces obesity by alleviating gut microbiota dysbiosis and modulating lipid and energy metabolism. J. Sci. Food Agric. 2022, 102, 7039–7051. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; de Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendrich, S.J.; Koralnik, L.R.; Bonner, M.; Goetz, D.; Joe, P.; Lee, J.; Mueller, B.; Robinson-Papp, J.; Gonen, O.; Clemente, J.C.; et al. Patient-reported exposures and outcomes link the gut-brain axis and inflammatory pathways to specific symptoms of severe mental illness. Psychiatry Res. 2022, 312, 114526. [Google Scholar] [CrossRef]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.C.; Lawlor, D.A.; Richardson, S.S. It’s the mother!: How assumptions about the causal primacy of maternal effects influence research on the developmental origins of health and disease. Soc. Sci. Med. 2018, 213, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Kumagaya, R.; Kakizaki, T.; Fujihara, K.; Wakamatsu, K.; Yanagawa, Y. Loss of Glutamate Decarboxylase 67 in Somatostatin-Expressing Neurons Leads to Anxiety-Like Behavior and Alteration in the Akt/GSK3β Signaling Pathway. Front. Behav. Neurosci. 2019, 13, 131. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, K.; Wen, Y.; Chen, X.; Liu, H.; Qi, F.; Fu, Y.; Zhu, J.; Guan, S.; Liu, Z. Contribution of hippocampal BDNF/CREB signaling pathway and gut microbiota to emotional behavior impairment induced by chronic unpredictable mild stress during pregnancy in rats offspring. PeerJ 2022, 10, e13605. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.W.; Lee, J.J.; Corcoran, C.M.; Kimhy, D.; Kranz, T.M.; Malaspina, D. Considering the Microbiome in Stress-Related and Neurodevelopmental Trajectories to Schizophrenia. Front. Psychiatry 2020, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, E.; Guzzardi, M.A.; Tripodi, M.; Panetta, D.; Selma-Royo, M.; Zega, A.; Telleschi, M.; Collado, M.C.; Iozzo, P. Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci. Rep. 2019, 9, 12609. [Google Scholar] [CrossRef] [Green Version]
- Lohuis, M.A.M.; Werkman, C.C.N.; Harmsen, H.J.M.; Tietge, U.J.F.; Verkade, H.J. Absence of Intestinal Microbiota during Gestation and Lactation Does Not Alter the Metabolic Response to a Western-type Diet in Adulthood. Mol. Nutr. Food Res. 2018, 63, e1800809. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.-H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C.; et al. Microbiota, metabolome, and immune alterations in obese mice fed a high-fat diet containing type 2 resistant starch. Mol. Nutr. Food Res. 2017, 61, 1700184. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [Green Version]
- del Pozo-Acebo, L.; Hazas, M.L.D.L.; Margollés, A.; Dávalos, A.; García-Ruiz, A. Eating microRNAs: Pharmacological opportunities for cross-kingdom regulation and implications in host gene and gut microbiota modulation. J. Cereb. Blood Flow Metab. 2021, 178, 2218–2245. [Google Scholar] [CrossRef]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocr. 2019, 55, 100797. [Google Scholar] [CrossRef] [PubMed]
- Coley, E.J.; Hsiao, E.Y. Malnutrition and the microbiome as modifiers of early neurodevelopment. Trends Neurosci. 2021, 44, 753–764. [Google Scholar] [CrossRef]
- Sun, X.; Fukami, T.; Li, T.; Desai, M.; Ross, M.G. Preferential development of neuropeptide Y/GABA circuit in hypothalamic arcuate nucleus in postnatal rats. Brain Res. 2016, 1635, 27–40. [Google Scholar] [CrossRef]
- Arruda-Carvalho, M.; Wu, W.-C.; Cummings, K.A.; Clem, R.L. Optogenetic Examination of Prefrontal-Amygdala Synaptic Development. J. Neurosci. 2017, 37, 2976–2985. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front. Microbiol. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [PubMed]
- Marques, T.M.; Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Ryan, C.A.; Stanton, C. Programming infant gut microbiota: Influence of dietary and environmental factors. Curr. Opin. Biotechnol. 2010, 21, 149–156. [Google Scholar] [CrossRef]
- Zubair, M.; Fatima, F.; Husain, F.M. Behavioral Abnormalities of Gut Microbiota and Progression of Dementia. In Current Thoughts on Dementia; Springer: Singapore, 2022; pp. 273–309. [Google Scholar]
- Glenny, E.M.; Fouladi, F.; Thomas, S.A.; Bulik-Sullivan, E.C.; Tang, Q.; Djukic, Z.; Trillo-Ordonez, Y.S.; Fodor, A.A.; Tarantino, L.M.; Bulik, C.M.; et al. Gut microbial communities from patients with anorexia nervosa do not influence body weight in recipient germ-free mice. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2015, 298 Pt B, 202–209. [Google Scholar] [CrossRef]
- Faucher, P.; Dries, A.; Mousset, P.; Leboyer, M.; Dore, J.; Beracochea, D. Synergistic effects of Lacticaseibacillus rhamnosus GG, glutamine, and curcumin on chronic unpredictable mild stress-induced depression in a mouse model. Benef. Microbes 2022, 13, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2016, 595, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Q.; Cai, M.; Xiao, B.; Zhan, Q.; Zeng, C. The Microbiota-Gut-Brain Axis and Epilepsy. Cell. Mol. Neurobiol. 2022, 42, 439–453. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Yang, H.; Rao, Y.; Miao, J.; Lu, X. Intestinal Microbiota as an Alternative Therapeutic Target for Epilepsy. Can. J. Infect. Dis. Med Microbiol. 2016, 2016, 9032809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Paik, D.; Ramirez, R.N.; Biggs, D.G.; Park, Y.; Kwon, H.-K.; Choi, G.B.; Huh, J.R. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4+ T cells. Immunity 2021, 55, 145–158.e7. [Google Scholar] [CrossRef]
- Sililas, P.; Huang, L.; Thonusin, C.; Luewan, S.; Chattipakorn, N.; Chattipakorn, S.; Tongsong, T. Association between Gut Microbiota and Development of Gestational Diabetes Mellitus. Microorganisms 2021, 9, 1686. [Google Scholar] [CrossRef]
- Tang, R.; Xiao, G.; Jian, Y.; Yuan, Q.; Jiang, C.; Wang, W. The Gut Microbiota Dysbiosis in Preeclampsia Contributed to Trophoblast Cell Proliferation, Invasion, and Migration via lncRNA BC030099/NF-κB Pathway. Mediat. Inflamm. 2022, 2022, 6367264. [Google Scholar] [CrossRef] [PubMed]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A.; et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr. Obes. 2022, 17, e12921. [Google Scholar] [CrossRef] [PubMed]
- Kar, F.; Hacioglu, C.; Kar, E.; Donmez, D.B.; Kanbak, G. Probiotics ameliorates LPS induced neuroinflammation injury on Aβ 1–42, APP, γ-β secretase and BDNF levels in maternal gut microbiota and fetal neurodevelopment processes. Metab. Brain Dis. 2022, 37, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.M.; Moreno, J.L.; Ibi, D.; Sikaroodi, M.; Kang, D.J.; Muñoz-Moreno, R.; Dalmet, S.S.; García-Sastre, A.; Gillevet, P.M.; Dozmorov, M.G.; et al. Gut microbiota manipulation during the prepubertal period shapes behavioral abnormalities in a mouse neurodevelopmental disorder model. Sci. Rep. 2020, 10, 4697. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.-Q.; Zheng, Q.-X.; Jiang, X.-M.; Chen, X.-Q.; Zhang, X.-Y.; Wu, J.-L. Probiotic Supplements Improve Blood Glucose and Insulin Resistance/Sensitivity among Healthy and GDM Pregnant Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evidence-Based Complement. Altern. Med. 2021, 2021, 9830200. [Google Scholar] [CrossRef]
- Lacroix, M.; Kina, E.; Hivert, M.-F. Maternal/Fetal Determinants of Insulin Resistance in Women During Pregnancy and in Offspring Over Life. Curr. Diabetes Rep. 2013, 13, 238–244. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Rajeswari, V.D. Gestational diabetes mellitus—A metabolic and reproductive disorder. Biomed. Pharmacother. 2021, 143, 112183. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Wang, S.; Harvey, L.; Martin, R.; van der Beek, E.M.; Knol, J.; Cryan, J.F.; Renes, I.B. Targeting the gut microbiota to influence brain development and function in early life. Neurosci. Biobehav. Rev. 2018, 95, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Chalazonitis, A.; Rao, M.; Sulzer, D. Similarities and differences between nigral and enteric dopaminergic neurons unravel distinctive involvement in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L.D.B.; de Oliveira, Y.; Carvalho, N.N.C.; Cavalcante, R.G.S.; Lira, M.M.P.; Nascimento, L.C.P.D.; Magnani, M.; Vidal, H.; Braga, V.D.A.; de Souza, E.L. Gut microbiota and probiotic intervention as a promising therapeutic for pregnant women with cardiometabolic disorders: Present and future directions. Pharmacol. Res. 2019, 145, 104252. [Google Scholar] [CrossRef] [PubMed]

| Metabolites | Function | Ref. |
|---|---|---|
| Short-chain fatty acids (e.g., butyric acid, propionic acid, acetic acid, valeric acid, isobutyric acid, isovaleric acid, and isocaproic acid) | Modulate BBB permeability; regulate microglia activation and neuroinflammation; regulate the activity of histone deacetylase | [18] [19] [20] [21] |
| Amino acid metabolites (e.g., GABA, serotonin, dopamine, “TRYP6”, norepinephrine, P-cresol) | Maintain normal neurotransmission and neurodevelopment; regulate the availability of vitamin B3 and NADP+ in the brain; regulate neurotoxicity and neurodegeneration; regulate myelination and differentiation to oligodendrocytes; increase oxidative stress | [22] [23] [24] [25] [26] |
| Trimethylamine N oxide | Disturb mitochondria function; increase synaptic damage; promote neuroinflammation | [27] [28] [29] [30] |
| Polyphenolic Metabolites | Modulate neuronal receptors;antioxidation; anti-inflammation | [31] [32] |
| Bacterial Amyloid Proteins | Induce α-syn-aggregates in the brain; enhance neuroinflammation | [33] [34] |
| Cholesterol Steroids hormones | Decarboxylation; dihydroxylation Deconjugations; oxidation reductions | [35] [36] |
| Gut Microbiota | Neurotransmitters | Functions | Ref |
|---|---|---|---|
| Staphylococcus Bacillus cereus, Proteus vulgaris, Serratia marcescens, Escherichia coli | Dopamine | Affect immune cells, cytokines productions by activated T cells; regulate microglial cell migration | [60,61,62,63,65] |
| Escherichia coli, Bacillus subtilis, Bacillus mycoides, Proteus vulgaris, Serratia marcescens | Norepinephrine | Neuroprotective effects by suppressing inflammatory genes; modulate excitatory and interneuronal responses | [66,67,68] |
| Lactobacillus Bifdobacterium, Streptococcus | GABA | Modulate the inhibitory balance; cytokine downregulations by proinflammatory immune cells | [69,70,71,72,73,74] |
| Candida, Streptococcus, Escherichia, Enterococcus, Pseudomonas | Serotonin | Suppress MHC class II expression; reduce proinflammatory cytokines generated by macrophages and lymphocytes; development of enteric and CNS neurons | [79,80,81,82,84,85,86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, F.; Fan, B.; Wang, R.; Ren, J.; Jia, S.; Wang, L.; Chen, Z.; Liu, X.-A. The Molecular Gut-Brain Axis in Early Brain Development. Int. J. Mol. Sci. 2022, 23, 15389. https://doi.org/10.3390/ijms232315389
Muhammad F, Fan B, Wang R, Ren J, Jia S, Wang L, Chen Z, Liu X-A. The Molecular Gut-Brain Axis in Early Brain Development. International Journal of Molecular Sciences. 2022; 23(23):15389. https://doi.org/10.3390/ijms232315389
Chicago/Turabian StyleMuhammad, Fahim, Bufang Fan, Ruoxi Wang, Jiayan Ren, Shuhui Jia, Liping Wang, Zuxin Chen, and Xin-An Liu. 2022. "The Molecular Gut-Brain Axis in Early Brain Development" International Journal of Molecular Sciences 23, no. 23: 15389. https://doi.org/10.3390/ijms232315389
APA StyleMuhammad, F., Fan, B., Wang, R., Ren, J., Jia, S., Wang, L., Chen, Z., & Liu, X.-A. (2022). The Molecular Gut-Brain Axis in Early Brain Development. International Journal of Molecular Sciences, 23(23), 15389. https://doi.org/10.3390/ijms232315389







