Abstract
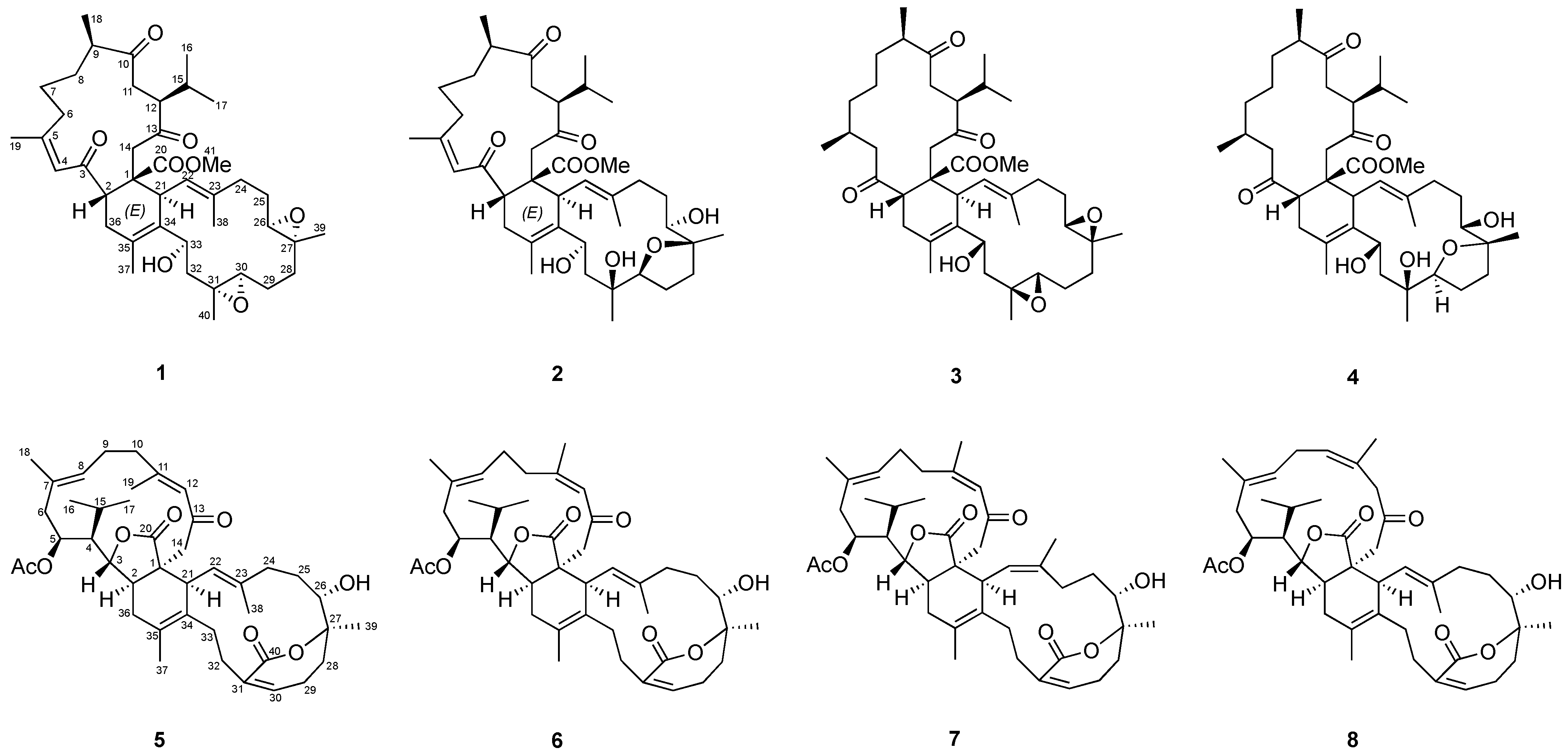
Biscembranoids are the distinctive tetraterpenoids owing a 14/6/14 membered tricyclic scaffold that have been mainly discovered in the soft corals, especially the genera Sarcophyton, Lobophytum and Sinularia. Recent findings have demonstrated the great anti-inflammatory potential of biscembranoid analogues in human neutrophils, motivating more chemical and biological explorations targeting these marine-derived natural products. In the current study, the chemical diversity of biscembranoids derived from the cultured-type Sarcophyton trocheliophorum von Marenzeller was illustrated through MS/MS molecular networking (MN) profiling approach. Based on the MN patterns, the prioritization of unknown biscembranoid derivatives was putatively analyzed. As a result, the biscembrane targeting isolation afforded two new metabolites, sarcotrochelides A (1) and B (2), along with six known analogues (3–8). Their structures and relative configurations were determined by spectroscopic methods. In vitro neutrophil inflammatory inhibition was further investigated for all isolates based on reduced superoxide anion (O2•−) generation detections. Compounds 5–8 showed significant dose-dependently inhibitory effects, suggesting the cruciality of 6,7-dihydrooxepin-2(5H)-one moiety and saturated γ-lactone ring in their reactive oxygen species (ROS)-dependent anti-inflammatory properties.
1. Introduction
Biscembranoids are the distinctive tetraterpenoids owing a 14/6/14 membered tricyclic scaffold that have been mainly discovered in the marine organisms, especially the soft corals belonging to the genus Sarcophyton [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. These secondary metabolites was first found in 1986 [1] and exhibited various bioactivities, ranging from anti-cancer, anti-inflammatory, neuroprotective, anti-microbial, and immunomodulatory activities. The anti-inflammatory effect accounts for the majority of bioactivities of the tetraterpene derivatives. Recent studies have also shown the great anti-inflammatory potential of biscembranoid analogues in human neutrophils, which has attracted more chemical and biological explorations targeting these marine-derived natural products.
Due to the promising pharmacological actions, higher quantity of potent marine-derived compounds is required for subsequent preclinical and clinical trials, but the yields of the secondary metabolites obtained from the wild-type marine organism are usually insufficient for these purposes. Therefore, marine aquaculture has emerged as an effective approach to maintain a sustainable, consistent, and reproducible supply of marine-derived natural products.
In the current study, a MS/MS molecular networking method has been utilized to assist in the discovery of new biscembranoids from the cultured soft coral Sarcophyton trocheliophorum. Based on the MN patterns and NMR spectra, the fraction that was putatively identified to contain biscembranoids was subjected to further purification steps. The biscembrane targeting isolation afforded two new metabolites, sarcotrochelide A (1) and B (2), along with six known analogues (3–8) (Figure 1). Their structures were determined by spectroscopic methods. In vitro neutrophil inflammatory inhibition was further investigated for all isolates based on ROS generation detections using luminol enhanced chemiluminescence. Compounds 5–8 showed significant dose-dependently inhibitory effects, suggesting the cruciality of 6,7-dihydrooxepin-2(5H)-one moiety in their anti-inflammatory properties.
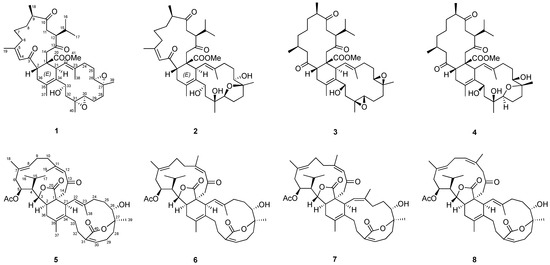
Figure 1.
The identified biscembranoids from the cultured soft coral Sarcophyton trocheliophorum.
2. Results and Discussion
2.1. Characterizing the Distribution of Anti-Inflammatory Biscembranoids Using Multi-informative Molecular Networking (MIMN)
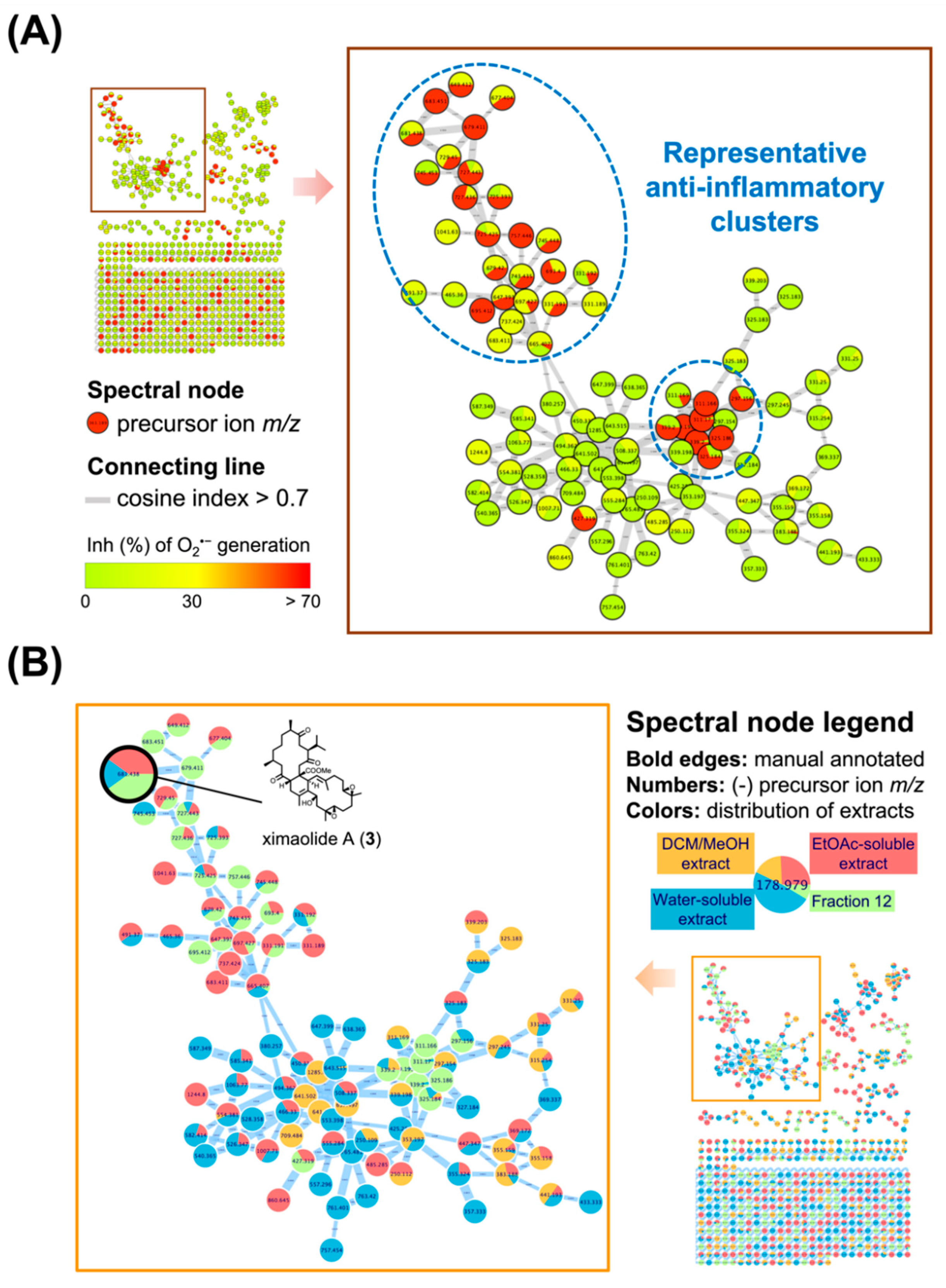
In order to facilitate the operating process for probing anti-inflammatory biscembranoids, a multi-informative molecular networking (MIMN) was applied [29]. In the primary extraction and fractionation part, the organic extract (using dichloromethane:methanol = 1:1) of the cultured-type Sarcophyton trocheliophorum was fractionationed into the EtOAc and the aqueous layers through liquid-liquid partitioning approaches. The chromatographic separation (normal phase) on EtOAc soluble residue further afforded 20 subfractions. Then the chemical and anti-inflammatory MIMN profiles of these fractions were constructed based on the MS/MS analysis and superoxide anion (O2•−) inhibitory assessments in activated neutrophils (Table 1), respectively. The followed-up clustering, classification, and annotation were performed on the GNPS platform (https://gnps.ucsd.edu, accessed on 17 April 2022).

Table 1.
Effects of crude samples on superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils.
Based on the MIMN analyzing results, the clusters of cembrane dimer (m/z 650–750) and monomer (m/z 290–350) exhibited the greatest anti-inflammatory potential with inhibition rate over 70% at the concentration of 10 μg/mL (Figure 2A). The insight MIMN patterns of metabolite distribution (Figure 2B) revealed that the fraction 12 from the EtOAc-soluble extract contained a variety of anti-inflammatory biscembranoids, resulting the further isolation targeting these characteristic tetraterpenoids from fraction 12.
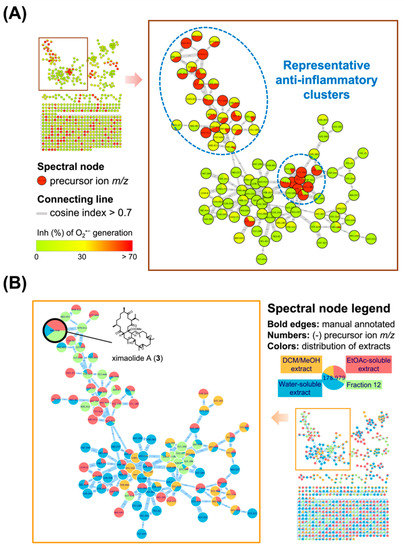
Figure 2.
The multi-informative molecular networking (MIMN) profiles illustrated the relationships between the metabolomic diversity and anti-inflammatory property of the Sarcophyton trocheliophorum extract. (A) The established MIMN is based on the inhibition of O2•− generation (10 μg/mL) in N-formylmethionyl-leucyl-phenylalanine (fMLF)-induced neutrophils and the spectral nodes were colored according to the levels of inhibition; (B) the MIMN spectral nodes are labeled according to the distributions of each extract and fractions.
2.2. Chemical Identification of Isolated Compounds
The target isolation of Fraction 12 of soft coral S. trocheliophorum yielded eight compounds (1–8), including six known ones (3–8) identified as ximaolide A (3) [9], methyl tortuoate D (4) [18], glaucumolide A (5) [20], glaucumolide B (6) [20], bistrochelide A (7) [18], and bistrochelide B (8) [18] by comparing their NMR spectroscopic data with those reported in the literature.
Sarcotrochelide A (1) was isolated as a white powder. The positive mode high resolution electrospray ionization mass spectrum (HRESIMS) showed a peak at m/z 681.4362, suggesting a molecular formula of C41H60O8 (calcd. for [C41H60O8 + H]+, 681.4366), and implying 12 degrees of unsaturation. The signal at 3417 cm−1 in the IR spectrum indicated the presence of the hydroxy group. A total of 41 carbons in the structure of compound 1 were deduced from the 13C NMR spectrum. The multiplicity of carbon signals was determined from distortionless enhancement by polarization transfer (DEPT) and heteronuclear single quantum coherence (HSQC) spectra, including nine methyls (including one methoxyl), eleven methylenes, ten methines, and eleven non-protonated carbons. The 1H and 13C NMR spectra signals (Table 2) showed three olefinic methyl groups [δH 1.89 (s); 1.74 (s); 1.62 (s); δC 26.3, 18.7, 17.3], two methyls attached to oxygen-bearing quaternary carbon [δH 1.25 (s); 1.25 (s); δC 16.3, 18.7], two methyls of an isopropyl group [δH 0.74 (d, J = 6.8 Hz); 0.95 (d, J = 6.8 Hz); δC 18.4, 21.2], and one methoxy group [δH 3.50, s; δC 51.3], two trisubstituted double bond [δH 6.59 (brs); 5.10 (d, J = 11.1 Hz); δC 126.7, CH; 126.7, CH; 134.0, C; 161.1, C]; one tetrasubstituted double bond (δC 130.4, C; 131.2, C); three oxygen-bearing methines [δH 2.29 (dd, J = 8.8, 4.1 Hz); 2.91 (dd, J = 6.1, 4.3 Hz); 4.79, (dd, J = 10.7, 2.1 Hz); δC 60.8, CH; 61.5, CH; 65.1, CH]; two oxygenated quaternary carbon (δC 59.4, 60.0), and four carbonyl carbons (δC 174.6, 203.3, 209.4, 214.8). The spectral analysis suggested the possible presence of a biscembranoid framework.

Table 2.
1H (600 MHz, CDCl3) and 13C (150 MHz, CDCl3) NMR data for 1.
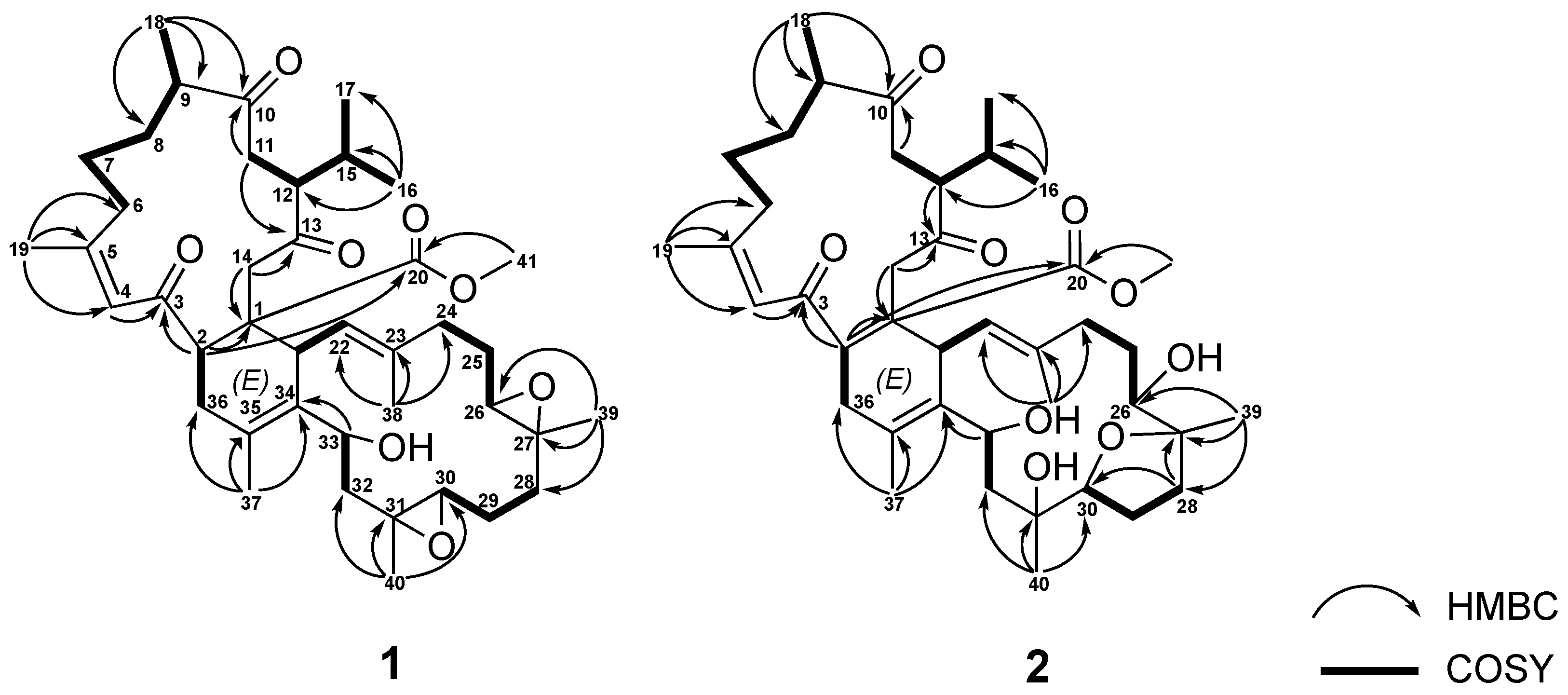
The correlations spectroscopy (COSY) spectrum (Figure 3) of 1 was applied to identify seven different spin systems from H-2 to H2-36; H2-6 via H2-7, H2-8, H-9, and H3-18; isopropyl protons H3-16 and H3-17 via H-15, H-12, and H-11; H-21 to H-22; H2-24 via H2-25 and H2-26; H2-28 via H2-29 and H-30; and H2-32 to H-33. These units were assembled by heteronuclear multiple bond correlation (HMBC) (Figure 3) of H3-16 to C-12, C-15, and C-17; H3-18 to C-8, C-9, and C-10; H3-19 to C-4, C-5, and C-6; H3-37 to C-34, C-35, and C-36; H3-38 to C-22, C-23, and C-24; H3-39 to C-26, C-27, and C-28; H3-40 to C-30, C-31, and C-32; H-2 to C-1, C-3, C-4, and C-20; H2-11 to C-10 and C-13; H2-14 to C-1; and H-33 to C-34. The above-mentioned group accounted for ten of the total twelve degrees of unsaturation, implying the presences of two additional rings. These were suggested to be two trisubstituted epoxide groups with methyl singlets at δH 1.24 (3H, s, H3-39) and 1.25 (3H, s, H3-40), epoxymethine multiplets at δH 2.91 (H-26) and 2.29 (H-30), and 13C NMR signals at 61.5 (C-26), 59.4 (C-27), 60.8 (C-30), and 60.0 (C-31). The gross structure of 1 was thus confirmed as shown in Figure 1, which possesses a biscembranoid skeleton similar to ximaolide A (3) [9].
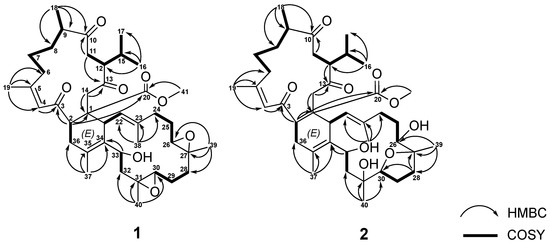
Figure 3.
The selected 1H-1H COSY and HMBC correlations of 1 and 2.
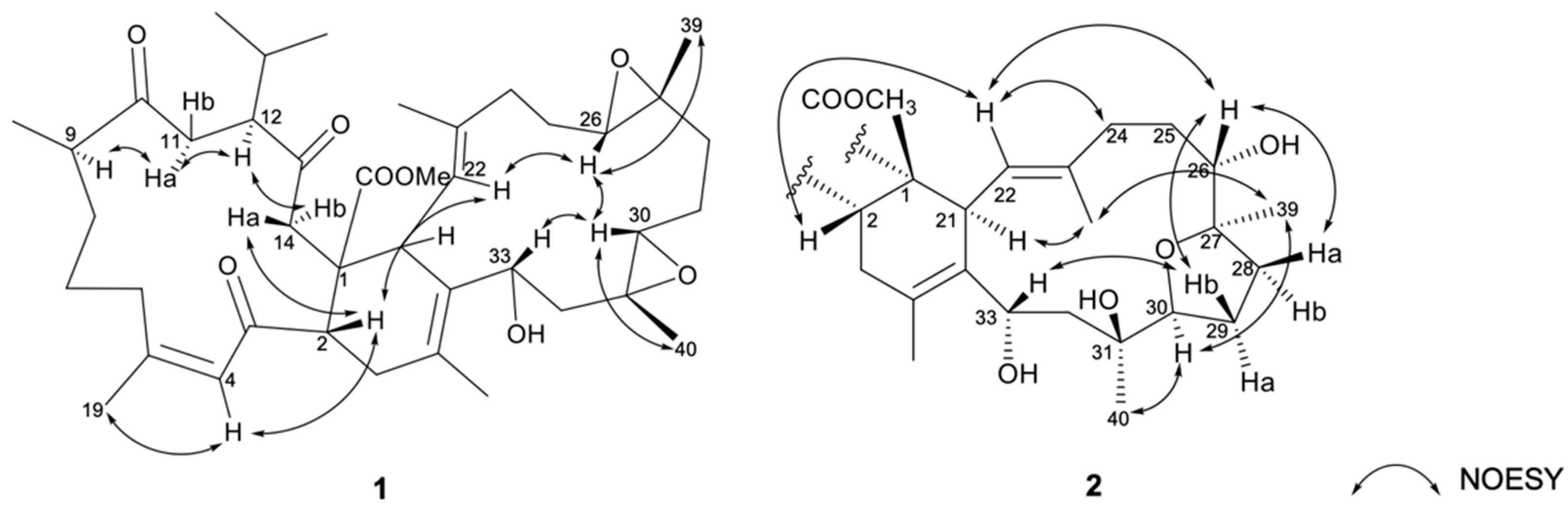
The relative stereochemistry of 1 was elucidated by correlations in the nuclear Overhauser effect relationships (NOESY) experiment. As shown in Figure 4, the NOESY correlations of H-4 (δH 6.59, br s) with H3-19 (δH 1.89, s), together with the obviously downfield-shifted methyl at C-19 (δC 26.3, CH3), suggested a cis geometry of C-4/C-5 trisubstituted double bond. Assuming the β-orientations of H-2 and H3-41 as previously reported, one of the methylene protons at C-14 (δH 3.05, d, J = 18.6 Hz) exhibited NOE correlations with H-2 and was assigned as H-14β, while the other (δH 2.71–2.77, m) was denoted as H-14α. The NOESY correlations observed between H-9 with H-11α (δH 2.71–2.80, m), H-11α (δH 2.71–2.80, m) with H-12 and H-14α, reflected the α-orientations of H-9 and H-12. Furthermore, the correlations of H-2 with H-22; H-22 with H-26; H-26 with H-30 and H3-39; H-30 with H-33 and H3-40, determined the β-orientation of the H-26, H-30, H-33, H3-39, and H3-40. Moreover, the 13C NMR signal of C-38 (δC 17.3, CH3) indicated the E geometry of the trisubstituted C-22/C-23 double bond. On the basis of the above observations and as the relative configurations of 1 determined as shown, the structure of compound 1 could be fully established as 1S*, 2S*, 9R*, 12S*, 21S*, 26R*, 27R*, 30R*, 31R*, 33R*.
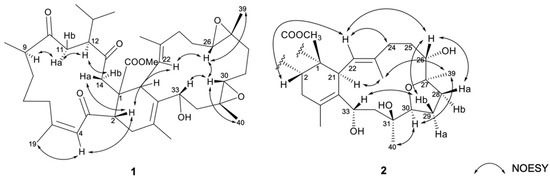
Figure 4.
The selected NOESY correlations of 1 and 2.
Sarcotrochelide B (2) was isolated as a white powder. Its formula was determined as C41H62O9 by the HRESIMS ion at m/z 699.4467 (calcd. for [C41H62O9 + H]+, 699.4472), indicating 11 indices of hydrogen deficiency. The hydroxy-containing structure of 2 was inferred from the IR signal at 3417 cm–1. 41 carbons, including 9 methyls, 11 methylenes, 10 methines, and 11 quaternary carbons, were revealed by the 13C NMR spectrum. The NMR signals (Table 3) showed three olefinic methyl groups (δH 1.89, s; 1.61, s; 1.72, s and δC 26.4; 18.1; 16.4, respectively), two methyl groups linked to oxygen-bearing quaternary carbons (δH 1.17, s; 1.19, s and δC 20.6; 21.4, respectively), one methoxy group (δH 3.50, s; δC 51.1), two methyls of an isopropyl group (δH 2.32–2.38, m; 0.74, d, J = 6.8 Hz; 0.98, d, J = 6.8 Hz; δc 28.6, CH;18.2, CH3; 21.4, CH3), two trisubstituted double bonds (δH 6.59, s; δc 126.4, CH; 161.4, C and δH 4.99, d, J= 10.8 Hz; δc 128.0, CH; 137.2, C, respectively), one tetrasubstituted double bond ( δc 125.3, C and 132.2, C), three oxymethines (δH 3.27, m; 3.96, dd, J= 10.3, 6.3 Hz; 5.05, d, J= 11.2 Hz and δc 74.1, CH; 88.4, CH; 67.5, CH, respectively), two oxygen-bearing quaternary carbons (δc 86.0, C and 76.3, C), and four carbonyl carbons (δc 202.9, C; 214.7, C; 208.7, C and 174.9,C). These findings established the biscembranoid scaffold of 2. The COSY signals (Figure 3) revealed seven different spin systems from H-2 to H2-36; H2-6 via H2-7, H2-8, H-9 and H3-18; H2-11 via H-12, H-15, H3-16 and H3-17; H-21 to H-22; H-24 via H2-25 and H-26; H2-28 via H2-29 and H-30; and H2-32 to H-33. These units were assembled by heteronuclear multiple bond correlation (HMBC) of H3-16 to C-12, C-15 and C-17; H3-18 to C-8, C-9 and C-10; H3-19 to C-4, C-5 and C-6; H3-37 to C-34, C-35 and C-36; H3-38 to C-22, C-23 and C-24; H3-39 to C-26, C-27 and C-28; H3-40 to C30, C-31 and C-32; H-2 to C-1, C-3, C-14 and C-20; H2-11 to C-10; H-12 to C-13; H2-14 to C-1, C-13 and C-20; and H-33 to C-34. The above-mentioned biscembrane framework put paid to ten of the total eleven unsaturated degrees, implying the existence of an additional ring. It was suggested to be of an ester ring between C-27 and C-30 established via an HMBC from H2-28 to C-27 and C-30. The gross structure of 2 was thereby confirmed, as shown in Figure 2, which possesses a biscembranoid skeleton similar to methyl tortuoate D (4) [18].

Table 3.
1H (600 MHz, CDCl3) and 13C (150 MHz, CDCl3) NMR data for 2.
The relative configurations of rings A and B of compound 2 were identical to those of co-occurring compound 1 as determined by the similar NOESY and the NMR data. NOESY correlations (Figure 4) of H3-40/H-30, H-30/H3-39, H3-39/H3-38, and H3-38/H-21 were observed, suggesting that H-21, H-30, H3-39, and H3-40 were co-facial and were assigned as α-orientations. In consequence, NOE correlations of H-2/H-22, H-22/H2-24, H-22/H-26, H-26/H-28a (δH 2.34–2.40, m), H-26/H-29b (δH 1.54–1.60, m), H-29b/H-33 suggested that H-26 and H-33 was β-oriented. Therefore, the structure of 1 was unambiguously elucidated as shown in Figure 2. On the basis of the above observations and as the relative configurations of 2 have been determined as shown, the structure of compound 2 could be fully established as 1S*, 2S*, 9R*, 12S*, 21S*, 26R*, 27R*, 30R*, 31S*, 33R*.
2.3. Bioactivities of the Biscembranoids
The activation effects of N-formyl-methionyl-leucyl-phenylalanine (fMLF) and pathogen-associated molecular patterns (PAMPs) on neutrophils can cause a series of inflammatory responses, such as respiratory burst (O2•− generation) and degranulation (elastase release) [30]. In order to evaluate the anti-inflammatory activities of the cultured soft coral S. trocheliophorum, the EtOAc, MeOH, and water-soluble extracts, and fraction 12 were assayed in fMLF-induced human neutrophils. All eight pure compounds obtained from fraction 12 were also evaluated for their anti-inflammatory effects in the same in vitro tests. The results showed that fraction 12 exhibited the highest inhibitory effects on superoxide anion generation and elastase release with IC50 5.45 and 7.48 μg/mL, respectively, among the crude samples (Table 1).
For pure derivatives, compound 6 displayed the strongest activity against superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils, followed by compounds 5, 7, and 8, respectively (Table 4).

Table 4.
Effects of compounds on superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils.
The significant difference in the anti-inflammatory effects of the isolates may be caused by some variations in their structures. The common characteristics of the four most active compounds is that they share a 6,7-dihydrooxepin-2(5H)-one moiety and a saturated γ-lactone ring. In addition, compound 6 possesses a 11Z and 22E double bonds instead of an E geometry of Δ11(12) and Δ22(23) in compound 5 and a Z geometry of Δ11(12) and Δ22(23) in compound 7. Additionally, when compared to compound 6, the 11,12-double bond was replaced by a 10,11-double bond in compound 8, which suggested that the reduced anti-inflammatory effect of compound 8 might be caused by this minor change.
3. Materials and Methods
3.1. General Experimental Procedures
The optical rotation was measured by a polarimeter JASCO P-2000 (JASCO, Tokyo, Japan). The infrared spectra were obtained on a FT-IR spectrophotometer, Nicolet™ iS™ 5 FTIR Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). UV spectra were collected by Spectrophotometer U-3310 UV-Vis (Hitachi, Ltd., Tokyo, Japan). The 1D and 2D NMR spectra were obtained on an Agilent 600 MHz DD2 NMR (Agilent, Santa Clara, CA, USA). The chloroform-d was used as the internal lock. HRESIMS data in positive mode were collected on a Waters LC/Q-TOF SYNAPT G2 (Waters Corporation, Milford, MA, USA) system. All isolations were purified by MPLC and HPLC. The former is Biotage® Isolera™ Systems (Biotage, Uppsala, Sweden), and the powder was filled in the flash column, Biotage® SNAP Cartridge KP-Sil 10 g (Biotage, Uppsala, Sweden), the latter is HPLC system Shimazu LC-2050 (Shimazu, Kyoto, Japan) with a Galaksil column EF-C18-H (5 μm, 120 Å, 10 × 250 mm, C18; Galak Chromatography, Wuxi, Jiangsu, China).
3.2. Non-Targeted Fragment Ions Collection Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
The acquisition of tandem mass spectral data was carried out based on a Waters SYNAPT G2 LC/Q-TOF (Waters Corporation, Milford, MA, USA) system. The extracts were filtered through a 0.45 μm membrane filter and dissolved in methanol to a final concentration of 5000 ppm for the analysis. For chromatographic part, a C18 column of Waters Acquity UPLC BEH (Waters, 1.7 µm, 2.1 mm × 100 mm) was used for the separation and was maintained in a 40 °C column oven. The analytes were eluated from the column by CH3CN (A, containing 0.1% formic acid)/water (W, containing 0.1% formic acid) gradient sequences: 0.01–25 min, 1–100% A; 25.01–30 min, 100% A, with the flow rate of 0.5 mL/min. Automatical injections were executed with injected volumes of 5 μL. The non-targeted MS1 and MS2 data were acquired within the range of m/z 100–2000. The automated data-dependent acquisition (DDA) approach was used for the acquisition of MS2 spectra, and five precursor ions were selected for further fragmentations with ramping of the collision energy from 10–50 eV. Finally, the finalization of MS data were conducted with the assistance of Waters MassFragment software (MassLynx4.1, Waters, MA, USA).
3.3. GNPS-Based Molecular Networking Analysis
A GNPS web-based platform (https://gnps.ucsd.edu, accessed on 17 April 2022) [31] was applied to analyze and output the MS/MS molecular networking data (job ID: edcb54569b0042fea7104782189e34af, 17 April 2022). The pre-processing of MS/MS raw data was conducted by converting into mzML file using Proteowizard MSConvert (Ver. 3, GitHub repository, Palo Alto, CA, USA). The conversions were uploaded to GNPS drive using WinSCP software (Ver. 5.21, SourceForge, San Diego, CA, USA) and performed molecule networking analysis. The MS/MS spectra were window-filtered according to the top five strongest ion peaks in the ±50 Da window throughout the spectrum. A molecular network was then created, in which the edges between nodes were kept if the cosine scores were above 0.70 and the separated consensus spectra shared at least four matched peaks. Then the appeared nodes in the network were annotated based on the experimental MS2 fragmentations of isolates. The data visualization was executed by Cytoscape 3.8.2 (Cytoscape 3.8.2, NRNB, San Diego, CA, USA) [32].
3.4. Animal Material, Extraction, and Isolation
Specimens of the wild-type S. trocheliophorum was originally collected by scuba diving from the coast of Pingtung, Taiwan, in 2015 (specimen No. 2015-07-ST). These corals were preserved and aquacultured in National Museum of Marine Biology and Aquarium (Pingtung, Taiwan). The aquaculture condition was mentioned below [33]: The collected wild corals were cut into several sub-strains of 4 to 5 cm, and these sub-strains are naturally placed and attached to porous tiles for domestication and cultivation. These soft corals were kept in cultured tanks (120 tons) with temperature controlled (25–28 °C) coolers and supported by natural light daily. The ecological environment was settled up with live sea rocks, live sea sands, snails, paracanthurus hepatus fishes, sea urchins, sea cucumbers, and other aquaculture soft corals, such as Briareum spp., Paralemnalia sp., Sarcophyton spp., and Sinularia spp. The specimens were then collected by hand in July 2020 and were kept in a −20 °C freezer until extraction. A voucher specimen (specimen no. 202007ST1) was deposited in the Graduate Institute of Pharmacognosy, Taipei Medical University.
The cultured-type S. trocheliophorum (1310 g, wet weight) was freeze-dried, then the dry material (106 g) was extracted exhaustively with dichloromethane and methanol (MeOH) to afford 38.3 g of residue after being dried under reduced pressure (Figure S51). The residue was partitioned with ethyl acetate (EtOAc) and water. The EtOAc soluble residue was subjected to silica gel flash chromatography column, using mixtures of n-hexane, EtOAc, and MeOH, with increasing polarity (n-hexane:EtOAc:MeOH, 100:0:0, 90:10:0, 84:16:0, 75:25:0, 65:35:0, 50:50:0, 0:100:0, 0:80:20) and the flow rate of 20–30 mL/min, to yield 20 fractions. After the MN-guided analyzing approach, fraction 12 was selected and then subjected to RP-HPLC, using acidic water (0.1% v/v acetic acid) and acetonitrile (CH3CN) with the ratio of 35:65 as the mobile phase for the isocratic mode elution, to afford glaucumolide A (5) (31.59 mg) (Figures S35–S37), glaucumolide B (6) (53.98 mg) (Figures S38–S40), and 13 other subfractions. Subfraction 1205 was purified with RP-HPLC, using 0.1% acetic acid solution:CH3CN (35:65) as the mobile phase for the isocratic mode elution, to yield sarcotrochelide A (1) (11.7 mg) and B (2) (2.05 mg). Subfraction 1211 was purified with RP-HPLC, using 0.1% acetic acid solution:MeOH (25:75) as the mobile phase for the isocratic mode elution, to yield bistrochelide B (8) (2.28 mg) (Figures S48–S50). Subfraction 1213 was purified with RP-HPLC, using 0.1% acetic acid solution: CH3CN (35:65) as the mobile phase for the isocratic mode elution, to yield bistrochelide A (7) (1.49 mg) (Figures S41–S47). Subfraction 1214 was purified with RP-HPLC, using 0.1% acetic acid solution:MeOH (25:75) as the mobile phase for the isocratic mode elution, to yield ximaolide A (3) (8.19 mg) (Figures S21–S27) and methyl tortuoate D (4) (2.29 mg) (Figures S28–S34).
Sarcotrochelide A (1) (Figures S1–S10 and S52): white powder; = +145.0 (c 0.01, MeOH); UV (MeOH) λmax 206 and 240 nm; IR (KBr) νmax 3417, 2929, 1741, 1704, 1668, 1604, 1434, 1385, 1254, 1207, 1111, 1032 cm−1; 13C and 1H NMR data, Table 2; HRESIMS m/z 681.4362 [M + H]+(calcd. for C41H60O8 + H, 681.4366).
Sarcotrochelide B (2) (Figures S11–S20 and S53): white powder; = +173.6 (c 0.01, MeOH); UV (MeOH) λmax 206 and 240 nm; IR (KBr) νmax 3417, 2923, 1738, 1701, 1667, 1601, 1416, 1372, 1257, 1206, 1110, 1060 cm−1; 13C and 1H NMR data, Table 3; HRESIMS m/z 699.4467 [M + H]+ (calcd. for C41H62O9 + H, 699.4472).
3.5. Preparation of Human Neutrophils
Venous blood sampling was performed on human donors (aged 20–30 years) according to an approved protocol (IRB No. 202002493A3). The purification of neutrophils was achieved according to a reported procedure [34].
3.6. Determination of Superoxide Anion (O2•−) Generation
Under the treatment of compounds 1–8, the O2•− generation of human neutrophils was determined by superoxidase dismutase (SOD) inhibitable reduction in ferricytochrome c as previously described [35].
3.7. Measurement of Elastase Release
MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide was used as a substrate in an elastase release assay to evaluate the degranulation of azurophilic granules as previously described [36].
3.8. Statistics
Statistical analysis was performed using Student’s t-test for calculations. p values < 0.05 were considered to be statistically significant.
4. Conclusions
The chemical investigation of the cultured soft coral Sarcophyton trocheliophorum led to the isolation of two novel metabolites 1–2, along with six known analogues of biscembranoid 3–8. The in vitro tests showed that compound 5–8 exhibited significant inhibitory effects on the superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils. The difference in their bioactivities suggested the importance of the lactone rings and geometry of double bonds in the structures.
The discovery of two novel biscembranoids demonstrated the chemical diversity of this type of metabolite in the aquaculture Sarcophyton trocheliophorum. In addition, the two most bioactive compounds, glaucumolide A (5) and glaucumolide B (6), were obtained with relatively high quantity, measured at 31.59 mg and 53.98 mg, respectively. The results suggested that aquaculture of soft coral could be a prolific and sustainable resource for the drug discovery and development [37].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415464/s1.
Author Contributions
N.B.A.N., L.-Y.C., B.-R.P., and K.-H.L. developed the experimental design; N.B.A.N. and L.-Y.C. performed the MS/MS analysis and isolation experiments; P.-J.C. and T.-L.H. completed the biological experiments; K.-H.L. provided reagent and analytical assessment; N.B.A.N., L.-Y.C., P.-J.C., T.-L.H., J.-H.S., C.-H.S., P.-T.Y., B.-R.P. and K.-H.L. participated in data interpretation; N.B.A.N., L.-Y.C., K.-H.L., B.-R.P. and M.E.-S., drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The grants that supported this work were from the Ministry of Education (DP2-111-21121-01-N-01-03), the National Science and Technology Council (MOST 111-2320-B-291-002, MOST 111-2321-B-255-001, and MOST 111-2320-B-038-040-MY3), and Taipei Medical University (TMU109-AE1-B15), Taiwan.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) at Chang Gung Memorial Hospital (protocol code No. 202002493A3) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, J.; Long, K.; Pang, T.; He, C.H.; Clardy, J. The structure of methyl isosartortuoate, a novel tetracyclic tetraterpenoid from the soft coral Sarcophyton tortuosum. J. Am. Chem. Soc. 1986, 108, 177–178. [Google Scholar] [CrossRef]
- Kusumi, T.; Igari, M.; Ishitsuka, M.O.; Ichikawa, A.; Itezono, Y.; Nakayama, N.; Kakisawa, H. A novel chlorinated biscembranoid from the marine soft coral Sarcophyton glaucum. J. Org. Chem. 1990, 55, 6286–6289. [Google Scholar] [CrossRef]
- Leone, P.A.; Bowden, B.F.; Carroll, A.R.; Coll, J.C.; Meehan, G.V. Studies of Australian soft corals, XLIX. a new biscembranoid and its probable biosynthetic precursors from the soft coral Sarcophyton tortuosum. J. Nat. Prod. 1993, 56, 521–526. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Tseng, H.-K.; Sheu, J.-H. A novel cytotoxic biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 1998, 39, 7121–7122. [Google Scholar] [CrossRef]
- Feller, M.; Rudi, A.; Berer, N.; Goldberg, I.; Stein, Z.; Benayahu, Y.; Schleyer, M.; Kashman, Y. Isoprenoids of the soft coral Sarcophyton glaucum: Nyalolide, a new biscembranoid, and other terpenoids. J. Nat. Prod. 2004, 67, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-M.; Lan, W.-J.; Su, J.-Y.; Zhang, G.-W.; Feng, X.-L.; Liang, Y.-J.; Yang, X.-P. Two new cytotoxic tetracyclic tetraterpenoids from the soft coral Sarcophyton tortuosum. J. Nat. Prod. 2004, 67, 1915–1918. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hashimoto, K.; Okamura, H.; Kurawaki, J.-I.; Nakatani, M.; Hou, D.-X.; Fujii, M.; Doe, M.; Morimoto, Y.; Takemura, K. Biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2006, 69, 1130–1133. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Benayahu, Y.; Kashman, Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic diels–alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007, 70, 1951–1954. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.-W.; Chen, P.; Yang, Y.-M.; Mollo, E.; Gavagnin, M.; Cimino, G. Biscembranoids and their probable biogenetic precursor from the Hainan soft coral Sarcophyton tortuosum. J. Nat. Prod. 2007, 70, 1158–1166. [Google Scholar] [CrossRef]
- Yan, X.-H.; Gavagnin, M.; Cimino, G.; Guo, Y.-W. Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahedron Lett. 2007, 48, 5313–5316. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.-W.; Mollo, E.; Gavagnin, M.; Cimino, G. Further new bis-cembranoids from the Hainan soft coral Sarcophyton tortuosum. Helv. Chim. Acta 2008, 91, 2069–2074. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hashimoto, K.; Yokogawa, Y.; Okamura, H.; Nakatani, M.; Doe, M.; Morimoto, Y.; Takemura, K. Cytotoxic biscembranes from the soft coral Sarcophyton glaucum. J. Nat. Prod. 2009, 72, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Huang, C.-Y.; Dai, C.-F.; Wen, Z.-H.; Sheu, J.-H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010, 51, 5764–5766. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones O-T, new biscembranoids and cembranoid from soft coral Lobophytum pauciflorum. Mar. Drugs 2010, 8, 2837–2848. [Google Scholar] [CrossRef]
- Yan, P.; Lv, Y.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones A-G, new isobiscembranoids from the soft coral Lobophytum pauciflorum. Org. Lett. 2010, 12, 2484–2487. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones U- Z1, biscembranoids from the Chinese soft coral Lobophytum pauciflorum. Chem. Biodivers. 2011, 8, 1724–1734. [Google Scholar] [CrossRef]
- Jia, R.; Kurtan, T.; Mandi, A.; Yan, X.H.; Zhang, W.; Guo, Y.W. Biscembranoids formed from an alpha,beta-unsaturated gamma-lactone ring as a dienophile: Structure revision and establishment of their absolute configurations using theoretical calculations of electronic circular dichroism spectra. J. Org. Chem. 2013, 78, 3113–3119. [Google Scholar] [CrossRef]
- Li, Y.F.; He, L.L.; Liu, H.L.; Liang, L.F.; Zhang, H.B.; Guo, Y.W. Structural revision of methyl tortuoate D, a bis-cembranoid from Hainan Sarcophyton tortuosum and its absolute stereochemistry. J. Asian Nat. Prod. Res. 2013, 15, 566–573. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Van Quang, N.; Van Minh, C.; Thuy Hang, D.T.; Le Tuan Anh, H.; Tai, B.H.; Yen, P.H.; Hoai, N.T.; Thung, D.C.; Van Kiem, P. Biscembranoids from the marine sponge Petrosia nigricans. Nat. Prod. Commun. 2013, 8, 1209–1212. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef]
- Nam, N.H.; Tung, P.T.; Ngoc, N.T.; Hanh, T.T.H.; Thao, N.P.; Thanh, N.V.; Cuong, N.X.; Thao, D.T.; Huong, T.T.; Thung, D.C.; et al. Cytotoxic biscembranoids from the soft coral Sarcophyton pauciplicatum. Chem. Pharm. Bull. 2015, 63, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, A.; Hertzer, C.; Kehraus, S.; Nietzer, S.; Rohde, S.; Schupp, P.J.; Wagele, H.; Konig, G.M. Secondary metabolome and its defensive role in the aeolidoidean Phyllodesmium longicirrum, (Gastropoda, Heterobranchia, Nudibranchia). Beilstein J. Org. Chem. 2017, 13, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zou, Y.H.; Ge, M.X.; Lou, L.L.; Xu, Y.S.; Ahmed, A.; Chen, Y.Y.; Zhang, J.S.; Tang, G.H.; Yin, S. Biscembranoids and cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2017, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Huang, C.Y.; Ahmed, A.F.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. New cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. [Google Scholar] [CrossRef]
- Sun, P.; Cai, F.Y.; Lauro, G.; Tang, H.; Su, L.; Wang, H.L.; Li, H.H.; Mandi, A.; Kurtan, T.; Riccio, R.; et al. Immunomodulatory biscembranoids and assignment of their relative and absolute configurations: Data set modulation in the density functional theory/nuclear magnetic resonance approach. J. Nat. Prod. 2019, 82, 1264–1273. [Google Scholar] [CrossRef]
- Huang, T.Y.; Huang, C.Y.; Chao, C.H.; Lin, C.C.; Dai, C.F.; Su, J.H.; Sung, P.J.; Wu, S.H.; Sheu, J.H. New biscembranoids sardigitolides A-D and known cembranoid-related compounds from sarcophyton digitatum: Isolation, structure elucidation, and bioactivities. Mar. Drugs 2020, 18, 452. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Cuadrado, C.; Gao, C.; Wu, Q.; Li, X.; Pang, T.; Daranas, A.H.; Guo, Y.; Li, X. Polyoxygenated anti-inflammatory biscembranoids from the soft coral Sarcophyton tortuosum and their stereochemistry. Chin. Chem. Lett. 2021, 32, 271–276. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones H-N, biscembranoids from the Chinese soft coral Lobophytum pauciflorum. Chem. Pharm. Bull. 2010, 58, 1591–1595. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Peng, B.-R.; Lai, G.-Y.; Weng, H.-J.; El-Shazly, M.; Su, C.-H.; Su, J.-H.; Sung, P.-J.; Liao, C.-P.; Lai, K.-H. Chemometric-guided exploration of marine anti-neurofibroma leads. Front. Mar. Sci. 2022, 9, 930736. [Google Scholar] [CrossRef]
- Lai, K.-H.; Chen, P.-J.; Chen, C.-C.; Yang, S.-H.; El-Shazly, M.; Chang, Y.-C.; Wu, Y.-H.; Wu, Y.-H.; Wang, Y.-H.; Hsieh, H.-L.; et al. Lophatherum gracile Brongn. attenuates neutrophilic inflammation through inhibition of JNK and calcium. J. Ethnopharmacol. 2021, 264, 113224. [Google Scholar] [CrossRef]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; van der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, E1743–E1752. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.R.; Lu, M.C.; El-Shazly, M.; Wu, S.L.; Lai, K.H.; Su, J.H. Aquaculture Soft Coral Lobophytum crassum as a Producer of Anti-Proliferative Cembranoids. Mar. Drugs 2018, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.R.; Lai, K.H.; Lee, G.H.; Yu, S.S.; Duh, C.Y.; Su, J.H.; Zheng, L.G.; Hwang, T.L.; Sung, P.J. Scalarane-type sesterterpenoids from the marine sponge Lendenfeldia sp. Alleviate inflammation in human neutrophils. Mar. Drugs 2021, 19, 561. [Google Scholar] [CrossRef]
- Lai, K.H.; Chen, Y.L.; Lin, M.F.; El-Shazly, M.; Chang, Y.C.; Chen, P.J.; Su, C.H.; Chiu, Y.C.; Illias, A.M.; Chen, C.C.; et al. Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lai, K.H.; Kumar, S.; Chen, P.J.; Wu, Y.H.; Lai, C.L.; Hsieh, H.L.; Sung, P.J.; Hwang, T.L. 1H NMR-Based Isolation of Anti-Inflammatory 9,11-Secosteroids from the Octocoral Sinularia leptoclados. Mar. Drugs 2020, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.B.A.; Chen, L.Y.; El-Shazly, M.; Peng, B.R.; Su, J.H.; Wu, H.C.; Lee, I.T.; Lai, K.H. Towards Sustainable Medicinal Resources through Marine Soft Coral Aquaculture: Insights into the Chemical Diversity and the Biological Potential. Mar. Drugs 2022, 20, 640. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).