Regulation of P-glycoprotein and Breast Cancer Resistance Protein Expression Induced by Focused Ultrasound-Mediated Blood-Brain Barrier Disruption: A Pilot Study
Abstract
:1. Introduction
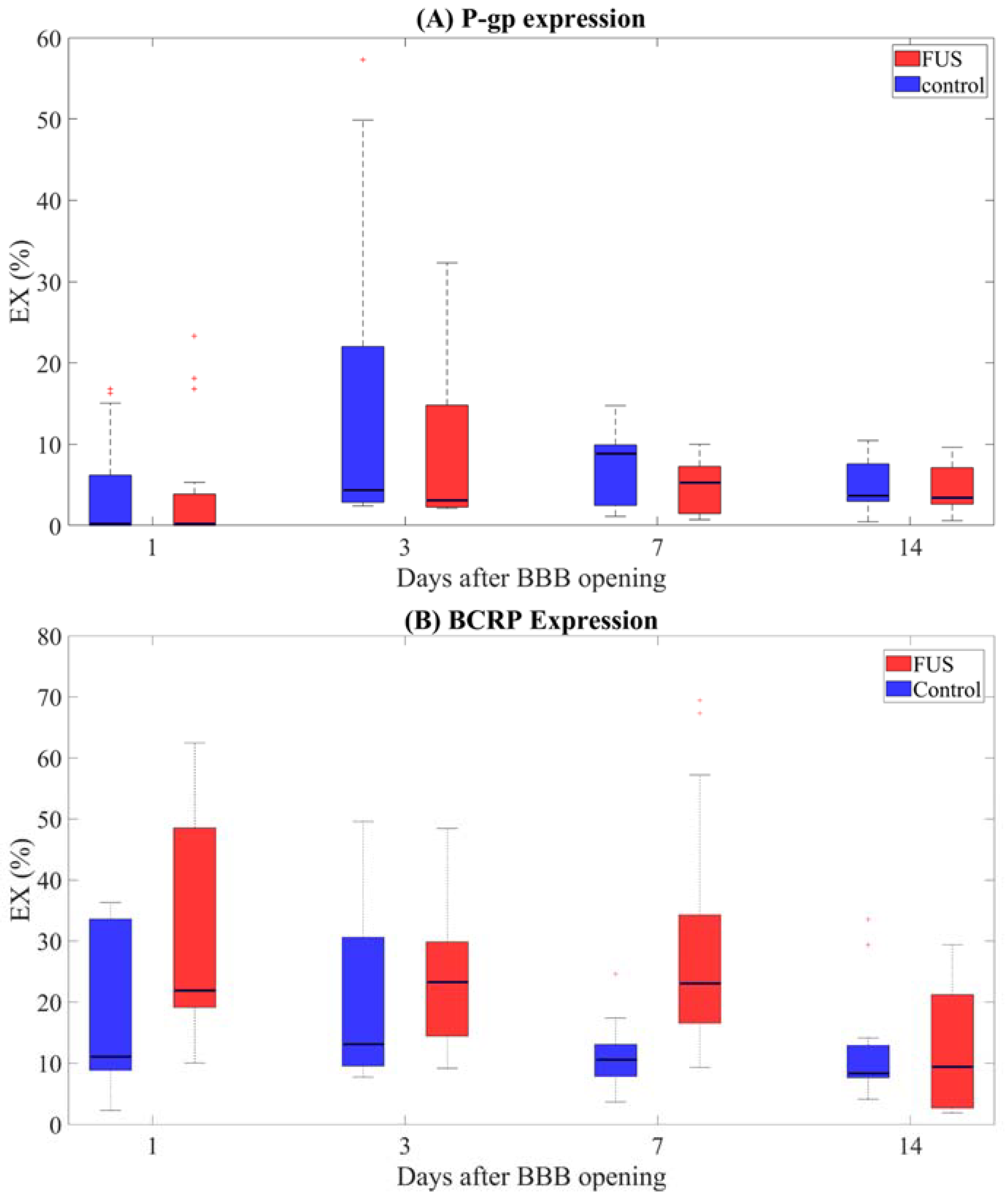
2. Results
- (i)
- Up to seven days after sonication, BCRP is significantly higher in the sonicated area compared with the contralateral brain regions (p-values fdr corrected <0.05, Table 1);
- (ii)
- Two weeks after sonication, BCRP expression in the treated and untreated regions was similar (p-value fdr corrected > 0.05 when comparing hemispheres at day 14);
- (iii)
- (iv)
3. Discussion and Conclusions
3.1. Implications for Drug Delivery Strategies
3.2. Study Limitations and Future Directions
4. Materials and Methods
4.1. Animals
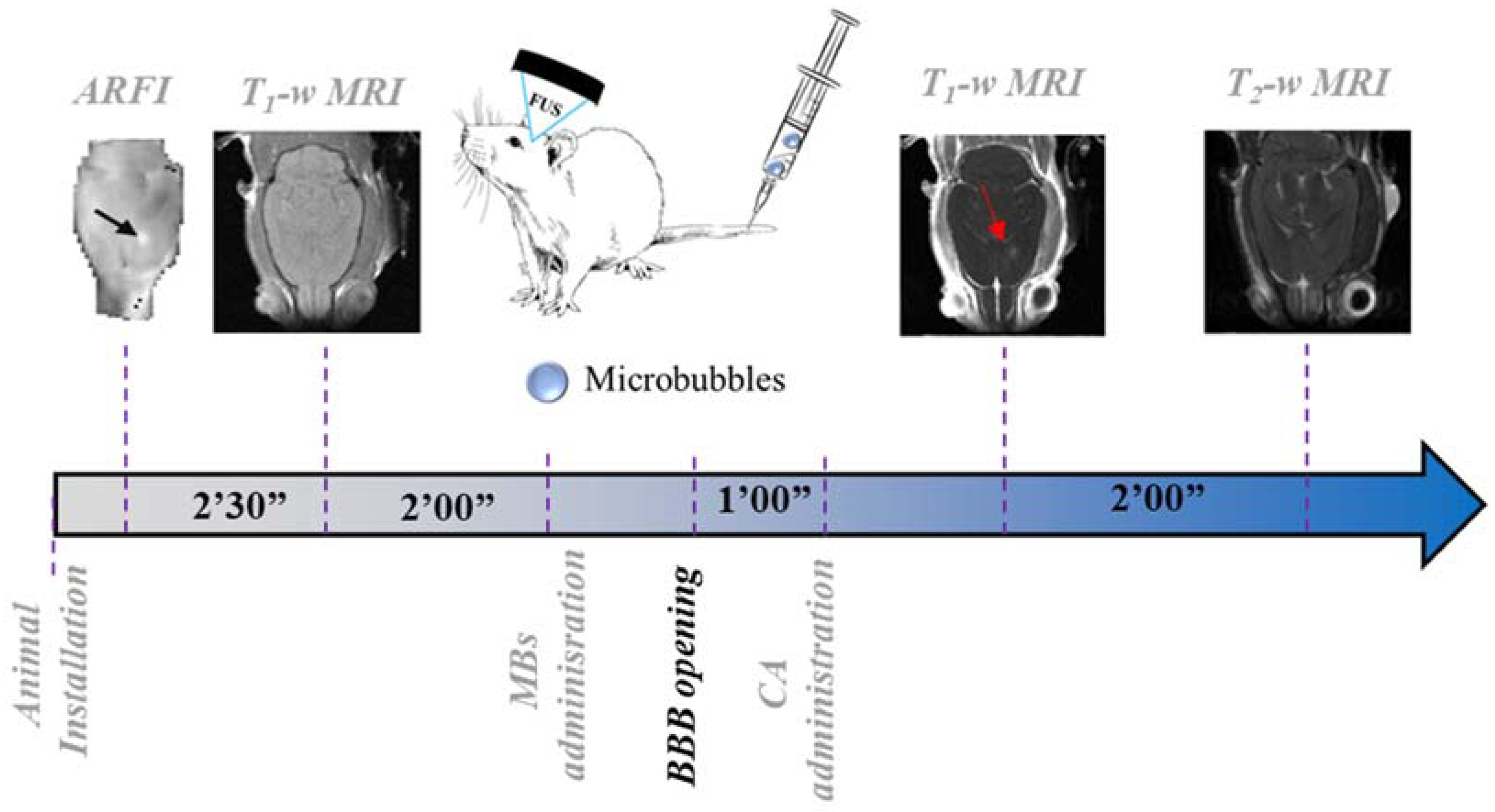
4.2. Experimental Protocol
4.3. Immunohistochemistry
- (i)
- Wash 2 × 5 min with PBS (concentration of 0.01 M);
- (ii)
- Application of antigen retrieval process (ac/eth) followed by blocking/permeabilization protocols (2 h);
- (iii)
- Wash with PBS;
- (iv)
- Incubation of the first antibody (1 h for P-gp/2 h for BCRP);
- (v)
- Wash 2 × 5 min with PBS;
- (vi)
- Incubation of the RECA-1 antibody for 1 h;
- (vii)
- Wash 2 × 5 min with PBS;
- (viii)
- Incubation for 1 h of secondary Dk anti-Rabbit Alexa647 and the secondary Dk anti-mouse Dylight488;
- (ix)
- Wash 3 × 5 min with PBS;
- (x)
- Application of ProLong with Dapi (mounting media, from Thermo Fisher, Waltham, MA, USA, ref P36931).
4.4. Histological Findings Evaluation
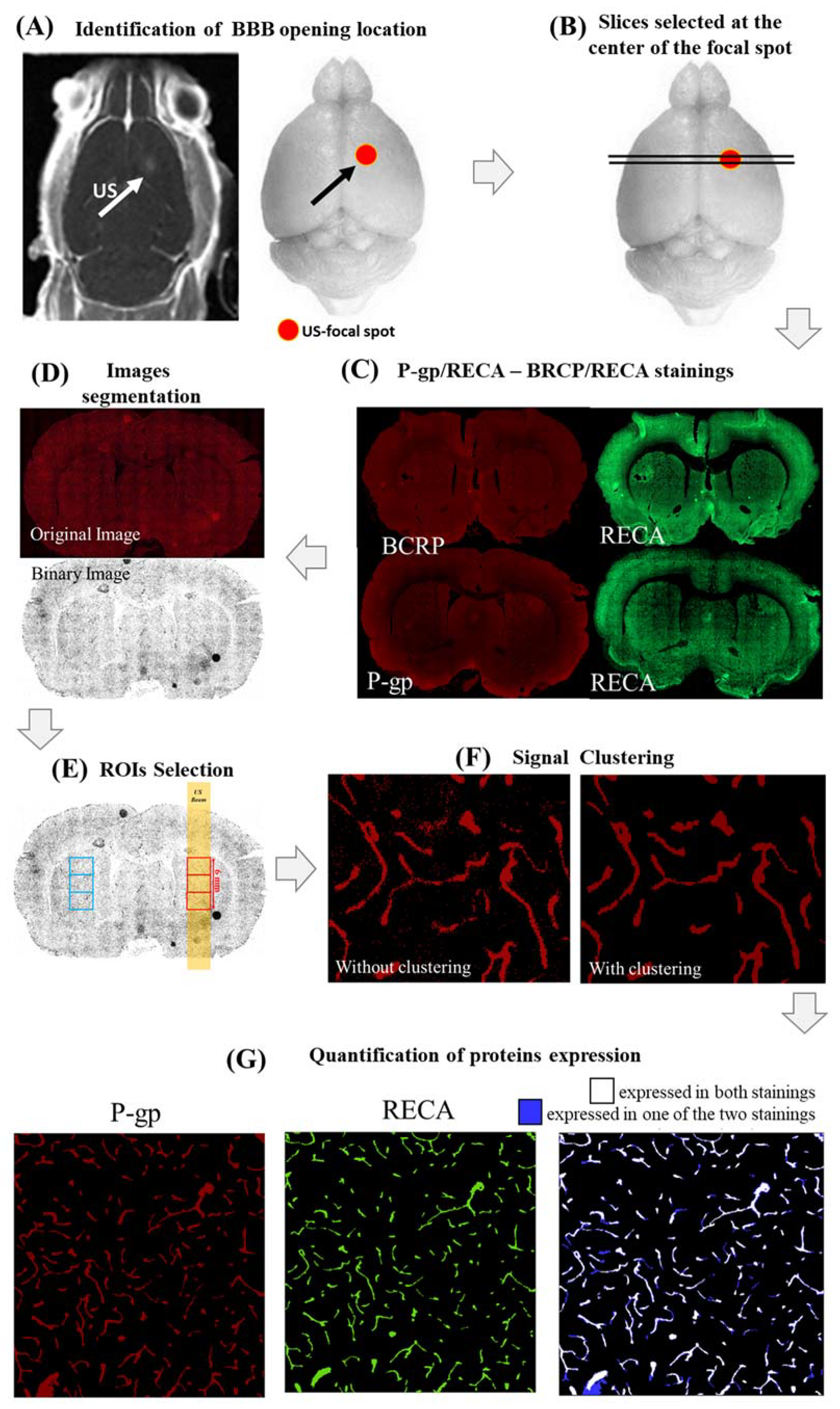
- (i)
- First, the center of the BBB opening was identified in all rat brains by looking at T1-w images, acquired immediately after FUS application and CA injection (Figure 5A);
- (ii)
- Four brain slices positioned around the center of the FUS focal spot were stained for both RECA-1/P-gp (2 slices) and RECA-1/BCRP (2 slices), as shown in Figure 5B,C;
- (iii)
- A custom MATLAB script (MathWorks, USA) thresholded and binarized the histological images for all stainings, as follows (Figure 5D):
- Distributions of P-gp/BCRP and RECA expressions were first fitted in the red and green color channels, respectively, through Gaussian functions. The mean (M) and the standard deviation (σ) of each of these distributions were calculated;
- Binarized images were obtained by excluding hyper/hypointense meaningless background voxels (i.e., with values lower than [M − 2.5σ] or higher than [M + 2.5σ]);
- (iv)
- For each slice and staining, three adjacent square regions of interest (ROIs) of 1000 voxels per side (about 2 mm) centered on the focal spot and the contra-lateral side were defined (Figure 5E). The ROIs were chosen to be large enough to cover the ultrasound spot dimensions (2 mm wide, 6 mm long). Finally, this led us to 6 analyzed ROIs per staining and per rat (18 ROIs in total per single hemisphere/time point). Our sample size was similar to that used in [24] so that we had sufficient statistical power to assess the effects of FUS on the expression of both proteins;
- (v)
- In order to remove the remaining spurious noisy voxels, the voxels in each of these ROIs were clustered using a custom MATLAB code (Figure 5F). The value of the suprathreshold voxel among eight neighbors was assigned to one cluster. This technique was used for all BCRP, P-gp, and RECA-1 stainings;
- (vi)
- The expressions of P-gp and BCRP were defined as the percentage of voxels in each ROI expressing both proteins (P-gp or BCRP) and RECA-1. These voxels are depicted in blue in Figure 5G for a representative ROI in which both P-gp and RECA-1 were expressed.
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, D.S.; Bauer, B.; Hartz, A.M.S. Modulation of P-Glycoprotein at the Blood-Brain Barrier: Opportunities to Improve CNS Pharmacotherapy. Pharmacol Rev. 2008, 60, 196–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laksitorini, M.; Prasasty, V.D.; Kiptoo, P.K.; Siahaan, T.J. Pathways and Progress in Improving Drug Delivery through the Intestinal Mucosa and Blood-Brain Barriers. Ther. Deliv. 2014, 5, 1143–1163. [Google Scholar] [CrossRef] [Green Version]
- Begley, D.J. ABC Transporters and the Blood-Brain Barrier. Curr. Pharm. Des. 2004, 10, 1295–1312. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-Brain Barrier Active Efflux Transporters: ATP-Binding Cassette Gene Family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, W.M.; Elsinga, P.H.; Gasca-Salas, C.; Versluis, M.; Martínez-Fernández, R.; Dierckx, R.A.J.O.; Borra, R.J.H.; Luurtsema, G. Focused Ultrasound for Opening Blood-Brain Barrier and Drug Delivery Monitored with Positron Emission Tomography. J. Control. Release 2020, 324, 303–316. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Ohlfest, J.R.; Elmquist, W.F. The Role of the Breast Cancer Resistance Protein (ABCG2) in the Distribution of Sorafenib to the Brain. J. Pharmacol. Exp. Ther. 2011, 336, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kang, J.; Kwon, N.-Y.; Sivaraman, A.; Naik, R.; Jin, S.-Y.; Oh, A.R.; Shin, J.-H.; Na, Y.; Lee, K.; et al. Dual Inhibition of P-Gp and BCRP Improves Oral Topotecan Bioavailability in Rodents. Pharmaceutics 2021, 13, 559. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging-Guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Marty, B.; Larrat, B.; Van Landeghem, M.; Robic, C.; Robert, P.; Port, M.; Le Bihan, D.; Pernot, M.; Tanter, M.; Lethimonnier, F.; et al. Dynamic Study of Blood-Brain Barrier Closure after Its Disruption Using Ultrasound: A Quantitative Analysis. J. Cereb. Blood Flow Metab. 2012, 32, 1948–1958. [Google Scholar] [CrossRef]
- Samiotaki, G.; Konofagou, E.E. Dependence of the Reversibility of Focused- Ultrasound-Induced Blood-Brain Barrier Opening on Pressure and Pulse Length in Vivo. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 2257–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Samiotaki, G.; Wang, S.; Acosta, C.; Chen, C.C.; Konofagou, E.E. Acoustic Cavitation-Based Monitoring of the Reversibility and Permeability of Ultrasound-Induced Blood-Brain Barrier Opening. Phys. Med. Biol. 2015, 60, 9079–9094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, A.; Mériaux, S.; Larrat, B. About the Marty Model of Blood-Brain Barrier Closure after Its Disruption Using Focused Ultrasound. Phys. Med. Biol. 2019, 64, 14NT02. [Google Scholar] [CrossRef]
- Konofagou, E.E.; Tung, Y.-S.; Choi, J.; Deffieux, T.; Baseri, B.; Vlachos, F. Ultrasound-Induced Blood-Brain Barrier Opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular Mechanisms of the Blood-Brain Barrier Opening Induced by Ultrasound in Presence of Microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Blood-Brain Barrier Disruption Induced by Focused Ultrasound and Circulating Preformed Microbubbles Appears to Be Characterized by the Mechanical Index. Ultrasound Med. Biol. 2008, 34, 834–840. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Akula, J.D.; McDannold, N.J. Targeted and Reversible Blood-Retinal Barrier Disruption via Focused Ultrasound and Microbubbles. PLoS ONE 2012, 7, e42754. [Google Scholar] [CrossRef]
- Samiotaki, G.; Vlachos, F.; Tung, Y.-S.; Konofagou, E.E. A Quantitative Pressure and Microbubble-Size Dependence Study of Focused Ultrasound-Induced Blood-Brain Barrier Opening Reversibility In Vivo Using MRI. Magn. Reason. Med. 2012, 67, 769–777. [Google Scholar] [CrossRef]
- Fan, C.-H.; Yeh, C.-K. Microbubble-Enhanced Focused Ultrasound-Induced Blood–Brain Barrier Opening for Local and Transient Drug Delivery in Central Nervous System Disease. J. Med. Ultrasound 2014, 22, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, H.A.; Flament, J.; Valette, J.; Cafarelli, A.; Aron Badin, R.; Hantraye, P.; Larrat, B. Feedback Control of Microbubble Cavitation for Ultrasound-Mediated Blood–Brain Barrier Disruption in Non-Human Primates under Magnetic Resonance Guidance. J. Cereb. Blood Flow Metab. 2019, 39, 1191–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Lee, E.-H.; Han, M.; An, S.-H.; Park, J. Diminished Expression of P-Glycoprotein Using Focused Ultrasound Is Associated With JNK-Dependent Signaling Pathway in Cerebral Blood Vessels. Front. Neurosci. 2019, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.-Z.; McDannold, N. Effects on P-Glycoprotein Expression after Blood-Brain Barrier Disruption Using Focused Ultrasound and Microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front. Oncol. 2022, 12, 903059. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood–Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [Green Version]
- Goutal, S.; Gerstenmayer, M.; Auvity, S.; Caillé, F.; Mériaux, S.; Buvat, I.; Larrat, B.; Tournier, N. Physical Blood-Brain Barrier Disruption Induced by Focused Ultrasound Does Not Overcome the Transporter-Mediated Efflux of Erlotinib. J. Control. Release 2018, 292, 210–220. [Google Scholar] [CrossRef]
- Rabkin, D.; Chhieng, D.C.; Miller, M.B.; Jennings, T.; Feustel, P.; Steiniger, J.; Parnes, S.M. P-Glycoprotein Expression in the Squamous Cell Carcinoma of the Tongue Base. Laryngoscope 1995, 105, 1294–1299. [Google Scholar] [CrossRef]
- Iorio, A.L.; da Ros, M.; Fantappiè, O.; Lucchesi, M.; Facchini, L.; Stival, A.; Becciani, S.; Guidi, M.; Favre, C.; de Martino, M.; et al. Blood-Brain Barrier and Breast Cancer Resistance Protein: A Limit to the Therapy of CNS Tumors and Neurodegenerative Diseases. Anticancer Agents Med. Chem. 2016, 16, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Roberts, J.W.; Powlovich, L.; Sheybani, N.; LeBlang, S. Focused ultrasound for the treatment of glioblastoma. J. Neurooncol. 2022, 157, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Larrat, B.; Pernot, M.; Aubry, J.-F.; Dervishi, E.; Sinkus, R.; Seilhean, D.; Marie, Y.; Boch, A.-L.; Fink, M.; Tanter, M. MR-Guided Transcranial Brain HIFU in Small Animal Models. Phys. Med. Biol. 2010, 55, 365–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstenmayer, M.; Fellah, B.; Magnin, R.; Selingue, E.; Larrat, B. Acoustic Transmission Factor through the Rat Skull as a Function of Body Mass, Frequency and Position. Ultrasound Med. Biol. 2018, 44, 2336–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnin, R.; Rabusseau, F.; Salabartan, F.; Mériaux, S.; Aubry, J.-F.; Le Bihan, D.; Dumont, E.; Larrat, B. Magnetic Resonance-Guided Motorized Transcranial Ultrasound System for Blood-Brain Barrier Permeabilization along Arbitrary Trajectories in Rodents. J. Ther. Ultrasound 2015, 3, 22. [Google Scholar] [CrossRef]


| P-gp Expression | BCRP Expression | ||
|---|---|---|---|
| Days After Sonication | p-Value | Days After Sonication | p-Value |
| Day 1 | 0.88 | Day 1 | 0.028 * |
| Day 3 | 0.46 | Day 3 | 0.5 |
| Day 7 | 0.22 | Day 7 | 2.52 × 10−4 * |
| Day 14 | 0.93 | Day 14 | 0.93 |
| P-gp Expression | BCRP Expression | ||
|---|---|---|---|
| Comparison | p-Value | Comparison | p-Value |
| Day 1/Day 3 | 6.8 × 10−3 * | Day 1/Day 3 | 0.51 |
| Day 1/Day 7 | 6.8 × 10−3 * | Day 1/Day 7 | 1.00 |
| Day 1/Day 14 | 0.02 * | Day 1/Day 14 | 3.0 × 10−3 * |
| Day 3/Day 7 | 0.51 | Day 3/Day 7 | 0.50 |
| Day 3/Day 14 | 0.51 | Day 3/Day 14 | 1.36 × 10−2 * |
| Day 7/Day 14 | 0.93 | Day 7/Day 14 | 3.00 × 10−3 * |
| P-gp Expression | BCRP Expression | ||
|---|---|---|---|
| Comparison | p-Value | Comparison | p-Value |
| Day 1/Day 3 | 4.80 × 10−3 * | Day 1/Day 3 | 0.74 |
| Day 1/Day 7 | 1.10 × 10−2 * | Day 1/Day 7 | 0.32 |
| Day 1/Day 14 | 0.064 | Day 1/Day 14 | 0.26 |
| Day 3/Day 7 | 0.48 | Day 3/Day 7 | 0.104 |
| Day 3/Day 14 | 0.51 | Day 3/Day 14 | 0.064 |
| Day 7/Day 14 | 0.36 | Day 7/Day 14 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, A.; Geffroy, F.; Kamimura, H.A.S.; Novell, A.; Tournier, N.; Mériaux, S.; Larrat, B. Regulation of P-glycoprotein and Breast Cancer Resistance Protein Expression Induced by Focused Ultrasound-Mediated Blood-Brain Barrier Disruption: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 15488. https://doi.org/10.3390/ijms232415488
Conti A, Geffroy F, Kamimura HAS, Novell A, Tournier N, Mériaux S, Larrat B. Regulation of P-glycoprotein and Breast Cancer Resistance Protein Expression Induced by Focused Ultrasound-Mediated Blood-Brain Barrier Disruption: A Pilot Study. International Journal of Molecular Sciences. 2022; 23(24):15488. https://doi.org/10.3390/ijms232415488
Chicago/Turabian StyleConti, Allegra, Francoise Geffroy, Hermes A. S. Kamimura, Anthony Novell, Nicolas Tournier, Sébastien Mériaux, and Benoit Larrat. 2022. "Regulation of P-glycoprotein and Breast Cancer Resistance Protein Expression Induced by Focused Ultrasound-Mediated Blood-Brain Barrier Disruption: A Pilot Study" International Journal of Molecular Sciences 23, no. 24: 15488. https://doi.org/10.3390/ijms232415488
APA StyleConti, A., Geffroy, F., Kamimura, H. A. S., Novell, A., Tournier, N., Mériaux, S., & Larrat, B. (2022). Regulation of P-glycoprotein and Breast Cancer Resistance Protein Expression Induced by Focused Ultrasound-Mediated Blood-Brain Barrier Disruption: A Pilot Study. International Journal of Molecular Sciences, 23(24), 15488. https://doi.org/10.3390/ijms232415488








