Lipopolysaccharide-Induced Functional Alteration of P-glycoprotein in the Ex Vivo Rat Inner Blood–Retinal Barrier
Abstract
1. Introduction
2. Results
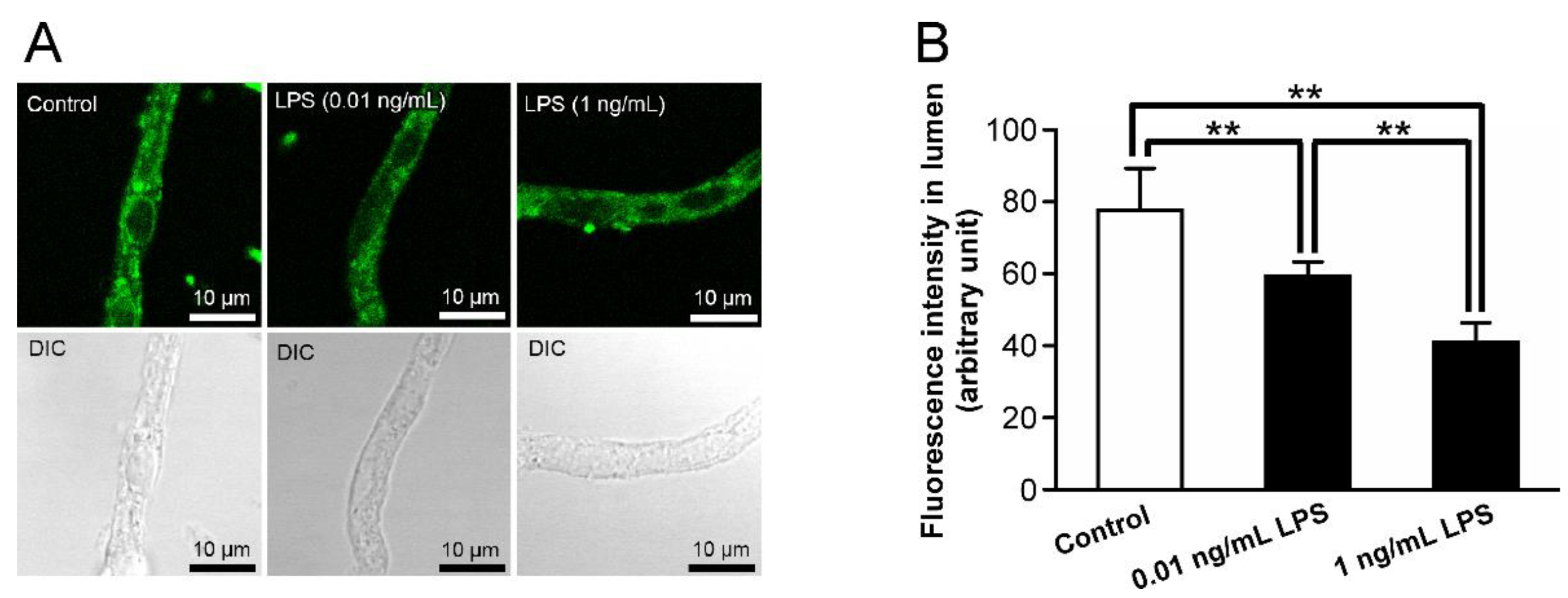
2.1. Alterations in P-gp Activity in the Retinal Capillaries after LPS Treatment
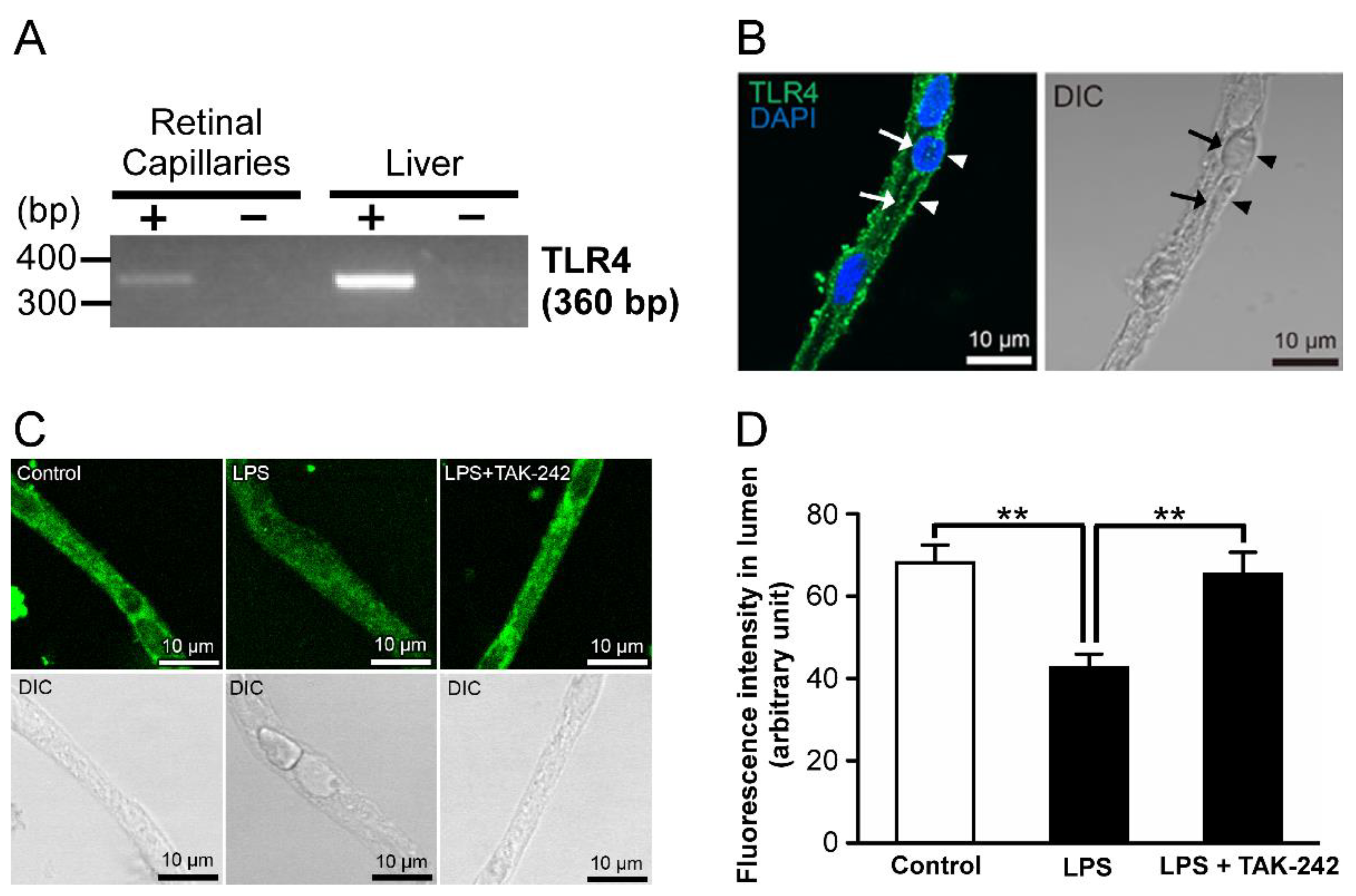
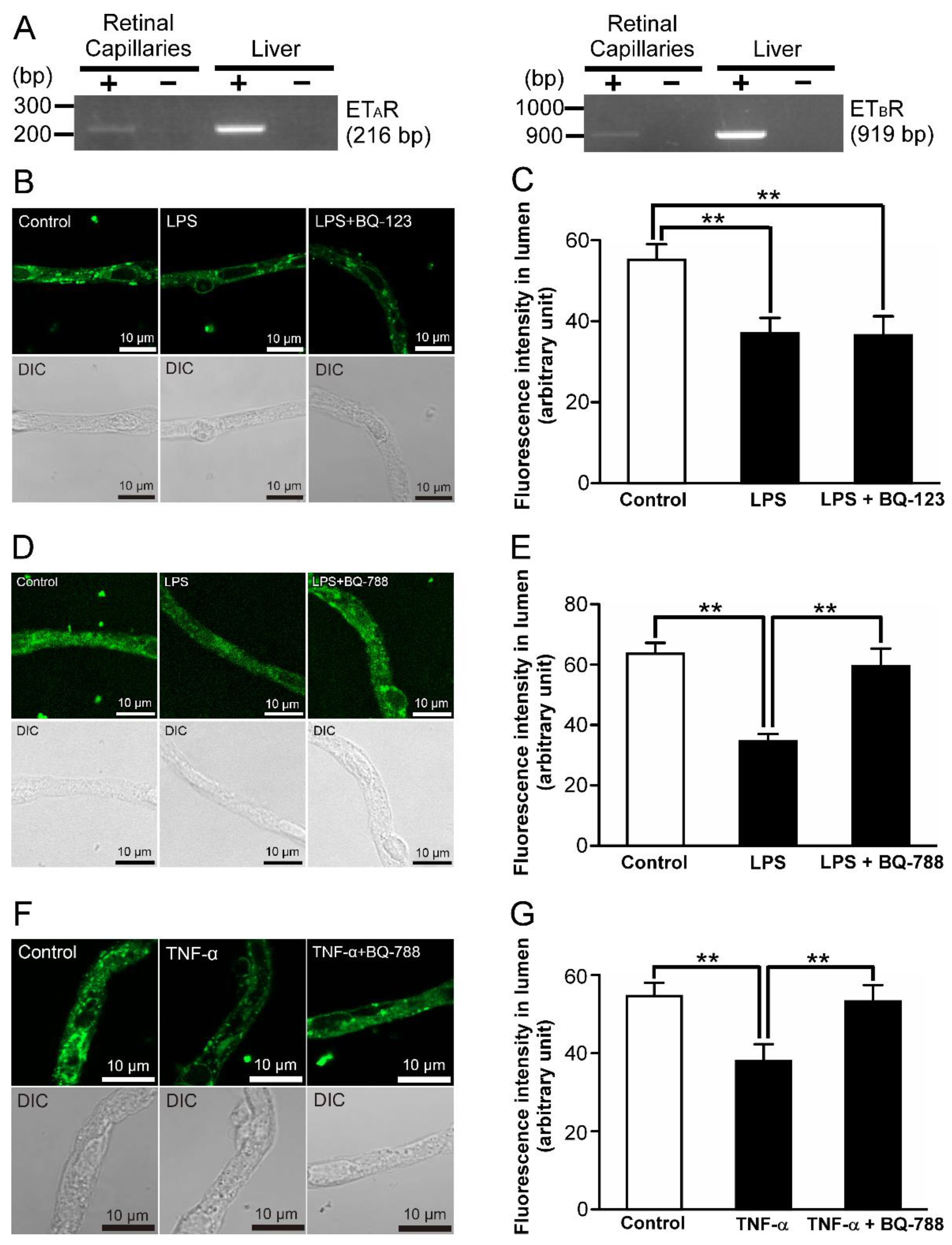
2.2. Relationship of Toll-like Receptor 4 (TLR4) with LPS-Mediated P-gp Attenuation in the Retinal Capillaries
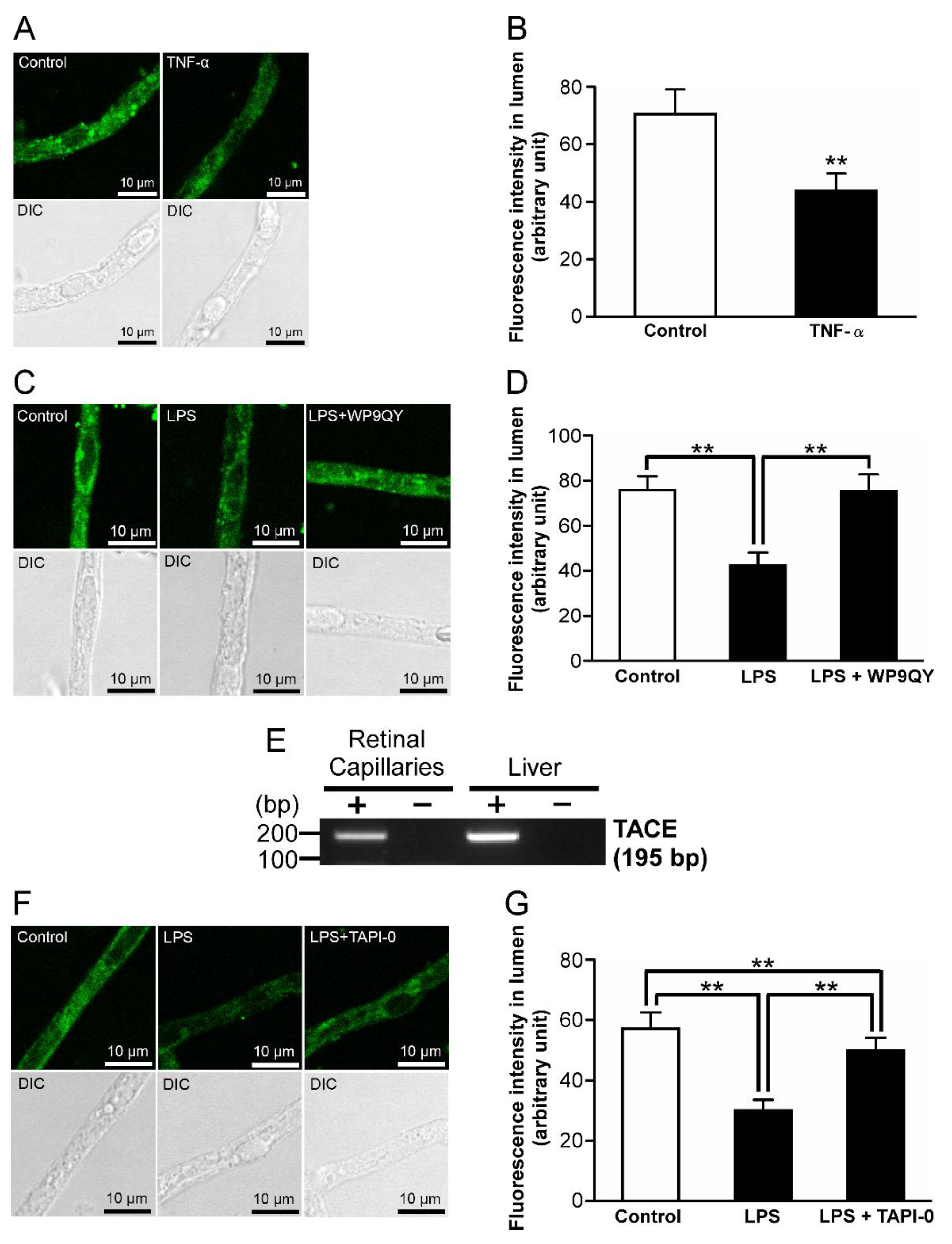
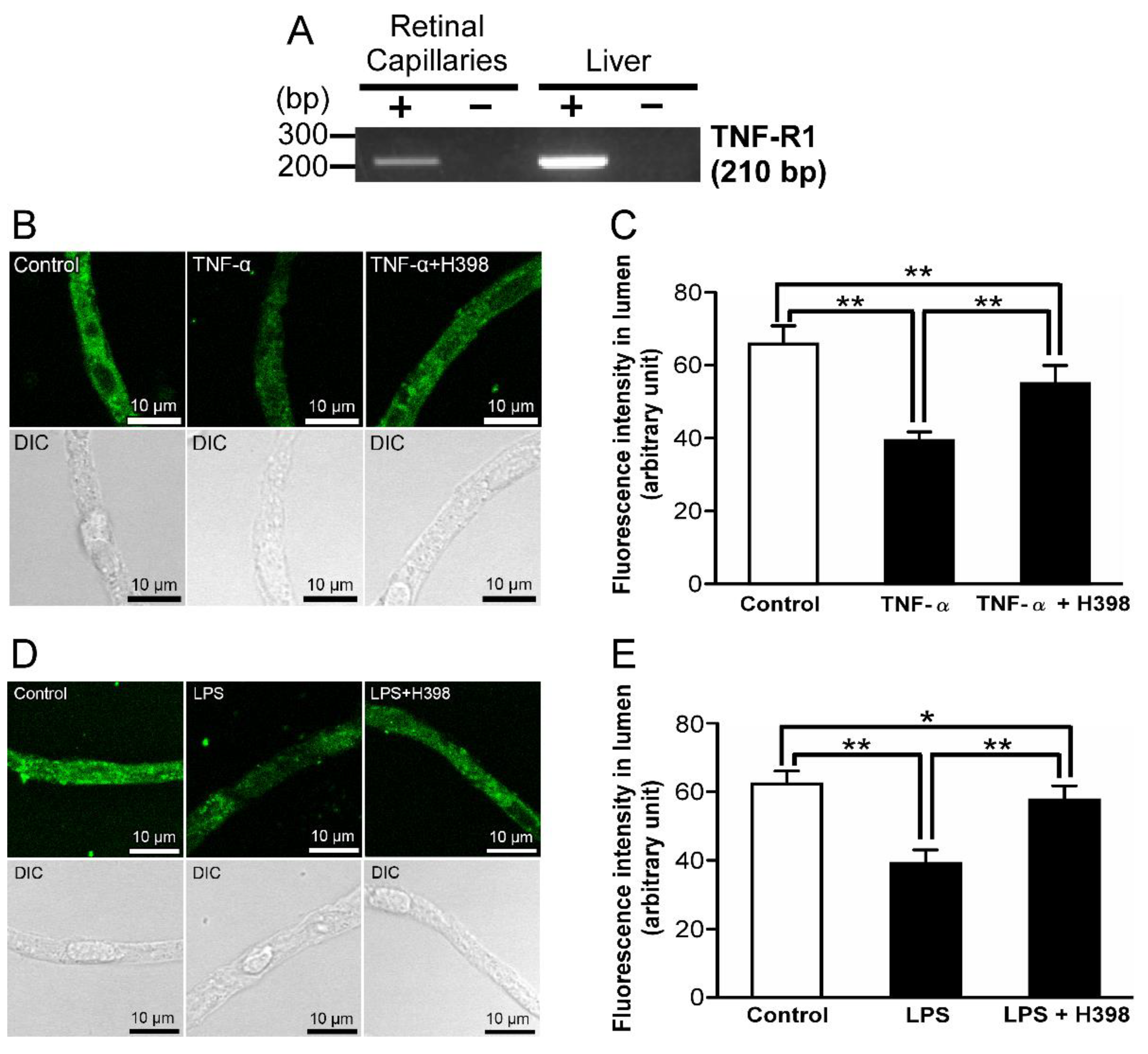
2.3. P-gp Attenuation by Tumor Necrosis Factor-α (TNF-α) in the Retinal Capillaries under LPS-Treated Conditions
2.4. Effect of an Inhibitor of TNF-Receptor 1 (TNF-R1) on LPS-Induced Attenuation of P-gp-Mediated Transport Activities in the Retinal Capillaries
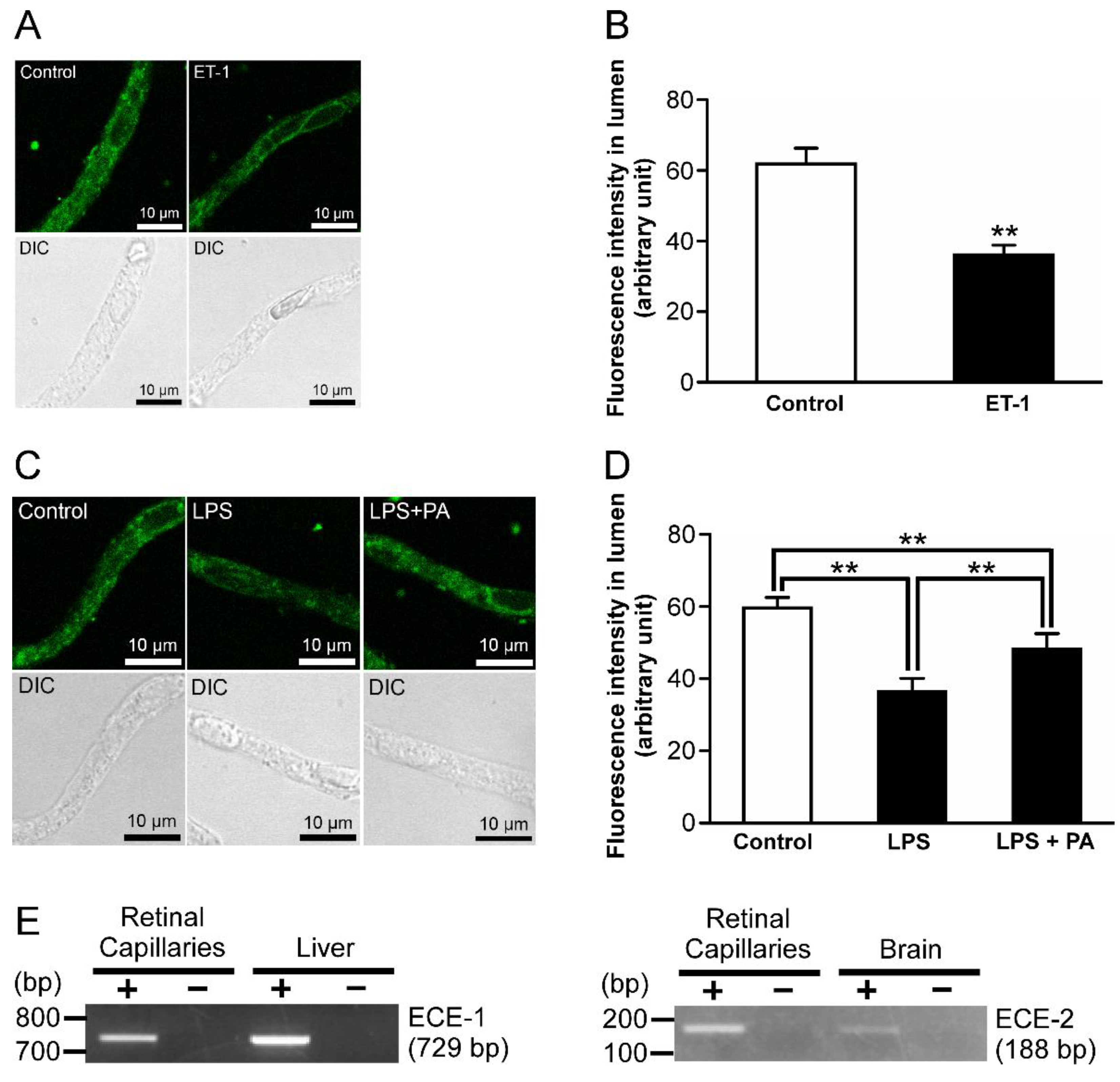
2.5. P-gp Attenuation by Endothelin-1 (ET-1) in the Retinal Capillaries under LPS-Treated Conditions
2.6. ETB Receptor-Mediated P-gp Attenuation in the Retinal Capillaries under LPS-Treated Conditions
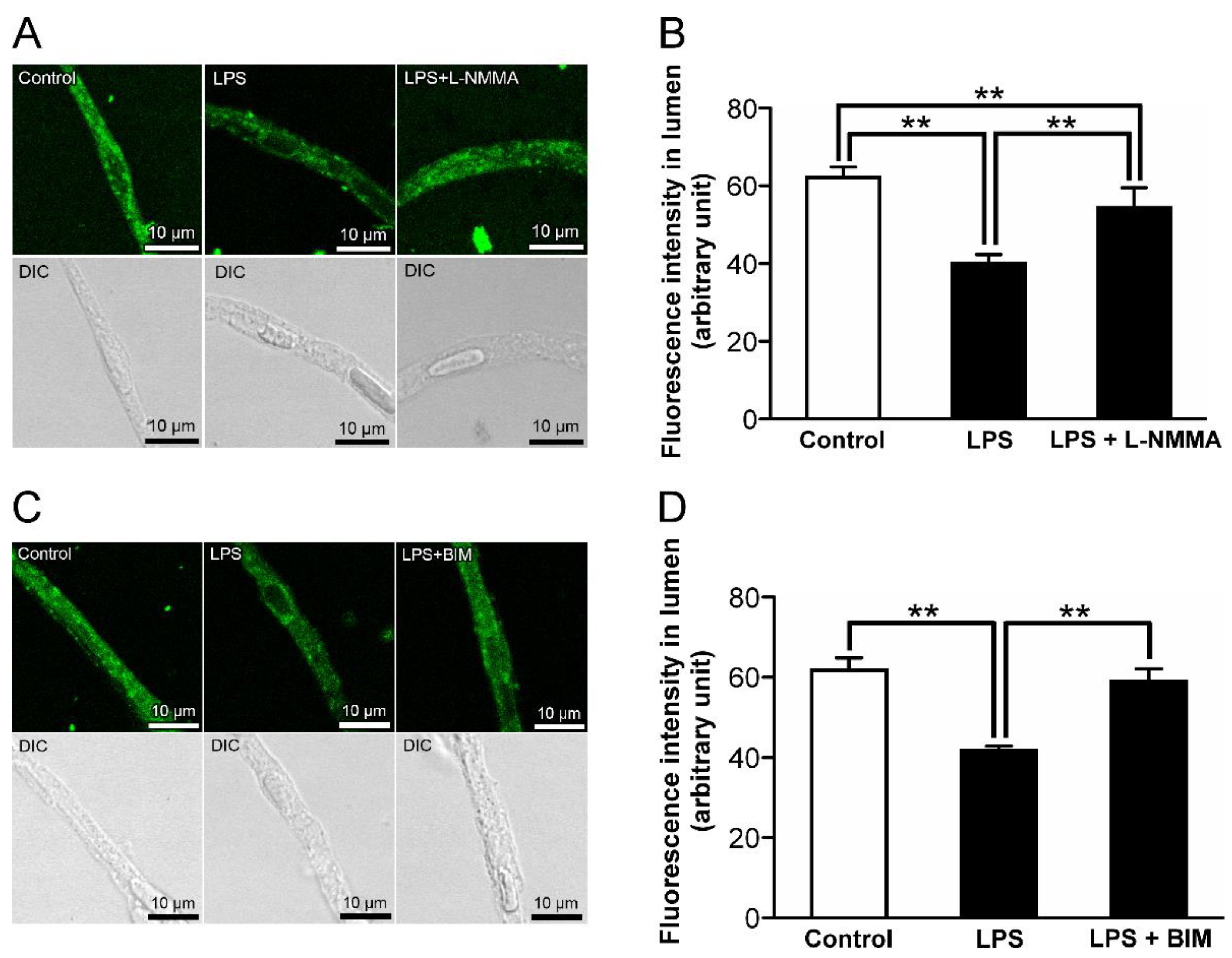
2.7. The Effects of Inhibitors for Nitric Oxide Synthase (NOS) and Protein Kinase C (PKC) on the Decrease in P-gp-Mediated Transport Activity in the Retinal Capillaries by LPS
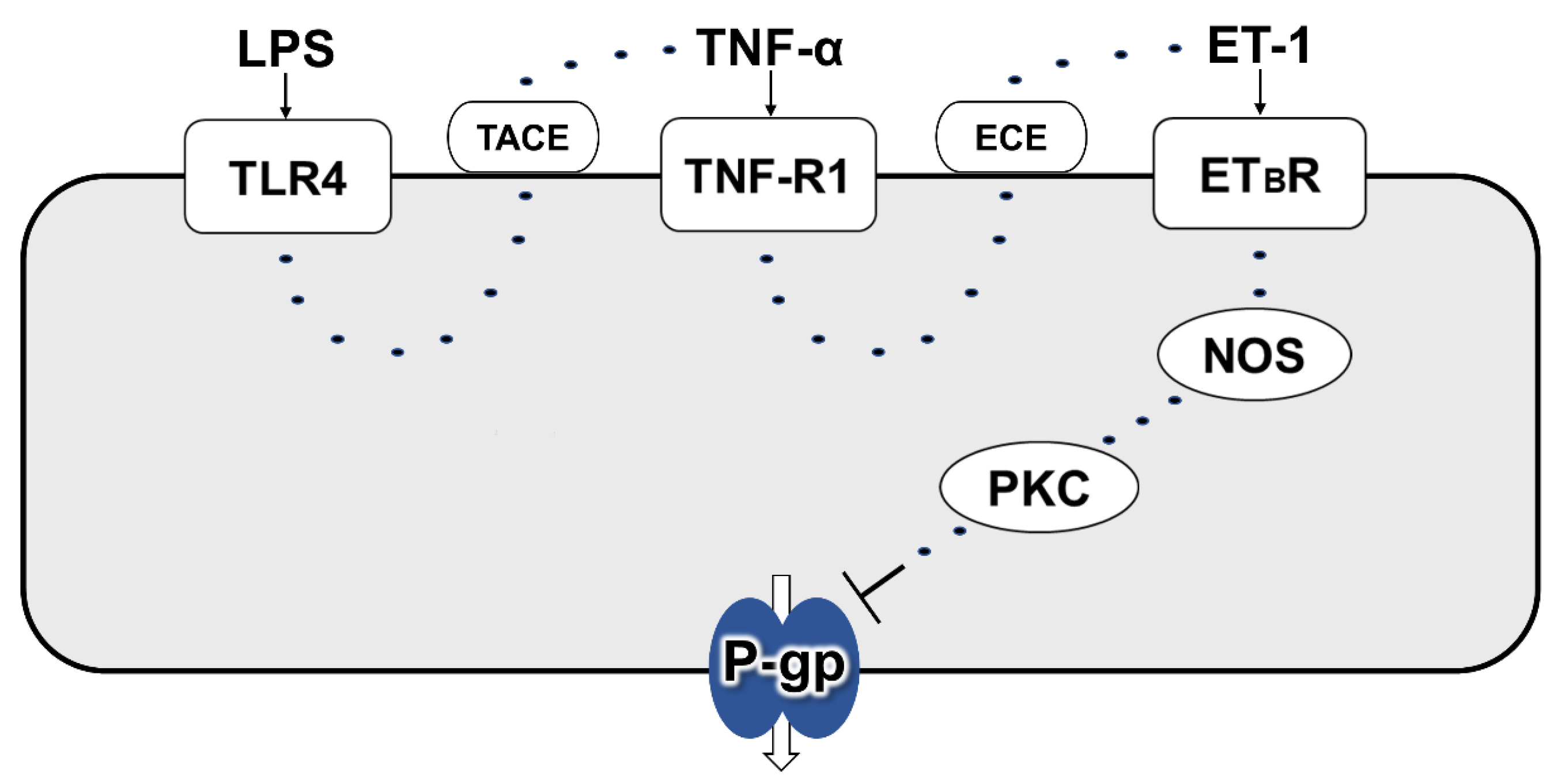
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Reagents and Supplies
4.3. Isolation of Retinal Capillaries
4.4. P-gp Transport Assay
4.5. mRNA Expression Analysis
4.6. Immunostaining
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marzolini, C.; Paus, E.; Buclin, T.; Kim, R.B. Polymorphisms in human MDR1 (P-glycoprotein): Recent advances and clinical relevance. Clin. Pharmacol. Ther. 2004, 75, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.F. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharm. Sci. 2004, 25, 423–429. [Google Scholar] [CrossRef]
- Hosoya, K.; Tomi, M. Advances in the cell biology of transport via the inner blood-retinal barrier: Establishment of cell lines and transport functions. Biol. Pharm. Bull. 2005, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Yamamoto, A.; Akanuma, S.; Tachikawa, M. Lipophilicity and transporter influence on blood-retinal barrier permeability: A comparison with blood-brain barrier permeability. Pharm Res. 2010, 27, 2715–2724. [Google Scholar] [CrossRef]
- Fujii, S.; Setoguchi, C.; Kawazu, K.; Hosoya, K. Impact of P-glycoprotein on blood-retinal barrier permeability: Comparison of blood-aqueous humor and blood-brain barrier using mdr1a knockout rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4650–4658. [Google Scholar] [CrossRef]
- Maines, L.W.; Antonetti, D.A.; Wolpert, E.B.; Smith, C.D. Evaluation of the role of P-glycoprotein in the uptake of paroxetine, clozapine, phenytoin and carbamazapine by bovine retinal endothelial cells. Neuropharmacology 2005, 49, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Akanuma, S.; Ohishi, Y.; Yoshida, Y.; Bauer, B.; Kubo, Y.; Inouye, M.; Hosoya, K. Freshly isolated retinal capillaries to determine efflux transporter function at the inner BRB. J. Control. Release 2022, 343, 434–442. [Google Scholar] [CrossRef]
- Sukhai, M.; Yong, A.; Pak, A.; Piquette-Miller, M. Decreased expression of P-glycoprotein in interleukin-1β and interleukin-6 treated rat hepatocytes. Inflamm. Res. 2001, 50, 362–370. [Google Scholar] [CrossRef]
- Ando, H.; Nishio, Y.; Ito, K.; Nakao, A.; Wang, L.; Zhao, Y.L.; Kitaichi, K.; Takagi, K.; Hasegawa, T. Effect of endotoxin on P-glycoprotein-mediated biliary and renal excretion of rhodamine-123 in rats. Antimicrob. Agents Chemother. 2001, 45, 3462–3467. [Google Scholar] [CrossRef]
- Moriguchi, J.; Kato, R.; Nakagawa, M.; Hirotani, Y.; Ijiri, Y.; Tanaka, K. Effects of lipopolysaccharide on intestinal P-glycoprotein expression and activity. Eur. J. Pharm. 2007, 565, 220–224. [Google Scholar] [CrossRef]
- Kato, R.; Moriguchi, J.; Irie, T.; Nakagawa, M.; Kusukawa, Y.; Matsumura, H.; Ijiri, Y.; Tanaka, K. Effects of lipopolysaccharide on P-glycoprotein expression and activity in the liver and kidneys. Eur. J. Pharm. 2010, 636, 155–158. [Google Scholar] [CrossRef]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Terasaki, T. Actin filament-associated protein 1 (AFAP-1) is a key mediator in inflammatory signaling-induced rapid attenuation of intrinsic P-gp function in human brain capillary endothelial cells. J. Neurochem. 2017, 141, 247–262. [Google Scholar] [CrossRef]
- Gomes, J.M.G.; Costa, J.A.; Alfenas, R.C.G. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef]
- Qin, X.; Zou, H. The role of lipopolysaccharides in diabetic retinopathy. BMC Ophthalmol. 2022, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Szabady, R.L.; Louissaint, C.; Lubben, A.; Xie, B.; Reeksting, S.; Tuohy, C.; Demma, Z.; Foley, S.E.; Faherty, C.S.; Llanos-Chea, A.; et al. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Investig. 2018, 128, 4044–4056. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Kotwani, A. Role of endocannabinoids in the progression of diabetic retinopathy. Diabetes Metab. Res. Rev. 2016, 32, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, A.; Bjersing, J.; Shestakov, A.; Bokarewa, M.I. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell Mol. Med. 2010, 14, 1419–1431. [Google Scholar] [CrossRef]
- Moser, V.A.; Uchoa, M.F.; Pike, C.J. TLR4 inhibitor TAK-242 attenuates the adverse neural effects of diet-induced obesity. J. Neuroinflammation 2014, 15, 306. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Ru, J.H.; Lopes-Virella, M.F.; Lyons, T.J.; Huang, Y. Interaction of palmitate and LPS regulates cytokine expression and apoptosis through sphingolipids in human retinal microvascular endothelial cells. Exp. Eye Res. 2019, 178, 61–71. [Google Scholar] [CrossRef]
- Iwai-Shimada, M.; Takahashi, T.; Kim, M.S.; Fujimura, M.; Ito, H.; Toyama, T.; Naganuma, A.; Hwang, G.-W. Methylmercury induces the expression of TNF-alpha selectively in the brain of mice. Sci. Rep. 2016, 6, 38294. [Google Scholar] [CrossRef]
- Bzowska, M.; Jura, N.; Lassak, A.; Black, R.A.; Bereta, J. Tumour necrosis factor-alpha stimulates expression of TNF-alpha converting enzyme in endothelial cells. Eur. J. Biochem. 2004, 271, 2808–2820. [Google Scholar] [CrossRef]
- Kagawa, K.; Nakano, A.; Miki, H.; Oda, A.; Amou, H.; Takeuchi, K.; Nakamura, S.; Harada, T.; Fujii, S.; Yata, K.; et al. Inhibition of TACE activity enhances the susceptibility of myeloma cells to TRAIL. PLoS ONE 2012, 7, e31594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.; Liu, J.; Yang, Y.; Cao, Q.; Huo, X.; Ma, S.; Hu, J.; Pavalko, F.M.; Liu, Q. Hydroxy-safflower yellow A inhibits the TNFR1-mediated classical NF-κB pathway by inducing shedding of TNFR1. Phytother. Res. 2016, 30, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Barisic, S.; Strozyk, E.; Peters, N.; Walczak, H.; Kulms, D. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: Its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell Death Differ 2008, 15, 1681–1690. [Google Scholar] [CrossRef]
- Ros, J.; Leivas, A.; Jimenez, W.; Morales, M.; Bosch-Marce, M.; Arroyo, V.; Rivera, F.; Rodés, J. Effect of bacterial lipopolysaccharide on endothelin-1 production in human vascular endothelial cells. J. Hepatol. 1997, 26, 81–87. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.G.; Palomo, M.A.; Moore, W.M.; Stern, M.K. Phosphoramidon blocks the pressor activity of porcine big endothelin-1-(1-39) in vivo and conversion of big endothelin-1-(1-39) to endothelin-1-(1-21) in vitro. Proc. Natl. Acad. Sci. USA 1991, 88, 703–707. [Google Scholar] [CrossRef]
- Tanfin, Z.; Leiber, D.; Robin, P.; Oyeniran, C.; Breuiller-Fouche, M. Endothelin-1: Physiological and pathological roles in myometrium. Int. J. Biochem. Cell Biol. 2011, 43, 299–302. [Google Scholar] [CrossRef]
- Nambi, P.; Pullen, M.; Wu, H.L.; Lee, D.; Saunders, D.; Heys, R.; Aiyar, N.; Leber, J.; Elliott, J.; Brooks, D.; et al. Nonpeptide endothelin receptor antagonists. VII: Binding characteristics of [3H]SB 209670, a novel nonpeptide antagonist of endothelin receptors. J. Pharm. Exp. 1996, 277, 1567–1571. [Google Scholar]
- Okada, M.; Nishikibe, M. BQ-788, a selective endothelin ET(B) receptor antagonist. Cardiovasc. Drug Rev. 2002, 20, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, S.; Miller, D.S.; Smits, P.; Russel, F.G.; Masereeuw, R. Role of NO in endothelin-regulated drug transport in the renal proximal tubule. Am. J. Physiol. Ren. Physiol. 2002, 282, F458–F464. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.M.; Bauer, B.; Fricker, G.; Miller, D.S. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol. Pharm. 2004, 66, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.L.; Tacy, T.A.; Fields, S.; Ofenstein, J.P.; Aranda, J.V.; Clyman, R.I. Combined treatment with a nonselective nitric oxide synthase inhibitor (l-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatr. Res. 2005, 58, 1216–1221. [Google Scholar] [CrossRef]
- Wojciechowski, W.; Li, H.; Marshall, S.; Dell’Agnola, C.; Espinoza-Delgado, I. Enhanced expression of CD20 in human tumor B cells is controlled through ERK-dependent mechanisms. J. Immunol. 2005, 174, 7859–7868. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Suzuki, H.; Sugiyama, Y. Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm. Res. 2001, 18, 1660–1668. [Google Scholar] [CrossRef]
- Hartz, A.M.; Bauer, B.; Fricker, G.; Miller, D.S. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol. Pharm. 2006, 69, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, S.; Peters, J.G.; Louisse, J.; Sagar, S.; Russel, F.G.; Masereeuw, R. Regulation of P-glycoprotein in renal proximal tubule epithelial cells by LPS and TNF-alpha. J. Biomed. Biotechnol. 2010, 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Xiao, X.; Yu, Y. Endotoxin-induced uveitis promotes retinal endothelial cell injury and Muller cell proliferation. Int. J. Clin. Exp. Med. 2018, 11, 1608–1614. [Google Scholar]
- Bauer, B.; Hartz, A.M.; Miller, D.S. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol. Pharm. 2007, 71, 667–675. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Chieli, E.; Romiti, N.; Rodeiro, I.; Garrido, G. In vitro modulation of ABCB1/P-glycoprotein expression by polyphenols from Mangifera indica. Chem. Biol. Interact. 2010, 186, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, X.; Xiu, M.; He, F.; Xing, J.; Min, D.; Guo, F. The regulation of Jmjd3 upon the expression of NF-κB downstream inflammatory genes in LPS activated vascular endothelial cells. Biochem. Biophys Res. Commun. 2017, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Stoll, L.L.; Denning, G.M.; Weintraub, N.L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arter. Thromb. Vasc. Biol. 2004, 24, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
| Molecules | Gene Bank Accession No. | Primer Sequence (Upper, Sense Primer; Lower, Antisense Primer) | Product Size (bp) |
|---|---|---|---|
| TLR4 | NM_019178 | 5′-CAT GTC CAT CGG TTG ATC T-3′ 5′-ACT TGG CAG AGC CAA CTG ACC AAA G-3′ | 360 |
| TACE | NM_020306 | 5′-GAG CCA TCT GAA GAG TTT GTC CGT C-3′ 5′-CCA CGA GGT GTT CCG GTA TAT GTC A-3′ | 195 |
| TNF-R1 | NM_013091 | 5′-CTG CCA CGC AGG ATT CTT TCT AAG C-3′ 5′-GGA TAT CGG CAC AGT AGA CTG ATG C-3′ | 210 |
| ECE-1 | NM_053596 | 5′-GAG GAT CTG GTG GAC TCA CTC TCC-3′ 5′-CGT CTT CAG GTA ATA GTC TCT-3′ | 729 |
| ECE-2 | NM_001002815 | 5′-TTA ACC GTA CGG AAC CAA GC-3′ 5′-GCC AAG GGC ATC ATC TGT AT-3′ | 188 |
| ETAR | NM_012550 | 5′-GAA GTC GTC CGT GGG CAT CA-3′ 5′-CTG TGC TGC TCG CCC TTG TA-3′ | 216 |
| ETBR | NM_017333 | 5′-AGC TGG TGC CCT TCA TAC AGA AGG C-3′ 5′-TGC ACA CCT TTC CGC AAG CAC G-3′ | 919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daikohara, K.; Akanuma, S.-i.; Kubo, Y.; Hosoya, K.-i. Lipopolysaccharide-Induced Functional Alteration of P-glycoprotein in the Ex Vivo Rat Inner Blood–Retinal Barrier. Int. J. Mol. Sci. 2022, 23, 15504. https://doi.org/10.3390/ijms232415504
Daikohara K, Akanuma S-i, Kubo Y, Hosoya K-i. Lipopolysaccharide-Induced Functional Alteration of P-glycoprotein in the Ex Vivo Rat Inner Blood–Retinal Barrier. International Journal of Molecular Sciences. 2022; 23(24):15504. https://doi.org/10.3390/ijms232415504
Chicago/Turabian StyleDaikohara, Kiyotaka, Shin-ichi Akanuma, Yoshiyuki Kubo, and Ken-ichi Hosoya. 2022. "Lipopolysaccharide-Induced Functional Alteration of P-glycoprotein in the Ex Vivo Rat Inner Blood–Retinal Barrier" International Journal of Molecular Sciences 23, no. 24: 15504. https://doi.org/10.3390/ijms232415504
APA StyleDaikohara, K., Akanuma, S.-i., Kubo, Y., & Hosoya, K.-i. (2022). Lipopolysaccharide-Induced Functional Alteration of P-glycoprotein in the Ex Vivo Rat Inner Blood–Retinal Barrier. International Journal of Molecular Sciences, 23(24), 15504. https://doi.org/10.3390/ijms232415504






