Synthesis, Characterization of Low Molecular Weight Chitosan Selenium Nanoparticles and Its Effect on DSS-Induced Ulcerative Colitis in Mice
Abstract
:1. Introduction
2. Results
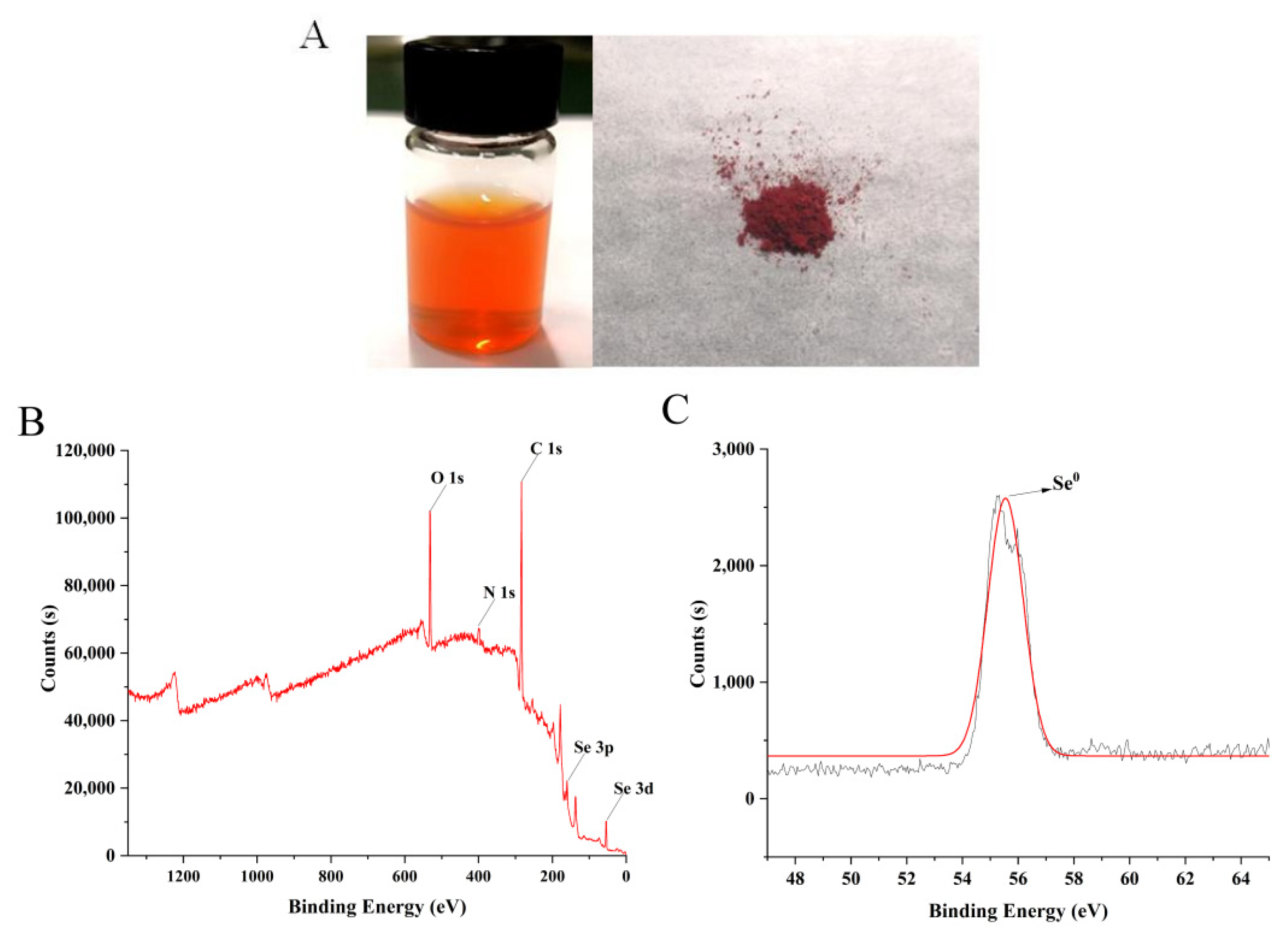
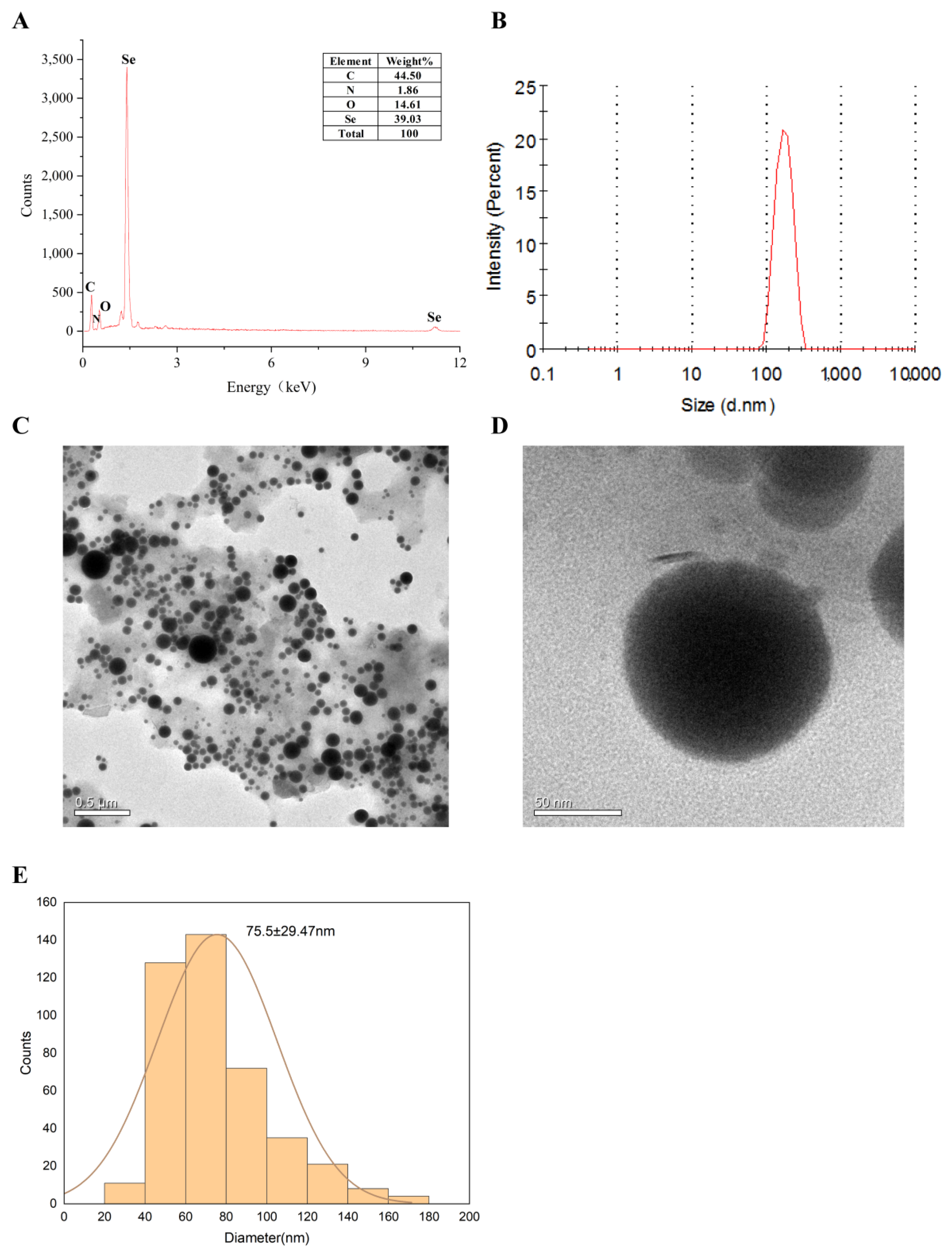
2.1. Characterization of LCS-SeNPs
2.1.1. Valence State of Selenium
2.1.2. Particle Size and Morphology of LCS-SeNPs
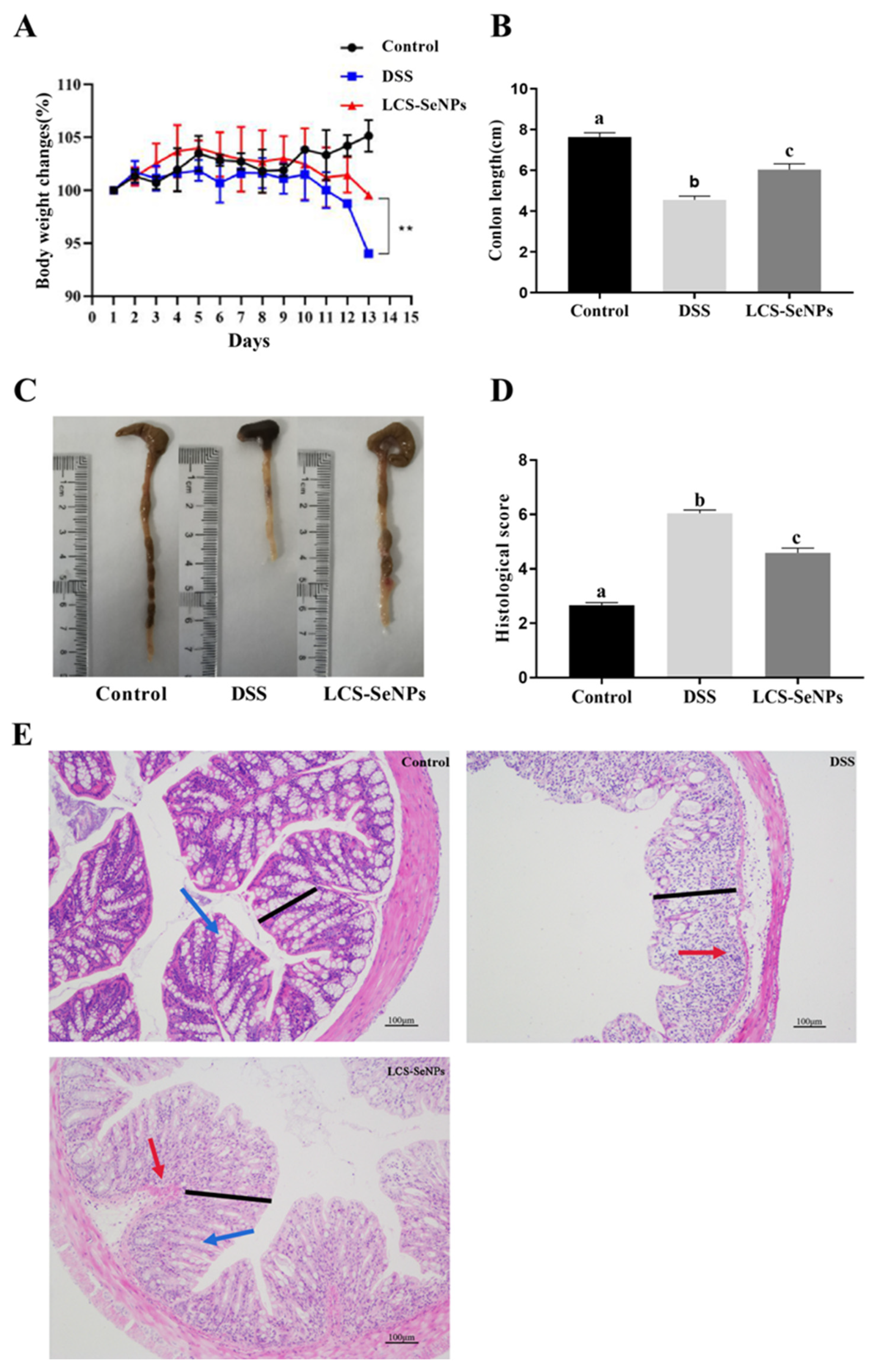
2.2. LCS-SeNPs Altered the Physical Signs and Colon Histopathological Scores in DSS-Induced Colitis Mice
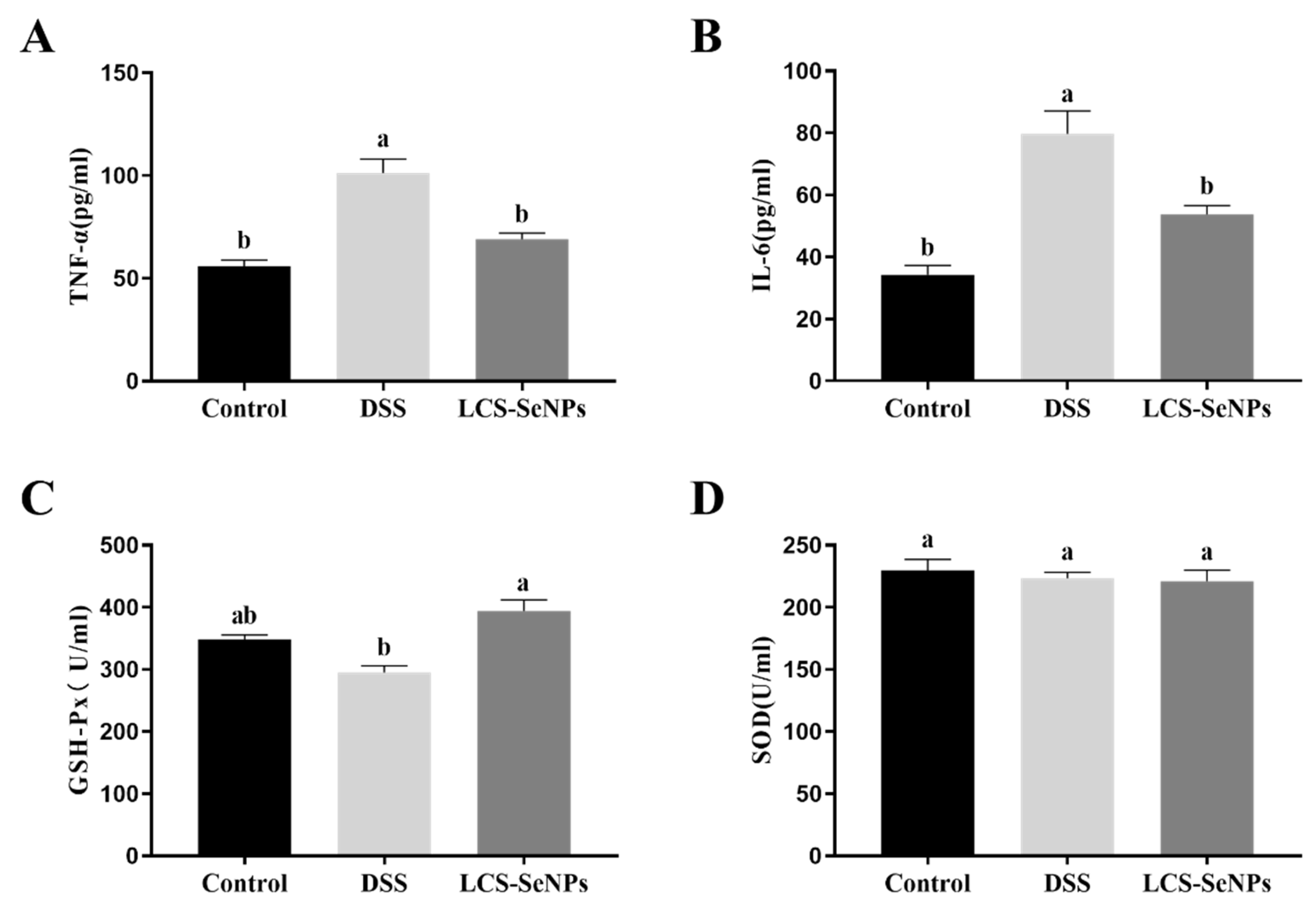
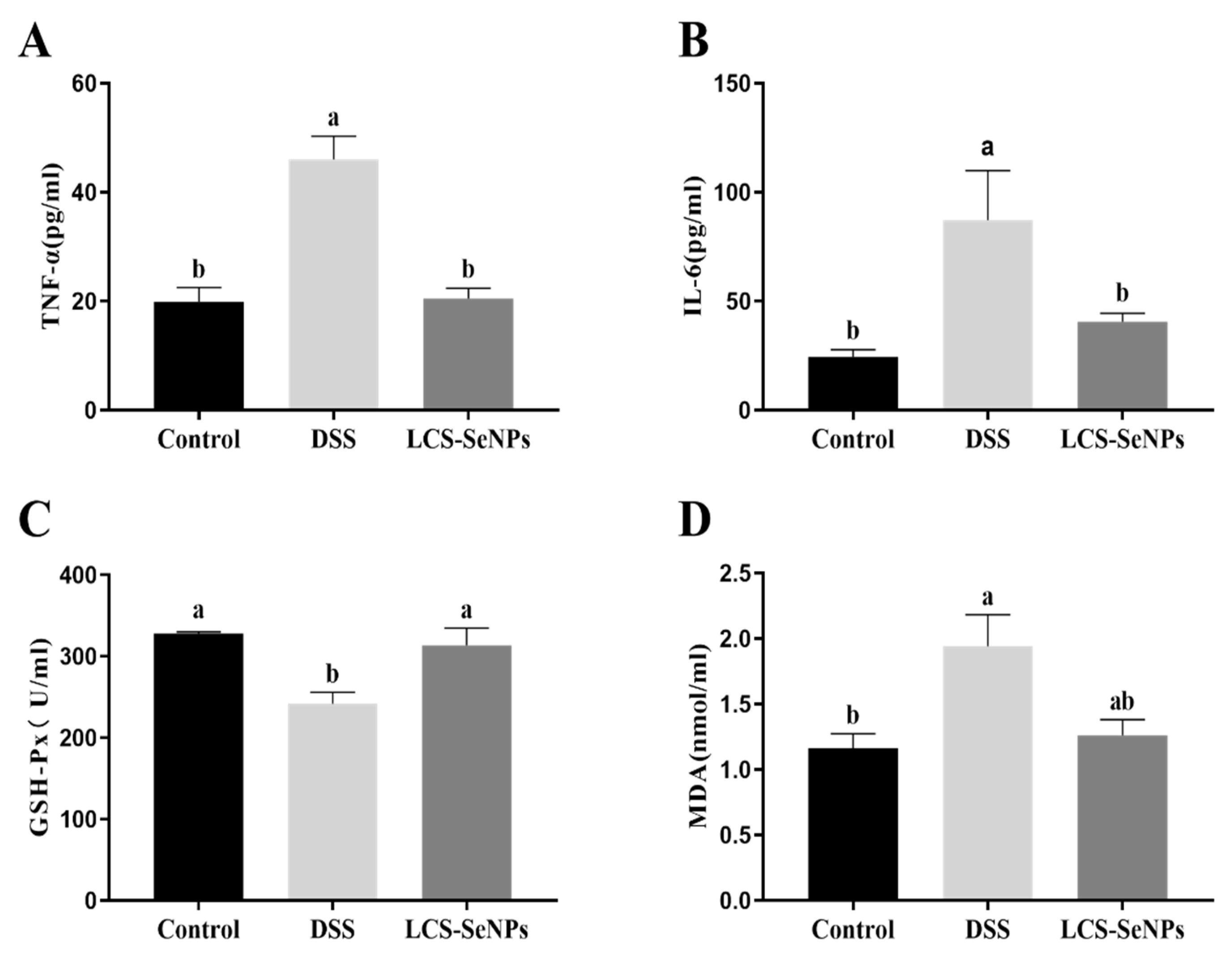
2.3. Effects of LCS-SeNPs on Serum Inflammatory Cytokines Levels and Antioxidant Capacity of DSS-Induced Colitis Mice
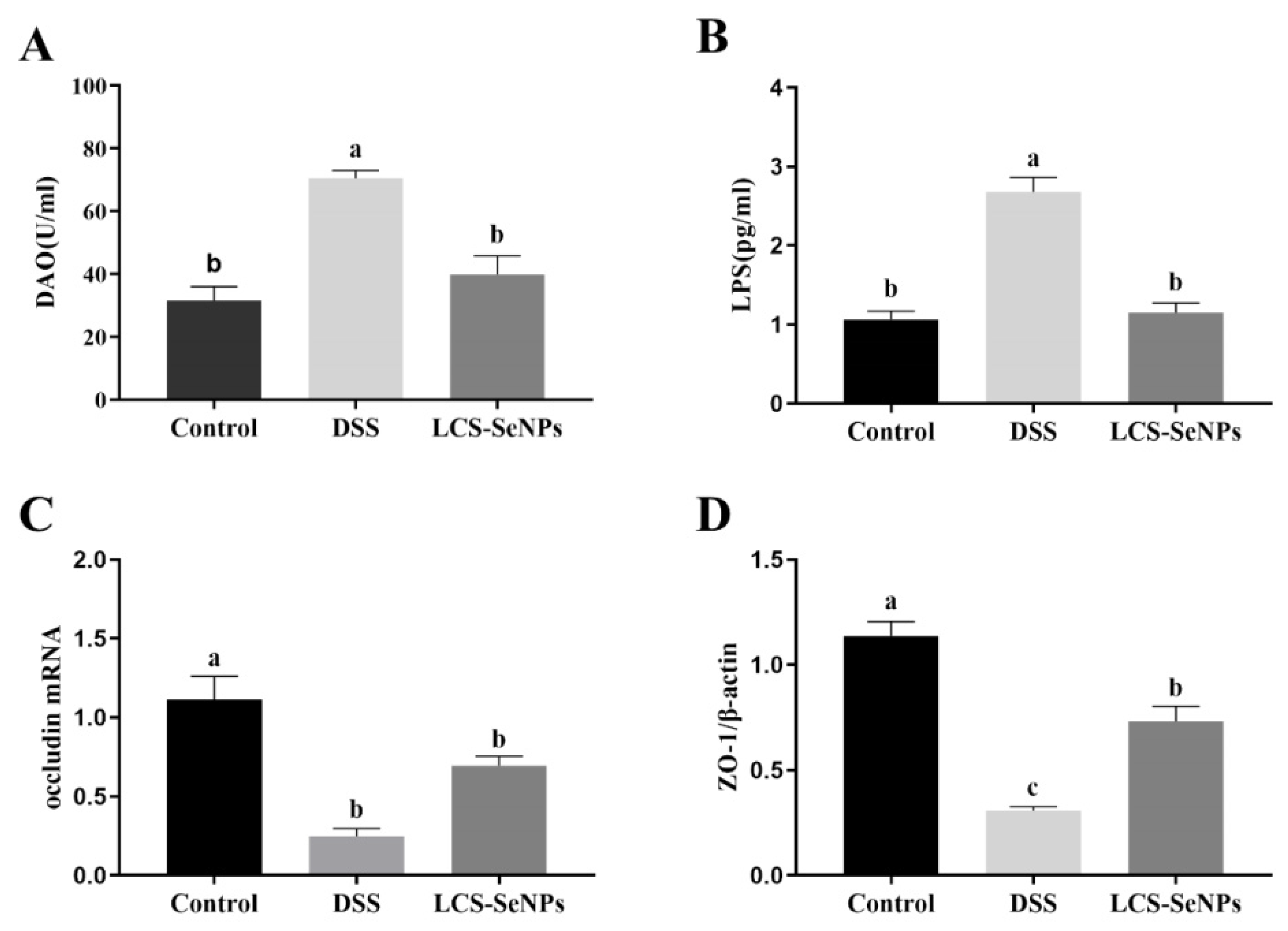
2.4. LCS-SeNPs Altered Intestinal Permeability in DSS-Induced Colitis Mice
2.5. Effects of LCS-SeNPs on Inflammatory Factors and Antioxidant Capacity of Colon Tissue in DSS-Induced Colitis Mice
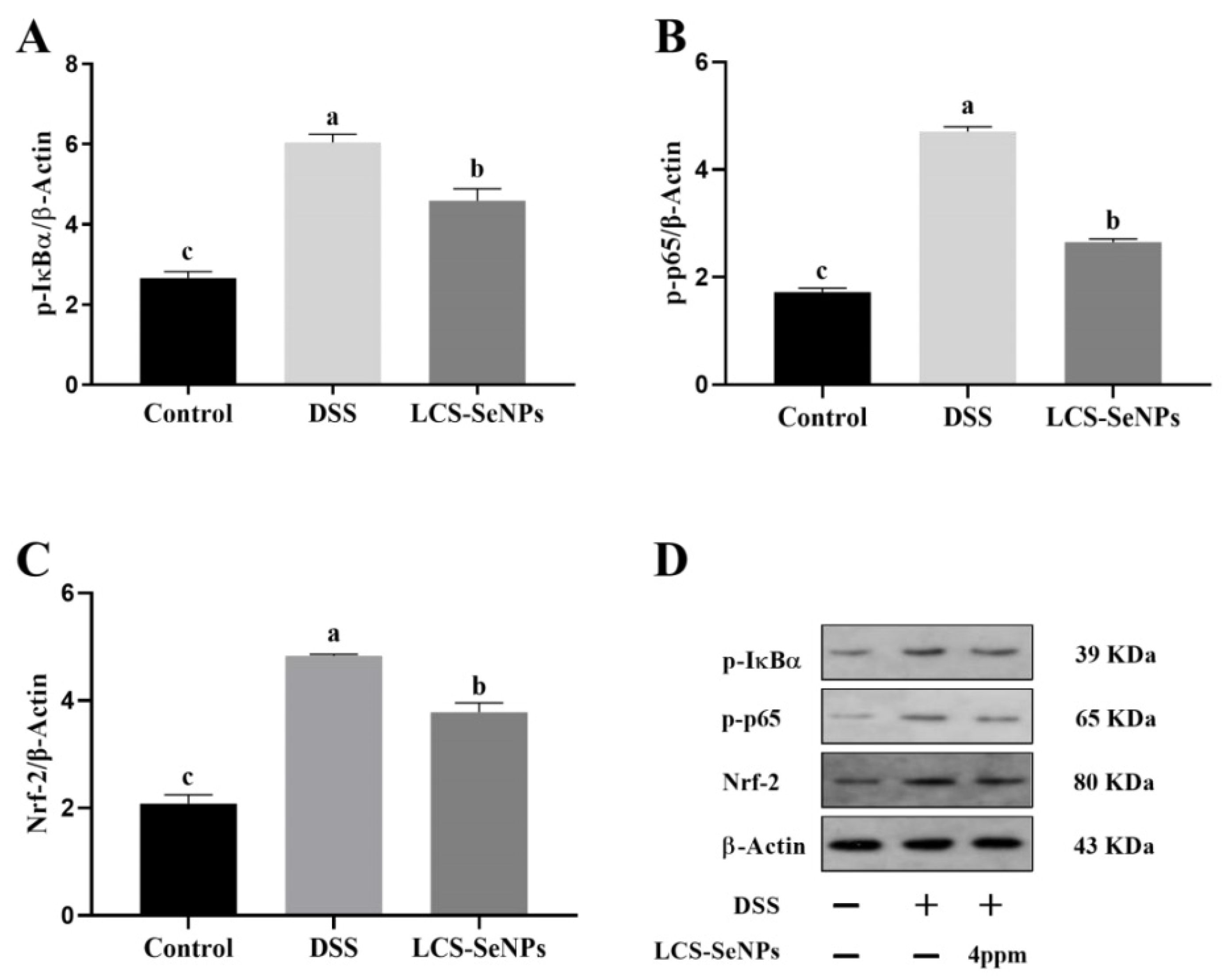
2.6. Effects of LCS-SeNPs on the Nrf-2 Signaling Pathways in DSS-Induced Colitis Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of LCS-SeNPs
4.3. Characterization and Measurements
4.4. Experimental Animals
4.5. Histopathological Analysis
4.6. Detection of Cytokines in Serum
4.7. Detection of Cytokines Levels in Colon
4.8. Determination of Tight Junction Protein mRNA Expression in Colon
4.9. Western Blotting Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gheorghe, C.; Pascu, O.; Gheorghe, L.; Iacob, R.; Dumitru, E.; Tantau, M.; Vadan, R.; Goldis, A.; Balan, G.; Iacob, S.; et al. Epidemiology of inflammatory bowel disease in adults who refer to gastroenterology care in Romania: A multicentre study. Eur. J. Gastroenterol Hepatol. 2004, 16, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Szigethy, E.; McLafferty, L.; Goyal, A. Inflammatory bowel disease. Child Adolesc. Psychiatr. Clin. N. Am. 2010, 19, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Cheng, W.H.; Qin, W. Preparation, characterization and evaluation of selenite-loaded chitosan/TPP nanoparticles with or without zein coating. Carbohydr. Polym. 2010, 82, 942–951. [Google Scholar] [CrossRef]
- Mattaveewong, T.; Wongkrasant, P.; Chanchai, S.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-kappa B and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.L.; Huang, S.Y.; Huang, H.L. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Zhang, G.; Jia, P.; Cheng, G.; Jiao, S.; Ren, L.; Ji, S.; Hu, T.; Liu, H.; Du, Y. Enhanced immune response to inactivated porcine circovirus type 2 (PCV2) vaccine by conjugation of chitosan oligosaccharides. Carbohydr. Polym. 2017, 166, 64–72. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Triggiani, V.; Tafaro, E.; Giagulli, V.A.; Sabbà, C.; Resta, F.; Licchelli, B.; Guastamacchia, E. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 277–294. [Google Scholar] [CrossRef]
- Wu, H.Y.; Xia, Y.M.; Chen, X.S. Selenium deficiency and thyroid hormone metabolism and function. Sheng Li Ke Xue Jin Zhan 1995, 26, 12–16. (In Chinese) [Google Scholar]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Li, T.Y.; Xu, H.P. Selenium-Containing Nanomaterials for Cancer Treatment. Cell Rep. Phys. Sci. 2020, 1, 100111. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, B. Synthesis, characterization and antioxidant activity of selenium modified polysaccharides from Hohenbuehelia serotina. Int. J. Biol. Macromol. 2018, 120, 1362–1368. [Google Scholar] [CrossRef]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Geerling, B.J.; Badart-Smook, A. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur. J. Clin. Nutr. 2000, 54, 514–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, C.W.; Kshipra, S.; Motley, A.K.; Lintel, M.K.; Elena, M.; Bradley, A.M.; Ning, W.; Poindexter, S.V.; Bobak, P.; Reddy, V.K. Dietary Selenium Deficiency Exacerbates DSS-Induced Epithelial Injury and AOM/DSS-Induced Tumorigenesis. PLoS ONE 2013, 8, e67845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liljana, G.; Bishop, K.S.; Yeo, H.D.; Morgan, A.R.; Fraser, A.G.; Jiun, L.W.; Nishi, K.; Bobbi, C.; Ferguson, L.R. Selenium, Selenoprotein Genes and Crohn’s Disease in a Case-Control Population from Auckland, New Zealand. Nutrients 2012, 4, 1247–1259. [Google Scholar]
- Yang, H.; Zhu, C.; Yuan, W.; Wei, X.; Liu, C.; Huang, J.; Yuan, M.; Wu, Y.; Ling, Q.; Hoffmann, P.R.; et al. Mannose-rich Oligosaccharides-functionalized selenium nanoparticles mediates Macrophage reprogramming and inflammation resolution in ulcerative colitis. Chem. Eng. J. 2022, 435, 131715. [Google Scholar] [CrossRef]
- Sakr, T.M.; Korany, M.; Katti, K.V. Selenium nanomaterials in biomedicine—An overview of new opportunities in nanomedicine of selenium. J. Drug Deliv. Sci. Technol. 2018, 46, 223–233. [Google Scholar] [CrossRef]
- Huang, Y.; Su, E.; Ren, J.; Qu, X. The recent biological applications of selenium-based nanomaterials. Nano Today 2021, 38, 101205. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, U.; Sharma, R.; Gupta, M.; Vyas, S.P. Is nanotechnology a boon for oral drug delivery? Drug Discov. Today 2014, 19, 1530–1546. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Mehta, S.K. Selenium nanomaterials: An overview of recent developments in synthesis, properties, and potential applications. Prog. Mater. Sci. 2016, 83, 270–329. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J. Se (IV) triggers faster Te (IV) reduction by soil isolates of heterotrophic aerobic bacteria: Formation of extracellular SeTe nanospheres. Microb. Cell Factories 2014, 13, 168. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [Green Version]
- Versantvoort, C.H.; Withoff, S.; Broxterman, H.J.; Kuiper, C.M.; Scheper, R.J.; Mulder, N.H.; de Vries, E.G. Resistance-associated factors in human small-cell lung-carcinoma GLC4 sub-lines with increasing adriamycin resistance. Int. J. Cancer. 1995, 61, 375–380. [Google Scholar] [CrossRef]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium nanoparticles (SeNP) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J. Surg. 2020, 18, 81. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, J.; Liu, P.; Ji, D.; Zhang, L.; Zhang, M.; Li, Y.; Xiao, Y. Preparation and in vitro evaluation of multi-target-directed selenium-chondroitin sulfate nanoparticles in protecting against the Alzheimer’s disease. Int. J. Biol. Macromol. 2020, 142, 265–276. [Google Scholar] [CrossRef]
- Ren, S.-X.; Zhang, B.; Lin, Y.; Ma, D.-S.; Yan, H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. 2019, 25, 991. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yan, H.; Ma, L.; Zhang, H.; Ren, D.F. Preparation and characterization of selenium nanoparticles decorated by Spirulina platensis polysaccharide. J. Food Biochem. 2020, 44, e13363. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, M.Z.; Liang, X.Y.; Nag, A.; Zeng, Q.Z.; Yuan, Y. Fabrication of food grade zein-dispersed selenium dual-nanoparticles with controllable size, cell friendliness and oral bioavailability. Food Chem. 2023, 398, 133878. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Ke, Y.; Liu, Y.; Shen, Y.; Zhang, L.; Li, C.; Liu, A.; Shen, L.; Hu, X.; Wu, H.; et al. Synthesis and antidiabetic properties of chitosan-stabilized selenium nanoparticles. Colloids Surf B Biointerfaces 2018, 170, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.Y.; Wang, Y.Y.; Wang, M.; Yan, J.K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Luo, X.; Wang, M.; Wang, Z.; Guo, J.; Kong, F.; Bi, Y. Synthesis, characterization, in vitro antioxidant and hypoglycemic activities of selenium nanoparticles decorated with polysaccharides of Gracilaria lemaneiformis. Int. J. Biol. Macromol. 2021, 193, 923–932. [Google Scholar] [CrossRef]
- Khiralla, G.; Elhariry, H.; Selim, S.M. Chitosan-stabilized selenium nanoparticles attenuate acrylamide-induced brain injury in rats. J. Food Biochem. 2020, 44, e13413. [Google Scholar] [CrossRef]
- Shao, C.; Yu, Z.; Luo, T.; Zhou, B.; Song, Q.; Li, Z.; Yu, X.; Jiang, S.; Zhou, Y.; Dong, W.; et al. Chitosan-Coated Selenium Nanoparticles Attenuate PRRSV Replication and ROS/JNK-Mediated Apoptosis in vitro. Int. J. Nanomed. 2022, 17, 3043–3054. [Google Scholar] [CrossRef]
- Lara, H.H.; Guisbiers, G.; Mendoza, J.; Mimun, L.C.; Vincent, B.A.; Lopez-Ribot, J.L.; Nash, K.L. Synergistic antifungal effect of chitosan-stabilized selenium nanoparticles synthesized by pulsed laser ablation in liquids against Candida albicans biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation. Pharmaceutics. 2020, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Chen, Y.; Sun, H.; Liu, X.; Leng, X. Physicochemical stability and functional properties of selenium nanoparticles stabilized by chitosan, carrageenan, and gum Arabic. Carbohydr. Polym. 2021, 255, 117379. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Han, W.; Li, C.; Zhang, Z.; Hu, B.; Chen, S.; Cui, P.; Luo, S.; Tang, Z.; et al. Structure characterization of Oudemansiella radicata polysaccharide and preparation of selenium nanoparticles to enhance the antioxidant activities. LWT 2021, 146, 111469. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Fan, P.; Dong, X.; Zhu, K.; Liu, X.; Zhang, N.; Cao, Y. Dioscin prevents DSS-induced colitis in mice with enhancing intestinal barrier function and reducing colon inflammation. Int. Immunopharmacol. 2021, 99, 108015. [Google Scholar] [CrossRef]
- Talapphet, N.; Palanisamy, S.; Li, C.; Ma, N.; Prabhu, N.M.; You, S. Polysaccharide extracted from Taraxacum platycarpum root exerts immunomodulatory activity via MAPK and NF-κB pathways in RAW264.7 cells. J. Ethnopharmacol. 2021, 281, 114519. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free. Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. 2017, 4, 33–46. [Google Scholar]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Vachharajani, T.J.; Work, J.; Issekutz, A.C.; Granger, D.N. Heme oxygenase modulates selectin expression in different regional vascular beds. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1613–H1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, E.; Wieczorek, E.; Jablonska, E.; Janasik, B.; Fendler, W.; Wasowicz, W. Association between plasma selenium level and Nrf2 target genes expression in humans. J. Trace Elem. Med. Biol. 2015, 30, 102–106. [Google Scholar] [CrossRef]
- Alves Júnior, E.B.; de Oliveira Formiga, R.; de Lima Serafim, C.A.; Cristina Araruna, M.E.; de Souza Pessoa, M.L.; Vasconcelos, R.C.; de Carvalho, T.G.; de Jesus, T.G.; Araújo, A.A.; de Araujo Junior, R.F.; et al. Estragole prevents gastric ulcers via cytoprotective, antioxidant and immunoregulatory mechanisms in animal models. Biomed. Pharmacother. 2020, 130, 110578. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Lv, T.; Wang, R.; Zhang, N.; Peng, H.; Diao, W. Salidroside attenuates dextran sulfate sodium-induced colitis in mice via SIRT1/FoxOs signaling pathway. Eur. J. Pharmacol. 2019, 861, 172591. [Google Scholar] [CrossRef]
- Lei, L.; Yang, J.; Zhang, J.; Zhang, G. The lipid peroxidation product EKODE exacerbates colonic inflammation and colon tumorigenesis. Redox Biol. 2021, 42, 101880. [Google Scholar] [CrossRef]
- Shi, X.D.; Tian, Y.Q.; Wu, J.L.; Wang, S.Y. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit. Rev. Food. Sci. Nutr. 2021, 61, 2225–2236. [Google Scholar] [CrossRef]
- Checker, R.; Patwardhan, R.S.; Sharma, D.; Menon, J.; Thoh, M. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. Free. Radic. Biol. Med. 2012, 53, 1421–1430. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell. Mol. Biol. 2018, 335, 41–84. [Google Scholar] [PubMed]
| Gene | Gene Accession Number | Primer Sequence 5’-3’ | Product Size (bp) |
|---|---|---|---|
| ZO-1 | XM_006540785 | F: TACCTCTTGAGCCTTGAACTT R: CGTGCTGATGTGCCATAATA | 248 |
| Occludin | XM_011244634 | F: GCCCAGGCTTCTGGATCTATGT R: GGGGATCAACCACACAGTAGTGA | 124 |
| β-actin | NM_007393 | F: AGTGTGACGTTGACATCCGT R: GCAGCTCAGTAACAGTCCGC | 298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.-J.; Ye, D.-T.; Zheng, J.; Xue, Y.-R.; Lin, L.; Zhao, Y.-D.; Miao, W.-H.; Song, Y.; Wen, Z.-S.; Zheng, B. Synthesis, Characterization of Low Molecular Weight Chitosan Selenium Nanoparticles and Its Effect on DSS-Induced Ulcerative Colitis in Mice. Int. J. Mol. Sci. 2022, 23, 15527. https://doi.org/10.3390/ijms232415527
Peng S-J, Ye D-T, Zheng J, Xue Y-R, Lin L, Zhao Y-D, Miao W-H, Song Y, Wen Z-S, Zheng B. Synthesis, Characterization of Low Molecular Weight Chitosan Selenium Nanoparticles and Its Effect on DSS-Induced Ulcerative Colitis in Mice. International Journal of Molecular Sciences. 2022; 23(24):15527. https://doi.org/10.3390/ijms232415527
Chicago/Turabian StylePeng, Shu-Jiang, Da-Tian Ye, Jie Zheng, Ya-Ru Xue, Lin Lin, Ya-Dong Zhao, Wen-Hua Miao, Yan Song, Zheng-Shun Wen, and Bin Zheng. 2022. "Synthesis, Characterization of Low Molecular Weight Chitosan Selenium Nanoparticles and Its Effect on DSS-Induced Ulcerative Colitis in Mice" International Journal of Molecular Sciences 23, no. 24: 15527. https://doi.org/10.3390/ijms232415527





