Obesity and Risk for Lymphoma: Possible Role of Leptin
Abstract
:1. Introduction
2. Obesity and Lymphoma
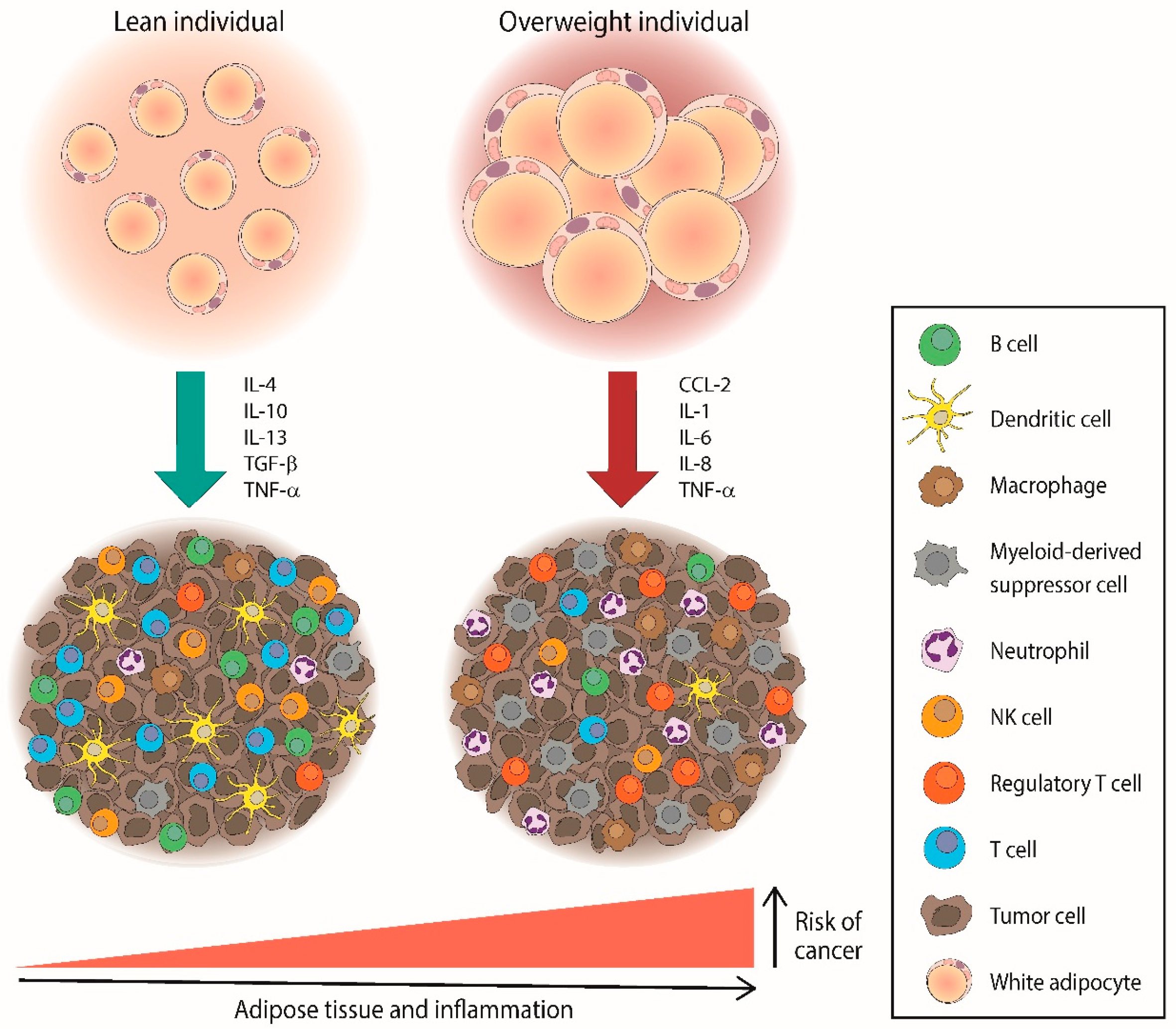
2.1. The Relationship between Obesity and Lymphoma
2.2. Molecular Mechanisms Underlying the Association between Obesity and Lymphomas
3. Leptin and Lymphoma
3.1. Leptin Signaling in Lymphoma
3.2. Leptin and LEPR Genes in Lymphoma
3.3. Serum Leptin and LEPR Expression in Lymphoma
3.4. Linking Leptin, Lymphoma, and Obesity
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, J.; Chang, C.; Pan, H.; Zhu, F.; Xiao, Y.; Liu, T.; Nie, X.; Wu, G.; Zhang, L. Epidemiologic characteristics of malignant lymphoma in Hubei, China: A single-center 5-year retrospective study. Medicine 2018, 97, e12120. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.A.; Ascani, S.; Leoncini, L.; Sabattini, E.; Zinzani, P.L.; Piccaluga, P.P.; Pileri, A., Jr.; Giunti, M.; Falini, B.; Bolis, G.B.; et al. Hodgkin’s lymphoma: The pathologist’s viewpoint. J. Clin. Pathol. 2002, 55, 162–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanas, G.; Ge, W.; Quek, R.G.W.; Keeven, K.; Nersesyan, K.; Jon, E.A. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: Population-level projections for 2020–2025. Leuk. Lymphoma 2022, 63, 54–63. [Google Scholar] [CrossRef]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Prim. 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Hong, F.; Gordon, L.I.; Fisher, R.I.; Bartlett, N.L.; Connors, J.M.; Gascoyne, R.D.; Wagner, H.; Gospodarowicz, M.; Cheson, B.D.; et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: A comprehensive analysis from the North American intergroup trial E2496. Br. J. Haematol. 2013, 161, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenclever, D.; Diehl, V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Pineros, M.; Znaor, A.; Marcos-Gragera, R.; Steliarova-Foucher, E.; Bray, F. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control 2019, 30, 489–499. [Google Scholar] [CrossRef]
- Chiu, B.C.; Hou, N. Epidemiology and etiology of non-hodgkin lymphoma. Cancer Treat. Res. 2015, 165, 1–25. [Google Scholar] [CrossRef]
- Dave, S.S. Host factors for risk and survival in lymphoma. Hematology 2010, 2010, 255–258. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Obesity and risk of non-Hodgkin’s lymphoma: A meta-analysis. Int. J. Cancer 2007, 121, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Willett, E.V.; Morton, L.M.; Hartge, P.; Becker, N.; Bernstein, L.; Boffetta, P.; Bracci, P.; Cerhan, J.; Chiu, B.C.; Cocco, P.; et al. Non-Hodgkin lymphoma and obesity: A pooled analysis from the InterLymph Consortium. Int. J. Cancer 2008, 122, 2062–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheich, S.; Enssle, J.C.; Mucke, V.T.; Acker, F.; Aspacher, L.; Wolf, S.; Wilke, A.C.; Weber, S.; Brunnberg, U.; Serve, H.; et al. Obesity is associated with an impaired survival in lymphoma patients undergoing autologous stem cell transplantation. PLoS ONE 2019, 14, e0225035. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Vilarino-Garcia, T.; Sanchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a new mutation in the mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 1997, 272, 6093–6096. [Google Scholar] [CrossRef] [Green Version]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Margalet, V.; Martin-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Gonzalez-Yanes, C. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martin-Romero, C.; Perez-Perez, A.; Gonzalez-Yanes, C.; Sanchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Vilarino-Garcia, T.; Fernandez-Riejos, P.; Martin-Gonzalez, J.; Segura-Egea, J.J.; Sanchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Deck, C.A.; Honeycutt, J.L.; Cheung, E.; Reynolds, H.M.; Borski, R.J. Assessing the Functional Role of Leptin in Energy Homeostasis and the Stress Response in Vertebrates. Front. Endocrinol. 2017, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [Green Version]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Rogoz, S. Role of leptin in autoimmune diseases. Maedica 2013, 8, 68–74. [Google Scholar]
- Martin-Gonzalez, J.; Perez-Perez, A.; Sanchez-Jimenez, F.; Diaz-Parrado, E.M.; de Miguel, M.; Sanchez-Margalet, V.; Segura-Egea, J.J. Leptin promotes dentin sialophosphoprotein expression in human dental pulp. J. Endod. 2015, 41, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Maymo, J.; Duenas, J.L.; Varone, C.; Sanchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Perez, A.; Toro, A.; Vilarino-Garcia, T.; Maymo, J.; Guadix, P.; Duenas, J.L.; Fernandez-Sanchez, M.; Varone, C.; Sanchez-Margalet, V. Leptin action in normal and pathological pregnancies. J. Cell Mol. Med. 2018, 22, 716–727. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Vilarino-Garcia, T.; Guadix, P.; Duenas, J.L.; Sanchez-Margalet, V. Leptin and Nutrition in Gestational Diabetes. Nutrients 2020, 12, 1970. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Garcia-Galey, A.; Tami, M.; Del Pino, P.; Carmona, I.; Lopez, S.; Alba, G.; Sanchez-Margalet, V. Role of Leptin in Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Ortiz-Garcia, G.; Serrano, A.; Moreno-Ramirez, D.; Sanchez-Margalet, V. Possible Role of Leptin in Atopic Dermatitis: A Literature Review. Biomolecules 2021, 11, 1642. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, F.; Perez-Perez, A.; de la Cruz-Merino, L.; Sanchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Lopez-Saavedra, A.; Sanchez-Jimenez, F.; Perez-Perez, A.; Castineiras, J.; Virizuela-Echaburu, J.A.; de la Cruz-Merino, L.; Sanchez-Margalet, V. Leptin, Both Bad and Good Actor in Cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Wolk, A.; Gridley, G.; Svensson, M.; Nyren, O.; McLaughlin, J.K.; Fraumeni, J.F.; Adam, H.O. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Willett, E.V.; Roman, E. Obesity and the risk of Hodgkin lymphoma (United Kingdom). Cancer Causes Control 2006, 17, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.; Glaser, S.L.; Clarke, C.A.; Dorfman, R.F.; Mann, R.B.; DiGiuseppe, J.A.; Chang, E.T.; Ambinder, R.F. Body size, physical activity, and risk of Hodgkin’s lymphoma in women. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1095–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; senior statistician Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Wolk, A. Body mass index and risk of non-Hodgkin’s and Hodgkin’s lymphoma: A meta-analysis of prospective studies. Eur. J. Cancer 2011, 47, 2422–2430. [Google Scholar] [CrossRef]
- Hidayat, K.; Li, H.J.; Shi, B.M. Anthropometric factors and non-Hodgkin’s lymphoma risk: Systematic review and meta-analysis of prospective studies. Crit. Rev. Oncol. Hematol. 2018, 129, 113–123. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Tzanninis, I.G.; Riza, E.; Dimopoulos, M.A. Anthropometric characteristics, physical activity and risk of hematological malignancies: A systematic review and meta-analysis of cohort studies. Int. J. Cancer 2019, 145, 347–359. [Google Scholar] [CrossRef]
- Moller, H.; Mellemgaard, A.; Lindvig, K.; Olsen, J.H. Obesity and cancer risk: A Danish record-linkage study. Eur. J. Cancer 1994, 30, 344–350. [Google Scholar] [CrossRef]
- Engeland, A.; Tretli, S.; Hansen, S.; Bjorge, T. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. Am. J. Epidemiol. 2007, 165, 44–52. [Google Scholar] [CrossRef]
- Lim, U.; Morton, L.M.; Subar, A.F.; Baris, D.; Stolzenberg-Solomon, R.; Leitzmann, M.; Kipnis, V.; Mouw, T.; Carroll, L.; Schatzkin, A.; et al. Alcohol, smoking, and body size in relation to incident Hodgkin’s and non-Hodgkin’s lymphoma risk. Am. J. Epidemiol. 2007, 166, 697–708. [Google Scholar] [CrossRef]
- Ingham, R.R.; Reagan, J.L.; Dalia, S.; Furman, M.; Merhi, B.; Nemr, S.; Zarrabi, A.; Mitri, J.; Castillo, J.J. The Relationship Between Obesity and Lymphoma: A Meta-Analysis of Prospective Cohort Studies. Blood 2011, 118, 5198. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Wing, A.L.; Hyde, R.T. Characteristics in youth indicative of adult-onset Hodgkin’s disease. J. Natl. Cancer Inst. 1977, 58, 1489–1491. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Wing, A.L.; Hyde, R.T. Characteristics in youth predictive of adult-onset malignant lymphomas, melanomas, and leukemias: Brief communication. J. Natl. Cancer Inst. 1978, 60, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Strongman, H.; Brown, A.; Smeeth, L.; Bhaskaran, K. Body mass index and Hodgkin’s lymphoma: UK population-based cohort study of 5.8 million individuals. Br. J. Cancer 2019, 120, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Fernberg, P.; Odenbro, A.; Bellocco, R.; Boffetta, P.; Pawitan, Y.; Adami, J. Tobacco use, body mass index and the risk of malignant lymphomas--a nationwide cohort study in Sweden. Int. J. Cancer 2006, 118, 2298–2302. [Google Scholar] [CrossRef]
- Pan, S.Y.; Mao, Y.; Ugnat, A.M.; the Canadian Cancer Registries Epidemiology Research Group. Physical activity, obesity, energy intake, and the risk of non-Hodgkin’s lymphoma: A population-based case-control study. Am. J. Epidemiol. 2005, 162, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.C.; Soni, L.; Gapstur, S.M.; Fought, A.J.; Evens, A.M.; Weisenburger, D.D. Obesity and risk of non-Hodgkin lymphoma (United States). Cancer Causes Control 2007, 18, 677–685. [Google Scholar] [CrossRef]
- Rapp, K.; Schroeder, J.; Klenk, J.; Stoehr, S.; Ulmer, H.; Concin, H.; Diem, G.; Oberaigner, W.; Weiland, S.K. Obesity and incidence of cancer: A large cohort study of over 145,000 adults in Austria. Br. J. Cancer 2005, 93, 1062–1067. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, K.; Du, X.; Shi, B.M. Body fatness at a young age and risks of eight types of cancer: Systematic review and meta-analysis of observational studies. Obes. Rev. 2018, 19, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.Y.; Johnson, K.C.; Ugnat, A.M.; Wen, S.W.; Mao, Y.; Canadian Cancer Registries Epidemiology Research Group. Association of obesity and cancer risk in Canada. Am. J. Epidemiol. 2004, 159, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Holly, E.A.; Lele, C.; Bracci, P.M.; McGrath, M.S. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay Area, California. Am. J. Epidemiol. 1999, 150, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Yoon, Y.S.; Shin, S.A. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 2005, 23, 4742–4754. [Google Scholar] [CrossRef]
- Kelta, M.; Zekri, J.; Abdelghany, E.; Rehman, J.U.; Khan, Z.A.; Al-Saadi, R.; Dada, R. High-dose chemotherapy and peripheral hematopoietic stem cell transplantation in relapsed/refractory Hodgkin’s lymphoma. Tumori J. 2018, 104, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Dahi, P.B.; Lee, J.; Devlin, S.M.; Ruiz, J.; Maloy, M.; Rondon-Clavo, C.; Petrlik, E.; Tamari, R.; Shah, G.; Scordo, M.; et al. Toxicities of high-dose chemotherapy and autologous hematopoietic cell transplantation in older patients with lymphoma. Blood Adv. 2021, 5, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Leiba, M.; Leiba, A.; Keinan-Boker, L.; Avigdor, A.; Derazne, E.; Levine, H.; Kark, J.D. Adolescent weight and height are predictors of specific non-Hodgkin lymphoma subtypes among a cohort of 2,352,988 individuals aged 16 to 19 years. Cancer 2016, 122, 1068–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goy, A.; Ramchandren, R.; Ghosh, N.; Munoz, J.; Morgan, D.S.; Dang, N.H.; Knapp, M.; Delioukina, M.; Kingsley, E.; Ping, J.; et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood 2019, 134, 1024–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Palazon-Carrion, N.; Martin Garcia-Sancho, A.; Nogales-Fernandez, E.; Carnicero-Gonzalez, F.; Rios-Herranz, E.; de la Cruz-Vicente, F.; Rodriguez-Garcia, G.; Fernandez-Alvarez, R.; Rueda Dominguez, A.; et al. Circulating myeloid-derived suppressor cells and regulatory T cells as immunological biomarkers in refractory/relapsed diffuse large B-cell lymphoma: Translational results from the R2-GDP-GOTEL trial. J. Immunother. Cancer 2021, 9, e002323. [Google Scholar] [CrossRef]
- Colditz, G.A.; Peterson, L.L. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin. Chem. 2018, 64, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Dossus, L.; Marant-Micallef, C.; His, M. Obesity and Cancer. Bull. Cancer 2019, 106, 635–646. [Google Scholar] [CrossRef]
- Skibola, C.F.; Holly, E.A.; Forrest, M.S.; Hubbard, A.; Bracci, P.M.; Skibola, D.R.; Hegedus, C.; Smith, M.T. Body mass index, leptin and leptin receptor polymorphisms, and non-hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2004, 13, 779–786. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Bernstein, L.; Severson, R.K.; Davis, S.; Colt, J.S.; Blair, A.; Hartge, P. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control 2005, 16, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Kane, E.; Skibola, C.F.; Bracci, P.M.; Cerhan, J.R.; Costas, L.; Smedby, K.E.; Holly, E.A.; Maynadie, M.; Novak, A.J.; Lightfoot, T.J.; et al. Non-Hodgkin Lymphoma, Body Mass Index, and Cytokine Polymorphisms: A Pooled Analysis from the InterLymph Consortium. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1061–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.T.; Hjalgrim, H.; Smedby, K.E.; Akerman, M.; Tani, E.; Johnsen, H.E.; Glimelius, B.; Adami, H.O.; Melbye, M. Body mass index and risk of malignant lymphoma in Scandinavian men and women. J. Natl. Cancer Inst. 2005, 97, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Bowzyk Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-Hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Janney, C.A.; Vachon, C.M.; Habermann, T.M.; Kay, N.E.; Potter, J.D.; Sellers, T.A.; Folsom, A.R. Anthropometric characteristics, physical activity, and risk of non-Hodgkin’s lymphoma subtypes and B-cell chronic lymphocytic leukemia: A prospective study. Am. J. Epidemiol. 2002, 156, 527–535. [Google Scholar] [CrossRef]
- Boyle, T.; Connors, J.M.; Gascoyne, R.D.; Berry, B.R.; Sehn, L.H.; Bashash, M.; Spinelli, J.J. Physical activity, obesity and survival in diffuse large B-cell and follicular lymphoma cases. Br. J. Haematol. 2017, 178, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Basirat, M.; Rabiei, M.; Bashardoust, N. Incidence of Head and Neck Lymphoma in Guilan Province, Iran. Asian Pac. J. Cancer Prev. 2016, 17, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Lukanova, A.; Bjor, O.; Kaaks, R.; Lenner, P.; Lindahl, B.; Hallmans, G.; Stattin, P. Body mass index and cancer: Results from the Northern Sweden Health and Disease Cohort. Int. J. Cancer 2006, 118, 458–466. [Google Scholar] [CrossRef]
- Willett, E.V.; Skibola, C.F.; Adamson, P.; Skibola, D.R.; Morgan, G.J.; Smith, M.T.; Roman, E. Non-Hodgkin’s lymphoma, obesity and energy homeostasis polymorphisms. Br. J. Cancer 2005, 93, 811–816. [Google Scholar] [CrossRef]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2018, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Naik, A.; Monjazeb, A.M.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1940. [Google Scholar] [CrossRef] [Green Version]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Kroenke, C.H.; Caan, B.J. The Obesity Paradox in Cancer: How Important Is Muscle? Annu. Rev. Nutr. 2018, 38, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, H.; Andreasson, A.; Carlsson, A.C.; Jerkeman, M.; Carlsten, M. Body composition measurements and risk of hematological malignancies: A population-based cohort study during 20 years of follow-up. PLoS ONE 2018, 13, e0202651. [Google Scholar] [CrossRef]
- Barberio, A.M.; Alareeki, A.; Viner, B.; Pader, J.; Vena, J.E.; Arora, P.; Friedenreich, C.M.; Brenner, D.R. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Matos, A.; Marinho-Dias, J.; Ramalheira, S.; Oliveira, M.J.; Bicho, M.; Ribeiro, R. Mechanisms underlying the association between obesity and Hodgkin lymphoma. Tumour Biol. 2016, 37, 13005–13016. [Google Scholar] [CrossRef]
- Hosgood, H.D.; Gunter, M.J.; Murphy, N.; Rohan, T.E.; Strickler, H.D. The Relation of Obesity-Related Hormonal and Cytokine Levels With Multiple Myeloma and Non-Hodgkin Lymphoma. Front. Oncol. 2018, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFkappaB-signaling pathway in cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, P.J.; Ruland, J. Aberrant NF-kappaB signaling in lymphoma: Mechanisms, consequences, and therapeutic implications. Blood 2007, 109, 2700–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-kappaB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaji, S.; Ahmed, M.; Lorence, E.; Yan, F.; Nomie, K.; Wang, M. NF-kappaB signaling and its relevance to the treatment of mantle cell lymphoma. J. Hematol. Oncol. 2018, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Rauch, D.A.; Harding, J.C.; Ratner, L.; Wickline, S.A.; Pan, H. Targeting NF-kappaB with Nanotherapy in a Mouse Model of Adult T-Cell Leukemia/Lymphoma. Nanomaterials 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Ghosh, S.; Ashcraft, K. An IL-6 link between obesity and cancer. Front. Biosci. 2013, 5, 461–478. [Google Scholar] [CrossRef]
- Burger, R. Impact of interleukin-6 in hematological malignancies. Transfus. Med. Hemotherapy 2013, 40, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Voorzanger, N.; Touitou, R.; Garcia, E.; Delecluse, H.J.; Rousset, F.; Joab, I.; Favrot, M.C.; Blay, J.Y. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996, 56, 5499–5505. [Google Scholar] [PubMed]
- Zhang, L.; Yang, J.; Qian, J.; Li, H.; Romaguera, J.E.; Kwak, L.W.; Wang, M.; Yi, Q. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood 2012, 120, 3783–3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, W.S.; Park, C. Interleukin-6 mediates resistance to PI3K-pathway-targeted therapy in lymphoma. BMC Cancer 2019, 19, 936. [Google Scholar] [CrossRef] [Green Version]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Muller, A. The IL-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef] [PubMed]
- Asou, H.; Said, J.W.; Yang, R.; Munker, R.; Park, D.J.; Kamada, N.; Koeffler, H.P. Mechanisms of growth control of Kaposi’s sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 1998, 91, 2475–2481. [Google Scholar] [CrossRef] [Green Version]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, H.S.; Kawada, T.; Kim, J.H.; Lim, D.; Hubbard, N.E.; Kwon, B.S.; Erickson, K.L.; Yu, R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Miyata-Takata, T.; Takata, K.; Toji, T.; Goto, N.; Kasahara, S.; Takahashi, T.; Tari, A.; Noujima-Harada, M.; Miyata, T.; Sato, Y.; et al. Elevation of serum interleukins 8, 4, and 1beta levels in patients with gastrointestinal low-grade B-cell lymphoma. Sci. Rep. 2015, 5, 18434. [Google Scholar] [CrossRef] [Green Version]
- Manfroi, B.; McKee, T.; Mayol, J.F.; Tabruyn, S.; Moret, S.; Villiers, C.; Righini, C.; Dyer, M.; Callanan, M.; Schneider, P.; et al. CXCL-8/IL8 Produced by Diffuse Large B-cell Lymphomas Recruits Neutrophils Expressing a Proliferation-Inducing Ligand APRIL. Cancer Res. 2017, 77, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Abreu, M.; Miranda, M.; Castro, M.; Fernandes, I.; Cabral, R.; Santos, A.H.; Fonseca, S.; Rodrigues, J.; Leander, M.; Lau, C.; et al. IL-31 and IL-8 in Cutaneous T-Cell Lymphoma: Looking for Their Role in Itch. Adv. Hematol. 2021, 2021, 5582581. [Google Scholar] [CrossRef]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef]
- Tobin, R.P.; Jordan, K.R.; Kapoor, P.; Spongberg, E.; Davis, D.; Vorwald, V.M.; Couts, K.L.; Gao, D.; Smith, D.E.; Borgers, J.S.W.; et al. IL-6 and IL-8 Are Linked With Myeloid-Derived Suppressor Cell Accumulation and Correlate With Poor Clinical Outcomes in Melanoma Patients. Front. Oncol. 2019, 9, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betsch, A.; Rutgeerts, O.; Fevery, S.; Sprangers, B.; Verhoef, G.; Dierickx, D.; Beckers, M. Myeloid-derived suppressor cells in lymphoma: The good, the bad and the ugly. Blood Rev. 2018, 32, 490–498. [Google Scholar] [CrossRef]
- Dutta, P.; Sarkissyan, M.; Paico, K.; Wu, Y.; Vadgama, J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018, 170, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Husson, H.; Carideo, E.G.; Cardoso, A.A.; Lugli, S.M.; Neuberg, D.; Munoz, O.; de Leval, L.; Schultze, J.; Freedman, A.S. MCP-1 modulates chemotaxis by follicular lymphoma cells. Br. J. Haematol. 2001, 115, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Shi, Z.H.; Wang, X.; Gu, K.S.; Zhai, Z.M. Prognostic significance of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in diffuse large B cell lymphoma. Ann. Hematol. 2019, 98, 413–422. [Google Scholar] [CrossRef]
- Kitai, R.; Ishisaka, K.; Sato, K.; Sakuma, T.; Yamauchi, T.; Imamura, Y.; Matsumoto, H.; Kubota, T. Primary central nervous system lymphoma secretes monocyte chemoattractant protein 1. Med. Mol. Morphol. 2007, 40, 18–22. [Google Scholar] [CrossRef]
- Ghanbari, M.; Momen Maragheh, S.; Aghazadeh, A.; Mehrjuyan, S.R.; Hussen, B.M.; Abdoli Shadbad, M.; Dastmalchi, N.; Safaralizadeh, R. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int. Immunopharmacol. 2021, 96, 107765. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Oelmann, E.; Stein, H.; Berdel, W.E.; Herbst, H. Expression of Interleukin-1 and Interleukin-1 Receptors Type 1 and Type 2 in Hodgkin Lymphoma. PLoS ONE 2015, 10, e0138747. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Gottschlich, A.; Endres, S.; Kobold, S. Therapeutic Strategies for Targeting IL-1 in Cancer. Cancers 2021, 13, 477. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun 2010, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Segui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahmus, J.; Rosario, M.; Clarke, K. Risk of Lymphoma Associated with Anti-TNF Therapy in Patients with Inflammatory Bowel Disease: Implications for Therapy. Clin. Exp. Gastroenterol. 2020, 13, 339–350. [Google Scholar] [CrossRef]
- Calip, G.S.; Patel, P.R.; Adimadhyam, S.; Xing, S.; Wu, Z.; Sweiss, K.; Schumock, G.T.; Lee, T.A.; Chiu, B.C. Tumor necrosis factor-alpha inhibitors and risk of non-Hodgkin lymphoma in a cohort of adults with rheumatologic conditions. Int. J. Cancer 2018, 143, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Song, W.K.; Cho, A.R.; Yoon, Y.H. Highly suspected primary intraocular lymphoma in a patient with rheumatoid arthritis treated with etanercept: A case report. BMC Ophthalmol. 2018, 18, 156. [Google Scholar] [CrossRef] [Green Version]
- van de Woestijne, A.P.; Monajemi, H.; Kalkhoven, E.; Visseren, F.L. Adipose tissue dysfunction and hypertriglyceridemia: Mechanisms and management. Obes. Rev. 2011, 12, 829–840. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Margalet, V.; Martin-Romero, C. Human leptin signaling in human peripheral blood mononuclear cells: Activation of the JAK-STAT pathway. Cell Immunol. 2001, 211, 30–36. [Google Scholar] [CrossRef]
- Martin-Romero, C.; Sanchez-Margalet, V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: Possible role of Sam68. Cell Immunol. 2001, 212, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Saeidi, J.; Azimi-Nejad, M.; Hashemy, S.I. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol. 2019, 42, 243–260. [Google Scholar] [CrossRef]
- Ding, B.B.; Yu, J.J.; Yu, R.Y.; Mendez, L.M.; Shaknovich, R.; Zhang, Y.; Cattoretti, G.; Ye, B.H. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 2008, 111, 1515–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Wang, K.B.; Rui, L. STAT3 Activation and Oncogenesis in Lymphoma. Cancers 2019, 12, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnadasan, R.; Bifulco, C.; Kim, J.; Rodov, S.; Zieske, A.W.; Vanasse, G.J. Overexpression of SOCS3 is associated with decreased survival in a cohort of patients with de novo follicular lymphoma. Br. J. Haematol. 2006, 135, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Cho-Vega, J.H.; Rassidakis, G.Z.; Amin, H.M.; Tsioli, P.; Spurgers, K.; Remache, Y.K.; Vega, F.; Goy, A.H.; Gilles, F.; Medeiros, L.J. Suppressor of cytokine signaling 3 expression in anaplastic large cell lymphoma. Leukemia 2004, 18, 1872–1878. [Google Scholar] [CrossRef] [Green Version]
- Maurer, B.; Nivarthi, H.; Wingelhofer, B.; Pham, H.T.T.; Schlederer, M.; Suske, T.; Grausenburger, R.; Schiefer, A.I.; Prchal-Murphy, M.; Chen, D.; et al. High activation of STAT5A drives peripheral T-cell lymphoma and leukemia. Haematologica 2020, 105, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, C.; Jiang, B.; Hu, X.; Zhang, W.; Chan, J.K.; Xiao, W.; Lack, N.; Alkan, C.; Williams, J.C.; Avery, K.N.; et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.; Hohaus, S.; Petrucci, G.; Cenci, T.; Pierconti, F.; Massini, G.; Teofili, L.; Leone, G.; Larocca, L.M. Phosphorylated STAT5 represents a new possible prognostic marker in Hodgkin lymphoma. Am. J. Clin. Pathol. 2008, 129, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Scheeren, F.A.; Diehl, S.A.; Smit, L.A.; Beaumont, T.; Naspetti, M.; Bende, R.J.; Blom, B.; Karube, K.; Ohshima, K.; van Noesel, C.J.; et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: Evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood 2008, 111, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Spolski, R.; Kovanen, P.E.; Suzuki, T.; Bollenbacher, J.; Pise-Masison, C.A.; Radonovich, M.F.; Lee, S.; Jenkins, N.A.; Copeland, N.G.; et al. Stat5 synergizes with T cell receptor/antigen stimulation in the development of lymphoblastic lymphoma. J. Exp. Med. 2003, 198, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Shipp, M.A.; Ross, K.N.; Tamayo, P.; Weng, A.P.; Kutok, J.L.; Aguiar, R.C.; Gaasenbeek, M.; Angelo, M.; Reich, M.; Pinkus, G.S.; et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat. Med. 2002, 8, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Voena, C.; Conte, C.; Ambrogio, C.; Boeri Erba, E.; Boccalatte, F.; Mohammed, S.; Jensen, O.N.; Palestro, G.; Inghirami, G.; Chiarle, R. The tyrosine phosphatase Shp2 interacts with NPM-ALK and regulates anaplastic lymphoma cell growth and migration. Cancer Res. 2007, 67, 4278–4286. [Google Scholar] [CrossRef] [Green Version]
- Karaca Atabay, E.; Mecca, C.; Wang, Q.; Ambrogio, C.; Mota, I.; Prokoph, N.; Mura, G.; Martinengo, C.; Patrucco, E.; Leonardi, G.; et al. Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma. Blood 2022, 139, 717–731. [Google Scholar] [CrossRef]
- Vega, G.G.; Aviles-Salas, A.; Chalapud, J.R.; Martinez-Paniagua, M.; Pelayo, R.; Mayani, H.; Hernandez-Pando, R.; Martinez-Maza, O.; Huerta-Yepez, S.; Bonavida, B.; et al. P38 MAPK expression and activation predicts failure of response to CHOP in patients with Diffuse Large B-Cell Lymphoma. BMC Cancer 2015, 15, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louissaint, A., Jr.; Schafernak, K.T.; Geyer, J.T.; Kovach, A.E.; Ghandi, M.; Gratzinger, D.; Roth, C.G.; Paxton, C.N.; Kim, S.; Namgyal, C.; et al. Pediatric-type nodal follicular lymphoma: A biologically distinct lymphoma with frequent MAPK pathway mutations. Blood 2016, 128, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramis-Zaldivar, J.E.; Gonzalez-Farre, B.; Nicolae, A.; Pack, S.; Clot, G.; Nadeu, F.; Mottok, A.; Horn, H.; Song, J.Y.; Fu, K.; et al. MAPK and JAK-STAT pathways dysregulation in plasmablastic lymphoma. Haematologica 2021, 106, 2682–2693. [Google Scholar] [CrossRef]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef] [Green Version]
- Kuo, A.H.; Stoica, G.E.; Riegel, A.T.; Wellstein, A. Recruitment of insulin receptor substrate-1 and activation of NF-kappaB essential for midkine growth signaling through anaplastic lymphoma kinase. Oncogene 2007, 26, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Urso, B.; Ilondo, M.M.; Holst, P.A.; Christoffersen, C.T.; Ouwens, M.; Giorgetti, S.; Van Obberghen, E.; Naor, D.; Tornqvist, H.; De Meyts, P. IRS-4 mediated mitogenic signalling by insulin and growth hormone in LB cells, a murine T-cell lymphoma devoid of IGF-I receptors. Cell. Signal. 2003, 15, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pongas, G.; Cheson, B.D. PI3K signaling pathway in normal B cells and indolent B-cell malignancies. Semin. Oncol. 2016, 43, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cai, Y.; Wang, W.; Liu, Z.; Wei, P.; Bi, R.; Chen, W.; Sun, M.; Zhou, X. Frequent copy number variations of PI3K/AKT pathway and aberrant protein expressions of PI3K subunits are associated with inferior survival in diffuse large B cell lymphoma. J. Transl. Med. 2014, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, S.; Clear, A.; Bodor, C.; Maharaj, L.; Lee, A.; Calaminici, M.; Matthews, J.; Iqbal, S.; Auer, R.; Gribben, J.; et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 2013, 121, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Barrientos, J.C. Oral PI3K-delta, gamma Inhibitor for the Management of People with Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: A Narrative Review on Duvelisib. OncoTargets Ther. 2021, 14, 2109–2119. [Google Scholar] [CrossRef]
- Huang, D.; Song, T.L.; Nairismagi, M.L.; Laurensia, Y.; Pang, W.L.; Zhe, D.C.M.; Wong, E.K.Y.; Wijaya, G.G.; Tan, J.; Tan, S.H.; et al. Evaluation of the PIK3 pathway in peripheral T-cell lymphoma and NK/T-cell lymphoma. Br. J. Haematol. 2020, 189, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Raso, G.M.; Pacilio, M.; Esposito, E.; Coppola, A.; Di Carlo, R.; Meli, R. Leptin potentiates IFN-gamma-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A.1. Br. J. Pharmacol. 2002, 137, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Conde, J.; Scotece, M.; Lopez, V.; Gomez, R.; Lago, F.; Pino, J.; Gomez-Reino, J.J.; Gualillo, O. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE 2012, 7, e52533. [Google Scholar] [CrossRef] [Green Version]
- Suzukawa, M.; Koketsu, R.; Baba, S.; Igarashi, S.; Nagase, H.; Yamaguchi, M.; Matsutani, N.; Kawamura, M.; Shoji, S.; Hebisawa, A.; et al. Leptin enhances ICAM-1 expression, induces migration and cytokine synthesis, and prolongs survival of human airway epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L801-811. [Google Scholar] [CrossRef]
- Terol, M.J.; Lopez-Guillermo, A.; Bosch, F.; Villamor, N.; Cid, M.C.; Rozman, C.; Campo, E.; Montserrat, E. Expression of the adhesion molecule ICAM-1 in non-Hodgkin’s lymphoma: Relationship with tumor dissemination and prognostic importance. J. Clin. Oncol. 1998, 16, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.C.; Agrawal, S.; Chaperot, L.; Giroux, C.; Gressin, R.; Le Marc’Hadour, F.; Favre, M.; Sotto, J.J.; Bensa, J.C.; Plumas, J. Quantification of cellular adhesion molecules on malignant B cells from non-Hodgkin’s lymphoma. Leukemia 1999, 13, 1428–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gu, J.J.; Yang, L.; Tsai, P.C.; Guo, Y.; Xue, K.; Xia, Z.; Liu, X.; Lv, F.; Cao, J.; et al. The adhesion molecule ICAM-1 in diffuse large B-cell lymphoma post-rituximab era: Relationship with prognostic importance and rituximab resistance. Aging 2020, 13, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Syrigos, K.N.; Salgami, E.; Karayiannakis, A.J.; Katirtzoglou, N.; Sekara, E.; Roussou, P. Prognostic significance of soluble adhesion molecules in Hodgkin’s disease. Anticancer Res. 2004, 24, 1243–1247. [Google Scholar] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Choi, H.J.; Oh, C.H.; Oh, J.W.; Han, J.S. Leptin increases TNF-alpha expression and production through phospholipase D1 in Raw 264.7 cells. PLoS ONE 2014, 9, e102373. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.F.; Bottcher, C.; Letizia, M.; Yerinde, C.; Wu, H.; Freise, I.; Rodriguez-Sillke, Y.; Stoyanova, A.K.; Kreis, M.E.; Asbach, P.; et al. Leptin induces TNFalpha-dependent inflammation in acquired generalized lipodystrophy and combined Crohn’s disease. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczuk, A.; Wiecek, A.; Franek, E.; Kokot, F. Plasma concentration of leptin, neuropeptide Y and tumor necrosis factor alpha in patients with cancers, before and after radio- and chemotherapy. Pol. Arch. Med. Wewn. 2001, 106, 657–668. [Google Scholar]
- Conroy, S.M.; Maskarinec, G.; Morimoto, Y.; Franke, A.A.; Cooney, R.V.; Wilkens, L.R.; Goodman, M.T.; Hernadez, B.Y.; Le Marchand, L.; Henderson, B.E.; et al. Non-hodgkin lymphoma and circulating markers of inflammation and adiposity in a nested case-control study: The multiethnic cohort. Cancer Epidemiol. Biomark. Prev. 2013, 22, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, S.M.; Abdo, N.; Al-Eitan, L.N.; Al-Mistarehi, A.W.; Zahran, D.J.; Kewan, T.Z. LTA, LEP, and TNF-a Gene Polymorphisms are Associated with Susceptibility and Overall Survival of Diffuse Large B-Cell lymphoma in an Arab Population: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2783–2791. [Google Scholar] [CrossRef]
- Barbosa-Cortes, L.; Klunder-Klunder, M.; Lopez-Alarcon, M.; Marquez, H.R.; Lopez-Aguilar, E.; Tapia-Marcial, A. Nutritional status and cytokine concentration during chemotherapy in Mexican children: A longitudinal analysis. Nutrition 2019, 57, 46–51. [Google Scholar] [CrossRef] [PubMed]
- El Demerdash, D.M.; Tawfik, N.M.; Elazab, R.; El Sissy, M.H. The Association of Pre-diagnostic Inflammatory Markers and Adipokines and the Risk of Non-Hodgkin Lymphoma Development in Egypt. Indian J. Hematol. Blood Transfus. 2021, 37, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, X.; Liang, Y.; Rao, H.; Fang, X.; Jiang, W.; Lin, T.; Lin, T.; Huang, H. Fasting blood glucose is a novel prognostic indicator for extranodal natural killer/T-cell lymphoma, nasal type. Br. J. Cancer 2013, 108, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, R.; Yang, Y.; Cai, L.; Ding, S.; Xu, T.; Han, M.; Wu, X. Disruption of the Golgi protein Otg1 gene causes defective hormone secretion and aberrant glucose homeostasis in mice. Cell Biosci. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.; Arber, D.A.; Chang, K.L.; Wilson, C.S.; Weiss, L.M. Frequency of bcl-2 expression in non-Hodgkin’s lymphoma: A study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod. Pathol. 1998, 11, 864–869. [Google Scholar]
- Wagner, S.D.; Ahearne, M.; Ko Ferrigno, P. The role of BCL6 in lymphomas and routes to therapy. Br. J. Haematol. 2011, 152, 3–12. [Google Scholar] [CrossRef]
- Child, F.J.; Scarisbrick, J.J.; Calonje, E.; Orchard, G.; Russell-Jones, R.; Whittaker, S.J. Inactivation of tumor suppressor genes p15(INK4b) and p16(INK4a) in primary cutaneous B cell lymphoma. J. Investig. Dermatol. 2002, 118, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Medeiros, L.J.; Bueso-Ramos, C.E.; Tang, G.; Wang, S.; Oki, Y.; Desai, P.; Khoury, J.D.; Miranda, R.N.; Tang, Z.; et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod. Pathol. 2017, 30, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Zhang, X. The Spectrum of MYC Alterations in Diffuse Large B-Cell Lymphoma. Acta Haematol. 2020, 143, 520–528. [Google Scholar] [CrossRef]
- Sup, S.J.; Alemany, C.A.; Pohlman, B.; Elson, P.; Malhi, S.; Thakkar, S.; Steinle, R.; Hsi, E.D. Expression of bcl-2 in classical Hodgkin’s lymphoma: An independent predictor of poor outcome. J. Clin. Oncol. 2005, 23, 3773–3779. [Google Scholar] [CrossRef]
- Mahmoud, H.M.; El-Sakhawy, Y.N. Significance of Bcl-2 and Bcl-6 immunostaining in B-Non Hodgkin’s lymphoma. Hematol. Rep. 2011, 3, e26. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Zhang, L.; Guo, S.Q.; Li, X.W.; Qu, F.L.; Zhao, H.F.; Zhang, L.Y.; Sun, B.C.; You, J.; Zhang, Y.Z. Coexpression of MYC and BCL-2 predicts prognosis in primary gastrointestinal diffuse large B-cell lymphoma. World J. Gastroenterol. 2015, 21, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, A.M.; Rotaru, I.; Olar, L.; Patrascu, S.; Ghilusi, M.C.; NeamTu, S.D.; Nacea, J.G.; Gluhovschi, A. The prognostic role of Bcl-2, Ki67, c-MYC and p53 in diffuse large B-cell lymphoma. Rom. J. Morphol. Embryol. 2017, 58, 837–843. [Google Scholar] [PubMed]
- Li, L.; Zhang, X.; Zhang, T.; Song, Z.; Hu, G.; Li, W.; Li, L.; Qiu, L.; Qian, Z.; Zhou, S.; et al. Prognostic Significance of BCL-2 and BCL-6 Expression in MYC-positive DLBCL. Clin. Lymphoma Myeloma Leuk. 2018, 18, e381–e389. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Zhang, X.Y.; Li, Y.; Song, L.L.; Wang, L. Correlations of mouse lymphoma xenografts with the expressions of MMP-9 and Bcl-2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1176–1183. [Google Scholar] [CrossRef]
- Pileri, A.; Agostinelli, C.; Bertuzzi, C.; Grandi, V.; Maio, V.; Lastrucci, I.; Santucci, M.; Pimpinelli, N. Prognostic significance of Bcl-2 expression in primary cutaneous B-cell lymphoma: A reappraisal. Ital. J. Dermatol. Venerol. 2021, 156, 642–649. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Lam, Q.L.; Wang, S.; Ko, O.K.; Kincade, P.W.; Lu, L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc. Natl. Acad. Sci. USA 2010, 107, 13812–13817. [Google Scholar] [CrossRef] [Green Version]
- Michurina, S.V.; Ishchenko, I.Y.; Arkhipov, S.A.; Cherepanova, M.A.; Vasendin, D.V.; Zavjalov, E.L. Apoptosis in the liver of male db/db mice during the development of obesity and type 2 diabetes. Vavilov J. Genet. Breed. 2020, 24, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Plante, E.; Menaouar, A.; Danalache, B.A.; Yip, D.; Broderick, T.L.; Chiasson, J.L.; Jankowski, M.; Gutkowska, J. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 2015, 156, 1416–1428. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Q.; Ju, J.; Chen, W.; Zhao, X.; Zhang, Y.; Yang, Y. Effect of Astragalus Polysaccharides on Cardiac Dysfunction in db/db Mice with Respect to Oxidant Stress. Biomed. Res. Int. 2018, 2018, 8359013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sataranatarajan, K.; Ikeno, Y.; Bokov, A.; Feliers, D.; Yalamanchili, H.; Lee, H.J.; Mariappan, M.M.; Tabatabai-Mir, H.; Diaz, V.; Prasad, S.; et al. Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.D.; Wu, X.H.; Zhang, L.N. Effect of leptin on expression of calpain-1 and Bcl-2 and apoptosis in myocardial tissue of neonatal rats after asphyxia. Zhongguo Dang dai er ke za zhi 2016, 18, 1044–1049. [Google Scholar]
- Shimabukuro, M.; Wang, M.Y.; Zhou, Y.T.; Newgard, C.B.; Unger, R.H. Protection against lipoapoptosis of beta cells through leptin-dependent maintenance of Bcl-2 expression. Proc. Natl. Acad. Sci. USA 1998, 95, 9558–9561. [Google Scholar] [CrossRef]
- Brown, J.E.; Dunmore, S.J. Leptin decreases apoptosis and alters BCL-2: Bax ratio in clonal rodent pancreatic beta-cells. Diabetes/Metab. Res. Rev. 2007, 23, 497–502. [Google Scholar] [CrossRef]
- da Silva, S.V.; Salama, C.; Renovato-Martins, M.; Helal-Neto, E.; Citelli, M.; Savino, W.; Barja-Fidalgo, C. Increased leptin response and inhibition of apoptosis in thymocytes of young rats offspring from protein deprived dams during lactation. PLoS ONE 2013, 8, e64220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Perez, A.; Toro, A.R.; Vilarino-Garcia, T.; Guadix, P.; Maymo, J.L.; Duenas, J.L.; Varone, C.L.; Sanchez-Margalet, V. Leptin reduces apoptosis triggered by high temperature in human placental villous explants: The role of the p53 pathway. Placenta 2016, 42, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Cai, L.; Ding, C.; Wang, X.; Chen, H.; Wang, X.; Yan, J.; Lu, J. Leptin induces cell proliferation and reduces cell apoptosis by activating c-myc in cervical cancer. Oncol. Rep. 2013, 29, 2291–2296. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S.; Yun, H.; Son, D.J.; Tompkins, V.S.; Peng, L.; Chung, S.T.; Kim, J.S.; Park, E.S.; Janz, S. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol. Cancer 2010, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Uddin, S.; Bu, R.; Ahmed, M.; Hussain, A.R.; Ajarim, D.; Al-Dayel, F.; Bavi, P.; Al-kuraya, K.S. Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leuk. Lymphoma 2010, 51, 1305–1314. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Xing, X.M.; Ran, W.W. Expression and significance of leptin receptor, p-STAT3 and p-AKT in diffuse large B-cell lymphoma. Acta Histochem. 2014, 116, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, P.; Guo, F.; Liu, R.; Yang, Y.; Huang, C.; Shu, H.; Gong, J.; Cai, M. Genetic G2548A polymorphism of leptin gene and risk of cancer: A meta-analysis of 6860 cases and 7956 controls. J. BUON 2014, 19, 1096–1104. [Google Scholar] [PubMed]
- Lin, H.Y.; Shi, H.; Li, C.Y.; Chen, Q.C.; Huang, T.B.; Liu, P.C.; Lou, L.M. LEP and LEPR polymorphisms in non-Hodgkin lymphoma risk: A systematic review and pooled analysis. J. BUON 2015, 20, 261–268. [Google Scholar]
- Vasku, V.; Vasku, A.; Vasku, J.B. Pharmacogenetic contribution of leptin gene polymorphism in cutaneous T-cell lymphoma. Int. J. Clin. Exp. Pathol. 2009, 2, 163–168. [Google Scholar]
- Liu, P.; Shi, H.; Huang, C.; Shu, H.; Liu, R.; Yang, Y.; Gong, J.; Yang, Y.; Cai, M. Association of LEP A19G polymorphism with cancer risk: A systematic review and pooled analysis. Tumour Biol. 2014, 35, 8133–8141. [Google Scholar] [CrossRef]
- Liu, P.; Shi, H.; Liu, R.; Yang, Y.; Yang, Y.; Huang, C.; Shu, H.; Gong, J.; Cai, M. Lack of association between LEPR Q223R polymorphisms and cancer susceptibility: Evidence from a meta-analysis. J. BUON 2014, 19, 855–862. [Google Scholar]
- Yang, J.; Zhong, Z.; Tang, W.; Chen, J. Leptin rs2167270 G > A (G19A) polymorphism may decrease the risk of cancer: A case-control study and meta-analysis involving 19 989 subjects. J. Cell. Biochem. 2019, 120, 10998–11007. [Google Scholar] [CrossRef] [PubMed]
- Skibola, D.R.; Smith, M.T.; Bracci, P.M.; Hubbard, A.E.; Agana, L.; Chi, S.; Holly, E.A. Polymorphisms in ghrelin and neuropeptide Y genes are associated with non-Hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1251–1256. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Lopez, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef]
- Wang, Q.; Bing, C.; Al-Barazanji, K.; Mossakowaska, D.E.; Wang, X.M.; McBay, D.L.; Neville, W.A.; Taddayon, M.; Pickavance, L.; Dryden, S.; et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes 1997, 46, 335–341. [Google Scholar] [CrossRef]
- Goto, M.; Arima, H.; Watanabe, M.; Hayashi, M.; Banno, R.; Sato, I.; Nagasaki, H.; Oiso, Y. Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology 2006, 147, 5102–5109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.J.; Xu, H.Z.; Li, J.S.; Geng, L.Y.; Li, X.Y.; Zhou, X.X.; Wang, X. Leptin and its receptor in glucose metabolism of T-cell lymphoma. Oncol. Lett. 2018, 16, 5838–5846. [Google Scholar] [CrossRef]
- Bertolini, F.; Paolucci, M.; Peccatori, F.; Cinieri, S.; Agazzi, A.; Ferrucci, P.F.; Cocorocchio, E.; Goldhirsch, A.; Martinelli, G. Angiogenic growth factors and endostatin in non-Hodgkin’s lymphoma. Br. J. Haematol. 1999, 106, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, G.E.; Demir, M.; Harmandar, F.; Yesil, Y.; Turgut, B.; Vural, O. Leptin and resistin levels in serum of patients with hematologic malignancies: Correlation with clinical characteristics. Exp. Oncol. 2006, 28, 241–244. [Google Scholar]
- Petridou, E.T.; Sergentanis, T.N.; Dessypris, N.; Vlachantoni, I.T.; Tseleni-Balafouta, S.; Pourtsidis, A.; Moschovi, M.; Polychronopoulou, S.; Athanasiadou-Piperopoulou, F.; Kalmanti, M.; et al. Serum adiponectin as a predictor of childhood non-Hodgkin’s lymphoma: A nationwide case-control study. J. Clin. Oncol. 2009, 27, 5049–5055. [Google Scholar] [CrossRef] [Green Version]
- Petridou, E.T.; Dessypris, N.; Panagopoulou, P.; Sergentanis, T.N.; Mentis, A.F.; Pourtsidis, A.; Polychronopoulou, S.; Kalmanti, M.; Athanasiadou-Piperopoulou, F.; Moschovi, M. Adipocytokines in relation to Hodgkin lymphoma in children. Pediatr. Blood Cancer 2010, 54, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Okur, F.V.; Karadeniz, C.; Buyukpamukcu, M.; Oguz, A.; Yucel, A.; Cinaz, P.; Emir, S.; Varan, A. Clinical significance of serum vascular endothelial growth factor, endostatin, and leptin levels in children with lymphoma. Pediatr. Blood Cancer 2010, 55, 1272–1277. [Google Scholar] [CrossRef]
- Head, G.A. The oestrogen-leptin paradox. J. Physiol. 2015, 593, 1523. [Google Scholar] [CrossRef]
- Ren, J. Lessons from the leptin paradox in cardiac regulation--too much versus too little. J. Physiol. 2005, 565, 347. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Yaris, N.; Sozen, E.; Erduran, E.; Okten, A.; Orem, A.; Cakirbay, H. Bone mineral metabolism and its relationship to leptin levels in survivors of childhood leukemia and lymphoma. Pediatr. Hematol. Oncol. 2005, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Park, C.S.; Yoon, D.H.; Suh, C.; Huh, J. High concordance of gene expression profiling-correlated immunohistochemistry algorithms in diffuse large B-cell lymphoma, not otherwise specified. Am. J. Surg. Pathol. 2014, 38, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.; Tesson, B.; Mareschal, S.; Viailly, P.J.; Bohers, E.; Ruminy, P.; Etancelin, P.; Peyrouze, P.; Copie-Bergman, C.; Fabiani, B.; et al. Refining diffuse large B-cell lymphoma subgroups using integrated analysis of molecular profiles. EBioMedicine 2019, 48, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahwa, P.; Karunanayake, C.P.; Spinelli, J.J.; Dosman, J.A.; McDuffie, H.H. Ethnicity and incidence of Hodgkin lymphoma in Canadian population. BMC Cancer 2009, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, B.; Zimmermann, M.; Oschlies, I.; Niggli, F.; Mann, G.; Parwaresch, R.; Riehm, H.; Schrappe, M.; Reiter, A.; the BFM Group. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br. J. Haematol. 2005, 131, 39–49. [Google Scholar] [CrossRef]
- Wilson, L.D.; Hinds, G.A.; Yu, J.B. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin. Lymphoma Myeloma Leuk. 2012, 12, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chang, E.T.; Bassig, B.A.; Dai, M.; Qin, Q.; Gao, Y.; Zhang, Y.; Zheng, T. Body size and risk of Hodgkin’s lymphoma by age and gender: A population-based case-control study in Connecticut and Massachusetts. Cancer Causes Control 2013, 24, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Blansky, D.; Mantzaris, I.; Rohan, T.; Hosgood, H.D., 3rd. Influence of Rurality, Race, and Ethnicity on Non-Hodgkin Lymphoma Incidence. Clin. Lymphoma Myeloma Leuk. 2020, 20, 668–676.e665. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.; Perry, C.M. Metreleptin: First global approval. Drugs 2013, 73, 989–997. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, N.X.S.; Adiyaman, S.C.; Yuksel, B.D.; Ferrari, C.T.; Eldin, A.J.; Saydam, B.O.; Altay, C.; Sharma, P.; Bhave, N.; Little, A.; et al. Unusual Presentations of Lmna-Associated Lipodystrophy with Complex Phenotypes and Generalized Fat Loss: When the Genetic Diagnosis Uncovers Novel Features. AACE Clin. Case Rep. 2020, 6, e79–e85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.J.; Chan, J.L.; Jaffe, E.S.; Cochran, E.; DePaoli, A.M.; Gautier, J.F.; Goujard, C.; Vigouroux, C.; Gorden, P. Lymphoma in acquired generalized lipodystrophy. Leuk. Lymphoma 2016, 57, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esfandiari, N.H.; Rubenfire, M.; Neidert, A.H.; Hench, R.; Eldin, A.J.; Meral, R.; Oral, E.A. Diagnosis of acquired generalized lipodystrophy in a single patient with T-cell lymphoma and no exposure to Metreleptin. Clin. Diabetes Endocrinol. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]

| Reference | Type of Study | Leptin/LEPR Genes | Conclusions |
|---|---|---|---|
| [71] | Case-control | LEP 19AG, LEP 2548GA, LEP 2548AA, and LEPR Q223R | Polymorphism in LEP 19AG increased the risk of DLBCL and FL. Genetic interactions in LEPR 223RR, LEP 2548GA, or LEP 2548AA genes also increased the risk of NHL. |
| [80]. | Case-control | LEP 2548GA, LEP 2548AA, LEP 19AA, and LEPR 223Q>R | Obesity was associated with risk of NHL, especially DLBCL. The risk of NHL was increased by LEP 2548GA and LEP 2548AA genes and decreased by LEP 19AA, particularly in men younger than in 45 years olds with FL. Conversely, no associations were found between lymphoma risk and LEPR 223Q>R. |
| [204] | Case-control | LEP 2548GA | Cutaneous T-cell lymphoma patients with leptin genes involving AG or GG genotypes may respond better to topical steroids and phototherapy. |
| [202] | Meta-analysis | LEP 2548GA | Gene polymorphism may increase the risk of NHL, particularly in the homozygote co-dominant model and the additive genetic model of Caucasian populations. |
| [205] | Meta-analysis | LEP 19AG | Gene polymorphism was associated with lower NHL risk under the homozygous codominant model, recessive genetic model (especially among the Latin American population), and additive genetic model. |
| [206] | Meta-analysis | LEPR Q223R | Gene polymorphism did not affect the risk of NHL, although it may be significantly increased in Asian and African individuals. |
| [203] | Meta-analysis | LEP 2548GA, and LEP 19AG | LEP 2548GA polymorphism increases NHL susceptibility and LEP 19AG is associated with a decreased risk of NHL, especially FL. |
| [207] | Meta-analysis | LEP 19AG | LEP 19AG may decrease the risk of NHL, especially in Asians, Caucasians, and mixed populations. |
| [170] | Case-control | LEPR rs1327118G>C, and LEP rs2167270G>A (LEP 19AG) | LEP rs2167270 G>A polymorphism was associated with the decreased risk of DLBCL in the recessive mode models among the Jordanian Arab population. |
| Reference | Type of Study | Leptin/LEPR Levels or Expression | Conclusions |
|---|---|---|---|
| [213] | Case study | All patients: 23 (0–310) pg/mL. CR patients: 25 (0–310) pg/mL. PD patients: 21.5 (0–140) pg/mL. | Leptin levels were similar regardless of the response to treatment. |
| [168] | Case-control | Article not available * | After treatment, BMI, body weight and body fat mass decreased significantly. Also, low leptin levels were found before and after treatment compared with controls. |
| [214] | Case-control | Lymphoma patients: 16.4 ± 10.4 ng/mL. Controls: 10.3 ± 7.6 ng/mL. | Leptin levels were positively correlated with BMI but were not high in lymphoma patients at diagnosis. |
| [215] | Case-control | Patients: 6.0 ± 6.31 ng/mL. Controls: 5.9 ± 7.3 ng/mL. | There was no association between leptin levels and NHL in children. |
| [216] | Case-control | Patients: 8.2 ± 7.26 ng/mL. Controls: 7.5 ± 8.3 ng/mL. | There was no association between leptin levels and HL in children. |
| [200] | Case study | High LEPR expression in 39.8% of DLBCL patients | LEPR overexpression could be associated with DLBCL carcinogenesis via PI3K/AKT pathway. Also, leptin/LEPR signaling promoted the proliferation of DLBCL cells in vitro. |
| [217] | Case-control | Pre-treatment: 5.3 ± 1.56 ng/mL. Post-treatment: 9.8 ± 2.7 ng/mL. Controls: 6.7 ± 1.2 ng/mL. | Leptin levels were significantly lower in patients than in controls and increased in patients who achieved remission. |
| [169] | Case-control | Patients: 8.5 (3.8–17.1) ng/mL. Controls: 10.6 (5.2–21.8) ng/mL. | Serum leptin levels were significantly associated with NHL risk at diagnosis, but predicted a lower risk of FL. |
| [201] | Case-control | High LEPR expression in 45% of DLBCL patients | LEPR may promote JAK/STAT and PI3K/AKT signaling pathways and induce the phosphorylation of STAT3 and AKT, which may be involved in the prognosis of DLBCL. |
| [212] | Cases | Higher LEPR expression in tissues of T-cell lymphoma patients (58.3%) and in all cell lines, especially in MOLT-3 and Jurkat cell lines | LEPR overexpression was positively correlated with Glut1 expression. TCL MOLT-3 cell line demonstrated that leptin stimulated cell glucose uptake via promoting the recruitment and expression of Glut1. |
| [172] | Case-control | Patients: 4182.30 ± 246.95 pg/mL. Controls: 4782.00 ± 193.65 pg/mL | Leptin levels were significantly higher in women than in men and in obese patients compared with their non-obese counterparts, which increased the risk of NHL. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Cortegana, C.; Hontecillas-Prieto, L.; García-Domínguez, D.J.; Zapata, F.; Palazón-Carrión, N.; Sánchez-León, M.L.; Tami, M.; Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; et al. Obesity and Risk for Lymphoma: Possible Role of Leptin. Int. J. Mol. Sci. 2022, 23, 15530. https://doi.org/10.3390/ijms232415530
Jiménez-Cortegana C, Hontecillas-Prieto L, García-Domínguez DJ, Zapata F, Palazón-Carrión N, Sánchez-León ML, Tami M, Pérez-Pérez A, Sánchez-Jiménez F, Vilariño-García T, et al. Obesity and Risk for Lymphoma: Possible Role of Leptin. International Journal of Molecular Sciences. 2022; 23(24):15530. https://doi.org/10.3390/ijms232415530
Chicago/Turabian StyleJiménez-Cortegana, Carlos, Lourdes Hontecillas-Prieto, Daniel J. García-Domínguez, Fernando Zapata, Natalia Palazón-Carrión, María L. Sánchez-León, Malika Tami, Antonio Pérez-Pérez, Flora Sánchez-Jiménez, Teresa Vilariño-García, and et al. 2022. "Obesity and Risk for Lymphoma: Possible Role of Leptin" International Journal of Molecular Sciences 23, no. 24: 15530. https://doi.org/10.3390/ijms232415530
APA StyleJiménez-Cortegana, C., Hontecillas-Prieto, L., García-Domínguez, D. J., Zapata, F., Palazón-Carrión, N., Sánchez-León, M. L., Tami, M., Pérez-Pérez, A., Sánchez-Jiménez, F., Vilariño-García, T., de la Cruz-Merino, L., & Sánchez-Margalet, V. (2022). Obesity and Risk for Lymphoma: Possible Role of Leptin. International Journal of Molecular Sciences, 23(24), 15530. https://doi.org/10.3390/ijms232415530






