Association between Tumor Microbiome and Hypoxia across Anatomic Subsites of Head and Neck Cancers
Abstract
:1. Introduction
2. Results
2.1. Hypoxia Scores
2.2. Microbiome of the HNSCC According to Anatomic Subsites and Relationship with Hypoxia Scores
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primer 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Göttgens, E.L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, hypoxia and radiation response in head and neck cancer. Br. J. Radiol. 2019, 92, 20180047. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Nordsmark, M.; Overgaard, J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004, 43, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Cullin, N.; Antunes, C.A.; Straussman, R.; Stein-Thoeringer, C.K.; Elinav, E. Microbiome and cancer. Cancer Cell 2021, 39, 1317–1341. [Google Scholar] [CrossRef]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New Insights into the Cancer–Microbiome–Immune Axis: Decrypting a Decade of Discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef]
- Malla, R.R.; Marni, R.; Kumari, S.; Chakraborty, A.; Lalitha, P. Microbiome Assisted Tumor Microenvironment: Emerging Target of Breast Cancer. Clin. Breast Cancer 2022, 22, 200–211. [Google Scholar] [CrossRef]
- Wang, L.; Ganly, I. The oral microbiome and oral cancer. Clin. Lab. Med. 2014, 34, 711–719. [Google Scholar] [CrossRef]
- De Marco, F. Oxidative stress and HPV carcinogenesis. Viruses 2013, 5, 708–731. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Cobucci, R.N.O.; Jatobá, C.A.N.; de Medeiros Fernandes, T.A.A.; de Azevedo, J.W.V.; de Araújo, J.M.G. The role of the mediators of inflammation in cancer development. Pathol. Oncol. Res. POR 2015, 21, 527–534. [Google Scholar] [CrossRef]
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; MacIntyre, D.A.; Paraskevaidi, M.; Moscicki, A.-B.; Mahajan, V.; Smith, A.; Lee, Y.S.; Lyons, D.; Paraskevaidis, E.; Marchesi, J.R.; et al. The vaginal microbiota and innate immunity after local excisional treatment for cervical intraepithelial neoplasia. Genome Med. 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Moscicki, A.B. Vaginal microbiome and cervical cancer. Semin. Cancer Biol. 2022, 86 Pt 3, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Funchain, P.; Bebek, G.; Altemus, J.; Zhang, H.; Niazi, F.; Peterson, C.; Lee, W.T.; Burkey, B.B.; Eng, C. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef] [Green Version]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef]
- Belgioia, L.; Morbelli, S.D.; Corvò, R. Prediction of Response in Head and Neck Tumor: Focus on Main Hot Topics in Research. Front. Oncol. 2021, 10, 604965. Available online: https://www.frontiersin.org/article/10.3389/fonc.2020.604965 (accessed on 6 June 2022). [CrossRef]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Buffa, F.M.; Harris, A.L.; West, C.M.; Miller, C.J. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br. J. Cancer 2010, 102, 428–435. [Google Scholar] [CrossRef]
- Hoyd, R.; Wheeler, C.; Liu, Y.; Singh, M.S.J.; Muniak, M.; Denko, N.; Carbone, D.P.; Xiaokui, M.; Spakowicz, D. Exogenous sequences in tumors and immune cells (exotic): A tool for estimating the microbe abundances in tumor RNAseq data. BioRxiv 2022. [Google Scholar] [CrossRef]
- Hoyd, R.; Wheeler, C.; Spakowicz, D. Spakowiczlab/Tmesig, Initial Release (Version 0.1); Tumor MicroEnvironment Expression Signatures; Zenedo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 19 September 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
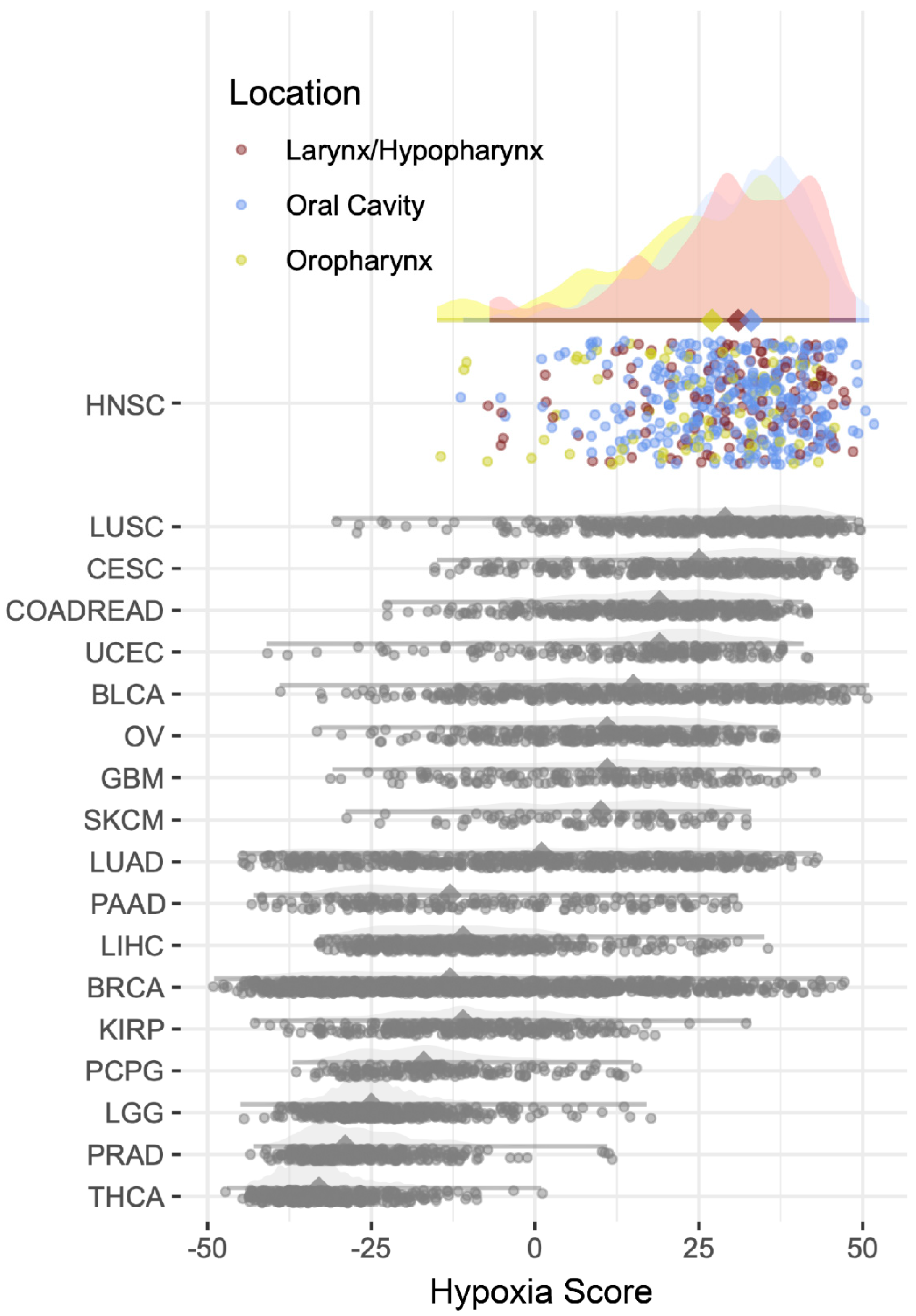

| Oral Cavity | Oropharynx | Larynx/ Hypopharynx | p | |
|---|---|---|---|---|
| Total “n” | 226 | 53 | 78 | |
| Age (mean (SD)) | 61.25 (13.27) | 58.00 (11.80) | 63.11 (9.34) | 0.067 |
| Sex = male (%) | 153 (67.7) | 44 (83.0) | 61 (78.2) | 0.034 |
| Hypoxia Score (mean (SD)) | 30.18(11.10) | 24.31 (14.13) | 29.53 (12.61) | 0.004 |
| HPV positive (%) | 29 (12.8) | 38 (71.7) | 8 (10.3) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, A.; Upadhyay, R.; Wheeler, C.; Hoyd, R.; Karivedu, V.; Gamez, M.E.; Valentin, S.; Vanputten, M.; Bhateja, P.; Bonomi, M.; et al. Association between Tumor Microbiome and Hypoxia across Anatomic Subsites of Head and Neck Cancers. Int. J. Mol. Sci. 2022, 23, 15531. https://doi.org/10.3390/ijms232415531
Dhakal A, Upadhyay R, Wheeler C, Hoyd R, Karivedu V, Gamez ME, Valentin S, Vanputten M, Bhateja P, Bonomi M, et al. Association between Tumor Microbiome and Hypoxia across Anatomic Subsites of Head and Neck Cancers. International Journal of Molecular Sciences. 2022; 23(24):15531. https://doi.org/10.3390/ijms232415531
Chicago/Turabian StyleDhakal, Aastha, Rituraj Upadhyay, Caroline Wheeler, Rebecca Hoyd, Vidhya Karivedu, Mauricio E. Gamez, Sasha Valentin, Meade Vanputten, Priyanka Bhateja, Marcelo Bonomi, and et al. 2022. "Association between Tumor Microbiome and Hypoxia across Anatomic Subsites of Head and Neck Cancers" International Journal of Molecular Sciences 23, no. 24: 15531. https://doi.org/10.3390/ijms232415531
APA StyleDhakal, A., Upadhyay, R., Wheeler, C., Hoyd, R., Karivedu, V., Gamez, M. E., Valentin, S., Vanputten, M., Bhateja, P., Bonomi, M., Konieczkowski, D. J., Baliga, S., Mitchell, D. L., Grecula, J. C., Blakaj, D. M., Denko, N. C., Jhawar, S. R., & Spakowicz, D. (2022). Association between Tumor Microbiome and Hypoxia across Anatomic Subsites of Head and Neck Cancers. International Journal of Molecular Sciences, 23(24), 15531. https://doi.org/10.3390/ijms232415531






