Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses
Abstract
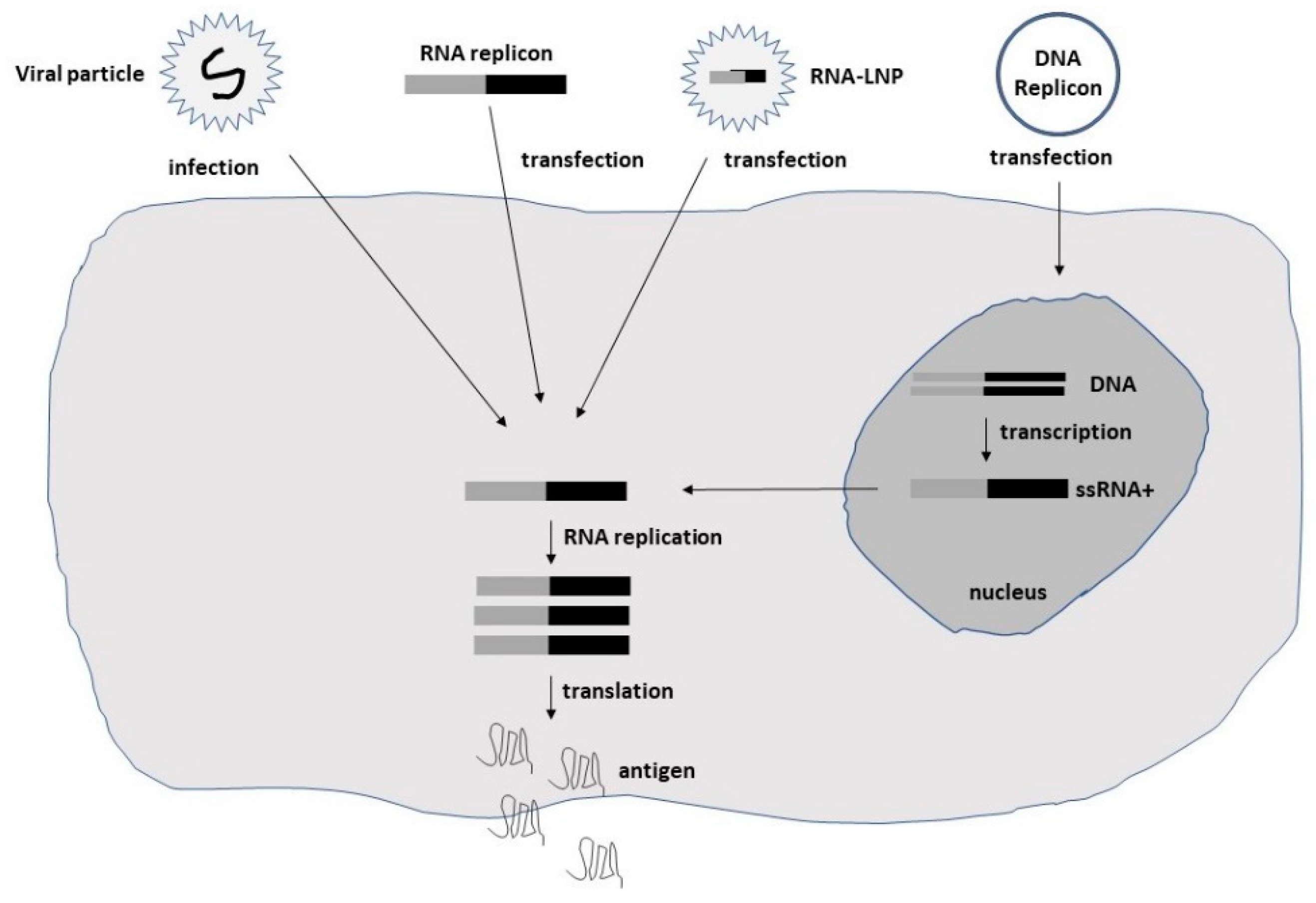
:1. Introduction
2. Characterization of Oncolytic Self-Replicating RNA Viruses
3. Preclinical Studies Using Oncolytic Self-Replicating RNA Viruses
4. Clinical Trials Using Oncolytic Self-Replicating RNA Viruses
5. Conclusions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Cancers. CA Cancer J. Clin. 2017, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.S.; Snook, A.E.; Marszalowicz, G.P.; Waldman, S.A. Immunotherapeutic Strategies to Target Prognostic and Predictive Markers of Cancer. Biomark. Med. 2013, 7, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundstrom, K.; Boulikas, T. Viral and Non-viral Vectors in Gene Therapy: Technology Development and Clinical Trials. Technol. Cancer Res. Treat. 2003, 2, 471–485. [Google Scholar] [CrossRef] [Green Version]
- Fortner, R.T.; Damms-Machado, A.; Kaaks, R. Systematic Review: Tumor-Associated Antigen Autoantibodies and Ovarian Cancer Early Detection. Gynecol. Oncol. 2017, 147, 465–480. [Google Scholar] [CrossRef]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody Therapy of Cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Liang, J.; Li, K.; Zhu, W.; Fu, L.; Wang, F.; Zheng, X.; Shi, H.; Wu, S.; et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl. Acad. Sci. USA 2014, 111, E4504–E4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, E.; Nemunaitis, J. Oncolytic viral therapies. Cancer Gene Ther. 2004, 11, 643–664. [Google Scholar]
- Young, B.A.; Spencer, J.F.; Ying, B.; Tollefson, A.E.; Toth, K.; Wold, W.S. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther. 2013, 20, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Li, J.M.; Kao, K.C.; Li., L.F.; Yang, T.M.; Wu, C.P.; Horng, Y.M.; Russelli, A. MicroRNA-145 regulates oncolytic herpes simplex virus-1 for selective killing of human non-small lung cancer cells. Virol. J. 2013, 10, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.M.; Besmer, D.M.; Moerdyk-Schauwecker, M.; Moestl, N.; Ornelles, D.A.; Mukherjee, P.; Grdzelishvili, V.Z. Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J. Virol. 2012, 86, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, H. Newcastle disease virus: A promising agent for tumor immunotherapy. Clin. Exp. Pharmacol. Physiol. 2012, 39, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, K.; Kilinc, M.O.; Chen, N.G.; Stritzker, J.; Buckel, L.; Zhang, Q.; A Szalay, A. Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68. J. Transl. Med. 2013, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francipane, M.G.; Douradinha, P.; Chinnici, C.M.; Russelli, G.; Conaldi, P.G.; Iannolo, G. Zika Virus: A New Therapeutic Candidate for Glioblastoma Treatment. Int. J. Mol. Sci. 2021, 22, 10996. [Google Scholar] [CrossRef]
- Pidelaserra-Marti, G.; Engeland, C.E. Mechanisms of oncolytic measles virus immunotherapy. Cytokine Growth Factor Rev. 2020, 56, 28–38. [Google Scholar] [CrossRef]
- Cripe, T.P.; Wang, P.-Y.; Marcato, P.; Mahler, Y.Y.; Lee, P.W. Targeting cancer-initiating cells with oncolytic viruses. Mol. Ther. 2009, 17, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Kirn, D. Gene therapy progress and prospects cancer: Oncolytic viruses. Gene Ther. 2008, 15, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Strong, J.E.; Coffrey, M.C.; Tang, D.; Sabinin, P.; Lee, P.W. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998, 17, 3351–3362. [Google Scholar] [CrossRef] [Green Version]
- Strauss, J.H.; Strauss, E.G. The Alphaviruses: Gene Expression, Replication, and Evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Pijlman, G.P.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicons: An RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Exp Opin Biol Ther. 2006, 6, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, O.; Errington-Mais, F.; Steele, L.; Hadac, E.; Jennings, V.; Scott, K.; Peach, H.; Phillips, R.M.; Bond, J.; Pandha, H.; et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013, 20, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Callaway, E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 2013, 8, 1583–1601. [Google Scholar] [CrossRef] [Green Version]
- Muhuri, M.; Gao, G. Oncolytic Alphavirus M1: A New and Promising Weapon to Fight Cancer. Human Gene Ther. 2021, 32, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yan, G. The Identification and Development of a Novel Oncolytic Virus: Alphavirus M1. Human Gene Ther. 2021, 32, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Unno, Y.; Shino, Y.; Kondo, F.; Igarashi, N.; Wang, G.; Shimura, R.; Yamaguchi, T.; Asano, T.; Saisho, H.; Sekiya, S.; et al. Oncolytic Viral Therapy for Cervical and Ovarian Cancer Cells by Sindbis Virus AR339 Strain. Clin. Cancer Res. 2005, 11, 4553–4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Määttä, A.M.; Mäkinen, K.; Ketola, A.; Liimatainen, T.; Yongabi, F.N.; Vähä-Koskela, M. Replication Competent Semliki Forest Virus Prolongs Survival in Experimental Lung Cancer. Int. J. Cancer 2008, 123, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Toribio, R.; Diaz-Lopez, I.; Berlanga, J.J.; Molina-Jimenez, F.; Majano, P.; Ventoso, I. Naturally Occurring and Engineered Alphaviruses Sensitive to Double-Stranded-RNA-Activated Protein Kinase Show Restricted Translation in Mammalian Cells, Increased Sensitivity to Interferon, and Marked Oncotropism. J. Virol. 2020, 94, e01630-19. [Google Scholar] [CrossRef]
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017, 214, 2843–2857. [Google Scholar] [CrossRef] [Green Version]
- Urbiola, C.; Santer, F.R.; Petersson, M.; Van Der Pluijm, G.; Horninger, W.; Erlmann, P.; Wollmann, G.; Kimpel, J.; Culig, Z.; Von Laer, D. Oncolytic activity of the rhabdovirus VSV-GP against prostate cancer. Int. J. Cancer 2018, 143, 1786–1796. [Google Scholar] [CrossRef]
- Le Boeuf, F.; Selman, M.; Son, H.H.; Bergeron, A.; Chen, A.; Tsang, J.; Butterwick, D.; Arulanandam, R.; Forbes, N.E.; Tzelepis, F.; et al. Oncolytic Maraba Virus MG1 as a Treatment for Sarcoma. Int. J. Cancer 2017, 141, 1257–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkilä, J.E.; Vähä-Koskela, M.; Ruotsalainen, J.; Martikainen, M.W.; Stanford, M.M.; McCart, J.A.; Bell, J.C.; Hinkkanen, A.E. Intravenously Administered Alphavirus Vector VA7 Eradicates Orthotopic Human Glioma Xenografts in Nude Mice. PLoS ONE 2010, 5, e8603. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, M.; Ruotsalainen, J.; Tuomela, J.; Härkönen, P.; Essand, M.; Heikkilä, J. Oncolytic alphavirus SFV-A7 efficiently eradicates subcutaneous and orthotopic human prostate tumours in mice. Br. J. Cancer 2017, 117, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martikainen, M.; Ramachandran, M.; Lugano, R.; Ma, J.; Martikainen, M.-M.; Dimberg, A.; Yu, D.; Merits, A.; Essand, M. IFN-1-tolerant oncolytic Semliki Forest virus in combination with anti-PD1 enhances T cell response against mouse glioma. Mol. Ther. Oncolytics 2021, 21, 37–46. [Google Scholar] [CrossRef]
- Ramachandran, M.; Yu, D.; Dyczynski, M.; Baskaran, S.; Zhang, L.; Lulla, A.; Shimura, E. Safe and Effective Treatment of Experimental Neuroblastoma and Glioblastoma Using Systematically Delivered Triple MicroRNA-Detargeted Oncolytic Semliki Forest Virus. Clin. Cancer Res. 2017, 23, 1519–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Liang, J.; Tan, J.; Guo, L.; Cai, J.; Hu, J.; Yan, G.; Liu, Y.; Zhang, M.J.; Song, D.; et al. Real-Time Visualization and Quantification of Oncolytic M1 Virus In Vitro and In Vivo. Hum. Gene Ther. 2021, 32, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, W.; Lin, Y.; Zhang, S.; Chen, M.X.; Gong, S.; He, S.; Hu, J.; Yan, G.; Liang, J. Systematic Characterization of the Biodistribution of the Oncolytic Virus M1. Hum. Gene Ther. 2020, 31, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, Y.; Lin, Y.; Liang, J.-K.; Zhong, W.-W.; Li, K.; Huang, W.-T.; Wang, D.-J.; Yan, G.-M.; Zhu, W.-B.; et al. Intravenous injections of the oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. 2018, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, K.; Zhu, W.-B.; Zhang, H.; Huang, W.-T.; Liu, X.-C.; Lin, Y.; Cai, J.; Yan, G.-M.; Qiu, J.-G.; et al. Suppression of CCDC6 sensitizes tumor to oncolytic virus M1. Neoplasia 2021, 23, 158–168. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.-W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Liang, L.; et al. Necroptotic Virotherapy of Oncolytic Alphavirus M1 Cooperated with Doxorubicin Displays Promising Therapeutic Efficacy in TNBC. Oncogene 2021, 40, 4783–4795. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Y.; He, C.; Hu, W.; Liu, W.; Huang, X.; Wu, J.; Xie, F.; Chen, C.; Wang, J.; et al. Combining Nanoknife with M1 oncolytic virus enhances anticancer activity in pancreatic cancer. Cancer Lett. 2021, 502, 9–24. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Zou, H.; Tian, X.; Hu, J.; Qiu, P.; Ferreira, L. Liposome Encapsulation of Oncolytic Virus M1 To Reduce Immunogenicity and Immune Clearance in Vivo. Mol. Pharm. 2019, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Kaid, M. Zika Virus Targets Glioblastoma Stem Cells through a SOX-2 Integrin αvβ5 Axis. Cell Stem Cell 2020, 26, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Mazzoccoli, L.; Jash, A.; Govero, J.; Bais, S.S.; Hu, T.; Fontes-Garfias, C.R.; Shan, C.; Okada, H.; Shresta, S.; et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight 2021, 6, e144619. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.O.; Granha, I.; Ferreira, R.S.; Bueno, H.D.S.; Okamoto, O.K.; Kaid, C.; Zatz, M. Effect of Serial Systemic and Intratumoral Injections of Oncolytic ZIKVBR in Mice Bearing Embryonal CNS Tumors. Viruses 2021, 13, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kaid, C.; Madi, R.A.D.S.; Astray, R.; Goulart, E.; Caires-Junior, L.C.; Mitsugi, T.G.; Moreno, A.C.R.; Castro-Amarante, M.F.; Pereira, L.R.; Porchia, B.F.M.M.; et al. Safety, Tumor Redaction, and Clinical Impact of Zika Virus Injection in Dogs with Advanced-Stage Brain Tumors. Mol. Ther. 2020, 28, 1276–1286. [Google Scholar] [CrossRef] [Green Version]
- Delafiori, J.; Lima, E.D.O.; Dabaja, M.Z.; Dias-Audibert, F.L.; de Oliveira, D.N.; Melo, C.F.O.R.; Morishita, K.N.; Sales, G.M.; Ruiz, A.L.T.G.; Da Silva, G.G.; et al. Molecular signatures associated with prostate cancer cell line (PC-3) exposure to inactivated Zika virus. Sci. Rep. 2019, 9, 15351. [Google Scholar] [PubMed] [Green Version]
- Lal, S.; Carrera, D.; Phillips, J.J.; Weiss, W.A.; Raffel, C. An oncolytic measles virus sensitive Group 3 medulloblastoma model in immune-competent mice. Neuro Oncol. 2018, 20, 1606–1615. [Google Scholar] [CrossRef] [Green Version]
- Studebaker, A.W.; Kreofsky, C.R.; Pierson, C.R.; Russell, S.J.; Glanis, E.; Raffel, C. Treatment of medulloblastoma with a modified measles virus. Neuro Oncol. 2010, 12, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo-Musibay, E.; Federspiel, M.J.; Galanis, E. Oncolytic measles virus strains have a significant antitumor activity against glioma stem cells. Gene Ther. 2013, 2, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Yoneda, M.; Kuraishi, T.; Hattori, S.; Inoue, Y.; Sato, H.; Kai, C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013, 20, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, S.A.; Al-Shammari, A.M.; Lateef, S.A. Attenuated measles vaccine strain have potent oncolytic activity against Iraqi patient derived breast cancer cell line. Saudi J. Biol. Sci. 2020, 27, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shi, J.; Sun, Z.; Zhu, D.; Xu, X. Attenuated measles virus overcomes radio and chemoresistance in human breast cancer cells by inhibiting the nonhomologous end joining pathway. Oncol. Rep. 2020, 44, 2253–2264. [Google Scholar]
- Zhao, D.; Chen, P.; Yang, H.; Wu, Y.; Zeng, X.; Zhao, Y.; Wen, Y.; Zhao, X.; Liu, X.; Wei, Y.; et al. Live attenuated measles virus vaccine induces apoptosis and promotes tumor regression in lung cancer. Oncol. Rep. 2013, 29, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisgerault, N.; Guillerme, J.-B.; Pouliquen, D.; Mesel-Lemoine, M.; Achard, C.; Combredet, C.; Fonteneau, J.-F.; Tangy, F.; Grégoire, M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed. Res. Int. 2013, 2013, 387362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.R.; Jacobson, B.A.; Belgum, H.; Raza, A.; Sadiq, A.; Drees, J.; Wang, H.; Jay-Dixon, J.; Etchison, R.; Federspiel, M.J.; et al. Measles vaccine strains for virotherapy of non-small cell lung carcinoma. J. Thorac. Oncol. 2014, 9, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammour, Y.; Ryabaya, O.; Shchetinina, Y.; Prokofeva, E.; Gavrilova, M.; Khochenkov, D.; Vorobyev, D.; Faizuloev, E.; Shohin, I.; Zverev, V.V.; et al. The susceptibility of human melanoma cells to infection with the Leningrad-16 vaccine strain of measles virus. Viruses 2020, 12, 173. [Google Scholar] [CrossRef] [Green Version]
- Awano, M.; Fujiyuki, T.; Shoji, K.; Amagai, Y.; Murakami, Y.; Furukawa, Y.; Sato, H.; Yoneda, M.; Kai, C. Measles virus selectively blind to signaling lymphocyte activity molecule has oncolytic efficacy against nectin-4 expressing pancreatic cells. Cancer Sci. 2016, 107, 1647–1652. [Google Scholar] [CrossRef] [Green Version]
- May, V.; Berchtold, S.; Berger, A.; Venturelli, S.; Burkard, M.; Leischner, C.; Malek, N.P.; Lauer, U.M. Chemovirotherapy for pancreatic cancer: Gemcitabine plus oncolytic measles vaccine virus. Oncol. Lett. 2019, 18, 5534–5542. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.M.; Leber, M.F.; Bossow, S.; Engeland, C.E.; Dessila, J.; Grossardt, C. MicroRNA–sensitive oncolytic measles virus for chemovirotherapy of pancreatic cancer. Mol. Ther. Oncolytics 2021, 21, 340–355. [Google Scholar] [CrossRef]
- Msaouel, P.; Iankov, I.D.; Allen, C.; Morris, J.C.; von Messling, V.; Cattaneo, R. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009, 69, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hasegawa, K.; Russell, S.J.; Sadelain, M.; Peng, K.-W. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate 2009, 69, 1128–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.A.; Zhang, L.; Cuong, B.K.; Van Tong, H.; Cuong, L.D.; Hang, N.T.; Wu, J. Combination of vaccine-strain measles and mumps viruses enhances oncolytic activity against human solid malignancies. Cancer Investig. 2018, 7, 106–117. [Google Scholar] [CrossRef]
- Ozduman, K.; Wollmann, G.; Piepmeier, J.M.; van den Pol, A.N. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J. Neurosci. 2008, 28, 1882–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollmann, G.; Rogulin, V.; Simon, I.; Rose, J.K.; van den Pol, A.N. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J. Virol. 2010, 84, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Ebert, O.; Harbaran, S.; Shinozaki, K.; Woo, S.L.C. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immunocompetent mice. Cancer Gene Ther. 2005, 12, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Day, G.L.; Bryan, M.L.; Northrup, S.A.; Lyles, D.S.; Westcott, M.M.; Stewart, J.H., 4th. Immune effects of M51R vesicular stomatitis virus treatment of carcinomatosis from colon cancer. J. Surg. Res. 2020, 245, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.-M.; Urbiola, C.; Das, K.; Spiesschaert, B.; Kimpel, J.; Heinemann, F.; Stierstorfer, B.; Müller, P.; Petersson, M.; Erlmann, P.; et al. The lytic activity of VSV.GP treatment dominates the therapeutic effects in a syngeneic model of lung cancer. Br. J. Cancer 2019, 121, 647–658. [Google Scholar] [CrossRef]
- Kimpel, J.; Urbiola, C.; Koske, I.; Tober, R.; Banki, Z.; Wollmann, G. The Oncolytic virus VSV-GP is effective against malignant melanoma. Viruses 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Yang, Y.; Kang, T.; Zhao, W.; Cheng, H.; Wu, Y.; Du, T.; Liu, B.; Li, Y.; Luo, F.; et al. Ovarian cancer therapy by VSVMP gene mediated by a paclitaxel-enhanced nanoparticle. ACS Appl. Mater. Interfaces 2017, 9, 39152–39164. [Google Scholar] [CrossRef]
- Galivo, F.; Diaz, R.M.; Wongthida, P.; Thompson, J.; Kottke, T.; Barber, G.; Melcher, A.; Vile, R. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010, 17, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Chen, X.; Wei, Y.; Zhao, J.; Fan, L.; Wen, Y.; Wu, H.; Zhao, X. Efficient inhibition of intraperitoneal human ovarian cancer growth and prolonged survival by gene transfer of vesicular stomatitis virus matrix protein in nude mice. Gynecol. Oncol. 2007, 104, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Wen, Y.J.; Yang, H.S.; Luo, H.; Fu, A.F.; Yang, F.; Dowdyl, E. Efficient cisplatin-resistant human ovarian cancer growth and prolonged survival by gene transferred vesicular stomatitis virus matrix protein in nude mice. Ann. Oncol. 2008, 19, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Fehl, D.J.; Ahmed, M. Curcumin promotes the oncolytic capacity of vesicular stomatitis virus for the treatment of prostate cancers. Virus Res. 2017, 228, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.G.; Zhang, L.; Bridle, B.W.; Stephenson, K.B.; Rességuier, J.; Hanson, S.; Chen, L.; Kazdhan, N.; Bramson, J.; Stojdl, D.F.; et al. Maraba virus as a potent oncolytic vaccine vector. Mol. Ther. 2014, 22, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Tahara, M.; Takishima, Y.; Miyamoto, S.; Nakatsu, Y.; Someya, K.; Sato, M.; Tani, K.; Takeda, M. Photocontrollable mononegaviruses. Proc. Natl. Acad. Sci. USA 2019, 116, 11587–11589. [Google Scholar] [CrossRef] [Green Version]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Msaouel, P.; Dispenzieri, A.; Galanis, E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: An overview. Curr. Opin. Mol. Ther. 2009, 11, 43–53. [Google Scholar]
- Viral Therapy in Treating Patients with Recurrent Glioblastoma Multiforme. Available online: www.clinicaltrials.govNCT00390299 (accessed on 7 November 2022).
- Crosby, E.J.; Hobeika, A.C.; Niedzwiecki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Iii, W.R.G.; Hartman, Z.C.; et al. Long-term Survival of Patients with Stage III colon Cancer Treated with VRP-CEA(6D), an Alphavirus Vector that Increases the CD8+ Effector Memory T Cell to Treg Ratio. J. Immunother. Cancer 2020, 8, e001662. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, D.; Devi, G.R.; Burnett, B.K.; Clay, T.M.; et al. An Alphavirus Vector Overcomes the Presence of Neutralizing Antibodies and Elevated Numbers of Tregs to Induce Immune Responses in Humans with Advanced Cancer. J. Clin. Investig. 2010, 120, 3234–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzerling, L.; Künzi, V.; Oberholzer, P.A.; Kündig, T.; Naim, H.; Dummer, R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumors. Blood 2005, 106, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Galanis, E.; Atherton, P.J.; Maurer, M.J.; Knutson, K.L.; Dowdy, S.C.; Cliby, W.A.; Haluska, P.; Long, H.J.; Oberg, A.; Aderca, I.; et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015, 75, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Intrapleural Measles Virus Therapy in Patients with Malignant Pleural Mesothelioma. Available online: www.clinicaltrials.govNCT01503177 (accessed on 7 November 2022).
- Vaccine Therapy in Treating Patients with Malignant Peripheral Nerve Sheath Tumor That Is Recurrent of Cannot Be Removed by Surgery. Available online: www.clinicaltrials.govNCT02700230 (accessed on 7 November 2022).
- Viral Therapy in Treating Patients with Recurrent of Metastatic Squamous Cell Carcinoma of the Head and Neck Cancer or Metastatic Breast Cancer. Available online: www.clinicaltrials.govNCT01846091 (accessed on 7 November 2022).
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Nijman, H. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 2020, 34, 3310–3322. [Google Scholar] [CrossRef] [Green Version]
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Rushing, L. A Phase I Dose Escalation Trial of Vaccine Replicon Particles (VRP) Expressing Prostate-specific Membrane Antigen (PSMA) in Subjects with Prostate Cancer. Vaccine 2013, 31, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Rooney, C.M.; Dispenzieri, A.; Peng, K.-W.; Russell, S.J. Vaccine-induced memory CD8(+) T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clin. Cancer Res. 2019, 25, 2725–2736. [Google Scholar]
- Maxmen, A. Ebola vaccine approved for use in ongoing outbreak. Nature 2017. [Google Scholar] [CrossRef]
- Li, M.L.; Stollar, V. Alphaviruses and apoptosis. Int. Rev. Immunol. 2004, 23, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- De Mare, A.; Lambeck, A.J.A.; Regts, J.; van Dam, G.M.; Nijman, H.W.; Snippe, H.; Wilschut, J.; Daemen, T. Viral vector-based prime-boost immunization regimens: A possible involvement of T-cell competition. Gene Ther. 2008, 15, 393–403. [Google Scholar] [CrossRef]
| Cancer | Oncolytic Virus | Gene(s) | Findings | Ref. |
|---|---|---|---|---|
| Alphaviruses | ||||
| GBM | SFV VA7 | EGFP, Rluc | Tumor eradication, long-term survival in mice | [31] |
| Lung A459 | SFV-VA7 | EGFP | Prolonged survival in mice | [26] |
| Prostate LNCaP | SFV-VA7 | EGFP | Tumor cell killing, tumor eradication in mice | [32] |
| GBM | SFV-AM6-124T | miR124 | Targeting GL261 gliomas, enhanced by anti-PD1 | [33] |
| GBM | SFV4miRT | miR124,125,134 | Prolonged survival in mice | [34] |
| Cervical | SIN AR339 | SIN AR339 | Tumor cell killing, tumor regression in mice | [25] |
| Ovarian | SIN AR339 | SIN AR339 | Tumor cell killing, tumor regression in mice | [25] |
| Liver | M1 | GFP | Targeting of liver tumors in mice | [35] |
| Glioma | M1 | M1 | Killing of malignant glioma cells in mice, rats | [36] |
| Bladder MIBC | M1 | GFP | Tumor growth inhibition in mice | [37] |
| Bladder | M1 | M1 | Oncolytic activity in mouse bladder tumor model | [38] |
| Breast TNBC | M1 | M1 + Dox | Reduced tumor growth in mice | [39] |
| Pancreatic | M1 | M1 + IRE | Superior tumor inhibition, prolonged survival | [40] |
| Liver | M1 | M-LPO | Inhibition of Hep3B cancer cell growth in vitro | [41] |
| Colorectal | M1 | M-LPO | Inhibition of LoVo cancer cell growth in vitro | [41] |
| Flaviviruses | ||||
| GBM | ZIKV | m-ZIKV | Prolonged survival in mice | [28] |
| MB, ependymoma | ZIKV | ZIKV | Infection and killing of GSCs | [42] |
| GBM | ZIKV | ZIKV + anti-PD1 | Synergistic effect on survival in mice | [43] |
| Embryonal CNS | ZIKV | ZIKVBR | Eradication of brain tumors, no effect on normal cells | [44] |
| Spontaneous CNS | ZIKV | ZIKVBR | Tumor eradication, prolonged survival in dogs | [45] |
| Prostate | ZIKV | ZVp | Metabolomics to identify PC-3 cancer cell markers | [46] |
| Measles viruses | ||||
| Medulloblastoma | MV | GFP | Complete tumor regression in mice | [47] |
| Medulloblastoma | MV | GFP | Significantly prolonged survival in mice | [48] |
| Glioma | MV | CEA, NIS | Cytopathic effects in GSC cell lines | [49] |
| Breast | MV | SLAMblind | Anti-tumor activity in mice | [50] |
| Breast | MV | MV | Infection, killing of MCF-7 and CAL-51 cancer cells | [51] |
| Breast | MV | MV-Edm | Re-sensitization of Dox and ironicizing radiation | [52] |
| Lung | MV | MV Hu-191 | Suppression of tumor growth in mice | [53] |
| Lung. colorectal | MV | MV-Schwarz | Repression of tumor growth in mice | [54] |
| Lung | MV | CEA | Tumor growth inhibition in mice | [55] |
| Melanoma | MV | MV L-16 | Killing of tumor cells, tumor inhibition in mice | [56] |
| Pancreatic | MV | SLAMBlind | Inhibition of tumor growth in mice | [57] |
| Pancreatic | MV | MV-SCD + Gem | Reduced tumor mass in pancreatic cell lines | [58] |
| Pancreatic | MV | MV-miR-148 | Delayed tumor growth, prolonged survival in mice | [59] |
| Prostate | MV | CEA | Delayed tumor growth, prolonged survival in mice | [60] |
| Prostate | MV | sc-Fv-PSMA | Killing of prostate cancer cells | [61] |
| Prostate | MV | MV + MuV | Superior anti-tumor activity, survival in mice | [62] |
| Rhabdoviruses | ||||
| Glioma, breast | VSV | VSVrp30a | Targeting and eradication of tumors in mice | [63] |
| Olfactory bulb | VSV | VSVrp30a | Tumor targeting, no damage to normal cells in mice | [63] |
| Glioblastoma | VSV | VSV-p1-GFP | Killing of tumor cells, not normal cells | [64] |
| Breast 4T1 | VSV | VSV(M51R)-LacZ | Lesions in breast cancer cells in mice | [65] |
| Colon CT-26 | VSV | VSV(M51R) | Prolonged survival in mice | [66] |
| Lung LLC-1 | VSV | VSV-LCMV GP | Tumor-to-tumor spread, killing of tumor cells | [67] |
| Melanoma | VSV | VSV-LCMV GP | Tumor regression, prolonged survival in mice | [68] |
| Ovarian | VSV | VSV-LCMV GP | Superior tumor regression with ruxolitinib | [69] |
| Melanoma | VSV | VSV-XN2-ΔG | Tumor regression in mice | [70] |
| Ovarian | VSV | VSVMP-p DNA | Tumor weight decrease, prolonged survival in mice | [71] |
| Ovarian | VSV | VSVMP-p DNA | 87–98% tumor regression, prolonged survival | [72] |
| Prostate | VSV | VSV(M51R) | Superior oncolysis after curcumin treatment | [73] |
| Melanoma | Maraba MG1 | hDCT + Ad-hDCT | Immune response after prime Ad-hCDT | [74] |
| Sarcoma | Maraba MG1 | MG1 | Protection against tumor challenges, cure in mice | [30] |
| Breast | MV, RABV | rMVEGFP-LDMV | Blue light induced tumor regression | [75] |
| Cancer | Oncolytic Virus | Phase | Findings | Ref |
|---|---|---|---|---|
| Ovarian | MV-CEA | I/II | No toxicity, SD in patients, 2-fold extended OS | [76] |
| GBM | MV-CEA | I | Study in progress | [77,78] |
| Colorectal | VEE-CEA | I | Antigen-specific responses, extended survival | [79] |
| Pancreatic | VEE-CEA | I | T cell responses, tumor toxicity, extended OS | [80] |
| CTCL | MV-EZ | I | Good safety, complete tumor regression | [81] |
| Ovarian | MV-NIS | I | SD in patients, significantly extended OS | [82] |
| Mesothelioma | MV-NIS | I | Study in progress | [83] |
| MPNST | MV-NIS | I | Study in progress | [84] |
| Head & Neck | MV-NIS | I | Study in progress | [85] |
| Myeloma | MV.NIS | I | Complete remission in one patient | [86] |
| Prostate | VEE-PSMA | I | Safe, but disappointingly weak immune response | [87] |
| Breast | VEE-HER2 | I | SD in 1 patient, PR in 2 patients | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundstrom, K. Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses. Int. J. Mol. Sci. 2022, 23, 15622. https://doi.org/10.3390/ijms232415622
Lundstrom K. Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses. International Journal of Molecular Sciences. 2022; 23(24):15622. https://doi.org/10.3390/ijms232415622
Chicago/Turabian StyleLundstrom, Kenneth. 2022. "Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses" International Journal of Molecular Sciences 23, no. 24: 15622. https://doi.org/10.3390/ijms232415622




