Antiulcerogenic Potential of the Ethanolic Extract of Ceiba speciosa (A. St.-Hil.) Ravenna Evaluated by In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Results
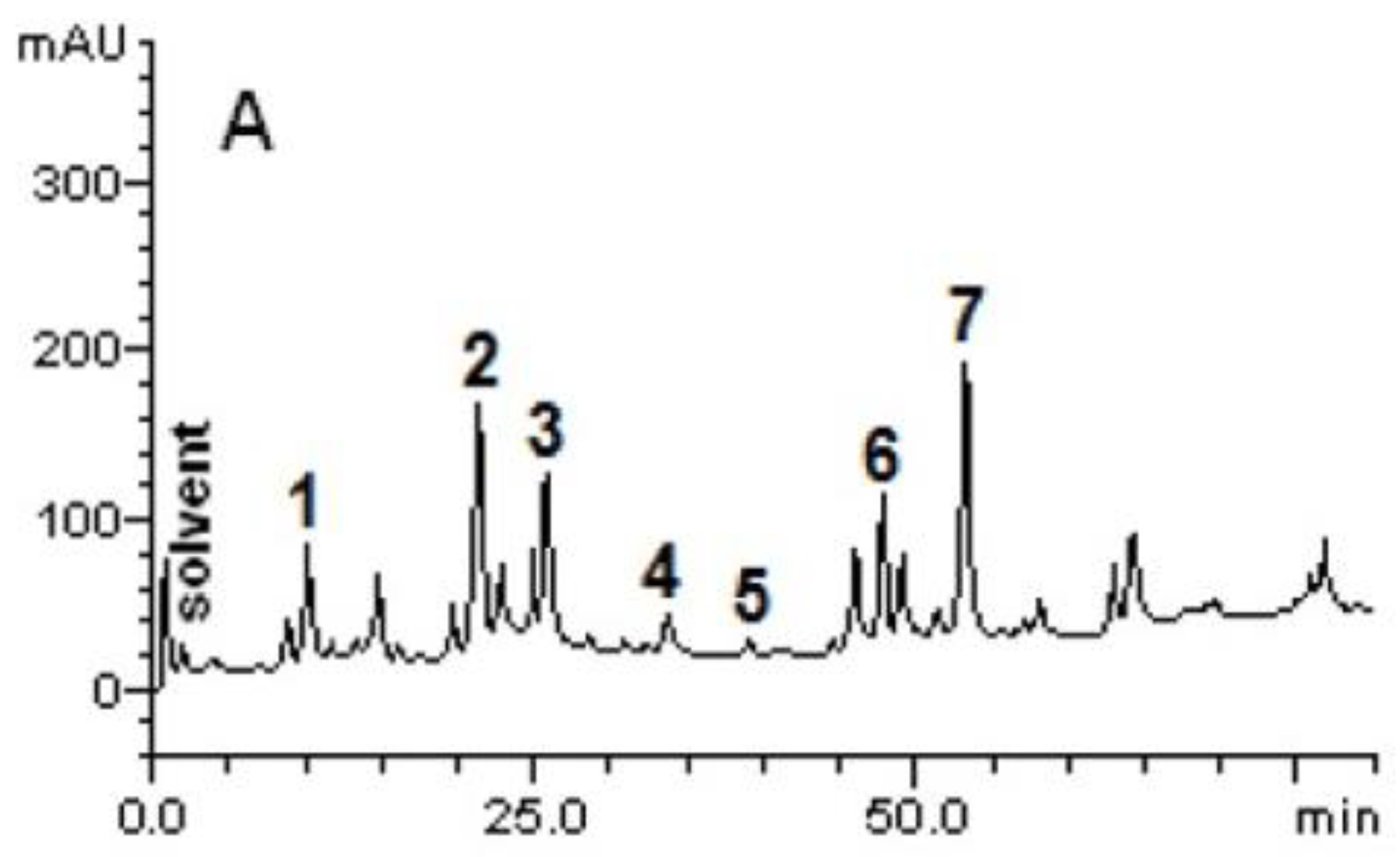
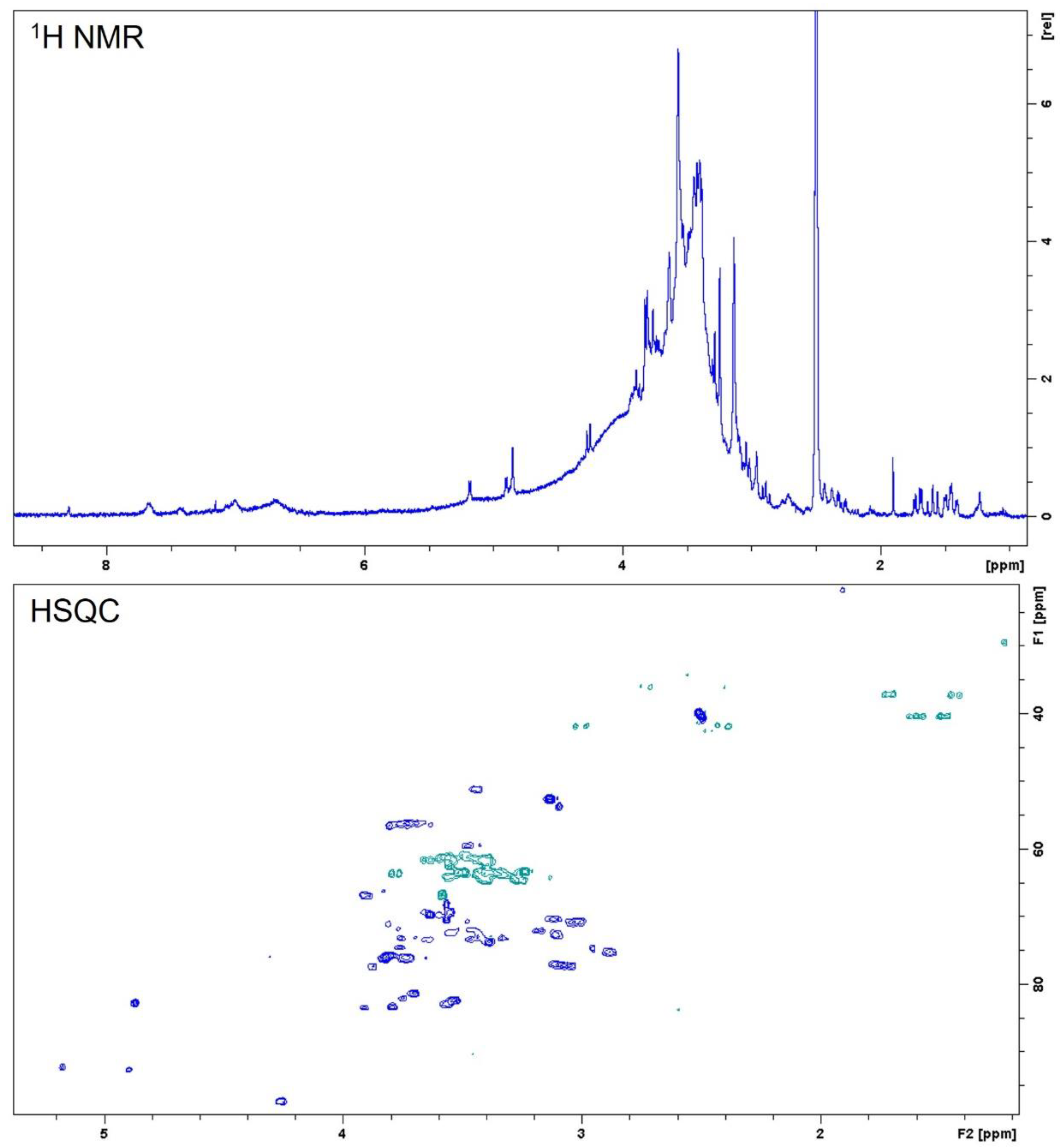
2.1. Phytoconstituents of C. speciosa Ethanolic Extract
2.2. Ceiba speciosa Antioxidant Activity
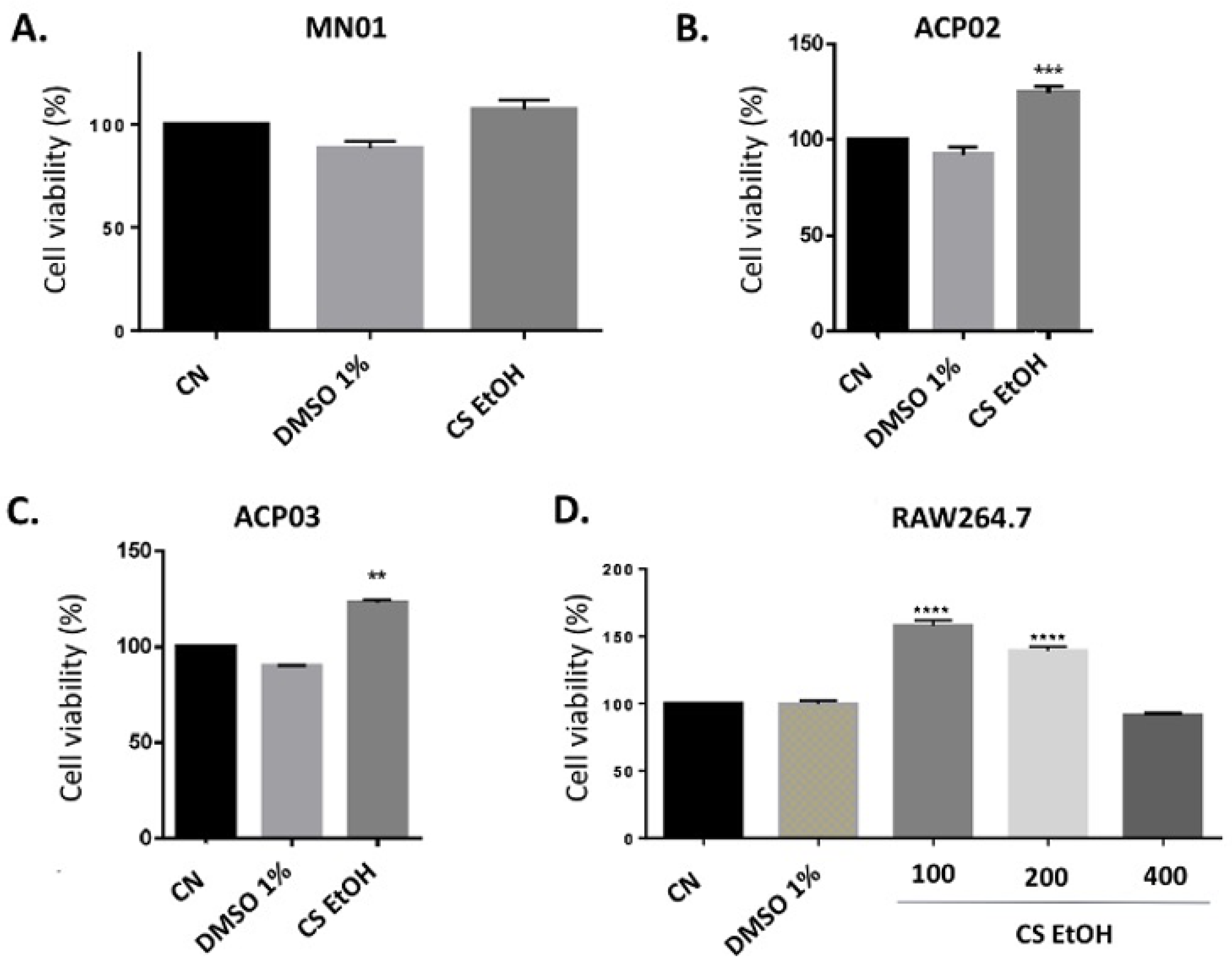
2.3. Cytotoxicity of C. speciosa Ethanolic Extract on Cell Lines
2.4. In Vitro Inhibitory Activity of the Kinases p38α, JAK3, and JNK3
2.5. In Vitro Anti-Inflammatory Potential
2.6. Ethanolic Extract of C. speciosa Decreases the Number of Leukocytes in Inflammatory Conditions
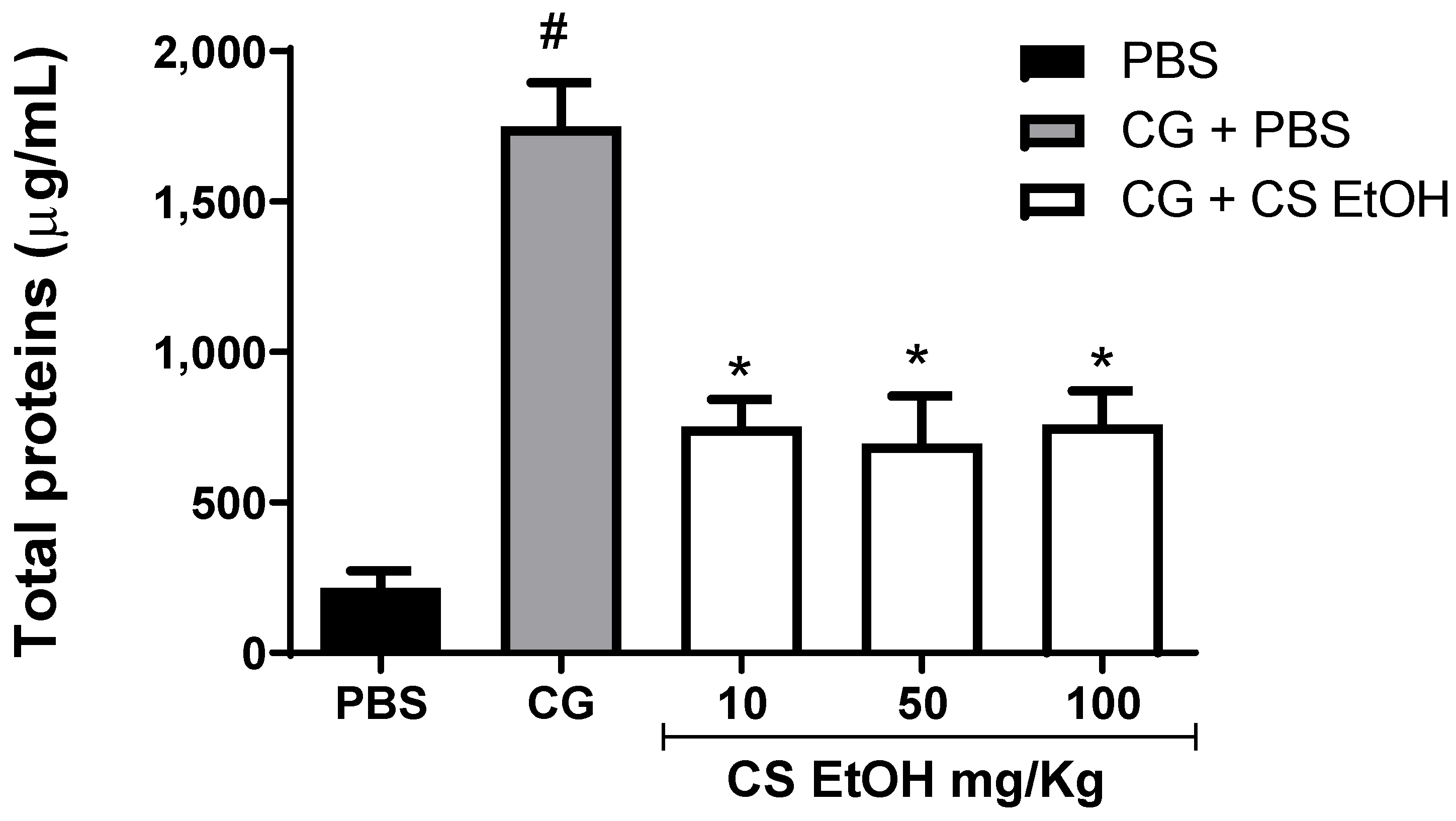
2.7. Ethanolic Extract of C. speciosa Inhibits Protein Extravasation into Inflamed Air Pouch
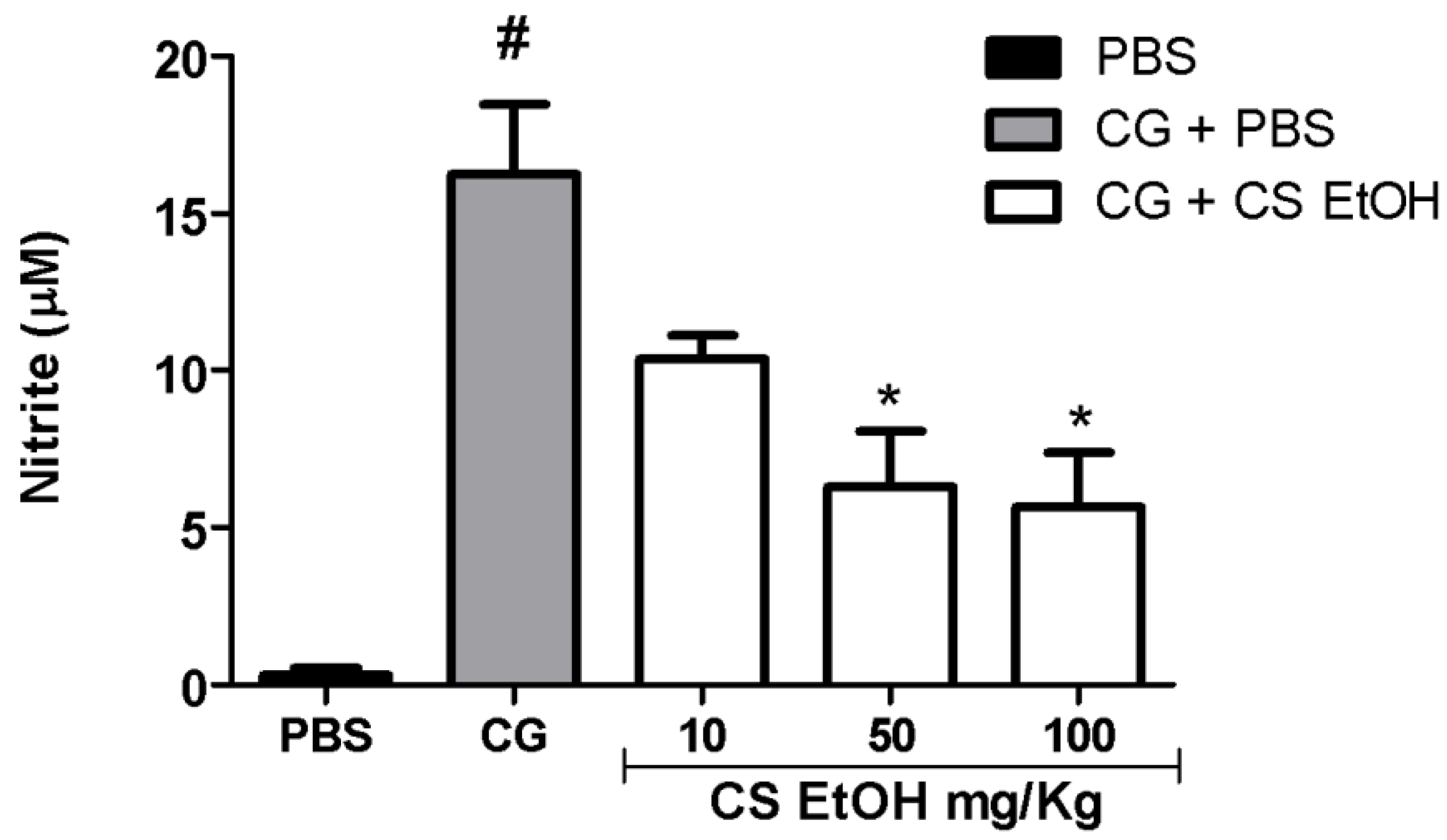
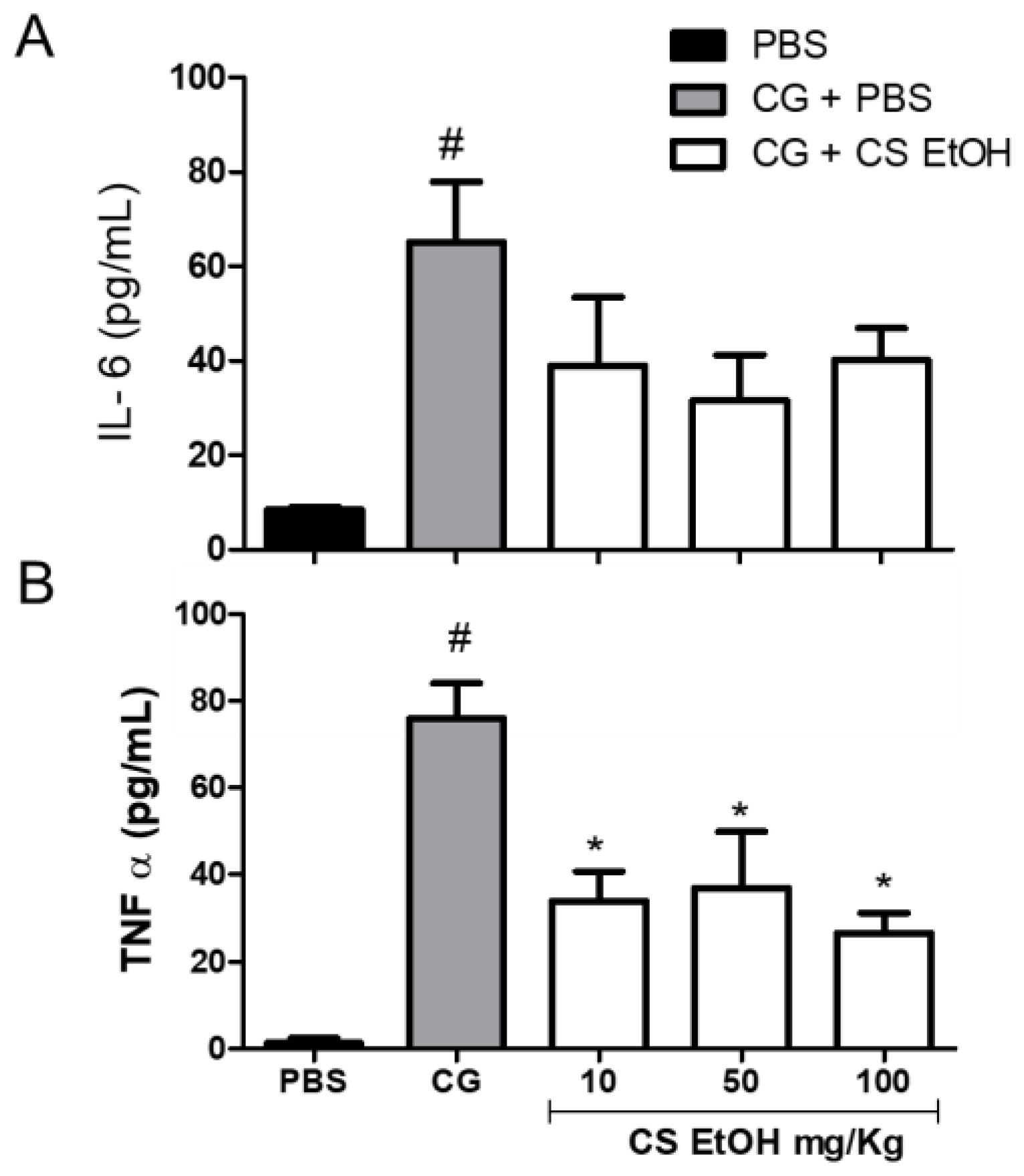
2.8. Ethanolic Extract of C. speciosa Inhibits NO Production, IL-6, and TNF-α in the Inflamed Air Pouches
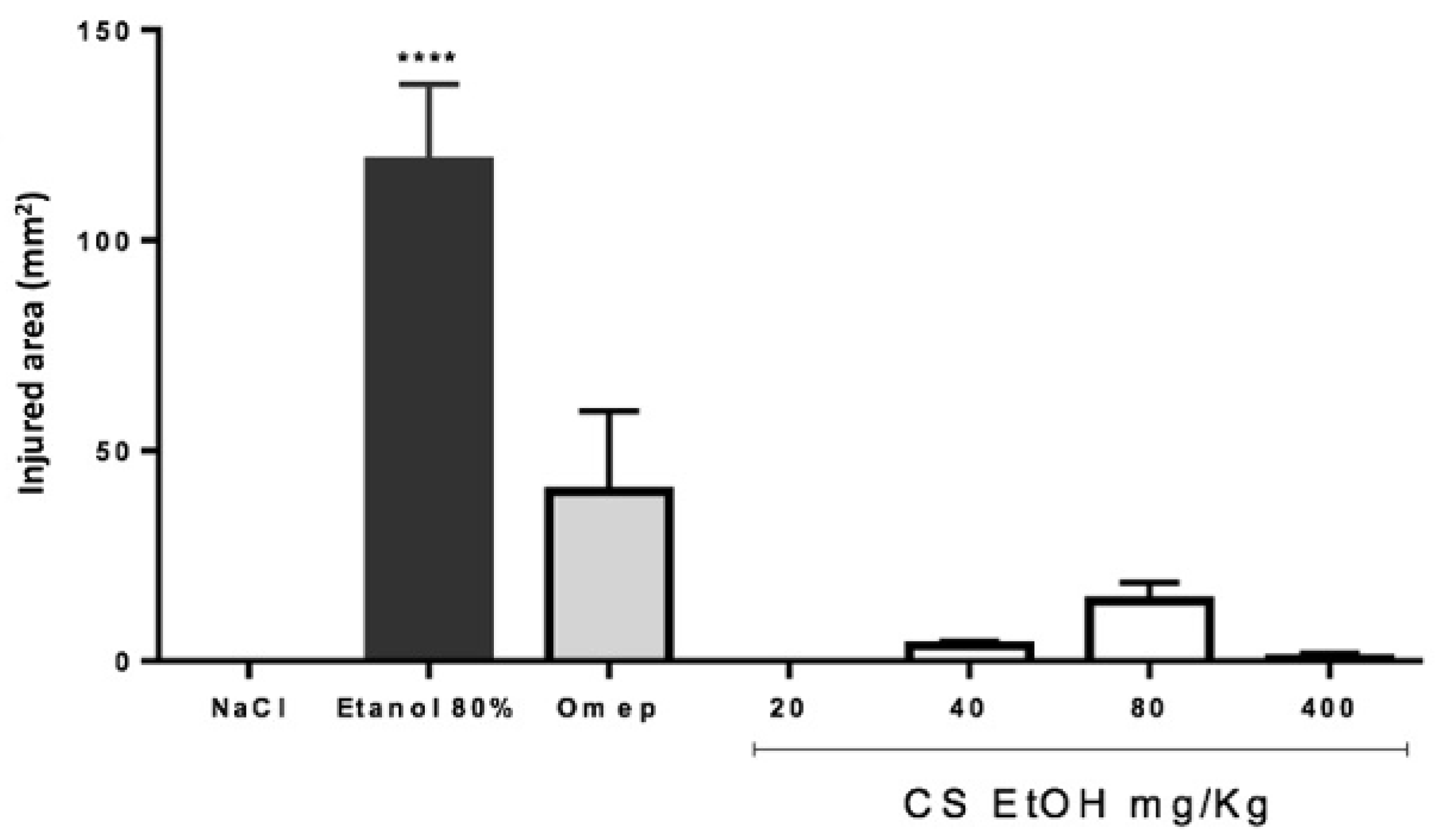
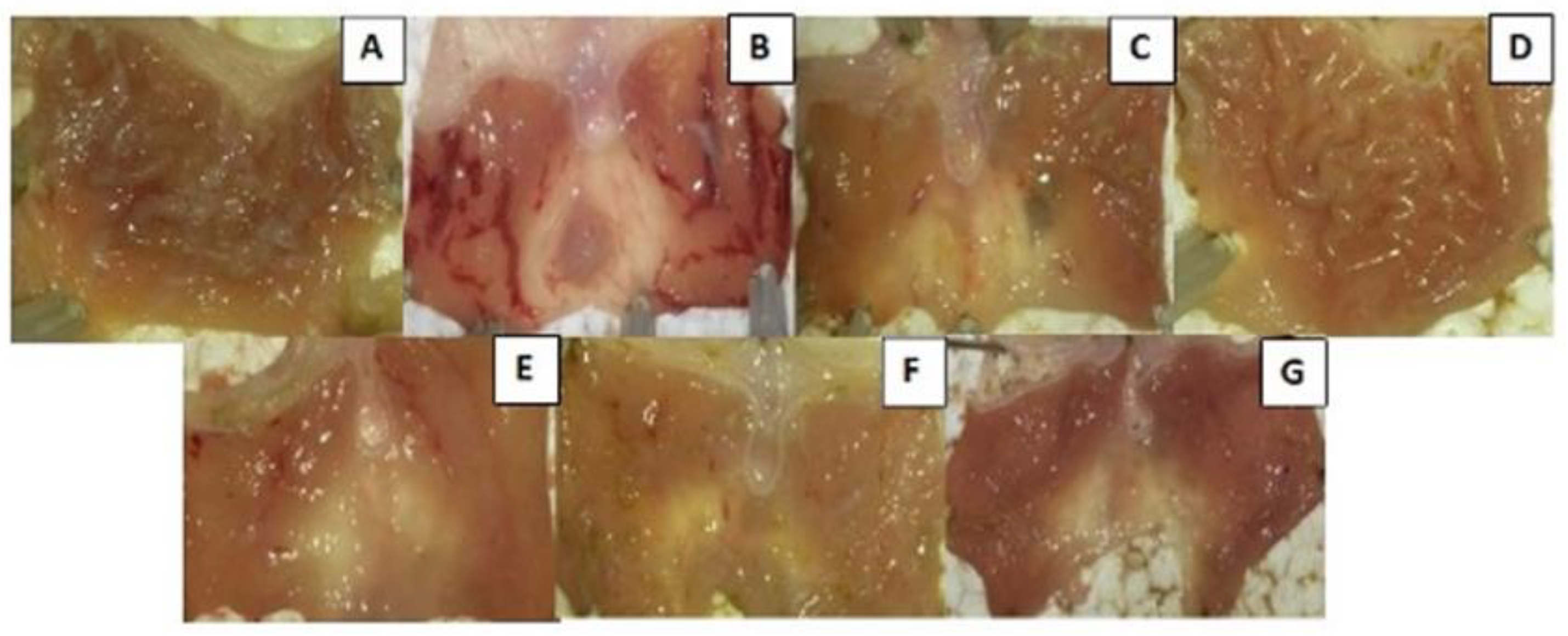
2.9. Antiulcerogenic Potential of the Ethanolic Extract in Wistar Rats with Ethanol-Induced Ulcer
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction Procedure
4.3. Chemical Characterization
4.3.1. Qualitative Phytochemical Screening
4.3.2. High-Performance Liquid Chromatography-Diode Array Detector
4.3.3. Nuclear Magnetic Resonance Analysis
4.4. Antioxidant Capacity of Ceiba speciosa Extract
4.4.1. Determination of Total Phenolic Content
4.4.2. Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
4.5. In Vitro Cell Assays
4.5.1. Cell Lines and Culture Conditions
4.5.2. Cell Viability Assessment
4.5.3. Human Peripheral Blood Mononuclear Cells (PBMC) Isolation
4.5.4. Anti-Inflammatory Potential
4.5.5. Cell-Free Kinase Assay
4.6. In Vivo Experiments
4.6.1. Animals
4.6.2. Air Pouch
4.6.3. Evaluation of Leukocyte Accumulation into the Air Pouch
4.6.4. Total Protein Quantification
4.6.5. Cytokines and Lipid Mediators’ Measurement
4.6.6. Antiulcerogenic Effect on a Model of Ethanol-Induced Ulcer in Wistar Rats
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, H.J.; Kim, H.; Song, J.; Lee, D. Gastroprotective Effects of Paeonia Extract Mixture HT074 against Experimental Gastric Ulcers in Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 3546258. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Padol, I.T.; Hunt, R.H. Peptic ulcer disease today. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Prostaglandins, NSAIDs, and Gastric Mucosal Protection: Why Doesn’t the Stomach Digest Itself? Physiol. Rev. 2019, 88, 1547–1565. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.; Rhee, M.H.; Sung, G.; Yoo, B.C.; Cho, J.Y. Functional Roles of p38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [Green Version]
- Mancini, A.D.; Di Battista, J.A. The cardinal role of the phospholipase A 2/cyclooxygenase-2/prostaglandin E synthase / prostaglandin E2 (PCPP) axis in inflammostasis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2011, 60, 1083–1092. [Google Scholar] [CrossRef]
- Tamaddonfard, E.; Erfanparast, A.; Abbas, A.; Imani, M. Safranal, a constituent of sa ff ron, exerts gastro-protective e ff ects against indomethacin-induced gastric ulcer. Life Sci. 2019, 224, 88–94. [Google Scholar] [CrossRef]
- Anosike, C.A.; Ofoegbu, R.E. Anti-ulcerogenic Activity of the Methanol Extract of Ceiba pentandra Stem Bark on Indomethacin and Ethanol-induced Ulcers in Rats. IJPS 2013, 3, 223–228. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-I.; Park, S.W.; Lim, J.J.; Sohn, S.I.; Shin, J.S.; Park, S.C.; Jang, Y.P.; Chung, E.K.; Lee, H.W.; Lee, K.T. Gastroprotective effects of the isopropanol extract of Artemisia princeps and its gastroretentive floating tablets on gastric mucosal injury. Acta Pharm. 2017, 67, 479–494. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; De, B.; Mazumder, J. Plants and phytochemicals for peptic ulcer: An overview. Pharmacogn. Rev. 2009, 3, 270–279. [Google Scholar]
- Anosike, C.A.; Ugwu, J.C.; Ojeli, P.C.; Abugu, S.C. Anti-ulcerogenic Effects and Anti-oxidative Properties of Ceiba pentandra Leaves on Alloxan-induced Diabetic Rats. Eur. J. Med. Plants 2014, 4, 458–472. [Google Scholar] [CrossRef]
- Dörr, J.A.; Bitencourt, S.; Bortoluzzi, L.; Alves, C.; Stoll, S.; Pinteus, S.; Boligon, A.A.; Christ, R.; Santos, V.; Laufer, S.; et al. In vitro activities of Ceiba speciosa (A. St.-Hil) Ravenna aqueous stem bark extract. Nat. Prod. Res. 2018, 33, 3441–3444. [Google Scholar] [CrossRef]
- Mohan, A.; Sagar, S.; Bhanu, P.; Talole, B. Phytochemical screening, flavonoid content and antioxidant activity of ethanolic extract of Ceiba pentandra. J. Ethnopharmacol. 2013, 4, 240–243. [Google Scholar] [CrossRef]
- Ladeji, O.; Omekarah, I.; Solomon, M. Hypoglycemic properties of aqueous bark extract of Ceiba pentandra in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2003, 84, 139–142. [Google Scholar] [CrossRef]
- Bhushan, G.; Kavimani, S.; Rajkapoor, B. Antiulcer activity of methanolic extract of Ceiba pentandra (Linn.) Gaertn. on rats. J. Pharm. Res. 2011, 4, 4132–4134. [Google Scholar]
- Khan, A.; Asadsaeed, M.; Ahmed Chaudhary, M.; Ahmad, Q.; Ansari, F. Antimicrobial, Anti-Inflammatory and Antipyretic Activity of Chorisia Speciosa Leaves. (Bombacaceae). IJBPAS 2015, 4, 6826–6838. [Google Scholar]
- Nasr, E.M.; Assaf, M.H.; Ramadan, M.A.; Darwish, F.M. Phytochemical and biological study of Chorisia speciosa A. St. Hil. Cultivated in Egypt. J. Pharmacogn. Phytochem. 2018, 7, 649–656. [Google Scholar]
- Dörr, J.A. Avaliação da Atividade Anti-Inflamatória e Antiulcerogênica de Extratos de Ceiba Speciosa (A. St-hill) Ravenna Em Modelos Experimentais In Vitro e In Vivo Avaliação da Atividade Anti-Inflamatória e Antiulcerogênica de Extratos de Ceiba Speciosa A.S. 2018. Available online: https://oasisbr.ibict.br/vufind/Search/Results?lookfor=ceiba&type=AllFields&limit=20 (accessed on 21 July 2021).
- Malheiros, C.K.C.; Silva, J.S.B.; Hofmann, T.C.; Messina, T.M.; Manfredini, V.; Piccoli, J.D.C.E.; Faoro, D.; Oliveira, L.F.S.; Machado, M.M.; Farias, F.M. Preliminary in vitro assessment of the potential toxicity and antioxidant activity of Ceiba speciosa (A. St.-Hill) ravenna (paineira). Braz. J. Pharm. Sci. 2017, 53, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Refaat, J.; Desoky, S.Y.; Ramadan, M.A.; Kamel, M.S. Bombacaceae: A Phytochemical Review. Pharm. Biol. 2013, 51, 100–130. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Poetsch, F.; Nahrstedt, A. Structure assignment of natural quinic acid derivatives using proton nuclear magnetic resonance techniques. Phytochem. Anal. 1998, 9, 177–185. [Google Scholar] [CrossRef]
- Flores-Parra, A.; Gutiérrez-Avella, D.M.; Contreras, R.; Khuong-Huu, F. 13C and 1H NMR investigations of quinic acid derivatives: Complete spectral assignment and elucidation of preferred conformations. Magn. Reson. Chem. 1989, 27, 544–555. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R.; Nigussie, D. Phytochemical investigation and nephroprotective potential of Sida cordata in rat. BMC Complement. Altern. Med. 2017, 17, 388. [Google Scholar] [CrossRef]
- Haule, E.E.; Moshi, M.J.; Nondo, R.S.O.; Mwangomo, D.T.; Mahunnah, R.L.A. A study of antimicrobial activity, acute toxicity and cytoprotective effect of a polyherbal extract in a rat ethanol-HCl gastric ulcer model. BMC Res Notes 2012, 5, 546. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Dinda, B.; Das, N.; Dinda, S.; Dinda, M.; Silsarma, I. The genus Sida L.—A traditional medicine: Its ethnopharmacological, phytochemical and pharmacological data for commercial exploitation in herbal drugs industry. J. Ethnopharmacol. 2015, 176, 135–176. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Číž, M.; Dvořáková, A.; Skočková, V.; Kubala, L. The role of dietary phenolic compounds in epigenetic modulation involved in inflammatory processes. Antioxidants 2020, 9, 691. [Google Scholar] [CrossRef]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans. Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Chisom, I.F.; Okereke, C.; Okeke, C. Comparative phytochemical and proximate analyses on Ceiba pentandra (L) and Bombax buonopozense (P) Beauv. J. Pharmacogn. Phytochem. 2014, 2, 162–167. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, E.-K.; Nawarathna, W.P.A.S.; Dong, X.; Shin, W.-B.; Park, J.-S.; Moon, S.-H.; Park, P.-J. Immune-stimulatory effects of Althaea rosea flower extracts through the MAPK Signaling Pathway in RAW264.7 Cells. Molecules 2017, 22, 679. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-W.; Kwon, O.K.; Jang, H.; Jeong, H.; Oh, S.R.; Lee, H.-K.; Han, S.B.; Ahn, K.S. A leaf methanolic extract of Wercklea insignis attenuates the lipopolysaccharide-induced inflammatory response by blocking the NF-κB signaling pathway in RAW 264.7 macrophages. Inflammation 2012, 35, 321–331. [Google Scholar] [CrossRef]
- Lima, A.L.A.; Alves, A.F.; Xavier, A.L.; Mozzini-Monteiro, T.; Oliveira, T.R.R.; Leite, F.C.; Matias, W.N.; Branco, M.V.S.C.; Souza, M.F.V.; Piuvezam, M.R. Anti-inflammatory activity and acute toxicity studies of hydroalcoholic extract of Herissantia tiubae. Rev. Bras. Farmacogn. 2016, 26, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.H.; Huang, H.P.; Huang, C.C.; Chen, J.H.; Wang, C.J. Hibiscus polyphenol-rich extract induces apoptosis in human gastric carcinoma cells via p53 phosphorylation and p38 MAPK/FasL cascade pathway. Mol. Carcinog. 2005, 43, 86–99. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, N.; Ramalingayya, G.V.; Setty, M.M.; Pai, K.S.R. Evaluation of Ceiba pentandra (L.) Gaertner bark extracts for in vitro cytotoxicity on cancer cells and in vivo antitumor activity in solid and liquid tumor models. Cytotechnology 2016, 68, 1909–1923. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2012, 1, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Pei, H.; Lan, T.; Tang, M.; Zhang, C.; Chen, L. Design and Synthesis of a Highly Selective JAK3 Inhibitor for the Treatment of Rheumatoid Arthritis. Arch. Pharm. 2017, 350, 1700194. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Rashid, F.; Bragg, J.; Ibdah, J.A. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Suzuki, A.; Tomiyasu, N.; Tsuruta, O.; Kitazaki, S.; Takeda, T.; Satoh, Y. Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease. Int. J. Mol. Med. 2006, 17, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.J.; Lin, C.M.; Lee, C.Y.; Shih, N.C.; Peng, S.F.; Tsuzuki, M.; Amagaya, S.; Huang, W.W.; Yang, J.S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol. Rep. 2013, 30, 925–932. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.Y.; Lee, E.G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.S.; Yoo, H.G.; Yoo, W.H. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–977. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Liu, T.; Guan, M.; Feng, X.; Dong, W.; Chu, X.; Liu, J.; Tian, X.; Ci, X.; et al. Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2012, 14, 209–216. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Wang, K.; Wang, Y.; Yin, W.; Li, L. P38/NF-κB/Snail Pathway Is Involved in Caffeic Acid-Induced Inhibition of Cancer Stem Cells-Like Properties and Migratory Capacity in Malignant Human Keratinocyte. PLoS ONE 2013, 8, e58915. [Google Scholar] [CrossRef] [Green Version]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d21–d26. [Google Scholar]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Fehr, S.; Unger, A.; Schaeffeler, E.; Herrmann, S.; Laufer, S.; Schwab, M.; Albrecht, W. Impact of p38 MAP kinase inhibitors on LPS-induced release of TNF-α in whole blood and primary cells from different species. Cell. Physiol. Biochem. 2015, 36, 2237–2249. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Brown, D.E.; Brewer, J.A.; Vogt, S.K.; Muglia, L.J. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 2007, 109, 4313–4319. [Google Scholar] [CrossRef] [Green Version]
- Juhn, S.K.; Jung, M.-K.; Hoffman, M.D.; Drew, B.R.; Preciado, D.A.; Sausen, N.J.; Jung, T.T.K.; Kim, B.H.; Park, S.-Y.; Lin, J.; et al. The Role of Inflammatory Mediators in the Pathogenesis of Otitis Media and Sequelae. Clin. Exp. Otorhinolaryngol. 2008, 1, 117. [Google Scholar] [CrossRef] [Green Version]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Fronza, M.; Muhr, C.; da Silveira, D.S.C.; Sorgi, C.A.; Rodrigues, S.F.D.P.; Farsky, S.H.P.; Paula-Silva, F.W.G.; Merfort, I.; Faccioli, L.H. Hyaluronidase decreases neutrophils infiltration to the inflammatory site. Inflamm. Res. 2016, 65, 533–542. [Google Scholar] [CrossRef]
- Marques, F.M.; Da Costa, M.R.; Vittorazzi, C.; Gramma, L.D.S.D.S.; Barth, T.; De Andrade, T.U.; Endringer, D.C.; Scherer, R.; Fronza, M. In Vitro and in Vivo Anti-inflammatory Effects of Struthanthus vulgaris. Planta Med. 2017, 83, 770–777. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chen, H.L.; Lan, X.Z.; Wu, Y.Y.; Ou, Y.W.; Chen, T.C.; Wu, W.T. The antioxidant activity and nitric oxide production of extracts obtained from the leaves of Chenopodium quinoa Willd. BioMedicine 2017, 7, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K.M. Anti-inflammatory and anti-neuropathic effects of a novel quinic acid derivative from Acanthus syriacus. Avicenna J. Phytomed. 2019, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric Mucosal Defense and Cytoprotection: Bench to Bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Repetto, M.G.; Llesuy, S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res. 2002, 35, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Rajeswari, G.; Sk, A.; Priyanka, B.; Rajaram, C.; Re, A. Evaluation of anti ulcer activity of ethanolic leaf extract of Ceiba pentandra in aspirin plus pylorus ligated wistar rats. Int. J. Res. Phytochem. Pharmacol. 2013, 3, 23–26. [Google Scholar]
- Kich, D.M.; Bitencourt, S.; Caye, B.; Faleiro, D.; Alves, C.; Silva, J.; Pinteus, S.; Mergener, M.; Majolo, F.; Boligon, A.A.; et al. Lymphocyte genotoxicity and protective effect of Calyptranthes tricona (Myrtaceae) against H2O2-induced cell death in MCF-7 cells. Mol. Cell. Biochem. 2017, 424, 35–43. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic 1965, 16, 144–158. [Google Scholar]
- Bauer, S.M.; Kubiak, J.M.; Rothbauer, U.; Laufer, S. From Enzyme to Whole Blood: Sequential Screening Procedure for Identification and Evaluation of p38 MAPK Inhibitors. In Kinase Screening and Profiling: Methods and Protocols, Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1360, pp. 123–148. ISBN 978-1-4939-3072-2. [Google Scholar]
- Goettert, M.; Luik, S.; Graeser, R.; Laufer, S.A. A direct ELISA assay for quantitative determination of the inhibitory potency of small molecules inhibitors for JNK3. J. Pharm. Biomed. Anal. 2011, 55, 236–240. [Google Scholar] [CrossRef]
- Goettert, M.; Shaalan, N.; Graeser, R.; Laufer, S.A. Development of a p38δ mitogen activated protein kinase ELISA assay for the quantitative determination of inhibitor activity. J. Pharm. Biomed. Anal. 2012, 66, 349–351. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Secundary Metabolites | CS EtOH | |
|---|---|---|
| Tannins | Hydrolysable | − |
| Condensed | + | |
| Alkaloids | + | |
| Flavonoids | Flavonols | + |
| Flavones | + | |
| Flavonones | + | |
| Coumarins | − | |
| Quinones | − | |
| Saponins | + | |
| Steroids/Triterpenes | − | |
| Compounds | CS EtOH (mg/g) |
|---|---|
| Gallic acid | 1.98 ± 0.02 d |
| Chlorogenic acid | 4.15 ± 0.01 b |
| Caffeic acid | 3.76 ± 0.04 c |
| Ellagic acid | 0.59 ± 0.01 e |
| Rutin | 0.17 ± 0.01 f |
| Quercetin | 2.03 ± 0.03 d |
| Kaempferol | 5.11 ± 0.05 a |
| Enzyme | CS EtOH | Standard |
|---|---|---|
| p38-α | 1.66 ± 0.24 µg/mL | 0.052 ± 0.02 µM (SB 203580) |
| JNK3 | 5.40 ± 0.21 µg/mL | 0.164 ± 0.06 µM (SP 600125) |
| JAK3 | 8.34 ± 0.83 ng/mL | 2.603 ± 0.05 nM (CP 690550) |
| Concentration of the Extract (µg/mL) | CS EtOH % Inibition |
|---|---|
| SB203580 | 88.3 ± 6.3 |
| 100 | 50.3 ± 10 |
| 50 | 33.3 ± 9 |
| 25 | 15.2 ± 0.5 |
| 12.5 | 13.8 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörr, J.A.; Majolo, F.; Bortoluzzi, L.; de Vargas, E.Z.; Silva, J.; Pasini, M.; Stoll, S.N.; da Rosa, R.L.; Figueira, M.M.; Fronza, M.; et al. Antiulcerogenic Potential of the Ethanolic Extract of Ceiba speciosa (A. St.-Hil.) Ravenna Evaluated by In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2022, 23, 15634. https://doi.org/10.3390/ijms232415634
Dörr JA, Majolo F, Bortoluzzi L, de Vargas EZ, Silva J, Pasini M, Stoll SN, da Rosa RL, Figueira MM, Fronza M, et al. Antiulcerogenic Potential of the Ethanolic Extract of Ceiba speciosa (A. St.-Hil.) Ravenna Evaluated by In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2022; 23(24):15634. https://doi.org/10.3390/ijms232415634
Chicago/Turabian StyleDörr, Juliana Andréa, Fernanda Majolo, Luísa Bortoluzzi, Evelin Zen de Vargas, Joana Silva, Manoela Pasini, Stefani Natali Stoll, Rafael Lopes da Rosa, Mariana Moreira Figueira, Márcio Fronza, and et al. 2022. "Antiulcerogenic Potential of the Ethanolic Extract of Ceiba speciosa (A. St.-Hil.) Ravenna Evaluated by In Vitro and In Vivo Studies" International Journal of Molecular Sciences 23, no. 24: 15634. https://doi.org/10.3390/ijms232415634
APA StyleDörr, J. A., Majolo, F., Bortoluzzi, L., de Vargas, E. Z., Silva, J., Pasini, M., Stoll, S. N., da Rosa, R. L., Figueira, M. M., Fronza, M., Beys-da-Silva, W. O., Martins, A., Gaspar, H., Pedrosa, R. P., Laufer, S., & Goettert, M. I. (2022). Antiulcerogenic Potential of the Ethanolic Extract of Ceiba speciosa (A. St.-Hil.) Ravenna Evaluated by In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 23(24), 15634. https://doi.org/10.3390/ijms232415634










