Degradation of Chemical Components of Thermally Modified Robinia pseudoacacia L. Wood and Its Effect on the Change in Mechanical Properties
Abstract
:1. Introduction
2. Resultes and Discussion
2.1. Mechanical Analysis
2.2. Static Analysis
2.3. Dynamic Bending
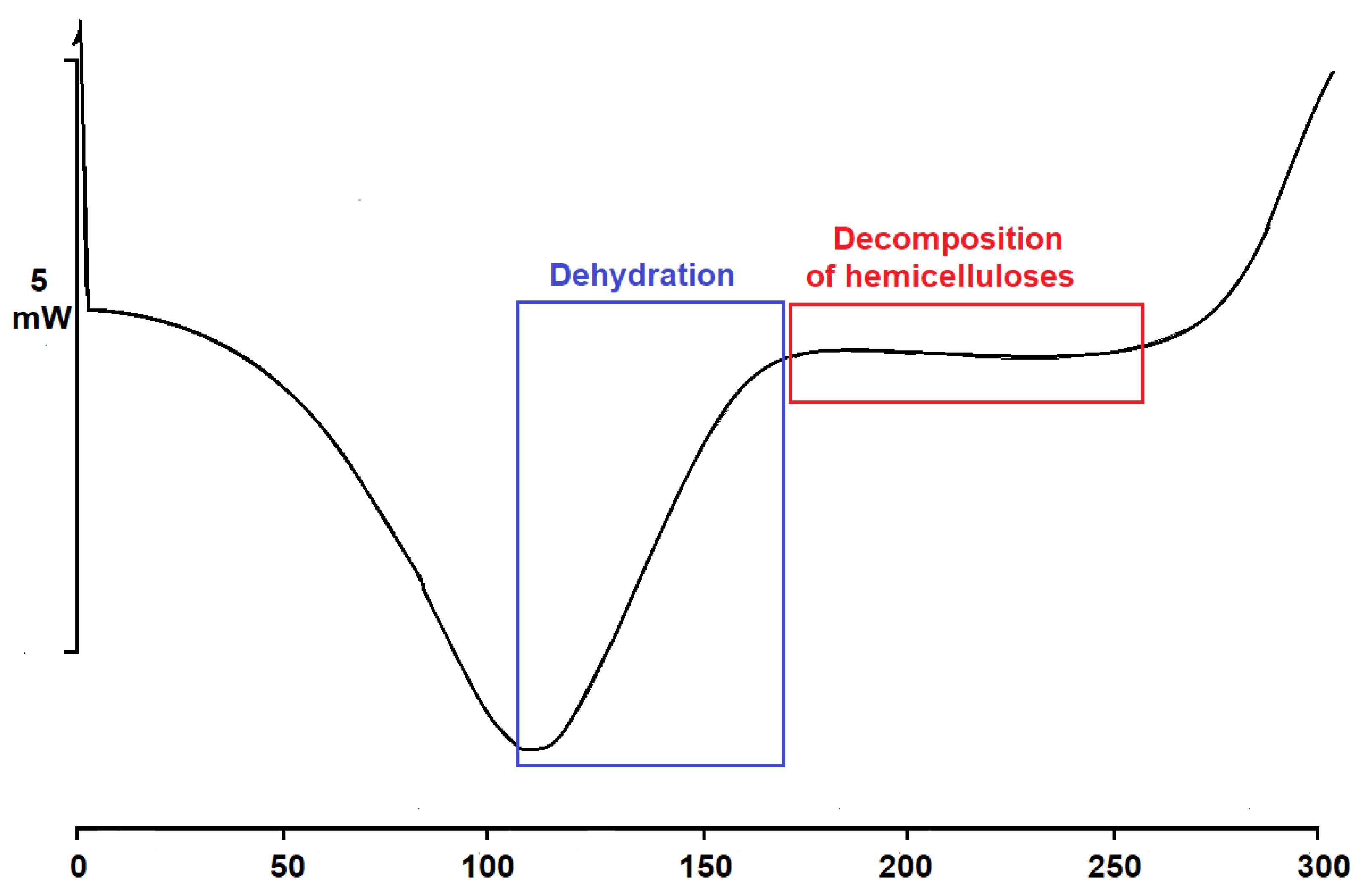
2.4. DSC Thermogram
2.5. Chemical Analysis
2.6. Cellulose Degree of Polymerization
2.7. Cellulose Crystallinity
3. Material and Methods
3.1. Materials
3.2. Thermal Modification
3.3. Experimental Methods
3.3.1. Mechanical Analysis
3.3.2. Chemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuong, V.M.; Li, J. Effect of Heat Treatment on the Change in Color and Dimensional Stability of Acacia Hybrid Wood. BioResources 2010, 5, 1257–1267. [Google Scholar]
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal, and Other Processes; John Eiley and Sons: Hoboken, NJ, USA, 2006; p. 260. [Google Scholar]
- Jones, D.; Sandberg, D.; Goli, G.; Todaro, L. Wood Modifiaction in Euope a State-of-the-Art about Processes Products and Applications; Firenze University Press: Florence, Italy, 2019; p. 113. [Google Scholar]
- Tran, V.C. Improvement of Dimensional Stability of Acacia mangium Wood by Heat Treatment: A case Study of Vietnam. J. For. Sci. 2013, 29, 109–115. [Google Scholar] [CrossRef]
- Wahab, R.; Edin, T.; Nasilah, M.; Sulaiman, M.S.; Mohd Ghani, R.S.; Ghani, M.; Razak, M. Monitoring Changes in the Colour, Strength and Chemical Properties of Oil Heat Treated 18-Years Old Cultivated Acacia mangium. In Recent Research Advances in Biology 4; University of Istanbul: Istanbul, Turkey, 2021; pp. 48–69. [Google Scholar]
- Desch, H.E.; Dinwoodie, J.M. Timber: Structure, Properties, Conversion and Use; CRC Press: Boca Raton, FL, USA, 1996; p. 306. [Google Scholar]
- Hon, D.N.S.; Siraishi, N. Wood and Cellulosic Chemistry; Taylor & Francis Group: Abingdon, UK, 2000; p. 928. [Google Scholar]
- Kosikova, B.; Hricovini, M.; Cosentino, C. Interfaction of lignin and polyssacharides in beach wood (Fagus sylvatica) during drying processes. Wood Sci. Technol. 1999, 33, 373–380. [Google Scholar]
- Wikberg, H.; Maunu, S.L. Characterisation of thermally modied hard- and softwoods by 13C CPMAS NMR. Carbohydr. Polym. 2004, 58, 461–466. [Google Scholar] [CrossRef]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Rautkari, L.; Hill, C.A.; Curling, S.; Jalaludin, Z.; Ormondroyd, G. What is the role of the accessibility of wood hydroxyl groups in controlling moisture content? J. Mater. Sci. 2013, 48, 6352–6356. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; p. 487. [Google Scholar]
- Salmén, L.; Burgert, I. Cell wall features with regard to mechanical performance. A Review COST Action E35 2004–2008: Wood Machining—Micromechanics and Fracture. Wood Res. Technol. Holzforsch. 2009, 63, 121–129. [Google Scholar] [CrossRef]
- Winandy, J.E.; Lebow, P.K. Modeling strength loss in wood by chemical composition. Part I. An individual component model for southern pine. Wood Fiber Sci. 2001, 33, 239–254. [Google Scholar]
- Rowell, R.M.; Ibach, R.E.; McSweeny, J.; Nilsson, T. Understanding decay resistance, dimensional stability and strength changes in heat treated and acetylated wood. In Proceedings of the 4th European Conference on Wood Modification, Stockholm, Sweden, 27–29 April 2009; pp. 489–502. [Google Scholar]
- Boonstra, M.J.; Acker, J.V.; Tjeerdsma, B.F.; Kegel, E.V. Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann. For. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Spink, C.H. Differential Scanning Calorimetry. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2009; pp. 115–141. [Google Scholar]
- Lee, C.H.; Yang, T.H.; Cheng, Y.W.; Lee, C.J. Effects of thermal modification on the surface and chemical properties of moso bamboo. Constr. Build. Mater. 2018, 178, 59–71. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Gašparík, M. Impact of thermal modification on the chemical changes and impact bending strength of European oak and Norway spruce. Compos. Struct. 2019, 216, 80–88. [Google Scholar] [CrossRef]
- Wahab, R.; Ghani, R.S.M.; Samsi, H.W.; Rasat, M.S.M. Colour, strength and chemical alteration of Acacia Mangium wood treated in oil heat treatment. Can. J. Pure Appl. Sci. 2017, 11, 4169–4181. [Google Scholar]
- Jebrane, M.; Pockrandt, M.; Cuccui, I.; Allegretti, O.; Uetimane, E.; Terziev, N. Comparative Study of Two Softwood Species Industrially Modified by Thermowood® and Therm-Vacuum Process. BioResources 2018, 13, 715–728. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Gašparík, M.; Todaro, L.; Jones, D.; Corleto, R.; Makovická Osvaldová, L.; Čekovská, H. The effect of synthetic and natural fire-retardants on burning and chemical characteristics of thermally modified teak (Tectona grandis L. f.) wood. Constr. Build. Mater. 2019, 200, 551–558. [Google Scholar] [CrossRef]
- Razak, W.; Izyan, K.; Tamer, A.T.; Aminuddin, M.; Othman, S.; Rafidah, S.; Farah, W.A. The effectiveness of hot oil treatment on cultivated 15 yer-old Acacia hybrid against Coriolus versicolours, Gloephyllum trabeum and Pycnoporus sanguineus. J. Sci. Malays. 2012, 41, 163–169. [Google Scholar]
- Gawron, J.; Grzeskiewicz, M.; Zawadzki, J.; Zielenkiewicz, T.; Radomski, A. The influence of time and temperature of beech wood (Fagus sylvatica L.) heat treatment in superheated steam on the carbohydrates content. Wood Res. 2011, 56, 213–220. [Google Scholar]
- Candelier, K.; Dumarcay, S.; Petrissans, A.; Desharnais, L.; Gerardin, P.; Petrissans, M. Comparisons of chemical composition and decay durability of heat treated wood cured under different inert atmosphere: Nitrogen and vacuum. Polym. Degrad. Stab. 2013, 98, 677–681. [Google Scholar] [CrossRef]
- Čabalová, I.; Zachar, M.; Kačík, F.; Tribulová, T. Impact of Thermal Loading on Selected Chemical and Morphological Properties of Spruce ThermoWood. BioResources 2019, 14, 387–400. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.; Zattera, A. Native Cellulose Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Tjeerdsma, B.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Als Roh Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Windeisen, E.; Wegener, G. Chemical characterization and comparison of thermally treated beech and ash wood. Mater. Sci. Forum 2009, 599, 143–158. [Google Scholar] [CrossRef]
- Čabalová, I.; Kačík, F.; Lagaňa, R.; Výbohová, E.; Bubeníková, T.; Čaňová, I.; Ďurkovič, J. Effect of thermal treatment on the chemical, physical, and mechanical properties of pedunculate oak (Quercus robur L.) wood. BioResources 2018, 13, 157–170. [Google Scholar] [CrossRef]
- Kubovský, D.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact in interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tribulová, T.; Kačík, F.; Evtuguin, D.V.; Čabalová, I.; Ďurkovič, J. The effects of transition metal sulfates on cellulose crystallinity during accelerated ageing of silver fir wood. Cellulose 2019, 26, 2625–2638. [Google Scholar] [CrossRef]
- Yuan, J.M.; Feng, Y.R.; He, L.P. Effect of thermal treatment on properties of ramie fibers. Polym. Degrad. Stab. 2016, 133, 303–311. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. 2021, 12, 100–168. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 185, 317–335. [Google Scholar] [CrossRef]
- Åkerholm, M.; Hinterstoisser, B.; Salmén, L. Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr. Res. 2004, 3, 569–578. [Google Scholar] [CrossRef]
- Nakao, T.; Tanaka, C.; Takahashi, A. Long-Term Changes in Degree of Crystallinity of Wood Cellulose. Holzforschung 1989, 43, 419–420. [Google Scholar]
- Kubojima, Y.; Okano, T.; Ohta, M. Vibrational properties of heat-treated green wood. J. Wood Sci. 2000, 46, 63–67. [Google Scholar] [CrossRef]
- Li, M.Y.; Cheng, S.C.; Li, D.; Wang, S.N.; Huang, A.M.; Sun, S.Q. Structural characterization of steam-heat treated Tectona grandis wood analyzed by FT-IR and 2D-IR correlation spectroscopy. Chin. Chem. Lett. 2015, 26, 221–225. [Google Scholar] [CrossRef]
- Lopes, J.Q.; Garcia, R.A.; Dias, S.N. Infrared spectroscopy of the surface of thermally-modified teak juvenile wood. Cienc. Y Technol. 2018, 20, 737–746. [Google Scholar] [CrossRef]
- Kačíková, D.; Kačík, F.; Čabalová, I.; Ďurkovič, J. Effects on thermal treatment on chemical, mechanical and colour traits in Norway spruce wood. Bioresour. Technol. 2013, 144, 669–674. [Google Scholar] [CrossRef]
- Nakano, T.; Miyazaki, J. Surface Fractal dimensionality and hygroscopicity for heated wood. Holzforschung 2003, 57, 289–294. [Google Scholar] [CrossRef]
- Seifert, K. Uber ein neues Verfahren zur Schnellbestimmung Der Rein-Cellulose. Das Pap. 1956, 10, 301–306. [Google Scholar]
- Wise, L.E.; Murphy, M.; D’Addieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–43. [Google Scholar]
- Kačík, F.; Solár, R. Analytical Chemistry of Wood; Technical University in Zvolen: Zvolen, Slovakia, 1999; p. 369. (In Slovak) [Google Scholar]
- Yildiz, S.; Gümüşkaya, E. The effects of thermal modification on crystalline structure of cellulose in soft and hardwood. Build. Environ. 2007, 42, 62–67. [Google Scholar] [CrossRef]
- Bhuiyan, M.T.R.; Hirai, N.; Sobue, N. Effect of intermittent heat treatment on crystallinity in wood cellulose. J. Wood Sci. 2001, 47, 336–341. [Google Scholar] [CrossRef]
- Creely, J.J.; Conrad, C.M. X-ray diffractometer thermal technique for study of structural changes in cellulosic compounds. Text. Res. J. 1962, 32, 184–189. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]





| Temperature, °C | Density, kg·m−3 | Static Bending | Dynamic Bending | ||
|---|---|---|---|---|---|
| Modulus of Elasticity, MPa | Limit of Proportionality, MPa | Modulus of Rupture, MPa | Impact Bending Strength, J·cm−2 | ||
| Unmodified (reference) | 790.2 (4.5) | 13,269.4 (8.8) | 106.6 (20.0) | 150.8 (15.4) | 10.8 (16.2) |
| 160 | 751.6 (4.9) | 12,324.6 (11.1) | 95.7 (19.5) | 124.6 (21.2) | 5.1 (45.1) |
| 180 | 755.4 (4.5) | 12,216.2 (7.7) | 73.7 (27.3) | 101.4 (18.6) | 6.6 (41.3) |
| 210 | 686.8 (5.1) | 10,755.3 (16.4) | 60.0 (28.1) | 82.8 (22.8) | 3.9 (52.5) |
| Variable | Degree of Thermal Modification | Density | MOE | LOP | MOR |
|---|---|---|---|---|---|
| Degree of thermal modification | – | 0.565 (0.000) | 0.469 (0.000) | 0.573 (0.000) | 0.692 (0.000) |
| Density | 0.565 (0.000) | – | 0.563 (0.000) | 0.440 (0.000) | 0.513 (0.000) |
| MOE | 0.469 (0.000) | 0.563 (0.000) | – | 0.547 (0.000) | 0.654 (0.000) |
| LOP | 0.573 (0.000) | 0.440 (0.000) | 0.547 (0.000) | – | 0.890 (0.000) |
| MOR | 0.692 (0.000) | 0.513 (0.000) | 0.654 (0.000) | 0.890 (0.000) | – |
| Variable | Degree of Thermal Modification | Density | Energy for IBS | IBS |
|---|---|---|---|---|
| Degree of thermal modification | – | 0.623 (0.000) | 0.732 (0.000) | 0.728 (0.000) |
| Density | 0.623 (0.000) | – | 0.522 (0.000) | 0.534 (0.000) |
| Energy for IBS | 0.732 (0.000) | 0.522 (0.000) | – | 0.999 (0.000) |
| IBS | 0.728 (0.000) | 0.534 (0.000) | 0.999 (0.000) | – |
| Sample/Wood Component | Ash | Extractives Ethanol-Toluene | Lignin | Cellulose | Holocellulose |
|---|---|---|---|---|---|
| Reference | 1.87 (0.000) | 5.69 (0.757) | 19.66 (1.524) | 41.10 (0.191) | 78.98 (0.249) |
| 160 °C | 1.73 (0.000) | 2.31 (0.074) | 24.38(5.089) | 32.69 (1.467) | 62.58 (1.609) |
| 180 °C | 1.59 (0.000) | 2.11 (0.046) | 30.10 (3.408) | 30.57 (1.566) | 57.70 (0.051) |
| 210 °C | 1.33 (0.000) | 1.55 (0.135) | 29.75 (1.825) | 28.80 (0.712) | 54.10 (0.133) |
| Sample/ Components | Hemicellulose | Pentoses | Hexoses |
|---|---|---|---|
| Reference | 37.88 (0.185) | 15.99 (0.054) | 21.89 (0.155) |
| 160 °C | 29.88 (1.427) | 12.41 (0.345) | 17.48 (1.274) |
| 180 °C | 27.13 (1.566) | 9.71 (0.009) | 17.43 (1.566) |
| 210 °C | 25.30 (0.711) | 7.60 (0.021) | 17.70 (0.709) |
| Sample/Modification Temperature | Reference | 160 °C | 180 °C | 210 °C |
|---|---|---|---|---|
| black locust | 280 (2) | 311 (2) | 347 (2) | 302 (3) |
| Sample/Parameter for Crystallinity Characterization | TCI | LOI | HBI |
|---|---|---|---|
| Reference | 1.406 | 0.507 | 1.528 |
| 160 °C | 1.494 | 0.547 | 1.167 |
| 180 °C | 1.742 | 0.588 | 1.156 |
| 210 °C | 1.911 | 0.649 | 0.845 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, A.; Hájková, K.; Jurczyková, T. Degradation of Chemical Components of Thermally Modified Robinia pseudoacacia L. Wood and Its Effect on the Change in Mechanical Properties. Int. J. Mol. Sci. 2022, 23, 15652. https://doi.org/10.3390/ijms232415652
Sikora A, Hájková K, Jurczyková T. Degradation of Chemical Components of Thermally Modified Robinia pseudoacacia L. Wood and Its Effect on the Change in Mechanical Properties. International Journal of Molecular Sciences. 2022; 23(24):15652. https://doi.org/10.3390/ijms232415652
Chicago/Turabian StyleSikora, Adam, Kateřina Hájková, and Tereza Jurczyková. 2022. "Degradation of Chemical Components of Thermally Modified Robinia pseudoacacia L. Wood and Its Effect on the Change in Mechanical Properties" International Journal of Molecular Sciences 23, no. 24: 15652. https://doi.org/10.3390/ijms232415652
APA StyleSikora, A., Hájková, K., & Jurczyková, T. (2022). Degradation of Chemical Components of Thermally Modified Robinia pseudoacacia L. Wood and Its Effect on the Change in Mechanical Properties. International Journal of Molecular Sciences, 23(24), 15652. https://doi.org/10.3390/ijms232415652






