Soybean CALCIUM-DEPENDENT PROTEIN KINASE17 Positively Regulates Plant Resistance to Common Cutworm (Spodoptera litura Fabricius)
Abstract
:1. Introduction
2. Results
2.1. Identification of Candidate Genes
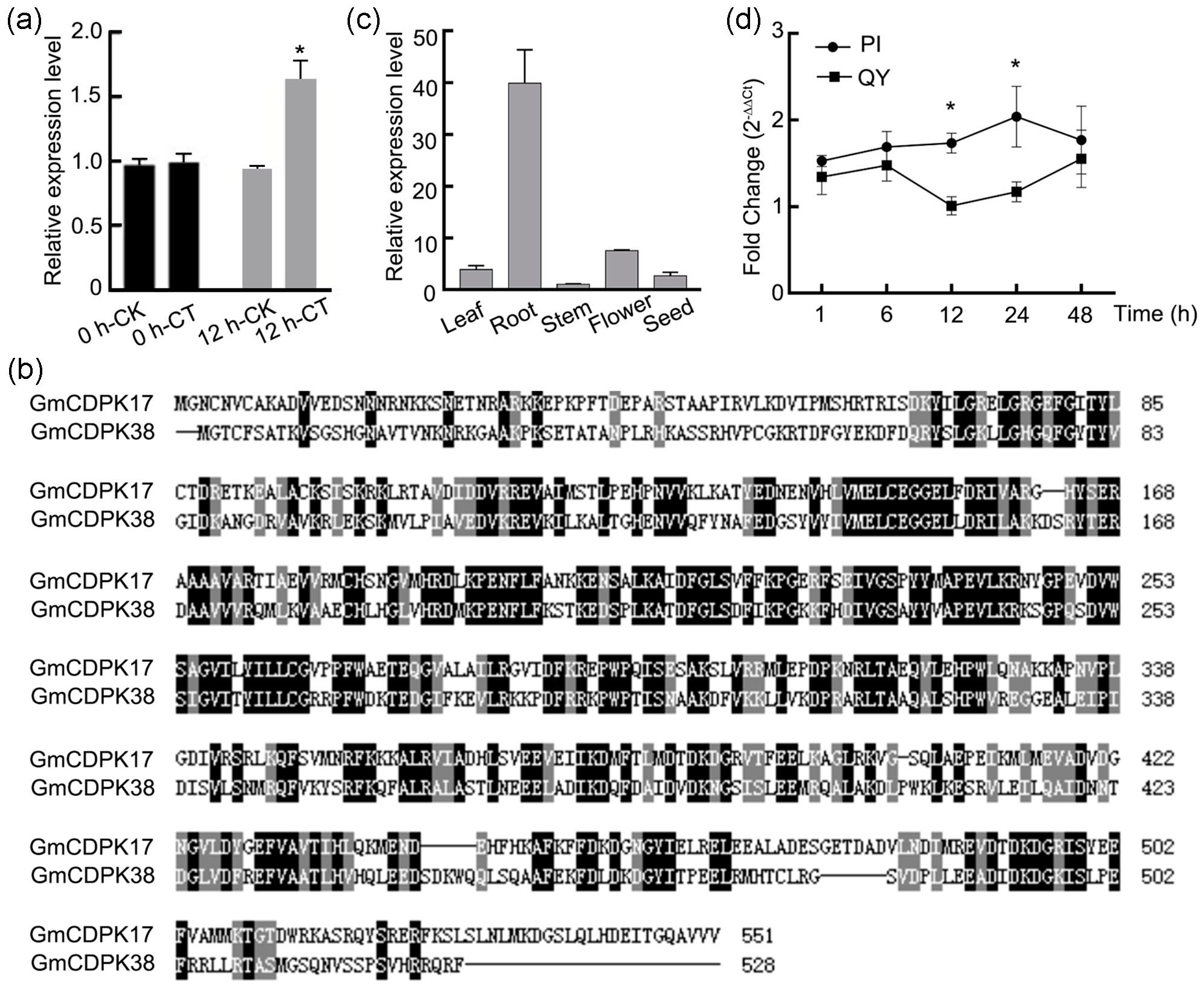
2.2. Cloning and Sequence Analysis of GmCDPK17
2.3. Expression Patterns of GmCDPK17 in Distinct Tissues and Leaves after CCW Attack
2.4. GmCDPK17 Localized to the Nucleus and Cytoplasm
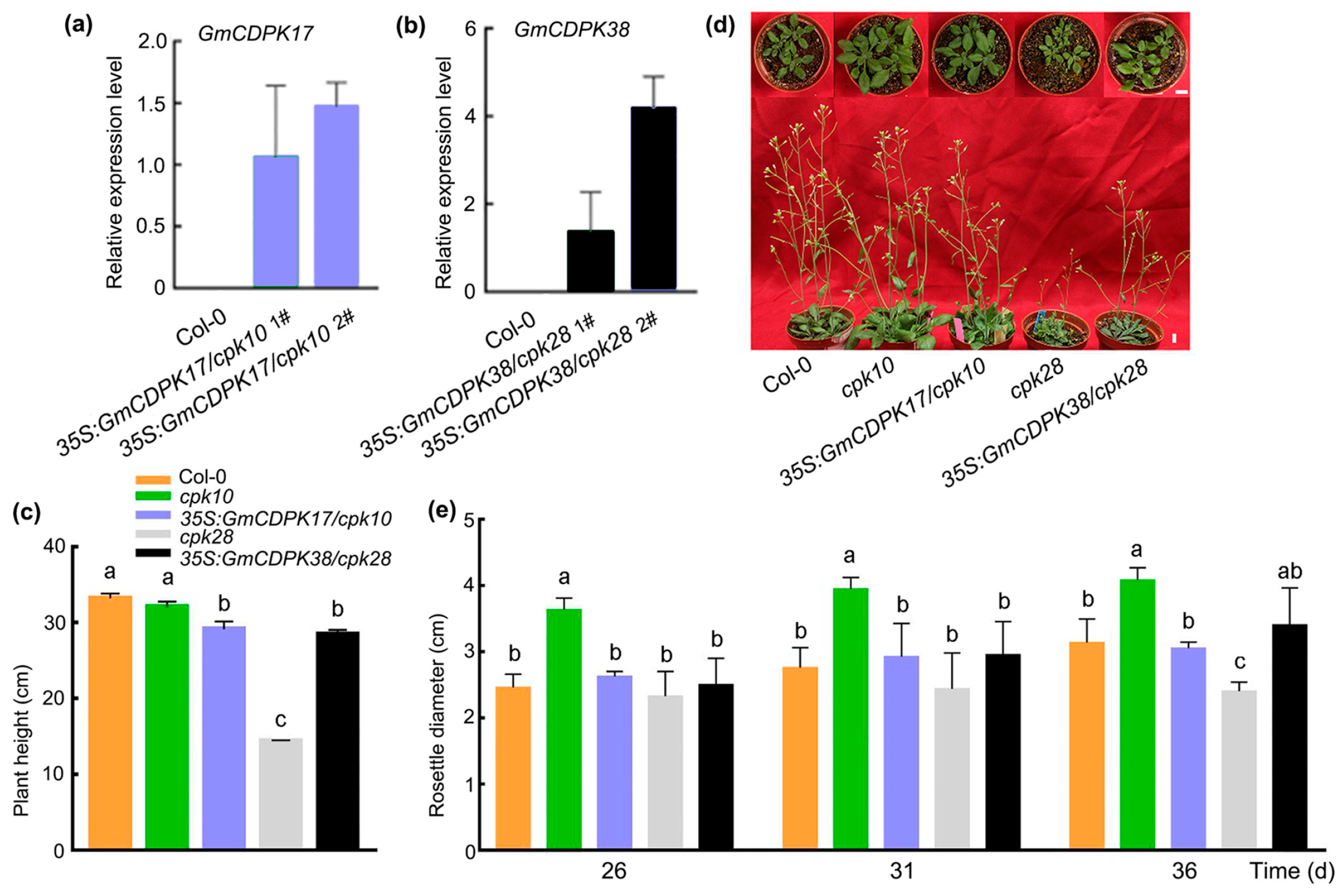
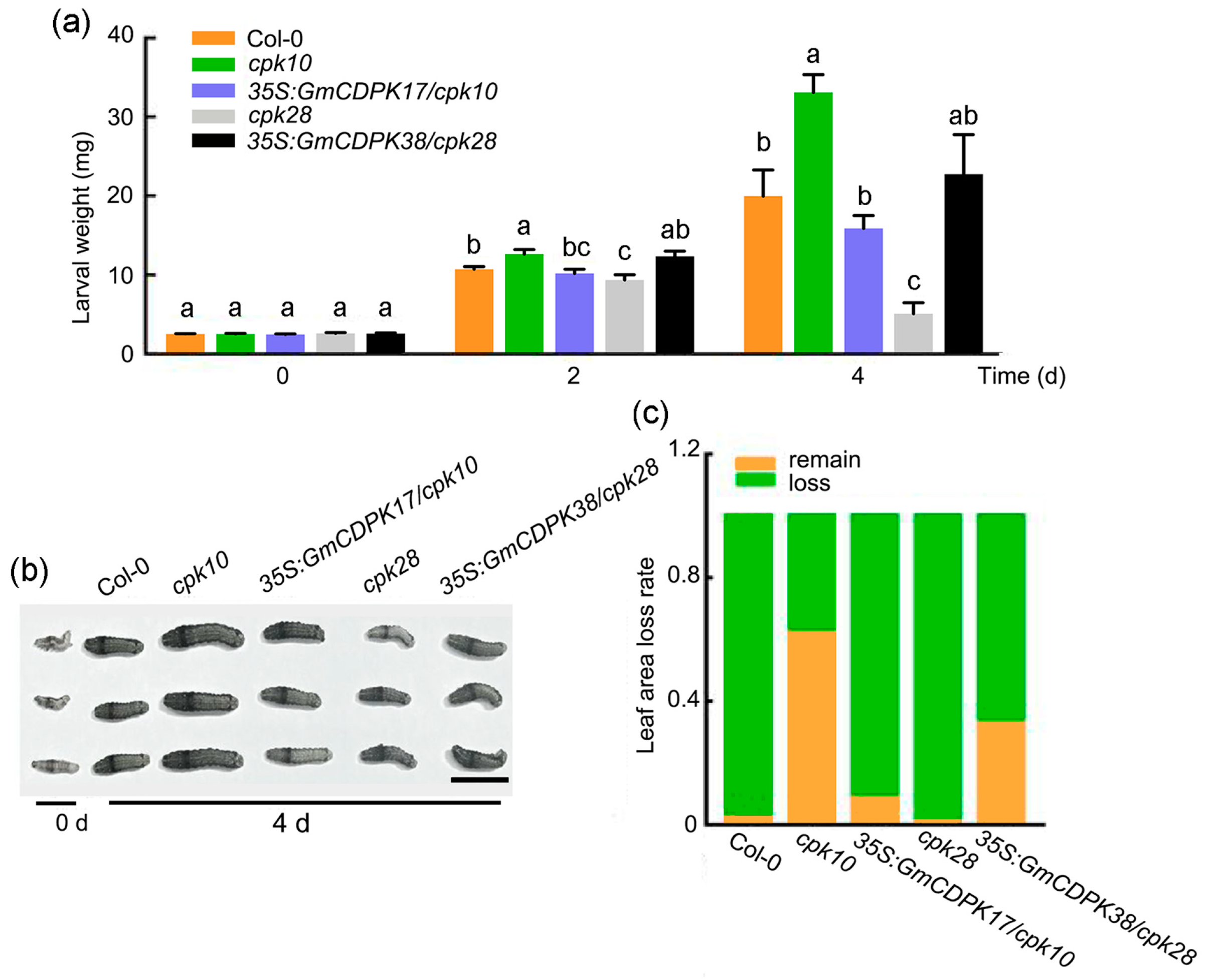
2.5. The Roles of GmCDPK17 in Arabidopsis Development and Resistance to CCW Differed from GmCDPK38
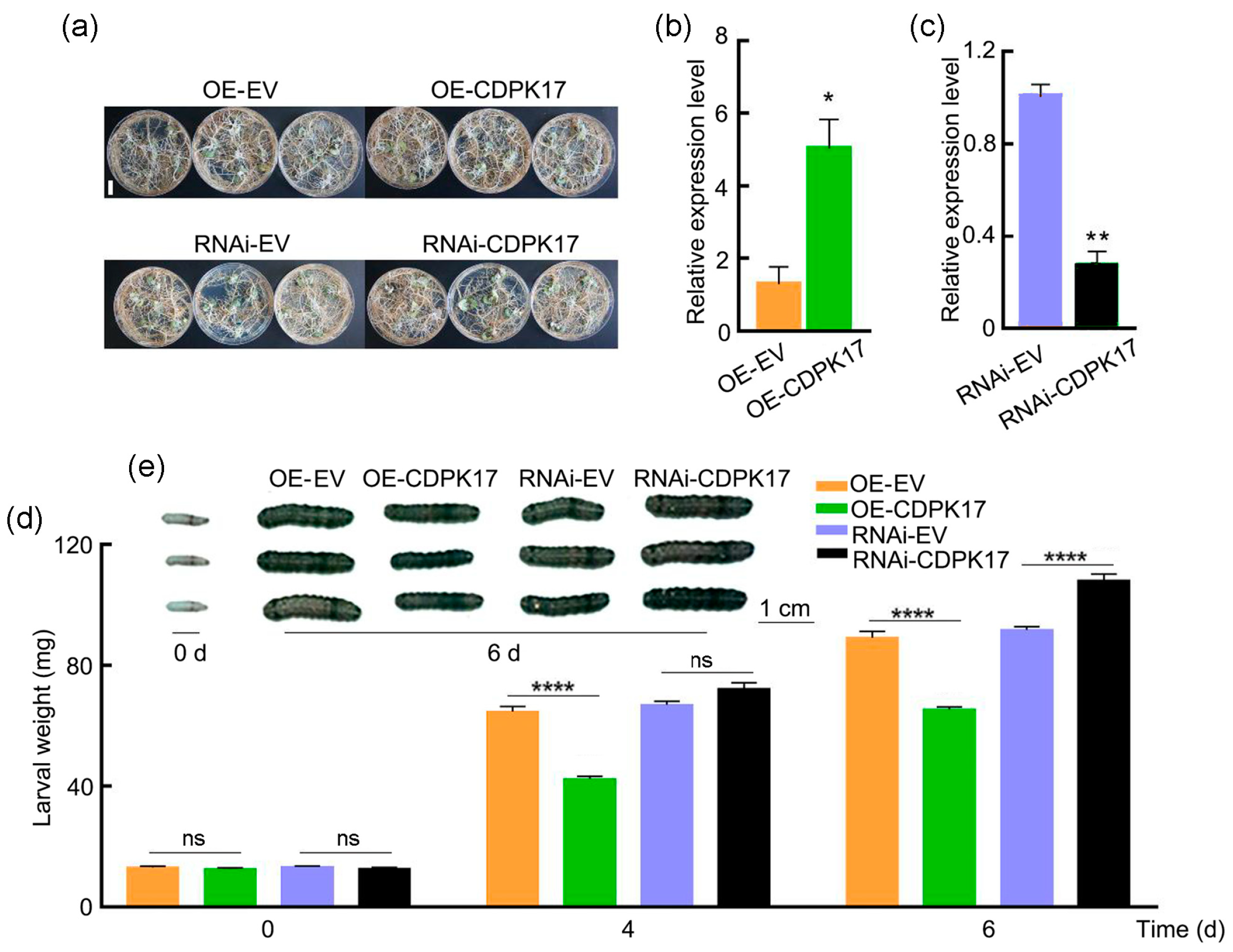
2.6. GmCDPK17 Positively Regulates Soybean Resistance to CCW
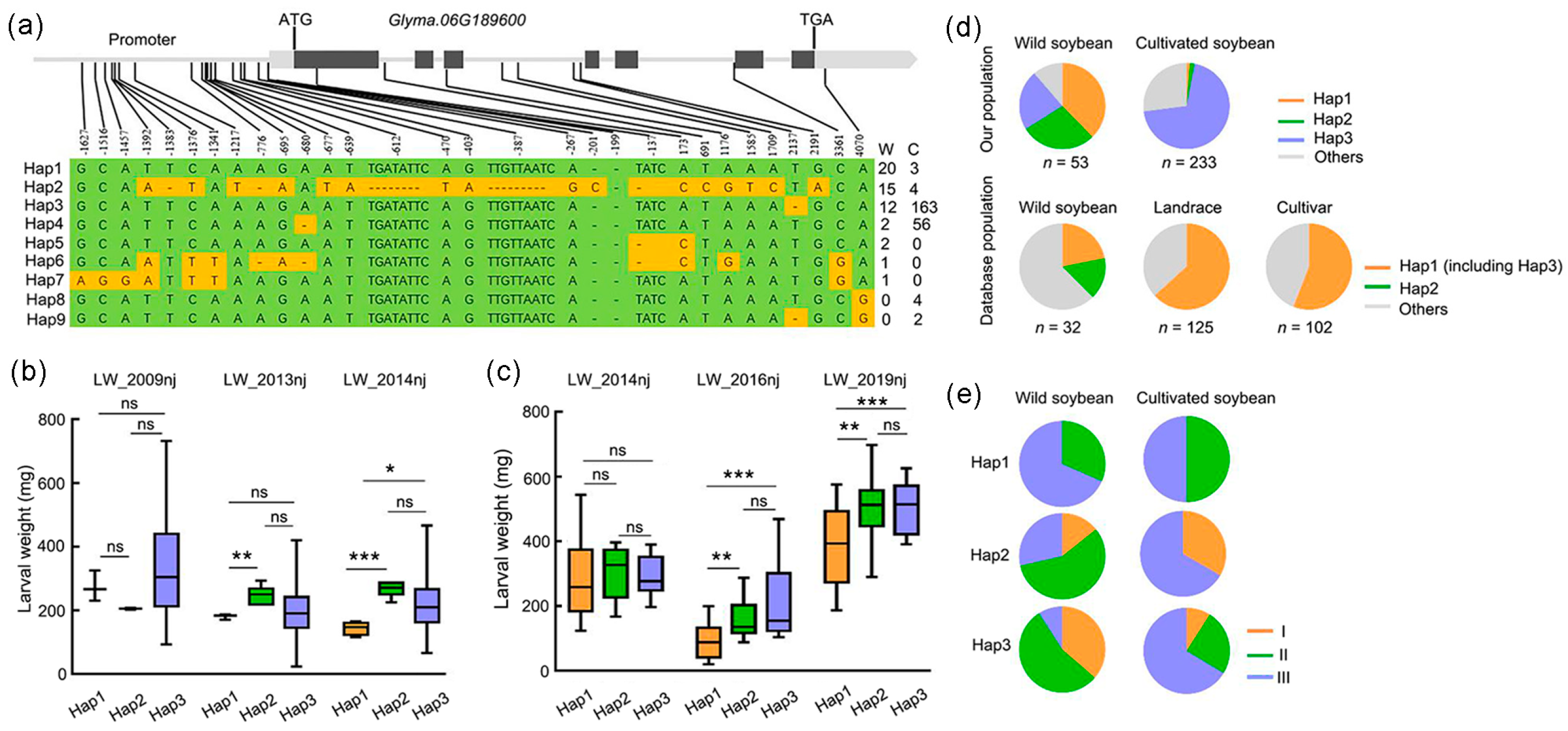
2.7. GmCDPK17 Underwent Selection during Soybean Domestication
2.8. Genetic Diversity of the GmCDPK17 Gene
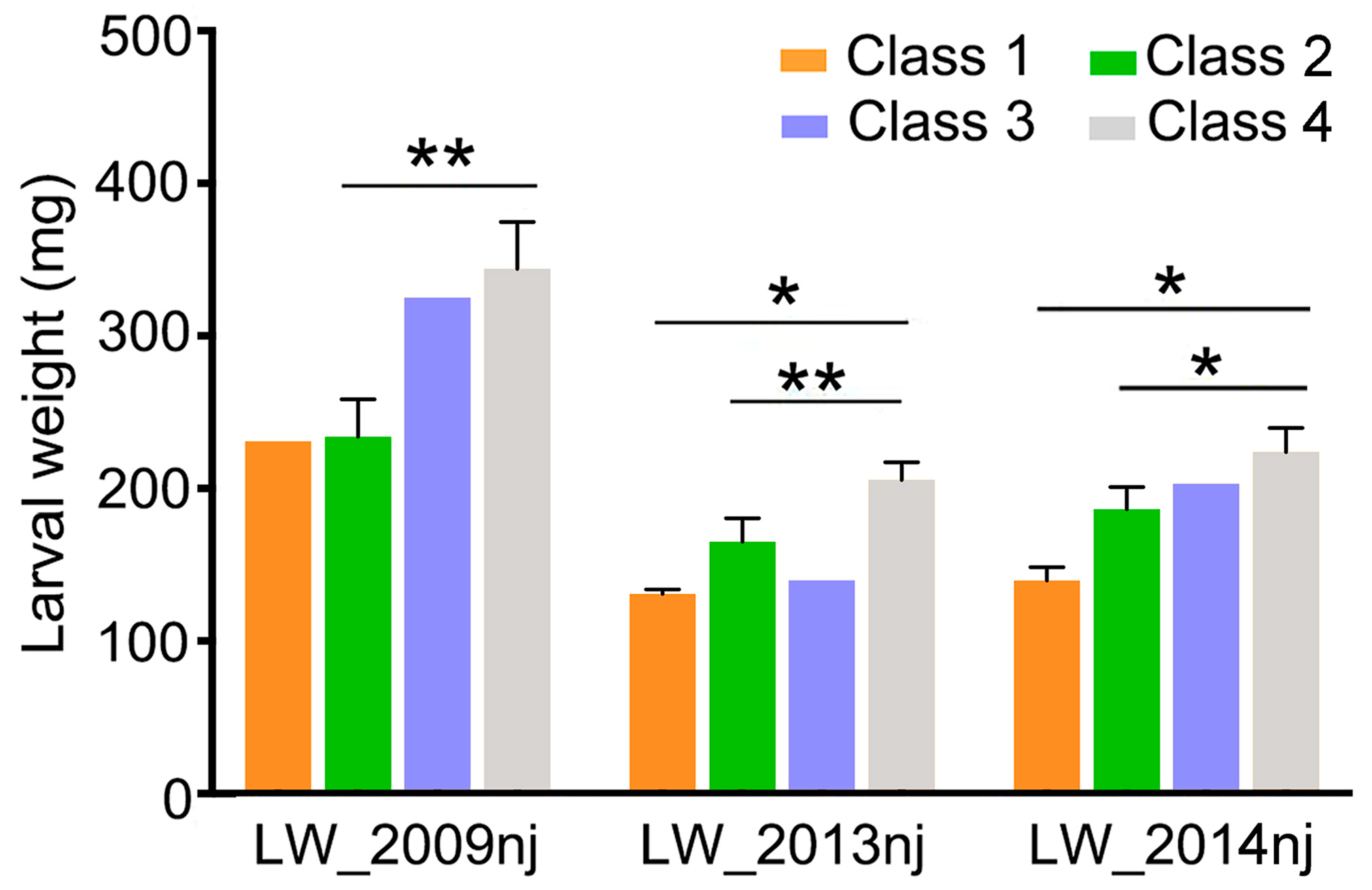
2.9. Soybean with Resistance Haplotypes of the GmCDPK17 and GmCDPK38 Genes Showed High Resistance to CCW
3. Discussion
3.1. GmCDPK17 Has a Different Function from GmCDPK38 in Soybean Insect Resistance
3.2. GmCDPK17 May Further Improve Soybean Insect Resistance via Polymerization with GmCDPK38
3.3. GmCDPK17 May Have a Role in Other Biological Processes
3.4. More Insect-Resistance Alleles May Exist in Accessions at Low Latitudes
4. Materials and Methods
4.1. Plant Materials and CCW Induction Treatments
4.2. Prediction of Candidate Genes within Loci and Their Tissue Expression
4.3. Cloning of GmCDPK17
4.4. Gene Expression Analysis
4.5. Subcellular Localization
4.6. Constitutive Expression of GmCDPK17 and GmCDPK38 in Arabidopsis Mutants
4.7. Overexpression and Suppression of GmCDPK17 in Soybean Hairy Roots
4.8. Insect Force-Feeding and Free-Feeding Trials
4.9. GmCDPK17 Sequence Diversity Analysis
4.10. Phenotypic Data of the Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; Gai, J. A study on leaf-feeding insect species on soybeans in Nanjing area. Soybean Sci. 1997, 16, 12–20. [Google Scholar]
- Cardona, E.V.; Ligat, C.S.; Subang, M.P. Life history of common cutworm, Spodoptera litura Fabricius (Moctuidae: Lepidoptera) in Benguet. BSU Res. J. 2007, 56, 73–84. [Google Scholar]
- Wu, J.; Wu, Q.; Wu, Q.; Gai, J.; Yu, D. Constitutive overexpression of AOS-like gene from soybean enhanced tolerance to insect attack in transgenic tobacco. Biotechnol. Lett. 2008, 30, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, H.; Wu, J.; Wang, D.; Wang, Y.; Zhang, L.; Huang, Z.; Yu, D. Soybean GmAOC3 promotes plant resistance to the common cutworm by increasing the expression of genes involved in resistance and volatile substance emission in transgenic tobaccos. J. Plant Biol. 2015, 58, 242–251. [Google Scholar] [CrossRef]
- Wang, H.; Ding, C.; Du, H.; Liu, H.; Wang, Y.; Yu, D. Identification of soybean MYC2-like transcription factors and overexpression of GmMYC1 could stimulate defense mechanism against common cutworm in transgenic tobacco. Biotechnol. Lett. 2014, 36, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, F.; Wang, X.; Zhang, M.; Zhang, R.; Wang, J.; Yu, D. Genome-wide analysis of terpene synthases in soybean: Functional characterization of GmTPS3. Gene 2014, 544, 83–92. [Google Scholar] [CrossRef]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.M.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef] [Green Version]
- Laule, O.; Fürholz, A.; Chang, H.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef] [Green Version]
- Gutensohn, M.; Orlova, I.; Nguyen, T.T.; Davidovich-Rikanati, R.; Ferruzzi, M.G.; Sitrit, Y.; Lewinsohn, E.; Pichersky, E.; Dudareva, N. Cytosolic monoterpene biosynthesis is supported by plastid-generated geranyl diphosphate substrate in transgenic tomato fruits. Plant J. 2013, 75, 351–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Chen, L.; Shen, X.; Yang, H.; Fang, Y.; Ouyang, W.; Mai, S.; Chen, H.; Chen, S.; et al. CRISPR/Cas9-mediated targeted mutagenesis of GmUGT enhanced soybean resistance against leaf-chewing insects through flavonoids biosynthesis. Front. Plant Sci. 2022, 13, 802716. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. How do plants “notice” attack by herbivorous arthropods? Biol. Rev. Camb. Philos. Soc. 2010, 85, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef] [Green Version]
- Haswell, E.S.; Peyronnet, R.; Barbier-Brygoo, H.; Meyerowitz, E.M.; Frachisse, J.M. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr. Biol. 2008, 18, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2010, 425, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, A.C.; Gribskov, M.; Harper, J.F. CDPKs—A kinase for every Ca2+ signal? Trends Plant Sci. 2000, 5, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ramachandiran, S.; Takezawa, D.; Wang, W.; Poovaiah, B.W. Functional domains of plant chimeric calcium/calmodulin-dependent protein kinase: Regulation by autoinhibitory and visinin-like domains. J. Biochem. 1997, 121, 984–990. [Google Scholar] [CrossRef]
- Hettenhausen, C.; Sun, G.; He, Y.; Zhuang, H.; Sun, T.; Qi, J.; Wu, J. Genome-wide identification of calcium-dependent protein kinases in soybean and analyses of their transcriptional responses to insect herbivory and drought stress. Sci. Rep. 2016, 6, 18973. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Lv, W.; Jiang, S.; Zhang, D.; Cai, G.; Pan, J.; Li, D. Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genom. 2013, 14, 433. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Willmann, M.R.; Chen, H.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhu, Y.; Tan, X.; Wang, X.; Wei, B.; Guo, H.; Zhang, Z.; Chen, X.; Zhao, G.; Kong, X.; et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 66, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-dependent protein kinases in plants: Evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, D.; Cai, L.; Wang, H.; Liu, X.; Du, H.; Yang, Z.; Zhang, H.; Hu, Z.; Huang, F.; et al. CALCIUM-DEPENDENT PROTEIN KINASE38 regulates flowering time and common cutworm resistance in soybean. Plant Physiol. 2022, 190, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Che, Z.; Zeng, X.; Zhou, X.; Sitoe, H.M.; Wang, H.; Yu, D. Genome-wide analysis of calcium-dependent protein kinases and their expression patterns in response to herbivore and wounding stresses in soybean. Funct. Integr. Genom. 2016, 16, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Matschi, S.; Werner, S.; Schulze, W.X.; Legen, J.; Hilger, H.H.; Romeis, T. Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development. Plant J. 2013, 73, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Matschi, S.; Shorinola, O.; Rovenich, H.; Matei, A.; Segonzac, C.; Malinovsky, F.G.; Rathjen, J.; MacLean, D.; Romeis, T.; et al. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 2014, 16, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Hettenhausen, C.; Baldwin, I.T.; Wu, J. Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound and herbivory-induced jasmonic acid accumulations. Plant Physiol. 2012, 159, 1591–1607. [Google Scholar] [CrossRef] [Green Version]
- Nie, L.; Wang, R.; Xia, Y.; Li, G. CDPK1, an Arabidopsis thaliana calcium-dependent protein kinase, is involved in plant defense response. Russ. J. Plant Physiol. 2015, 62, 866–874. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Takahashi, H.; Quadro, S.; Maffei, M.E.; Bossi, S.; Bertea, C.; Zebelo, S.A.; Muroi, A.; Ishihama, N.; Yoshioka, H.; et al. Regulation of Arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol. 2010, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Che, Z.; Zeng, X.; Zhang, G.; Wang, H.; Yu, D. Identification of single nucleotide polymorphisms in soybean associated with resistance to common cutworm (Spodoptera litura Fabricius). Euphytica 2016, 209, 49–62. [Google Scholar] [CrossRef]
- Bredow, M.; Bender, K.W.; Johnson Dingee, A.; Holmes, D.R.; Thomson, A.; Ciren, D.; Tanney, C.; Dunning, K.E.; Trujillo, M.; Huber, S.C.; et al. Phosphorylation-dependent subfunctionalization of the calcium-dependent protein kinase CPK28. Proc. Natl. Acad. Sci. USA 2021, 118, e2024272118. [Google Scholar] [CrossRef]
- Fan, R.; Li, X.; Wang, S.; Yu, D.; Wang, H. The soybean S-adenosylmethionine synthetase gene GmSAMS1 confers resistance to common cutworm in transgenic tobacco. Soybean Sci. 2018, 37, 268–274. [Google Scholar]
- Klümper, W.; Qaim, M. A meta-analysis of the impacts of genetically modified crops. PLoS ONE 2014, 9, e111629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef] [PubMed]
- Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. South Afr. J. Plant Soil 2007, 27, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and characterization of feld resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef]
- Zhu, S.; Walker, D.R.; Boerma, H.R.; All, J.N.; Parrott, W.A. Effects of defoliating insect resistance QTLs and a cry1Ac transgene in soybean near-isogenic lines. Theor. Appl. Genet. 2008, 116, 455–463. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, N.; Yang, G.; Hayashi, N.; Komatsu, S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: Comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 2005, 46, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Lv, X.; Xia, X.; Zhou, J.; Shi, K.; Yu, J.; Zhou, Y. Genome-wide identification and expression analysis of calcium-dependent protein kinase in tomato. Front. Plant Sci. 2016, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Wei, F.; Wang, C.; Wu, J.; Ratnasekera, D.; Liu, W.; Wu, W. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Himschoot, E.; Chen, J.; Boudsocq, M.; Geelen, D.; Friml, J.; Beeckman, T.; Vanneste, S. Constitutive active CPK30 interferes with root growth and endomembrane trafficking in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 862398. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, H.; Li, S.; Han, Z.; Li, B. Calcium signaling networks mediate nitrate sensing and responses in Arabidopsis. Plant Signal. Behav. 2021, 16, 1938441. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Hu, W.; Deng, X.; Zhang, Y.; Liu, X.; Zhao, X.; Luo, Q.; Jin, Z.; Li, Y.; Zhou, S.; et al. A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol. 2014, 14, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, R.; Qu, Y.; Guo, Y.; Yu, L.; Liu, Y.; Jiang, J.; Chen, J.; Ren, Y.; Liu, G.; Tian, L.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Sun, H.; Xu, D.; Chen, Q.; Liang, Y.; Wang, X.; Xu, G.; Tian, J.; Wang, C.; Li, D.; et al. ZmCCT9 enhances maize adaptation to higher latitudes. Proc. Natl. Acad. Sci. USA 2018, 115, E334–E341. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Shi, S.; Xu, M.; Cui, J. Current research on soybean pest management in China. Oil Crop Sci. 2018, 3, 215–227. [Google Scholar]
- Shi, S. Theory and Technology of Integrated Pest Management in Soybean; Jilin Publishing Group Co., Ltd.: Jilin, China, 2013; Volume 4, pp. 100–104. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.; Pennington, J. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Fan, R.; Wang, H.; Wang, Y.; Yu, D. Proteomic analysis of soybean defense response induced by cotton worm (Prodenia litura, Fabricius) feeding. Proteome Sci. 2012, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chu, S.; Zhang, H.; Zhu, Y.; Cheng, H.; Yu, D. Development and application of a novel genome-wide SNP array reveals domestication history in soybean. Sci. Rep. 2016, 6, 20728. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stable transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zeng, X.; Zhao, M.; Cui, X.; Wang, Q.; Yang, H.; Cheng, H.; Yu, D. Efficient targeted mutagenesis in soybean by TALENs and CRISPR/Cas9. J. Biotechnol. 2016, 217, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Dong, L.; Fang, C.; Liu, S.; Kong, L.; Cheng, Q.; Chen, L.; Su, T.; Nan, H.; Zhang, D.; et al. Stepwise selection on homeologous PRR genes controlling flowering and maturity during soybean domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gao, Z.; Fan, R.; Zhang, Y.; Qian, W.; Yu, D. Evaluation of resistance of soybean germplasm to common cutworm based on three resistance mechanisms. Soybean Sci. 2011, 30, 8–14. [Google Scholar]
- Du, H.; Li, X.; Ning, L.; Qin, R.; Du, Q.; Wang, Q.; Song, H.; Huang, F.; Wang, H.; Yu, D. RNA-Seq analysis reveals transcript diversity and active genes after common cutworm (Spodoptera litura Fabricius) attack in resistant and susceptible wild soybean lines. BMC Genom. 2019, 20, 237. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, X.; Su, F.; Liu, H.; Hu, D.; Huang, F.; Yu, D.; Wang, H. Soybean CALCIUM-DEPENDENT PROTEIN KINASE17 Positively Regulates Plant Resistance to Common Cutworm (Spodoptera litura Fabricius). Int. J. Mol. Sci. 2022, 23, 15696. https://doi.org/10.3390/ijms232415696
Wang H, Li X, Su F, Liu H, Hu D, Huang F, Yu D, Wang H. Soybean CALCIUM-DEPENDENT PROTEIN KINASE17 Positively Regulates Plant Resistance to Common Cutworm (Spodoptera litura Fabricius). International Journal of Molecular Sciences. 2022; 23(24):15696. https://doi.org/10.3390/ijms232415696
Chicago/Turabian StyleWang, Huiqi, Xiao Li, Fenglin Su, Hailun Liu, Dezhou Hu, Fang Huang, Deyue Yu, and Hui Wang. 2022. "Soybean CALCIUM-DEPENDENT PROTEIN KINASE17 Positively Regulates Plant Resistance to Common Cutworm (Spodoptera litura Fabricius)" International Journal of Molecular Sciences 23, no. 24: 15696. https://doi.org/10.3390/ijms232415696




