Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization
2.1.1. Microstructural Characterization
2.1.2. Morphology of Samples by Electron Microscope Analysis
2.2. Structural Investigation of PCL Polymer, CP, and dCP Powders as Well as dCP-PCL Composites by XRD and FT-IR Measurements
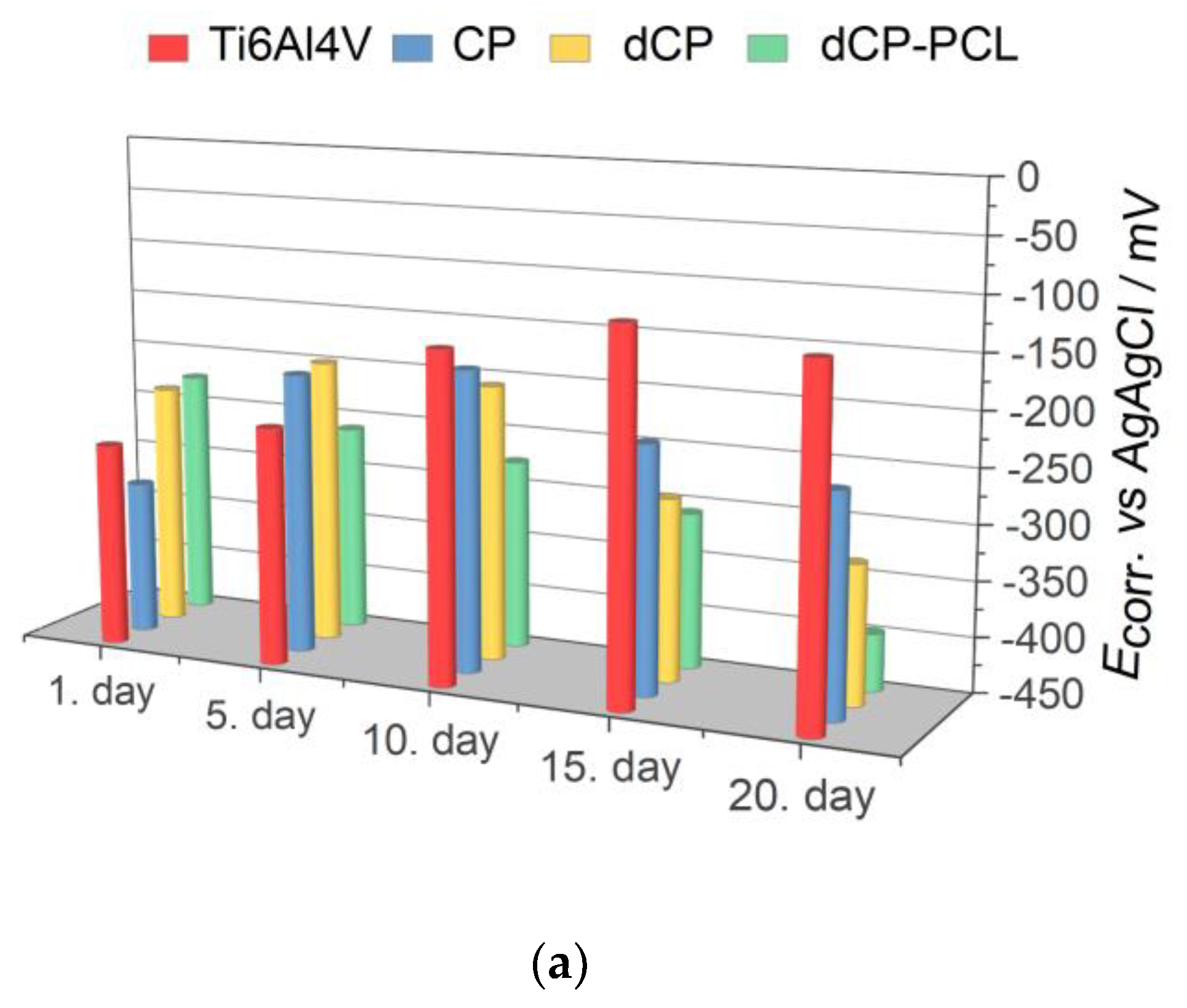
2.3. Corrosion Test, Biodegradability Characterization
3. Materials and Methods
3.1. Preparation of Nanocrystalline Calcium Phosphate (CP) Powders
3.2. Preparation of Biominerals Doped Nanocrystalline Apatite (dCP) Powders
3.3. Spin Coating of Powder and Composite Coatings
3.4. Characterization Methods
3.4.1. Microstructure Study
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. X-ray Diffraction Analysis
3.4.4. FT-IR Analysis
3.4.5. ICP-AES Measurements on CP and dCP Powders
3.4.6. Corrosion Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González–Carrasco, J.L.; Cifuentes Cuellar, S.C.; Lieblich Rodríguez, M. Chapter 5—Metals. In Bone Repair Biomaterials: Regeneration and Clinical Applications, 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing Series in Biomaterials; Elsevier: Sawston, UK, 2019; pp. 103–140. [Google Scholar]
- Yeung, K.W.; Wong, K.H. Biodegradable metallic materials for orthopaedic implantations: A review. Technol. Health Care 2012, 20, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanawa, T. Metal Ion release from metal implants. Mater. Eng. C 2004, 12, 745–752. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88. [Google Scholar] [CrossRef]
- Habraken, W.; Habinovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Goodrich, J.T.; Sandler, A.L.; Tepper, O. A review of reconstructive materials for use in craniofacial surgery bone fixation materials, bone substitutes, and distractors. Child’s Nerv. Syst. 2012, 28, 1577–1588. [Google Scholar] [CrossRef]
- Korytkin, A.A.; Orlinskaya, N.Y.; Novikova, Y.S.; Gerasimov, S.A.; Davydenko, D.V.; Kulakova, K.V.; Tverdokhlebov, S.I.; Bolbasov, E.N. Biocompatibility and Osseointegration of Calcium Phosphate-Coated and Non-Coated Titanium Implants with Various Porosities. Sovrem Tekhnologii Med. 2021, 13, 52–57. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Sulyok, A.; Kis, V.K.; Balázsi, K.; Mihály, J.; Balázsi, C. Preparation and morphological investigation on bioactive ion-modified carbonated hydroxyapatite biopolymer composite ceramics as coatings for orthopaedic implants. Ceram. Int. 2022, 48, 760–768. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Morphological, Chemical, and Biological Investigation of Ionic Substituted, Pulse Current Deposited Calcium Phosphate Coatings. Materials 2020, 13, 4690. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Calcium Phosphate Based Bioactive Ceramic Layers on Implant Materials Preparation, Properties and Biological Performance. Coatings 2020, 10, 823. [Google Scholar] [CrossRef]
- Wolf- Brandstetter, C.; Beutner, R.; Hess, R.; Bierbaum, S.; Wagner, K.; Scharnweber, D.; Gbureck, U.; Moseke, C. Multifunctional calcium phosphate based coatings on titanium implants with integrated trace elements. Biomed. Mater. 2020, 15, 025006. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Adzila, S.; Mustaffa, N.A.; Kanasan, N. Magnesium-doped calcium phosphate/sodium alginate biocomposite for bone implant application. J. Aust. Ceram. Soc. 2020, 56, 109–115. [Google Scholar] [CrossRef]
- Amaravathy, P.; Kumar, T.S.S. Bioactivity enhancement by Sr doped Zn-Ca-P coatings on biomedical magnesium alloy. J. Magnes. Alloys 2019, 7, 584–596. [Google Scholar] [CrossRef]
- Pemmer, B.; Roschger, A.; Wastl, A.; Hofstaetter, J.; Wobrauschek, P.; Simon, R.; Thaler, H.; Klaushofer, K.; Streli, C. Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone 2013, 57, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, S.; Ai, F.; Yan, J.; Zhou, K. Fabrication and Characterization of Sr-doped Hydroxyapatite Porous Scaffold. JOM 2021, 73, 1745–1753. [Google Scholar] [CrossRef]
- Yan, M.-D.; Ou, Y.-J.; Lin, Y.-J.; Liu, R.-M.; Fang, Y.; Wu, W.-L.; Zhou, L.; Yao, X.; Chen, J. Does the incorporation of strontium into calcium phosphate improve bone repair? A meta-analysis. BMC Oral Health 2022, 22, 62. [Google Scholar] [CrossRef]
- Kumar, A.; Gajraj, V.; Das, A.; Sen, D.; Xu, H.; Mariappan, C.R. Silver, Copper, Magnesium and Zinc Contained Electroactive Mesoporous Bioactive S53P4 Glass–Ceramics Nanoparticle for Bone Regeneration: Bioactivity, Biocompatibility and Antibacterial Activity. J. Inorg. Organomet Polym. 2022, 32, 2309–2321. [Google Scholar] [CrossRef]
- Uysal, I.; Yilmaz, B.; Evis, Z. Zn-doped hydroxyapatite in biomedical applications. J. Aust. Ceram. Soc. 2021, 57, 869–897. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Zhang, X.; Nune, K.C.; Zhao, Y.; Liu, N.; Misra, R.D.K.; Yang, K.; Tan, L.; Yan, J. The effect of different coatings on bone response and degradation behavior of porous magnesium-strontium devices in segmental defect regeneration. Bioact. Mater. 2021, 6, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Bohara, S.; Suthakorn, J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: A review. Biomater. Res. 2022, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Damerau, J.M.; Bierbaum, S.; Wiedemeier, D.; Korn, P.; Smeets, R.; Jenny, G.; Nadalini, J.; Stadlinger, B. A systematic review on the effect of inorganic surface coatings in large animal models and meta-analysis on tricalcium phosphate and hydroxyapatite on periimplant bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 157–175. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Mihály, J.; Balázsi, K.; Balázsi, C. Comparison of the Morphological and Structural Characteristic of Bioresorbable and Biocompatible Hydroxyapatite-Loaded Biopolymer Composites. Nanomaterials 2021, 11, 3194. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (∊-caprolactone) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9, 74–90. [Google Scholar] [CrossRef]
- Lam, C.X.; Hutmacher, D.W.; Schantz, J.T.; Teoh, H.S. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Rafique, I.; Lu, Z. Star-shaped polycaprolactone bearing mussel-inspired catechol end-groups as a promising bio-adhesive. Eur. Polym. J. 2020, 139, 110025. [Google Scholar] [CrossRef]
- Rezaei, A.; Mohammadi, M.R. In vitro study of hydroxyapatite/polycaprolactone (HA/PCL) nanocomposite synthesized by an in situ sol–gel process. Mater. Sci. Eng. C 2013, 33, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Kalantar, M.; Kharaziha, M.; LAmbrosio, L.; Grazia Raucci, M. Polycaprolactone/fluoride substituted-hydroxyapatite (PCL/FHA) nanocomposite coatings prepared by in-situ sol-gel process for dental implant applications. Prog. Org. Coat. 2020, 147, 105873. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Leonés Gil, A.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef] [PubMed]
- Amokrane, G.; Falentin-Daudré, C.; Ramtani, S.; Migonney, V. A Simple Method to Functionalize PCL Surface by Grafting Bioactive Polymers Using UV Irradiation. IRBM 2018, 39, 268–278. [Google Scholar] [CrossRef]
- Darren, S.; Holland, A.; Shanks, R. Poly(caprolactone) thin film preparation, morphology, and surface texture. J. Appl. Polym. Sci. 2006, 103, 1287–1294. [Google Scholar]
- Van, T.T.T.; Makkar, P.; Farwa, U.; Lee, B.T. Development of a novel polycaprolactone based composite membrane for periodontal regeneration using spin coating technique. J. Biomater. Sci. Polym. Ed. 2022, 33, 783–800. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Kukulka, E.C.; de Souza, J.R.; Borges, A.L.S.; Campos, T.M.B.; Thim, G.P.; de Vasconcellos, L.M.R. Development and characterization of PCL membranes incorporated with Zn-doped bioactive glass produced by electrospinning for osteogenesis evaluation. J. Polym. Res. 2022, 29, 370. [Google Scholar] [CrossRef]
- Ressler, A.; Bauer, L.; Prebeg, T.; Ledinski, M.; Hussainova, I.; Urlić, I.; Ivanković, M.; Ivanković, H. PCL/Si-Doped Multi-Phase Calcium Phosphate Scaffolds Derived from Cuttlefish Bone. Materials 2022, 15, 3348. [Google Scholar] [CrossRef]
- Yedekçi, B.; Tezcaner, A.; Yılmaz, B.; Demir, T.; Evis, Z. 3D porous PCL-PEG-PCL/strontium, magnesium and boron multi-doped hydroxyapatite composite scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2022, 125, 104941. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. A 2020, 7, 1546–1562. [Google Scholar] [CrossRef]
- Neščáková, Z.; Kaňková, H.; Galusková, D.; Galusek, D.; Boccaccini, A.R.; Liverani, L. Polymer (PCL) fibers with Zn-doped mesoporous bioactive glass nanoparticles for tissue regeneration. Int. J. Appl. Glass Sci. 2021, 12, 588–600. [Google Scholar] [CrossRef]
- Jinga, S.-I.; Costea, C.C.; Zamfirescu, A.I.; Banciu, A.; Banciu, D.D.; Busuioc, C. Composite Fiber Networks Based on Polycaprolactone and Bioactive Glass-Ceramics for Tissue Engineering Applications. Polymers 2020, 12, 1806. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, I.; Dziadek, M.; Kurowska, A.; Cholewa-Kowalska, K.; Ziąbka, M.; Menaszek, E.; Douglas, T.E. Electrospun polycaprolactone membranes with Zn-doped bioglass for nasal tissues treatment. J. Mater. Sci. Mater. Med. 2019, 30, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, L.; Antunović, M.; Gallego-Ferrer, G.; Ivanković, M.; Ivanković, H. PCL-Coated Multi-Substituted Calcium Phosphate Bone Scaffolds with Enhanced Properties. Materials 2021, 14, 4403. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Murugan, C.; Sundramoorthy, A.K. In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone-graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arabian J. Chem. 2018, 11, 959–969. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Wang, X.; Lai, Y.; Rui, G.; Lin, C. Hydroxyapatite-modified micro/nanostructured titania surfaces with different crystalline phases for osteoblast regulation. Bioact. Mater. 2021, 6, 1118–1129. [Google Scholar]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernández-Alfaro, F. Physicochemical characterization of barrier membranes for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef]
- Dennes, T.J.; Schwartz, J. A nanoscale Adhesion layer to promote cell attachment on PEEK. J. Am. Chem. Soc. 2009, 131, 3456–3457. [Google Scholar] [CrossRef]
- Boyan, B.; Sylvia, V.L.; Liu, Y.; Sagun, R.; Cochran, D.L.; Lohmann, C.H.; Dean, D.D.; Schwartz, Z. Surface roughness mediates its effects on osteoblasts via protein kinase A and phospholipase A2. Biomaterials 1999, 20, 2305–2310. [Google Scholar] [CrossRef]
- Du, J.; Wang, G.; Song, D.; Jiang, J.; Jiang, H.; Gao, J. In-vitro degradation behavior and biocompatibility of superhydrophilic hydroxyapatite coating on Mg-2Zn-Mn-Ca-Ce alloy. J. Mater. Res. Technol. 2022, 17, 2742–2754. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Tamai, N.; Murase, T.; Myoui, A. Interconnected porous hydroxyapatite ceramics for bone tissue engineering. J. R. Soc. Interface 2009, 6 (Suppl. 3), S341–S348. [Google Scholar] [CrossRef]
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [Google Scholar] [CrossRef]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ion release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: In vitro experiments and theoretical modelling study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of Titanium Implant Surface Topology on Bone Cell Attachment and Proliferation in vitro. Med. Devices 2022, 15, 103–119. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- de Azevedo Gonçalves Mota, R.; da Silva, E.; de Menezes, L. Polymer Nanocomposites Used as Scaffolds for Bone Tissue Regeneration. Mater. Sci. Appl. 2018, 9, 679–697. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, Y.; Sun, W.; Yang, X. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J. Dent. Sci. 2012, 7, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Eliaz, N.; Metok, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Lodoso-Torrecilla, I.; Klein Gunnewiek, R.; Grosfeld, E.C.; de Vries, R.B.M.; Habibović, P.; Jansen, J.A.; van den Beucken, J.J. Bioinorganic supplementation of calcium phosphate-based bone substitutes to improve in vivo performance: A systematic re-view and meta-analysis of animal studies. Biomater. Sci. 2020, 8, 4792–4809. [Google Scholar] [CrossRef]
- Boskey, A.L. Amorphous calcium phosphate: The contention of bone. J. Dent. Res. 1997, 76, 1433–1446. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Tzaphlidou, M.; Zaichick, V. Calcium, Phosphorus, Calcium–Phosphorus Ratio in Rib Bone of Healthy Humans. Biol. Trace Elem. Res. 2003, 3, 63–74. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Yin, M.; Yang, X.; Yuan, Q.; Ren, J.; Qu, X. Aptamer-Capped Multifunctional Mesoporous Strontium Hydroxyapatite Nanovehicle for Cancer-Cell-Responsive Drug Delivery and Imaging. Biomacromolecules 2012, 13, 4257–4263. [Google Scholar] [CrossRef]
- Specht, A.J.; Mostafaei, F.; Lin, Y.; Xu, J.; Nie, L.H. Measurements of Strontium Levels in Human Bone In Vivo Using Portable X-ray Fluorescence (XRF). Appl. Spectrosc. 2017, 71, 1962–1968. [Google Scholar] [CrossRef] [Green Version]
- Rattan, S.; Fawcett, D.; Poinern, G.E.J. Williamson-Hall based X-ray peak profile evaluation and nano-structural characterization of rod-shaped hydroxyapatite powder for potential dental restorative procedures. AIMS Mater. Sci. 2021, 8, 359–372. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Koerten, H.K.; van der Meulen, J. Degradation of calcium phosphate ceramics. J. Biomed. Mater. Res. 1999, 44, 78–86. [Google Scholar] [CrossRef]
- Al-Qasas, N.S.; Rohani, S. Synthesis of Pure Hydroxyapatite and the Effect of Synthesis Conditions on its Yield, Crystallinity, Morphology and Mean Particle Size. Sep. Sci. Technol. 2007, 40, 3187–3224. [Google Scholar] [CrossRef]
- Xue, J.J.; Shi, R.; Niu, Y.Z.; Gong, M.; Coates, P.; Crawford, A.; Chen, D.F.; Tian, W.; Zhang, L.Q. Fabrication of drug-loaded anti-infective guided tissue regeneration membrane with adjustable biodegradation property. Colloids Surf. B Biointerfaces 2015, 135, 846–854. [Google Scholar] [CrossRef]
- Baghali, M.; Ziyadi, H.; Faridi-Majidi, R. Fabrication and characterization of core–shell TiO2-containing nanofibers of PCL-zein by coaxial electrospinning method as an erythromycin drug carrier. Polym. Bull. 2022, 79, 1729–1749. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds, Hindawi Publishing Corporation. BioMed Res. Int. 2013, 2013, 490946. [Google Scholar]
- Slosarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744, 657–661. [Google Scholar] [CrossRef]
- Hong, S.I.; Lee, K.H.; Outslay, M.E. Ultrastructural analyses of nanoscale apatite biomimetically grown on organic template. J. Mater. Res. 2008, 23, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Macipe, A.; Rodriguez-Clemente, R.; Hidalgo-Lopez, A.; Arita, I.; Garcia-Garduno, M.V.; Rivera, E.; Castano, V.M. Wet Chemical Synthesis of Hydroxyapatite Particles from Nonstoichiometric Solutions. J. Mater. Synth. Process 1998, 6, 21–26. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Cazabou, S.; Grossin, D.; Brouillet, F.; Sarda, S. Surface properties of biomimetic nanocrystalline apatites; applications in biomaterials. Prog. Cryst. Growth Charact. Mater. 2014, 60, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Cansu Gurlek, A.C.; Sevinc, B.; Bayrak, E.; Erisken, C. Synthesis and characterization of polycaprolactone for anterior cruciate ligament regeneration. Mater. Sci. Eng. C 2017, 71, 820–826. [Google Scholar] [CrossRef]
- Nanni, G.; José, A.; Heredia-Guerrero, J.A.; Paul, U.C.; Dante, S.; Gianvito Caputo, G.; Canale, C.; Athanassiou, A.; Despina Fragouli, D.; Bayer, I.S. Poly(furfuryl alcohol)-Polycaprolactone Blends. Polymers 2019, 11, 1069. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, G.S.; Muńoz, P.A.R.; de Oliveira, C.F.P.; da Silva, L.C.E.; Malhotra, R.; Gonçalves, M.C.; ·Rosa, V.; ·Fechine, G.J.M. Polymer Nanocomposites Based on Poly(ε-caprolactone), Hydroxyapatite and Graphene Oxide. J. Polym. Environ. 2020, 28, 331–342. [Google Scholar] [CrossRef]
- Seri, O.; Siree, B. The Differentiating Polarization Curve Technique for the Tafel Parameter Estimation. Catalysts 2017, 7, 239. [Google Scholar] [CrossRef]
- Eken, T.Y.; Sarioglu, C.; Kucuk, I. Comparison of Tafel Extrapolation and Linear Polarization Resistance Readings for TRC 8006 Aluminium Alloys in 3.5 wt. % NaCI Aqueous Solution. J. Innov. Sci. Eng. 2018, 2, 19–24. [Google Scholar]
- Pawłowski, Ł.; Rosciszewska, M.; Majkowska-Marzec, B.; Jazdzewska, M.; Bartmanski, M.; Zielinski, A.; Tybuszewska, N.; Samsel, P. Influence of Surface Modification of Titanium and Its Alloys for Medical Implants on Their Corrosion Behavior. Materials 2022, 15, 7556. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Priyadarshini, B.; Stango, S.A.X.; Chellappa, M.; Geetha, M.; Vijayalakshmi, U. Preparation and characterisation of sol–gel-derived hydroxyapatite nanoparticles and its coatings on medical grade Ti-6Al-4V alloy for biomedical applications. Mater. Technol. 2017, 32, 800–814. [Google Scholar] [CrossRef]
- Shokri, N.; Safavi, M.S.; Etminanfar, M.; Walsh, F.C.; Khalil-Allafi, J. Enhanced corrosion protection of NiTi orthopedic implants by highly crystalline hydroxyapatite deposited by spin coating: The importance of pre-treatment. Mater. Chem. Phys. 2021, 259, 124041. [Google Scholar] [CrossRef]
- Pham, D.N.; Hiromoto, S.; Minho, O.; Kobayashi, E. Influence of substrate microstructure on hydroxyapatite coating and corrosion behavior of coated MgZn alloys. Surf. Coat. Technol. 2021, 421, 127414. [Google Scholar] [CrossRef]
- Uribe, R.; Uvillús, A.; Fernández, L.; Bonilla, O.; Jara, A.; González, G. Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium. Coatings 2022, 12, 440. [Google Scholar] [CrossRef]
- Kwok, C.T.; Wong, P.K.; Cheng, F.T.; Man, H.C. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Appl. Surf. Sci. 2013, 255, 6736–6744. [Google Scholar]
- Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Que, L.X.; Van Anh, N.; Hoang, T.; Lam, T.D. Controlling the electrodeposition, morphology and structure of hydroxyapatite coating on 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Castro, W.; Hoogewerff, J.; Latkoczy, C.; Almirall, J.R. Application of laser ablation (LA-ICP-SF-MS) for the elemental analysis of bone and teeth samples for discrimination purposes. Forensic Sci. Int. 2010, 195, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Golovanova, O.A.; Strunina, N.N.; Lemesheva, S.A.; Baisova, B.T. Determination of the elemental composition of human bone tissue by atomic emission spectral analysis. J. Appl. Spectrosc. 2011, 78, 157–160. [Google Scholar] [CrossRef]
- Coyte, R.M.; Harkness, J.S.; Darrah, T.H. The abundance of trace elements in human bone relative to bone type and bone pathology. GeoHealth 2022, 6, e2021GH000556. [Google Scholar] [CrossRef]
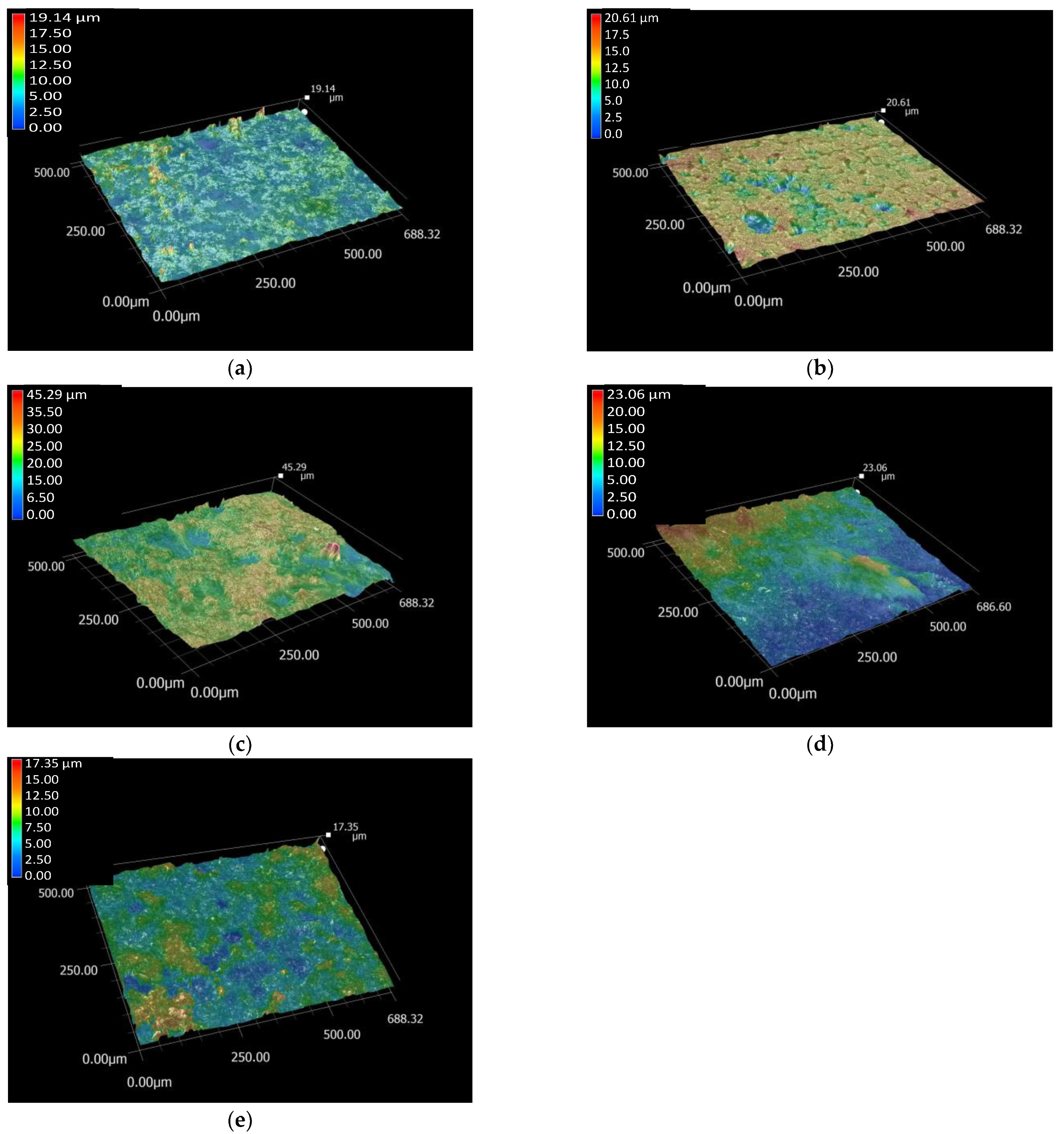
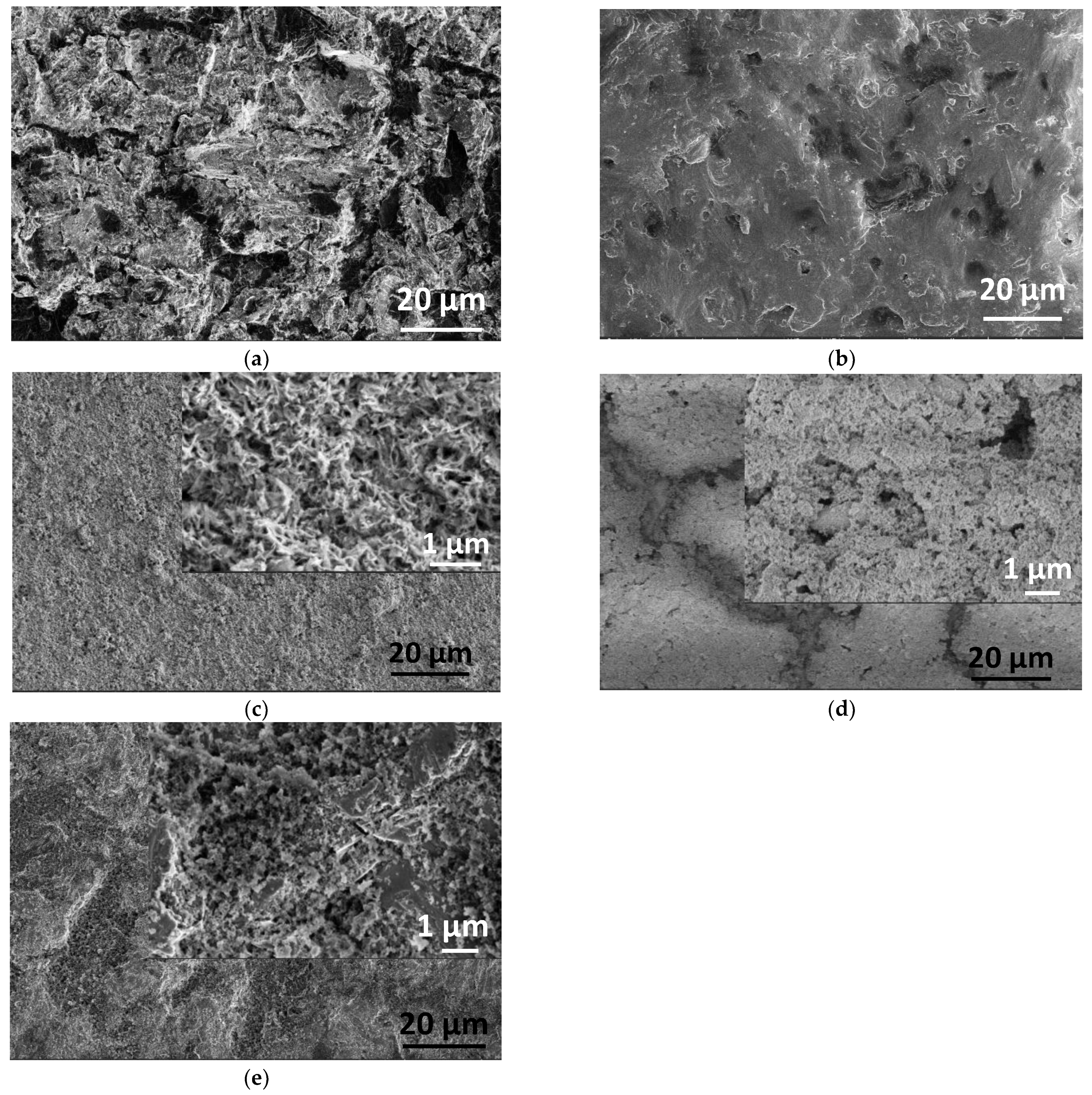





| Samples | Ra/µm | Rz/µm | Rz/Ra 1 |
|---|---|---|---|
| Ti6Al4V substrate | 0.87 ± 0.27 | 6.11 ± 2.88 | 7.02 |
| PCL layer | 1.34 ± 0.44 | 10.52 ± 3.29 | 7.85 |
| CP layer | 2.61 ± 0.53 | 15.10 ± 5.05 | 5.78 |
| dCP | 1.98 ± 0.49 | 9.92 ± 2.44 | 5.01 |
| dCP-PCL layer | 1.41 ± 0.38 | 7.10 ± 1.66 | 5.03 |
| Elements | Ca | P | Mg | Zn | Sr | Ca/P | (Ca + Mg + Zn + Sr)/P |
|---|---|---|---|---|---|---|---|
| CP | 43.02 | 23.59 | - | - | - | 1.82 | - |
| dCP | 40.82 | 23.19 | 1.61 | 0.46 | 0.28 | 1.76 | 1.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furko, M.; Horváth, Z.E.; Czömpöly, O.; Balázsi, K.; Balázsi, C. Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. Int. J. Mol. Sci. 2022, 23, 15737. https://doi.org/10.3390/ijms232415737
Furko M, Horváth ZE, Czömpöly O, Balázsi K, Balázsi C. Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. International Journal of Molecular Sciences. 2022; 23(24):15737. https://doi.org/10.3390/ijms232415737
Chicago/Turabian StyleFurko, Monika, Zsolt E. Horváth, Ottó Czömpöly, Katalin Balázsi, and Csaba Balázsi. 2022. "Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites" International Journal of Molecular Sciences 23, no. 24: 15737. https://doi.org/10.3390/ijms232415737
APA StyleFurko, M., Horváth, Z. E., Czömpöly, O., Balázsi, K., & Balázsi, C. (2022). Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. International Journal of Molecular Sciences, 23(24), 15737. https://doi.org/10.3390/ijms232415737









