Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities
Abstract
1. Introduction
2. ECM Proteins That Promote CM Proliferation and Cardiac Regeneration
3. ECM Proteins That Promote Endothelial Cell Proliferation and Cardiac Revascularization
4. Mechanical Properties of the ECM Controlling Regeneration
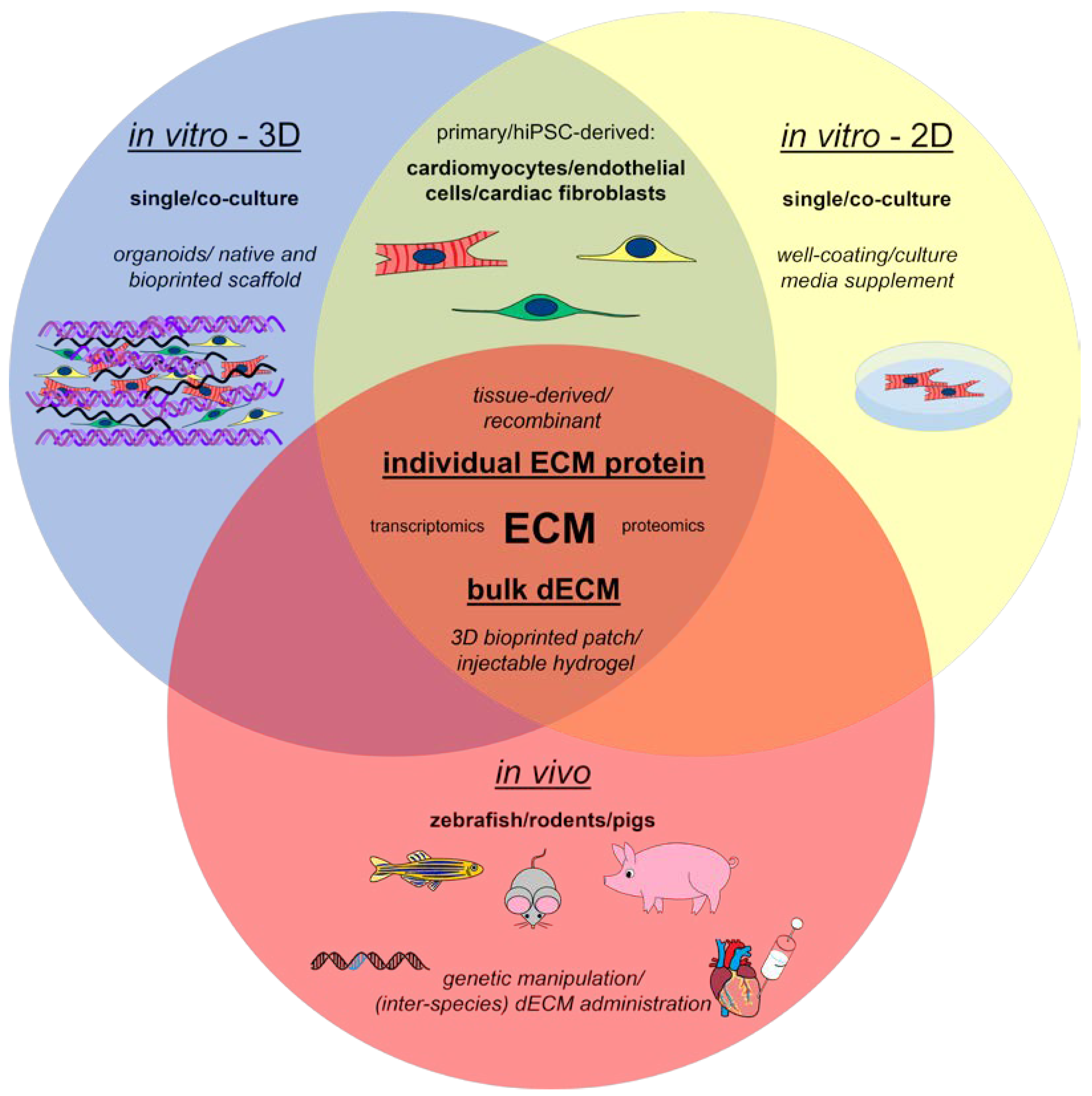
5. Strategies to Study the Role of ECM Proteins in Cardiac Regeneration in Experimental Models
5.1. In Vitro Models
5.2. Animal Models
5.2.1. Zebrafish
5.2.2. Rodents
5.2.3. Pigs
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Giacca, M.; Zacchigna, S. Harnessing the microRNA pathway for cardiac regeneration. J. Mol. Cell. Cardiol. 2015, 89, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, G.; Colliva, A.; Vuerich, R.; Pompilio, G.; Zacchigna, S. Biologics and cardiac disease: Challenges and opportunities. Trends Pharmacol. Sci. 2022, 43, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Baker, C.D.; Pritchett, E.M.; Burgos Villar, K.N.; Ashton, J.M.; Small, E.M. Characterizing Neonatal Heart Maturation, Regeneration, and Scar Resolution Using Spatial Transcriptomics. J. Cardiovasc. Dev. Dis. 2021, 9, 1. [Google Scholar] [CrossRef]
- Silva, A.C.; Pereira, C.; Fonseca, A.; Pinto-do, O.P.; Nascimento, D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2020, 8, 621644. [Google Scholar] [CrossRef] [PubMed]
- Sarohi, V.; Srivastava, S.; Basak, T. Comprehensive Mapping and Dynamics of Site-Specific Prolyl-Hydroxylation, Lysyl-Hydroxylation and Lysyl O-Glycosylation of Collagens Deposited in ECM During Zebrafish Heart Regeneration. Front. Mol. Biosci. 2022, 9, 892763. [Google Scholar] [CrossRef]
- Marin-Juez, R.; Marass, M.; Gauvrit, S.; Rossi, A.; Lai, S.L.; Materna, S.C.; Black, B.L.; Stainier, D.Y. Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 11237–11242. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef]
- Wirrig, E.E.; Snarr, B.S.; Chintalapudi, M.R.; O’Neal, J.L.; Phelps, A.L.; Barth, J.L.; Fresco, V.M.; Kern, C.B.; Mjaatvedt, C.H.; Toole, B.P.; et al. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev. Biol. 2007, 310, 291–303. [Google Scholar] [CrossRef]
- Sun, J.; Peterson, E.A.; Wang, A.Z.; Ou, J.; Smith, K.E.; Poss, K.D.; Wang, J. hapln1 Defines an Epicardial Cell Subpopulation Required for Cardiomyocyte Expansion During Heart Morphogenesis and Regeneration. Circulation 2022, 146, 48–63. [Google Scholar] [CrossRef]
- Bassat, E.; Mutlak, Y.E.; Genzelinakh, A.; Shadrin, I.Y.; Baruch Umansky, K.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Riabov Bassat, D.; et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017, 547, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Baehr, A.; Umansky, K.B.; Bassat, E.; Jurisch, V.; Klett, K.; Bozoglu, T.; Hornaschewitz, N.; Solyanik, O.; Kain, D.; Ferraro, B.; et al. Agrin Promotes Coordinated Therapeutic Processes Leading to Improved Cardiac Repair in Pigs. Circulation 2020, 142, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, J.; Hao, H.; Lin, H.; Wang, L.; Zhang, Y.; Chen, L.; Cao, S.; Huang, X.; Liao, W.; et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3beta/cyclin D1 signalling pathway. Cardiovasc. Res. 2017, 113, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.; del Monte, F.; Hajjar, R.J.; Chang, Y.S.; Lebeche, D.; Arab, S.; Keating, M.T. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007, 13, 962–969. [Google Scholar] [CrossRef]
- Ladage, D.; Yaniz-Galende, E.; Rapti, K.; Ishikawa, K.; Tilemann, L.; Shapiro, S.; Takewa, Y.; Muller-Ehmsen, J.; Schwarz, M.; Garcia, M.J.; et al. Stimulating myocardial regeneration with periostin Peptide in large mammals improves function post-myocardial infarction but increases myocardial fibrosis. PLoS ONE 2013, 8, e59656. [Google Scholar] [CrossRef]
- Lorts, A.; Schwanekamp, J.A.; Elrod, J.W.; Sargent, M.A.; Molkentin, J.D. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ. Res. 2009, 104, e1–e7. [Google Scholar] [CrossRef]
- Taniyama, Y.; Katsuragi, N.; Sanada, F.; Azuma, J.; Iekushi, K.; Koibuchi, N.; Okayama, K.; Ikeda-Iwabu, Y.; Muratsu, J.; Otsu, R.; et al. Selective Blockade of Periostin Exon 17 Preserves Cardiac Performance in Acute Myocardial Infarction. Hypertension 2016, 67, 356–361. [Google Scholar] [CrossRef]
- Wu, C.C.; Jeratsch, S.; Graumann, J.; Stainier, D.Y.R. Modulation of Mammalian Cardiomyocyte Cytokinesis by the Extracellular Matrix. Circ. Res. 2020, 127, 896–907. [Google Scholar] [CrossRef]
- Wang, J.; Karra, R.; Dickson, A.L.; Poss, K.D. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 2013, 382, 427–435. [Google Scholar] [CrossRef]
- Giacca, M.; Zacchigna, S. VEGF gene therapy: Therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012, 19, 622–629. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Fischer, J.W.; Gorski, D.J.; Harvey, R.P.; Kovacic, J.C. Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4: JACC Focus Seminar. J. Am.Coll. Cardiol. 2020, 75, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Loeys, B.; Mayr, M.; Rienks, M.; Verstraeten, A.; Kovacic, J.C. Extracellular Matrix in Vascular Disease, Part 2/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular Matrix, a Hard Player in Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1822. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Singelyn, J.M.; Salvatore, M.A.; Osborn, K.G.; Wang, J.J.; Sampat, U.; Kwan, O.L.; Strachan, G.M.; Wong, J.; Schup-Magoffin, P.J.; et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 2013, 5, 173ra125. [Google Scholar] [CrossRef] [PubMed]
- Singelyn, J.M.; Sundaramurthy, P.; Johnson, T.D.; Schup-Magoffin, P.J.; Hu, D.P.; Faulk, D.M.; Wang, J.; Mayle, K.M.; Bartels, K.; Salvatore, M.; et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 751–763. [Google Scholar] [CrossRef]
- Wassenaar, J.W.; Gaetani, R.; Garcia, J.J.; Braden, R.L.; Luo, C.G.; Huang, D.; DeMaria, A.N.; Omens, J.H.; Christman, K.L. Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J. Am. Coll. Cardiol. 2016, 67, 1074–1086. [Google Scholar] [CrossRef]
- Traverse, J.H.; Henry, T.D.; Dib, N.; Patel, A.N.; Pepine, C.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Chamberlin, P.; Christman, K.L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Korf-Klingebiel, M.; Reboll, M.R.; Grote, K.; Schleiner, H.; Wang, Y.; Wu, X.; Klede, S.; Mikhed, Y.; Bauersachs, J.; Klintschar, M.; et al. Heparan Sulfate-Editing Extracellular Sulfatases Enhance VEGF Bioavailability for Ischemic Heart Repair. Circ. Res. 2019, 125, 787–801. [Google Scholar] [CrossRef]
- Senk, A.; Djonov, V. Collagen fibers provide guidance cues for capillary regrowth during regenerative angiogenesis in zebrafish. Sci. Rep. 2021, 11, 19520. [Google Scholar] [CrossRef] [PubMed]
- Marin-Juez, R.; El-Sammak, H.; Helker, C.S.M.; Kamezaki, A.; Mullapuli, S.T.; Bibli, S.I.; Foglia, M.J.; Fleming, I.; Poss, K.D.; Stainier, D.Y.R. Coronary Revascularization During Heart Regeneration Is Regulated by Epicardial and Endocardial Cues and Forms a Scaffold for Cardiomyocyte Repopulation. Dev. Cell 2019, 51, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Wagh, G.; Mokalled, M.H.; Kontarakis, Z.; Dickson, A.L.; Rayrikar, A.; Gunther, S.; Poss, K.D.; Stainier, D.Y.R.; Patra, C. Ccn2a is an injury-induced matricellular factor that promotes cardiac regeneration in zebrafish. Development 2021, 148, dev193219. [Google Scholar] [CrossRef] [PubMed]
- El-Sammak, H.; Yang, B.; Guenther, S.; Chen, W.; Marin-Juez, R.; Stainier, D.Y.R. A Vegfc-Emilin2a-Cxcl8a Signaling Axis Required for Zebrafish Cardiac Regeneration. Circ. Res. 2022, 130, 1014–1029. [Google Scholar] [CrossRef]
- Ingason, A.B.; Goldstone, A.B.; Paulsen, M.J.; Thakore, A.D.; Truong, V.N.; Edwards, B.B.; Eskandari, A.; Bollig, T.; Steele, A.N.; Woo, Y.J. Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts. J. Thorac. Cardiovasc. Surg. 2018, 155, 1118–1127. [Google Scholar] [CrossRef]
- Notari, M.; Ventura-Rubio, A.; Bedford-Guaus, S.J.; Jorba, I.; Mulero, L.; Navajas, D.; Marti, M.; Raya, A. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 2018, 4, eaao5553. [Google Scholar] [CrossRef]
- Perestrelo, A.R.; Silva, A.C.; Oliver-De La Cruz, J.; Martino, F.; Horvath, V.; Caluori, G.; Polansky, O.; Vinarsky, V.; Azzato, G.; de Marco, G.; et al. Multiscale Analysis of Extracellular Matrix Remodeling in the Failing Heart. Circ. Res. 2021, 128, 24–38. [Google Scholar] [CrossRef]
- Corbin, E.A.; Vite, A.; Peyster, E.G.; Bhoopalam, M.; Brandimarto, J.; Wang, X.; Bennett, A.I.; Clark, A.T.; Cheng, X.; Turner, K.T.; et al. Tunable and Reversible Substrate Stiffness Reveals a Dynamic Mechanosensitivity of Cardiomyocytes. ACS Appl. Mater. Interfaces 2019, 11, 20603–20614. [Google Scholar] [CrossRef]
- Yahalom-Ronen, Y.; Rajchman, D.; Sarig, R.; Geiger, B.; Tzahor, E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife 2015, 4, e07455. [Google Scholar] [CrossRef]
- Crosby, C.O.; Valliappan, D.; Shu, D.; Kumar, S.; Tu, C.; Deng, W.; Parekh, S.H.; Zoldan, J. Quantifying the Vasculogenic Potential of Induced Pluripotent Stem Cell-Derived Endothelial Progenitors in Collagen Hydrogels. Tissue Eng. Part A 2019, 25, 746–758. [Google Scholar] [CrossRef]
- Mewhort, H.E.M.; Svystonyuk, D.A.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Guzzardi, D.G.; Park, D.S.; Kang, S.; Hollenberg, M.D.; Fedak, P.W.M. Bioactive Extracellular Matrix Scaffold Promotes Adaptive Cardiac Remodeling and Repair. JACC Basic Transl. Sci. 2017, 2, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Senapati, S.; Akinbote, A.; Gnanasambandam, B.; Park, P.S.; Senyo, S.E. Microenvironment stiffness requires decellularized cardiac extracellular matrix to promote heart regeneration in the neonatal mouse heart. Acta Biomater. 2020, 113, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Starchenko, A.; Williams, R.M.; Bonassar, L.J.; Reinhart-King, C.A. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013, 9, 4635–4644. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, N.; Liu, H.; Zhao, L.; Liu, J.; Wan, J.; Wu, W.; Lei, H.; Liu, R.; Han, M. Lysyl oxidase inhibition via beta-aminoproprionitrile hampers human umbilical vein endothelial cell angiogenesis and migration in vitro. Mol. Med. Rep. 2018, 17, 5029–5036. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Puig, A.; Mosquera, J.L.; Jimenez-Delgado, S.; Garcia-Pastor, C.; Jorba, I.; Navajas, D.; Canals, F.; Raya, A. Proteomics Analysis of Extracellular Matrix Remodeling During Zebrafish Heart Regeneration. Mol. Cell. Proteom. 2019, 18, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Didangelos, A.; Schoendube, F.A.; Drozdov, I.; Yin, X.; Fernandez-Caggiano, M.; Willeit, P.; Puntmann, V.O.; Aldama-Lopez, G.; Shah, A.M.; et al. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation 2012, 125, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Silva, A.C.; Oliveira, M.J.; McDevitt, T.C.; Barbosa, M.A.; Nascimento, D.S.; Pinto-do, O.P. Comparable Decellularization of Fetal and Adult Cardiac Tissue Explants as 3D-like Platforms for In Vitro Studies. J. Vis. Exp. 2019, 145, e56924. [Google Scholar] [CrossRef]
- Chen, W.C.; Wang, Z.; Missinato, M.A.; Park, D.W.; Long, D.W.; Liu, H.J.; Zeng, X.; Yates, N.A.; Kim, K.; Wang, Y. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci. Adv. 2016, 2, e1600844. [Google Scholar] [CrossRef]
- Williams, C.; Quinn, K.P.; Georgakoudi, I.; Black, L.D., 3rd. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 2014, 10, 194–204. [Google Scholar] [CrossRef]
- Bejleri, D.; Robeson, M.J.; Brown, M.E.; Hunter, J.; Maxwell, J.T.; Streeter, B.W.; Brazhkina, O.; Park, H.J.; Christman, K.L.; Davis, M.E. In vivo evaluation of bioprinted cardiac patches composed of cardiac-specific extracellular matrix and progenitor cells in a model of pediatric heart failure. Biomater. Sci. 2022, 10, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ansari, A.; Pierre, V.; Young, K.; Kothapalli, C.R.; von Recum, H.A.; Senyo, S.E. Injectable Extracellular Matrix Microparticles Promote Heart Regeneration in Mice with Post-ischemic Heart Injury. Adv. Healthc. Mater. 2022, 11, e2102265. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Sullivan, K.; Black, L.D. 3rd. Partially Digested Adult Cardiac Extracellular Matrix Promotes Cardiomyocyte Proliferation In Vitro. Adv. Healthc. Mater. 2015, 4, 1545–1554. [Google Scholar] [CrossRef]
- Ahmadi, A.; Thorn, S.L.; Alarcon, E.I.; Kordos, M.; Padavan, D.T.; Hadizad, T.; Cron, G.O.; Beanlands, R.S.; DaSilva, J.N.; Ruel, M.; et al. PET imaging of a collagen matrix reveals its effective injection and targeted retention in a mouse model of myocardial infarction. Biomaterials 2015, 49, 18–26. [Google Scholar] [CrossRef]
- Serpooshan, V.; Zhao, M.; Metzler, S.A.; Wei, K.; Shah, P.B.; Wang, A.; Mahmoudi, M.; Malkovskiy, A.V.; Rajadas, J.; Butte, M.J.; et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013, 34, 9048–9055. [Google Scholar] [CrossRef]
- Ozcebe, S.G.; Bahcecioglu, G.; Yue, X.S.; Zorlutuna, P. Effect of cellular and ECM aging on human iPSC-derived cardiomyocyte performance, maturity and senescence. Biomaterials 2021, 268, 120554. [Google Scholar] [CrossRef]
- Rosario-Quinones, F.; Magid, M.S.; Yau, J.; Pawale, A.; Nguyen, K. Tissue reaction to porcine intestinal Submucosa (CorMatrix) implants in pediatric cardiac patients: A single-center experience. Ann. Thorac. Surg. 2015, 99, 1373–1377. [Google Scholar] [CrossRef]
- Becker, L.M.; Chen, S.H.; Rodor, J.; de Rooij, L.; Baker, A.H.; Carmeliet, P. Deciphering Endothelial Heterogeneity in Health and Disease at Single Cell Resolution: Progress and Perspectives. Cardiovasc. Res. 2022, 118, e98–e100. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, K.; Feng, Q.; Liao, Y. Cardiac Organoids: A 3D Technology for Modeling Heart Development and Disease. Stem Cell Rev. Rep. 2022, 18, 2593–2605. [Google Scholar] [CrossRef]
- Kupfer, M.E.; Lin, W.H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat. Commun. 2020, 11, 4283. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 2021, 28, 2137–2152.e2136. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, F.; Boschetti, F.; Polvani, G.; Banfi, C.; Pesce, M.; Santoro, R. Aortic valve cell seeding into decellularized animal pericardium by perfusion-assisted bioreactor. J. Tissue Eng. Regen. Med. 2018, 12, 1481–1493. [Google Scholar] [CrossRef]
- Robertson, M.J.; Dries-Devlin, J.L.; Kren, S.M.; Burchfield, J.S.; Taylor, D.A. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS ONE 2014, 9, e90406. [Google Scholar] [CrossRef]
- Li, H.; Bao, M.; Nie, Y. Extracellular matrix-based biomaterials for cardiac regeneration and repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Izpisua Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Gamba, L.; Harrison, M.; Lien, C.L. Cardiac regeneration in model organisms. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 288. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Zhu, F.; Nair, R.R.; Fisher, E.M.C.; Cunningham, T.J. Humanising the mouse genome piece by piece. Nat. Commun. 2019, 10, 1845. [Google Scholar] [CrossRef]
- Sampaio-Pinto, V.; Rodrigues, S.C.; Laundos, T.L.; Silva, E.D.; Vasques-Novoa, F.; Silva, A.C.; Cerqueira, R.J.; Resende, T.P.; Pianca, N.; Leite-Moreira, A.; et al. Neonatal Apex Resection Triggers Cardiomyocyte Proliferation, Neovascularization and Functional Recovery Despite Local Fibrosis. Stem Cell Rep. 2018, 10, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Ma, F.F.; Zhang, W.; Li, Y.; Wei, F.Y.; Zhou, L. Qualitative research of alternatively splice variants of fibronectin in different development stage of mice heart. J. Thorac. Dis. 2015, 7, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Dasa, O.; Maden, M.; Vohra, R.; Batra, A.; Walter, G.; Yarrow, J.F.; Aranda, J.M., Jr.; Raizada, M.K.; Pepine, C.J. Functional heart recovery in an adult mammal, the spiny mouse. Int. J. Cardiol. 2021, 338, 196–203. [Google Scholar] [CrossRef]
- Mosala Nezhad, Z.; Poncelet, A.; de Kerchove, L.; Gianello, P.; Fervaille, C.; El Khoury, G. Small intestinal submucosa extracellular matrix (CorMatrix(R)) in cardiovascular surgery: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Hernandez, M.J.; Christman, K.L. Designing Acellular Injectable Biomaterial Therapeutics for Treating Myocardial Infarction and Peripheral Artery Disease. JACC Basic Transl. Sci. 2017, 2, 212–226. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Ferro, M.D.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Ingber, D.E. Can cancer be reversed by engineering the tumor microenvironment? Semin. Cancer Biol. 2008, 18, 356–364. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C. The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. BioEssays 2011, 33, 332–340. [Google Scholar] [CrossRef]
| ECM Protein | In Vitro/ In Vivo | 2D/3D | Cell Type | Species | Method | Effect of the ECM Protein and Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Hyaluronic acid | in vivo | zebrafish | hapln1 KO | ↑ CM proliferation | [10] | ||
| in vivo | mouse | hapln1 KO | ↑ CM proliferation | [9] | |||
| Periostin | in vitro + in vivo | 2D | neonatal CMs | mouse | periostin KO | ↑CM proliferation by activation of PI3K/GSK3β/cyclin D1 pathway, ↑ angiogenesis | [13] |
| in vitro + in vivo | 2D | adult CMs | rat | recombinant periostin | ↑ CM proliferation by activation of integrins and PI3K pathway, ↑ angiogenesis | [14] | |
| in vivo | pig | recombinant periostin | ↑ angiogenesis and fibrosis | [15] | |||
| in vitro + in vivo | 2D | neonatal CMs and FBs | rat, mouse | periostin KO, overexpression and recombinant periostin | No changes in cell proliferation | [16] | |
| in vitro + in vivo | 2D | cardiac FBs | rat | anti-periostin antibodies | ↑ fibrosis by TGF-β1 activation | [17] | |
| Agrin | in vitro + in vivo | 2D,3D | hiPSC-CMs, neonatal mouse CMs | mouse, human | recombinant agrin and agrin cKO mice | ↑ CM proliferation by DGC disassembly and Yap/ERK pathway | [11] |
| in vitro + in vivo | 2D | HUVECs | pig, mouse, human | recombinant agrin | ↑ cardiac function and angiogenesis | [12] | |
| Slit2, Nephronectin | in vitro + in vivo | 2D | neonatal CMs | rat | recombinant proteins | ↑ CM cytokinesis | [18] |
| Fibronectin | in vivo | zebrafish | fibronectin KO | ↑ CM mobilization | [19] | ||
| HSPGs | in vivo | mouse | sulfatase-1 and -2 KO and overexpression | ↓ angiogenesis by reduced VEGF availability | [30] | ||
| Ccn2a | in vivo | zebrafish | ccn2a KO, overexpression | ↑ CM proliferation by activation of Tgfβ/pSmad3 pathway | [33] | ||
| Emilin2a | in vivo | zebrafish | emilin2a KO, overexpression | ↑ CM and EC proliferation by activation of cxcl8a-cxcr1 pathway | [34] | ||
| Whole ECM | in vivo | pig | porcine dECM hydrogel | ↑ cardiac function | [25] | ||
| in vivo | rat | porcine dECM hydrogel | ↑CM proliferation | [26] | |||
| in vivo | rat | porcine dECM hydrogel | ↑ CM viability and angiogenesis | [27] | |||
| in vivo | human | Ventrigel (porcine) | Undefined (safety proven) | [28] | |||
| in vitro + in vivo | 2D | cardiac FBs, HUVECs | rat | CorMatrix (porcine) | ↑ angiogenesis | [41] | |
| in vitro + in vivo | 2D | neonatal CMs | rat | rat dECM | ↑CM proliferation | [50] | |
| in vitro | 2D | cardiac progenitors | human | zebrafish dECM | ↑ CM proliferation and angiogenesis byErbB2 pathway | [49] | |
| in vivo | rat | porcine dECM hydrogel | ↑ angiogenesis | [51] | |||
| in vitro + in vivo | 2D | HUVECs | human, rat | porcine dECM hydrogel | ↑ angiogenesis | [52] | |
| in vivo | mouse | dECM microparticles | ↑ CM proliferation | [53] | |||
| in vitro | 2D | neonatal CMs | rat | enzymatically digested rat ECM | ↑ CM proliferation | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.V.A.; Lorizio, D.; Vuerich, R.; Lippi, M.; Nascimento, D.S.; Zacchigna, S. Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 15783. https://doi.org/10.3390/ijms232415783
Vu TVA, Lorizio D, Vuerich R, Lippi M, Nascimento DS, Zacchigna S. Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities. International Journal of Molecular Sciences. 2022; 23(24):15783. https://doi.org/10.3390/ijms232415783
Chicago/Turabian StyleVu, Thi Van Anh, Daniela Lorizio, Roman Vuerich, Melania Lippi, Diana S. Nascimento, and Serena Zacchigna. 2022. "Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities" International Journal of Molecular Sciences 23, no. 24: 15783. https://doi.org/10.3390/ijms232415783
APA StyleVu, T. V. A., Lorizio, D., Vuerich, R., Lippi, M., Nascimento, D. S., & Zacchigna, S. (2022). Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities. International Journal of Molecular Sciences, 23(24), 15783. https://doi.org/10.3390/ijms232415783







