Mechanism Underlying Naringenin Hypocholesterolemic Effects: Involvement of Estrogen Receptor α Subtype
Abstract
:1. Introduction
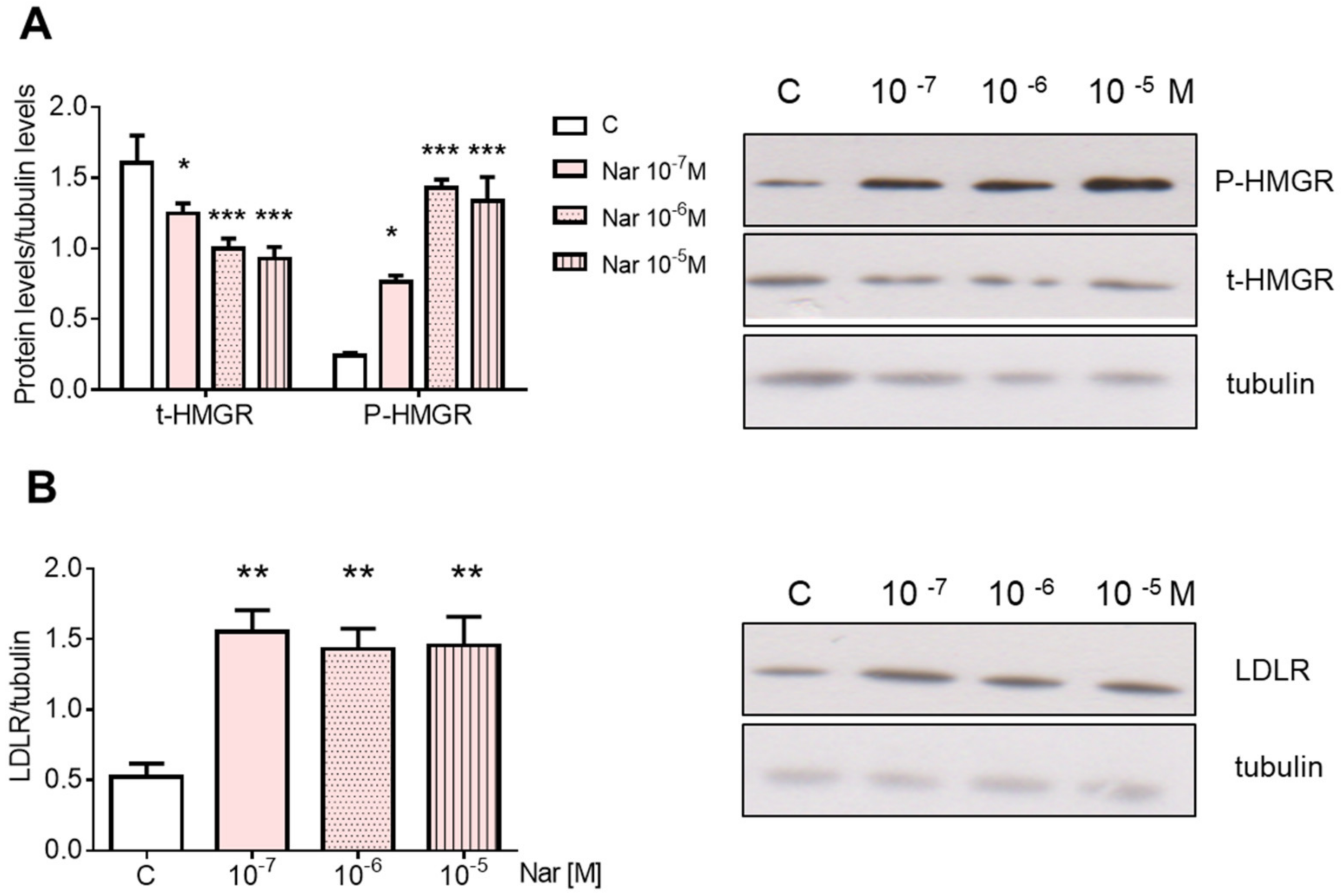
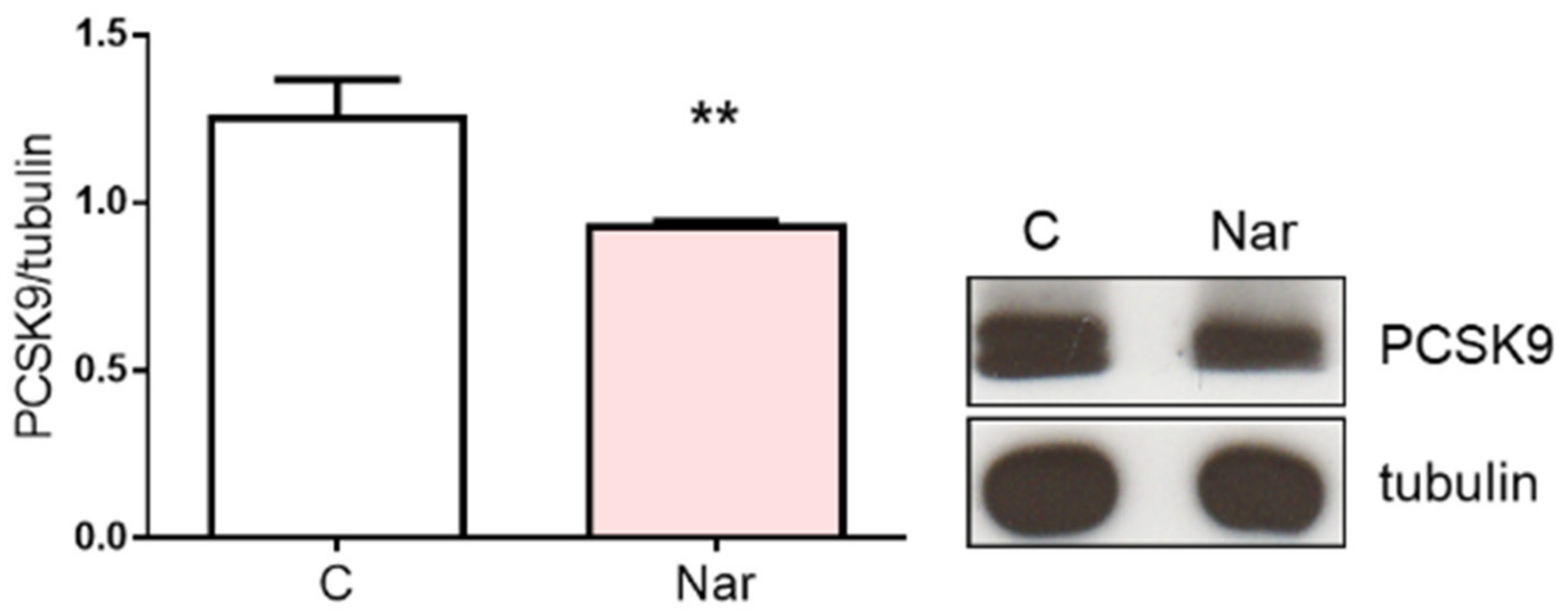
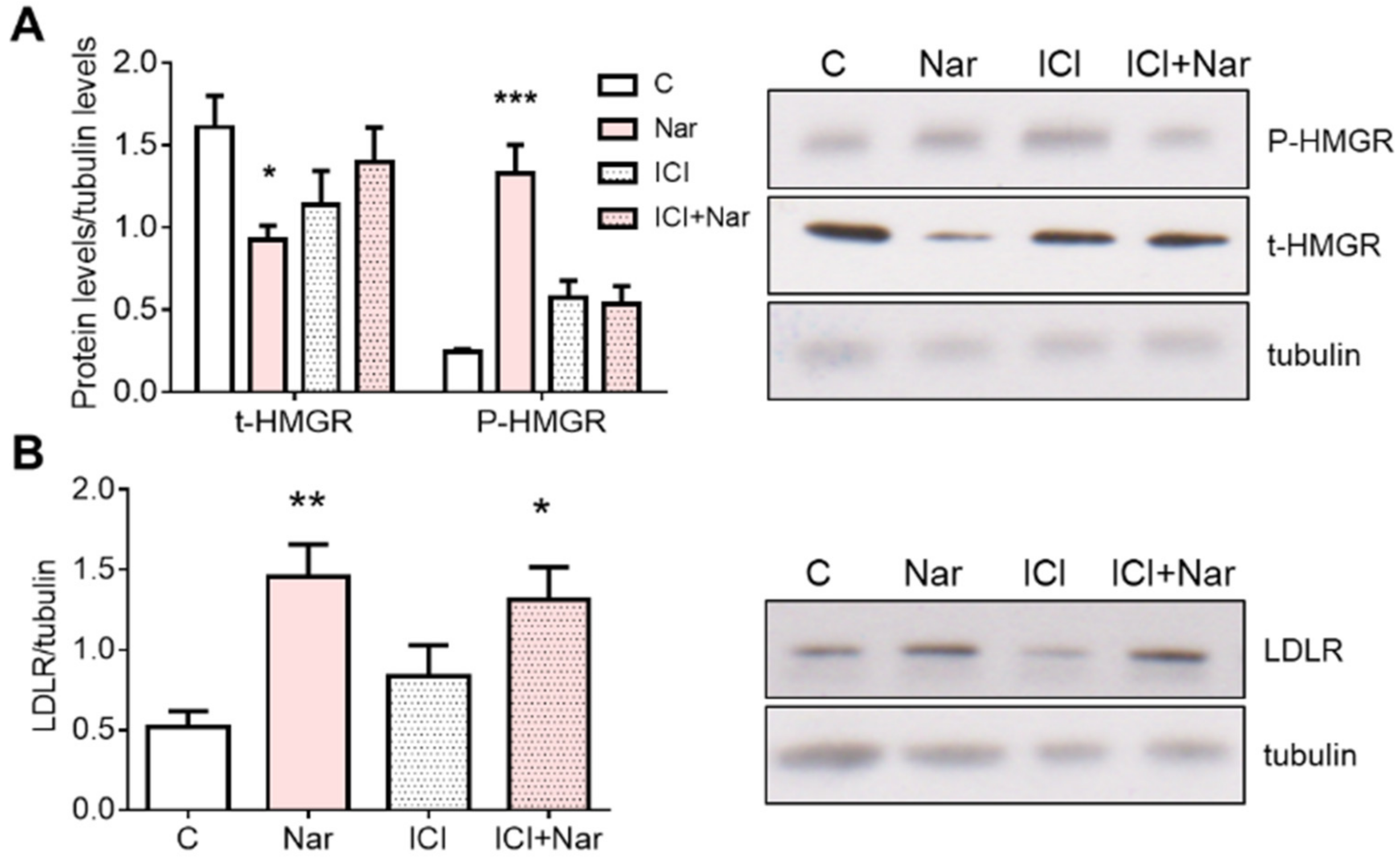
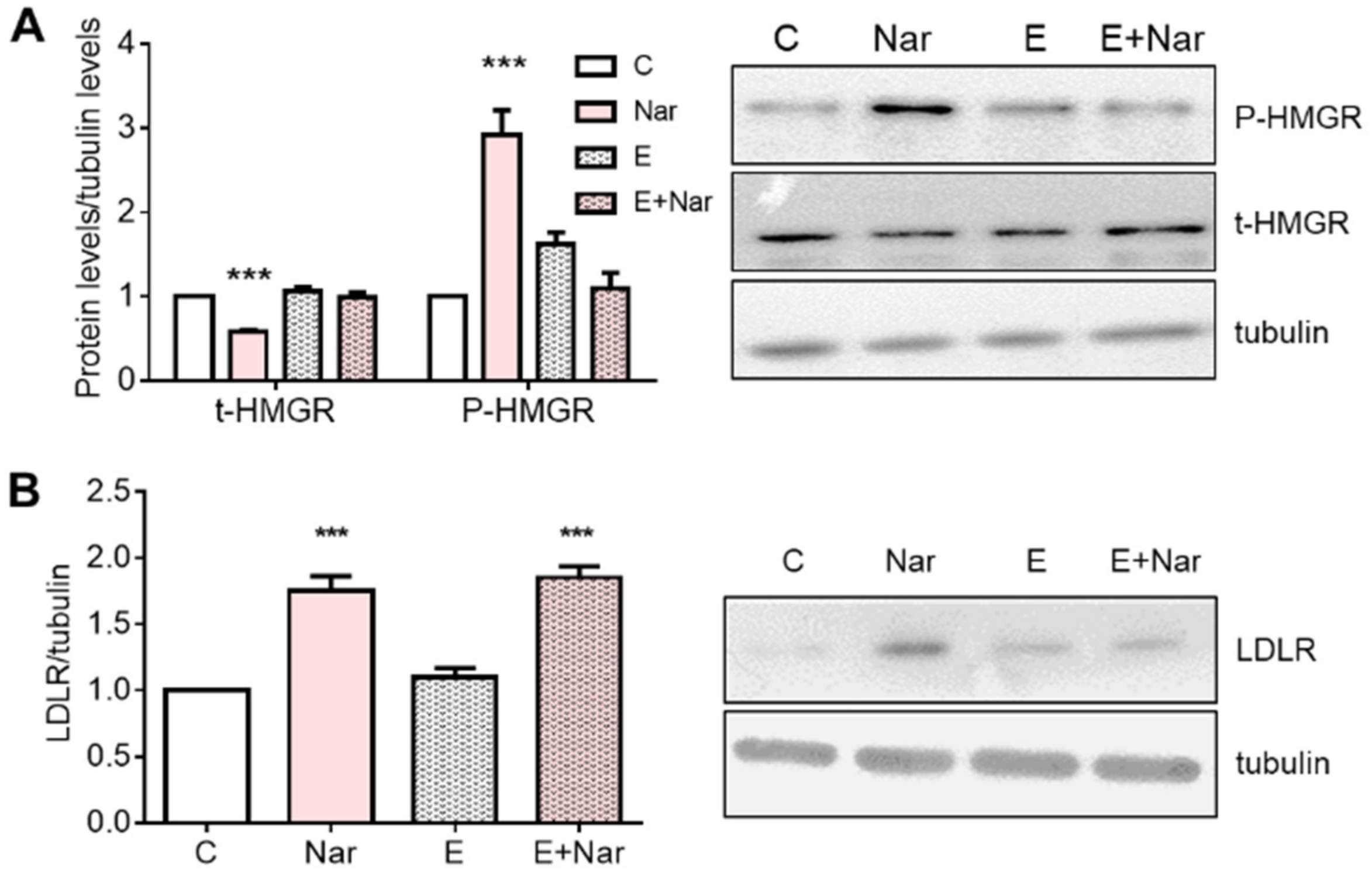
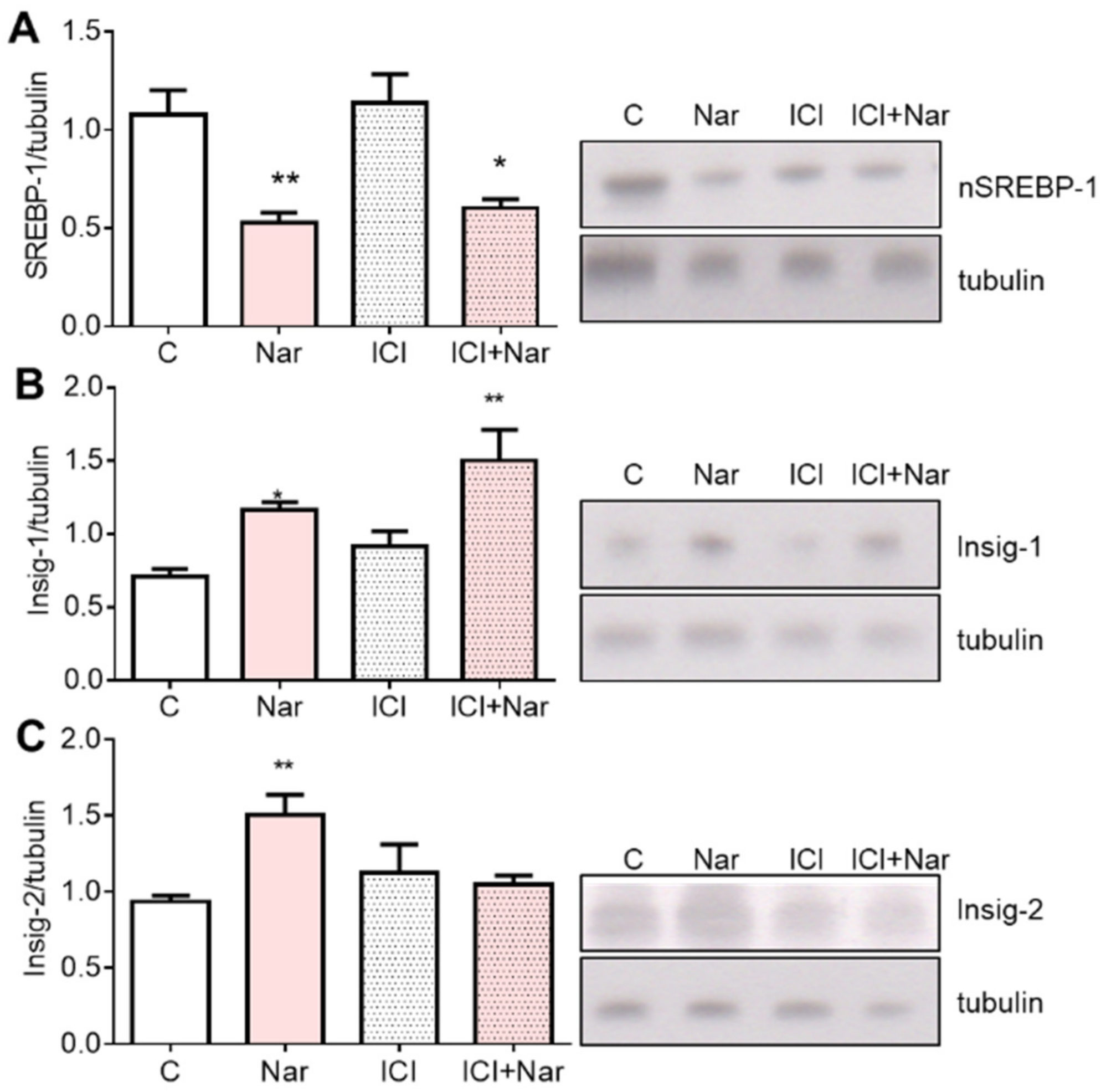
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- US Preventive Services Task Force. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 328, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Katselou, M.G.; Matralis, A.N.; Kourounakis, A.P. Multi-Target Drug Design Approaches for Multifactorial Diseases: From Neurodegenerative to Cardiovascular Applications. Curr. Med. Chem. 2014, 21, 2743–2787. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Kallend, D.; Wijngaard, P.L.; Kastelein, J.J. Inclisiran for the Treatment of Cardiovascular Disease: The ORION Clinical Development Program. Future Cardiol. 2018, 14, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Trapani, L.; Segatto, M.; Ascenzi, P.; Pallottini, V. Potential Role of Nonstatin Cholesterol Lowering Agents. IUBMB Life 2011, 63, 964–971. [Google Scholar] [CrossRef]
- Trapani, L. New Compounds Able to Control Hepatic Cholesterol Metabolism: Is It Possible to Avoid Statin Treatment in Aged People? WJH 2013, 5, 676. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Ascherio, A.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Hennekens, C.H.; Spiegelman, D.; Willett, W.C. Fruit and Vegetable Intake in Relation to Risk of Ischemic Stroke. JAMA 1999, 282, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, E.M.; Spence, J.D.; Jordan, J.; Wetmore, S.; Freeman, D.J.; Piché, L.A.; Serratore, P. HDL-Cholesterol-Raising Effect of Orange Juice in Subjects with Hypercholesterolemia. Am. J. Clin. Nutr. 2000, 72, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, L.J.; Borradaile, N.M.; de Dreu, L.E.; Huff, M.W. Secretion of Hepatocyte ApoB Is Inhibited by the Flavonoids, Naringenin and Hesperetin, via Reduced Activity and Expression of ACAT2 and MTP. J. Lipid Res. 2001, 42, 725–734. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Manthey, J.A.; Casaschi, A.; Theriault, A.G. Modulation of HepG2 Cell Net Apolipoprotein B Secretion by the Citrus Polymethoxyflavone, Tangeretin. Lipids 2004, 39, 143–151. [Google Scholar] [CrossRef]
- Borradaile, N.M.; de Dreu, L.E.; Barrett, P.H.R.; Huff, M.W. Inhibition of Hepatocyte ApoB Secretion by Naringenin: Enhanced Rapid Intracellular Degradation Independent of Reduced Microsomal Cholesteryl Esters. J. Lipid Res. 2002, 43, 1544–1554. [Google Scholar] [CrossRef]
- Borradaile, N.M.; de Dreu, L.E.; Barrett, P.H.R.; Behrsin, C.D.; Huff, M.W. Hepatocyte ApoB-Containing Lipoprotein Secretion Is Decreased by the Grapefruit Flavonoid, Naringenin, via Inhibition of MTP-Mediated Microsomal Triglyceride Accumulation. Biochemistry 2003, 42, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.T.T.; Takahashi, Y.; Ide, T. Activity and MRNA Levels of Enzymes Involved in Hepatic Fatty Acid Oxidation in Mice Fed Citrus Flavonoids. Nutrition 2006, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules 2021, 26, 5718. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The Nutritional Flavanone Naringenin Triggers Antiestrogenic Effects by Regulating Estrogen Receptor α-Palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef] [Green Version]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The Protective Role of Estrogen and Estrogen Receptors in Cardiovascular Disease and the Controversial Use of Estrogen Therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Billon-Galés, A.; Fontaine, C.; Douin-Echinard, V.; Delpy, L.; Berges, H.; Calippe, B.; Lenfant, F.; Laurell, H.; Guéry, J.-C.; Gourdy, P.; et al. Endothelial Estrogen Receptor-α Plays a Crucial Role in the Atheroprotective Action of 17β-Estradiol in Low-Density Lipoprotein Receptor–Deficient Mice. Circulation 2009, 120, 2567–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortmann, J.; Veit, M.; Zingg, S.; Di Santo, S.; Traupe, T.; Yang, Z.; Völzmann, J.; Dubey, R.K.; Christen, S.; Baumgartner, I. Estrogen Receptor-α But Not -β or GPER Inhibits High Glucose-Induced Human VSMC Proliferation: Potential Role of ROS and ERK. J. Clin. Endocrinol. Metab. 2011, 96, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Simoncini, T.; Genazzani, A.R.; Liao, J.K. Nongenomic Mechanisms of Endothelial Nitric Oxide Synthase Activation by the Selective Estrogen Receptor Modulator Raloxifene. Circulation 2002, 105, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Totta, P.; Acconcia, F.; Leone, S.; Cardillo, I.; Marino, M. Mechanisms of Naringenin-Induced Apoptotic Cascade in Cancer Cells: Involvement of Estrogen Receptor α and β Signalling. IUBMB Life 2004, 56, 491–499. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pallottini, V.; Marin, R.; Marino, M. Role of the Sex Hormone Estrogen in the Prevention of Lipid Disorder. Curr. Med. Chem. 2014, 21, 2734–2742. [Google Scholar] [CrossRef]
- Barros, R.P.A.; Gustafsson, J.-Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marinis, E.; Martini, C.; Trentalance, A.; Pallottini, V. Sex Differences in Hepatic Regulation of Cholesterol Homeostasis. J. Endocrinol. 2008, 198, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallottini, V.; Bulzomi, P.; Galluzzo, P.; Martini, C.; Marino, M. Estrogen Regulation of Adipose Tissue Functions: Involvement of Estrogen Receptor Isoforms. Infect. Disord.-Drug Targets 2008, 8, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Acconcia, F.; Trentalance, A. Biphasic Estradiol-Induced AKT Phosphorylation Is Modulated by PTEN via MAP Kinase in HepG2 Cells. MBoC 2003, 14, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Fiocchetti, M.; Nuzzo, M.T.; Totta, P.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin, a pro-Survival Player in Estrogen Receptor α-Positive Cancer Cells. Cell Death Dis. 2014, 5, e1449. [Google Scholar] [CrossRef] [Green Version]
- Lucero, D.; Dikilitas, O.; Mendelson, M.M.; Aligabi, Z.; Islam, P.; Neufeld, E.B.; Bansal, A.T.; Freeman, L.A.; Vaisman, B.; Tang, J.; et al. Transgelin: A New Gene Involved in LDL Endocytosis Identified by a Genome-Wide CRISPR-Cas9 Screen. J. Lipid Res. 2022, 63, 100160. [Google Scholar] [CrossRef]
- Huang, P.-P.; Zhu, W.-Q.; Xiao, J.-M.; Zhang, Y.-Q.; Li, R.; Yang, Y.; Shen, L.; Luo, F.; Dai, W.; Lian, P.-A.; et al. Alterations in Sorting and Secretion of Hepatic ApoA5 Induce Hypertriglyceridemia Due to Short-Term Use of Olanzapine. Front. Pharmacol. 2022, 13, 935362. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef]
- Segatto, M.; Di Giovanni, A.; Marino, M.; Pallottini, V. Analysis of the Protein Network of Cholesterol Homeostasis in Different Brain Regions: An Age and Sex Dependent Perspective. J. Cell. Physiol. 2013, 228, 1561–1567. [Google Scholar] [CrossRef]
- Espenshade, P.J.; Hughes, A.L. Regulation of Sterol Synthesis in Eukaryotes. Annu. Rev. Genet. 2007, 41, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. The SREBP Pathway: Regulation of Cholesterol Metabolism by Proteolysis of a Membrane-Bound Transcription Factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sever, N.; Yang, T.; Brown, M.S.; Goldstein, J.L.; DeBose-Boyd, R.A. Accelerated Degradation of HMG CoA Reductase Mediated by Binding of Insig-1 to Its Sterol-Sensing Domain. Mol. Cell 2003, 11, 25–33. [Google Scholar] [CrossRef]
- Peterson, A.S.; Fong, L.G.; Young, S.G. PCSK9 Function and Physiology. J. Lipid Res. 2008, 49, 1595–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Caro, G.; Borges, G.; van der Hooft, J.; Clifford, M.N.; Del Rio, D.; Lean, M.E.J.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange Juice (Poly)Phenols Are Highly Bioavailable in Humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, I.E.; Nabavi, S.F.; Daglia, M.; Tenore, G.C.; Mansouri, K.; Nabavi, S.M. Naringenin and Atherosclerosis: A Review of Literature. Curr Pharm Biotechnol 2015, 16, 245–251. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory Activity of Flavonoids from Prunus Davidiana and Other Flavonoids on Total ROS and Hydroxyl Radical Generation. Arch. Pharmacal Res. 2003, 26, 809–815. [Google Scholar] [CrossRef]
- Lee, C.-H.; Jeong, T.-S.; Choi, Y.-K.; Hyun, B.-H.; Oh, G.-T.; Kim, E.-H.; Kim, J.-R.; Han, J.-I.; Bok, S.-H. Anti-Atherogenic Effect of Citrus Flavonoids, Naringin and Naringenin, Associated with Hepatic ACAT and Aortic VCAM-1 and MCP-1 in High Cholesterol-Fed Rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, H.-J.; Lee, M.-K.; Jeon, S.-M.; Do, G.-M.; Kwon, E.-Y.; Cho, Y.-Y.; Kim, D.-J.; Jeong, K.-S.; Park, Y.B.; et al. Naringin Time-Dependently Lowers Hepatic Cholesterol Biosynthesis and Plasma Cholesterol in Rats Fed High-Fat and High-Cholesterol Diet. J. Med. Food 2006, 9, 582–586. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional Regulation of Human and Rat Hepatic Lipid Metabolism by the Grapefruit Flavonoid Naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The Inhibitory Effects of Bioactive Compounds of Tomato Juice Binding to Hepatic HMGCR: In Vivo Study and Molecular Modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D. Sterol Regulatory Element-Binding Proteins: Transcriptional Activators of Lipid Synthesis. Biochem. Soc. Trans. 2002, 30 Pt 6, 1091–1095. [Google Scholar] [CrossRef] [Green Version]
- Poirier, S.; Mayer, G. The Biology of PCSK9 from the Endoplasmic Reticulum to Lysosomes: New and Emerging Therapeutics to Control Low-Density Lipoprotein Cholesterol. Drug Des. Dev. Ther. 2013, 7, 1135–1148. [Google Scholar] [CrossRef] [Green Version]
- Cordero, A.; Olmo, M.R.F.; Quiroga, G.A.C.; Romero-Menor, C.; Fácila, L.; Seijas-Amigo, J.; Murillo, J.R.; Sandin, M.; Rodríguez-Mañero, M.; Mora, M.C.B.; et al. Effect of PCSK9 Inhibitors on Remnant Cholesterol and Lipid Residual Risk: The LIPID-REAL Registry. Eur. J. Clin. Invest. 2022, 52, e13863. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Dimsdale, J.E.; White, H.L.; Ritchie, J.B.; Criqui, M.H. Reduction in Blood Pressure with Statins: Results from the UCSD Statin Study, a Randomized Trial. Arch. Intern. Med. 2008, 168, 721–727. [Google Scholar] [CrossRef]
- Murugesan, N.; Woodard, K.; Ramaraju, R.; Greenway, F.L.; Coulter, A.A.; Rebello, C.J. Naringenin Increases Insulin Sensitivity and Metabolic Rate: A Case Study. J. Med. Food 2020, 23, 343–348. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Che, J.; Yao, W. Naringenin Protects against Hypertension by Regulating Lipid Disorder and Oxidative Stress in a Rat Model. Kidney Blood Press. Res. 2022, 47, 423–432. [Google Scholar] [CrossRef]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A pilot study on the nutraceutical properties of the Citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J. Funct. Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pallottini, V.; Martini, C.; Cavallini, G.; Donati, A.; Bergamini, E.; Notarnicola, M.; Caruso, M.G.; Trentalance, A. Modified HMG-CoA Reductase and LDLr Regulation Is Deeply Involved in Age-Related Hypercholesterolemia. J. Cell. Biochem. 2006, 98, 1044–1053. [Google Scholar] [CrossRef]



| Primary Antibody | Sources | Secondary Antibody | Sources |
|---|---|---|---|
| Anti-HMGCR | Upstate, Lake Placid, NY, USA | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti-Insig 1 | Novus Biologicals, Littleton, CO, USA | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti-Insig 2 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Rabbit anti-goat | Chemicon International, Temecula, Canada |
| Anti-SREBP 1 (N-terminal) | Abcam, Cambridge, UK | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti-SREBP 2 (N-terminal) | Abcam, Cambridge, UK | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti LDLR | Abcam, Cambridge, UK | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti PCSK9 (Anti NARC-1) | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti-tubulin | MP Biomedicals, Solon, OH, USA | Goat anti-mouse | UCS Diagnostic, Rome, Italy |
| Anti-AMPKα | Cell Signaling technology, Boston, MA USA | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti-P-AMPKα | Cell Signaling technology, Boston, MA USA | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
| Anti-Scap | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Rabbit anti-goat | UCS Diagnostic, Rome, Italy |
| Anti-PP2A | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Goat anti-rabbit | UCS Diagnostic, Rome, Italy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallottini, V.; Segatto, M.; Acconcia, F.; Fiocchetti, M.; Marino, M. Mechanism Underlying Naringenin Hypocholesterolemic Effects: Involvement of Estrogen Receptor α Subtype. Int. J. Mol. Sci. 2022, 23, 15809. https://doi.org/10.3390/ijms232415809
Pallottini V, Segatto M, Acconcia F, Fiocchetti M, Marino M. Mechanism Underlying Naringenin Hypocholesterolemic Effects: Involvement of Estrogen Receptor α Subtype. International Journal of Molecular Sciences. 2022; 23(24):15809. https://doi.org/10.3390/ijms232415809
Chicago/Turabian StylePallottini, Valentina, Marco Segatto, Filippo Acconcia, Marco Fiocchetti, and Maria Marino. 2022. "Mechanism Underlying Naringenin Hypocholesterolemic Effects: Involvement of Estrogen Receptor α Subtype" International Journal of Molecular Sciences 23, no. 24: 15809. https://doi.org/10.3390/ijms232415809







