Emerging Roles of Endocannabinoids as Key Lipid Mediators for a Successful Pregnancy
Abstract
1. Introduction
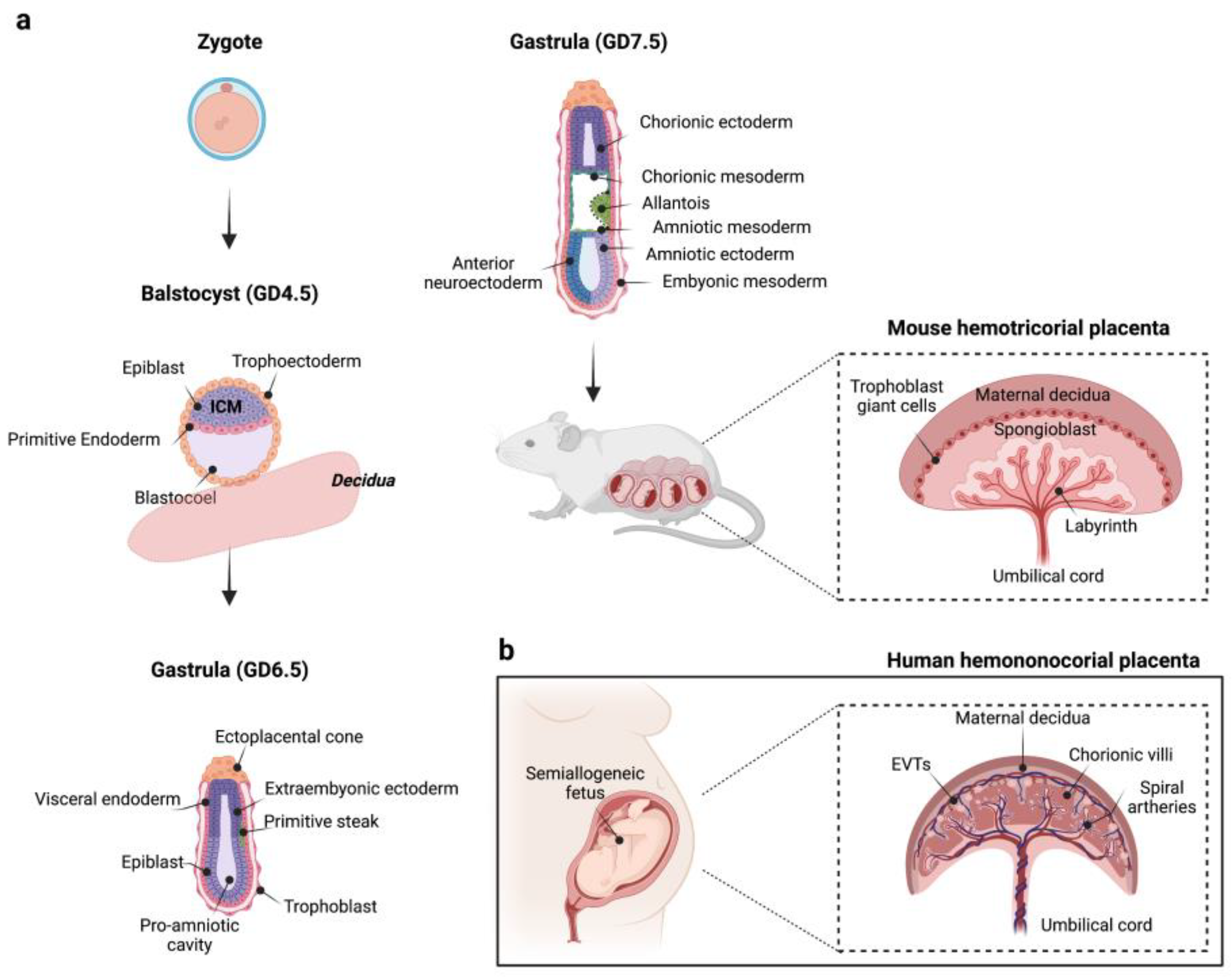
2. Formation and Development of the Maternal–Fetal Interface: An Overview
3. The Endocannabinoid System
4. The ECS: A Modulatory System for a Successful Pregnancy
4.1. Role of Endocannabinoids in the Early Gestational Processes
4.2. Endocannabinoids Regulate Placentation
5. The Role of Endocannabinoids in Low-Grade Inflammation and Maternal Immune Tolerance
5.1. Cannabinoids and T Lymphocytes
5.2. Cannabinoids and NK Cells
5.3. Cannabinoids and Decidual Macrophages
5.4. Endocannabinoids Regulate Nitric Oxide System in the Intrauterine Microenvironment: Insights from Inflammatory Conditions
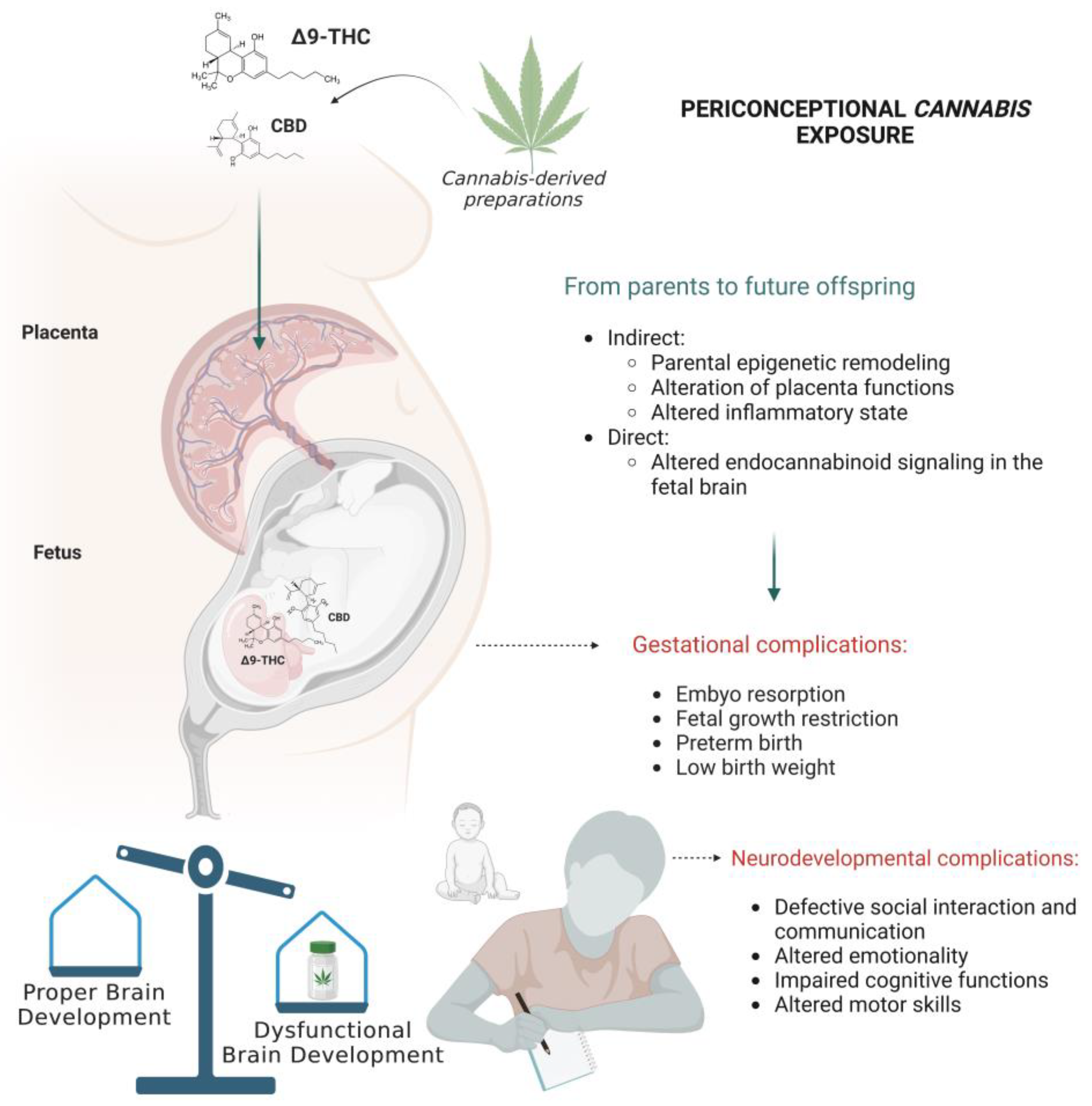
6. From Parent to Offspring: When Cannabis Threatens the Neurodevelopment Trajectories
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| ABHD | α,β-domain hydrolase |
| AEA | N-arachidonoylethanolamine (or Anandamide) |
| BBB | Blood–brain barrier |
| BCRP/ABCG2 | Breast cancer resistance protein/ATP-binding cassette subfamily member 2 |
| cAMP | cyclic Adenosine monophosphate |
| CB | G protein-coupled cannabinoid receptors (protein) |
| CBD | Cannabidiol |
| CD4 | Cluster of differentiation 4 |
| CD8 | Cluster of differentiation 8 |
| Cnr | Cannabinoid receptor (gene) |
| COX-2 | Cyclooxygenase-2 |
| CYP19A1 | Cytochrome P450 family 19 subfamily A member 1 |
| DAGL | Diacylglycerol lipase |
| ECS | Endocannabinoid system |
| eNOS | endothelial Nitric oxide synthase |
| EU | European Union |
| EVT | Extravillous trophoblasts |
| FA | Folic acid |
| FAAH | Fatty acid amide hydrolase |
| FABP | Fatty acid binding protein |
| FOXP3 | Forkhead box 3 |
| G-CSF | Granulocyte-macrophage colony-stimulating factor |
| GD | Gestational day |
| GPR | G protein-coupled receptor |
| hCG | Human chorionic gonadotropin |
| ICM | Inner cell mass |
| IFNγ | Interferon γ |
| IGFBP-1 | Insulin-like growth factor binding protein-1 |
| IL | Interleukin |
| iNOS | inducible Nitric oxide synthase |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharide |
| MAGL | Monoacylglycerol lipase |
| NAPE | N-acylphosphatidylethanolamine |
| NAPE-PLD | N-acylphosphatidylethanolamine specific phospholipase d-like |
| NAT | N-acyltransferase |
| NK cells | Natural killer cells |
| nNOS | neuronal Nitric oxide synthase |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NPRL3 | Nitrogen permease regulator-like 3 |
| OEA | N-oleoylethanolamide |
| PEA | N-palmitoylethanolamide |
| Peg10 | Paternally expressed 10 |
| PGE | Prostaglandin E |
| PIBF | Progesterone-induced blocking factor |
| PKA | Protein kinase A |
| Plag1 | Pleiomorphic Adenoma Gene-Like 1 |
| PPAR | Peroxisome proliferator-activated receptor |
| PRL | Prolactin |
| Reg | Regulatory |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGFβ | Tumor growth factor β |
| Th cells | T helper cells |
| TIM3 | T cell imunoglobulin and mucin 3 |
| TNFα | Tumor necrosis factor α |
| TRPV1 | Transient receptor potential vanilloid-1 |
| US | United States |
| VEGF | Vascular endothelial growth factor |
| WT | Wild-type |
| Δ9-THC | Δ9-tetrahydrocannabinol |
References
- Brown, Q.L.; Sarvet, A.L.; Shmulewitz, D.; Martins, S.S.; Wall, M.M.; Hasin, D.S. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. Jama 2017, 317, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Skelton, K.R.; Hecht, A.A.; Benjamin-Neelon, S.E. Association of Recreational Cannabis Legalization With Maternal Cannabis Use in the Preconception, Prenatal, and Postpartum Periods. JAMA Netw. Open 2021, 4, e210138. [Google Scholar] [CrossRef]
- Leung, J.; Chan, G.; Stjepanović, D.; Chung, J.Y.C.; Hall, W.; Hammond, D. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacology 2022, 239, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Rogers, C.E.; Lessov-Schlaggar, C.N.; Carter, E.B.; Lenze, S.N.; Grucza, R.A. Alcohol, Cigarette, and Cannabis Use Between 2002 and 2016 in Pregnant Women From a Nationally Representative Sample. JAMA Pediatr. 2019, 173, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Monitoring and Evaluating Changes in Cannabis Policies: Insights from the Americas; European Monitoring Centre for Drugs and Drug Addiction; Publications Office of the European Union: Luxembourg, 2020.
- Schrott, R.; Murphy, S.K. Cannabis use and the sperm epigenome: A budding concern? Environ. Epigenetics 2020, 6, dvaa002. [Google Scholar] [CrossRef]
- Corroon, J.M., Jr.; Mischley, L.K.; Sexton, M. Cannabis as a substitute for prescription drugs—A cross-sectional study. J. Pain Res. 2017, 10, 989–998. [Google Scholar] [CrossRef]
- Kitsantas, P.; Aljoudi, S.M.; Gimm, G. Marijuana use in Pregnant Women with Disabilities in the United States. Matern. Child Health J. 2022, 26, 242–249. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.-Y.; Goler, N.C.; Alexeeff, S.E.; Ridout, K.K.; Avalos, L.A. Association of Depression, Anxiety, and Trauma With Cannabis Use During Pregnancy. JAMA Netw. Open 2020, 3, e1921333. [Google Scholar] [CrossRef]
- Chang, J.C.; Tarr, J.A.; Holland, C.L.; De Genna, N.M.; Richardson, G.A.; Rodriguez, K.L.; Sheeder, J.; Kraemer, K.L.; Day, N.L.; Rubio, D.; et al. Beliefs and attitudes regarding prenatal marijuana use: Perspectives of pregnant women who report use. Drug Alcohol Depend. 2019, 196, 14–20. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Ray, G.T.; Alexeeff, S.E.; Adams, S.R.; Does, M.B.; Ansley, D.; Avalos, L.A. Rates of Prenatal Cannabis Use Among Pregnant Women Before and During the COVID-19 Pandemic. JAMA 2021, 326, 1745–1747. [Google Scholar] [CrossRef]
- Coleman-Cowger, V.H.; Oga, E.A.; Peters, E.N.; Mark, K. Prevalence and associated birth outcomes of co-use of Cannabis and tobacco cigarettes during pregnancy. Neurotoxicology Teratol. 2018, 68, 84–90. [Google Scholar] [CrossRef]
- Page, K.; Murray-Krezan, C.; Leeman, L.; Carmody, M.; Stephen, J.M.; Bakhireva, L.N. Prevalence of marijuana use in pregnant women with concurrent opioid use disorder or alcohol use in pregnancy. Addict. Sci. Clin. Pr. 2022, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Breit, K.R.; Rodriguez, C.G.; Lei, A.; Thomas, J.D. Combined vapor exposure to THC and alcohol in pregnant rats: Maternal outcomes and pharmacokinetic effects. Neurotoxicology Teratol. 2020, 82, 106930. [Google Scholar] [CrossRef] [PubMed]
- Carliner, H.; Brown, Q.L.; Sarvet, A.L.; Hasin, D.S. Cannabis use, attitudes, and legal status in the U.S.: A review. Prev. Med. 2017, 104, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lipnik-Štangelj, M.; Razinger, B. A regulatory take on cannabis and cannabinoids for medicinal use in the European Union. Arh. Hig. Rada. Toksikol. 2020, 71, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.; Freeman, T.P.; Kilian, C.; López-Pelayo, H.; Rehm, J. Public health monitoring of cannabis use in Europe: Prevalence of use, cannabis potency, and treatment rates. Lancet Reg. Health Eur. 2021, 10, 100227. [Google Scholar] [CrossRef]
- Cannabis legislation in Europe. European Monitoring Centre for Drugs and Drug Addiction; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Low-THC cannabis products in Europe. European Monitoring Centre for Drugs and Drug Addiction—EMCDDA; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Adams, S.R.; Wi, S.; Weisner, C.; Conway, A. Routes of cannabis administration among females in the year before and during pregnancy: Results from a pilot project. Addict. Behav. 2020, 100, 106125. [Google Scholar] [CrossRef]
- Maccarrone, M.; Rapino, C.; Francavilla, F.; Barbonetti, A. Cannabinoid signalling and effects of cannabis on the male reproductive system. Nat. Rev. Urol. 2021, 18, 19–32. [Google Scholar] [CrossRef]
- Kim, J.; de Castro, A.; Lendoiro, E.; Cruz-Landeira, A.; López-Rivadulla, M.; Concheiro, M. Detection of in utero cannabis exposure by umbilical cord analysis. Drug Test. Anal. 2018, 10, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, D.E.; Martin, B.R.; Gamagaris, Z.; Miller, N.; Fico, T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989, 44, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Baglot, S.L.; VanRyzin, J.W.; Marquardt, A.E.; Aukema, R.J.; Petrie, G.N.; Hume, C.; Reinl, E.L.; Bieber, J.B.; McLaughlin, R.J.; McCarthy, M.M.; et al. Maternal-fetal transmission of delta-9-tetrahydrocannabinol (THC) and its metabolites following inhalation and injection exposure during pregnancy in rats. J. Neurosci. Res. 2022, 100, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Corsi, D.J.; Donelle, J.; Sucha, E.; Hawken, S.; Hsu, H.; El-Chaâr, D.; Bisnaire, L.; Fell, D.; Wen, S.W.; Walker, M. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat. Med. 2020, 26, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Roncero, C.; Valriberas-Herrero, I.; Mezzatesta-Gava, M.; Villegas, J.L.; Aguilar, L.; Grau-López, L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod. Health 2020, 17, 25. [Google Scholar] [CrossRef]
- Paul, S.E.; Hatoum, A.S.; Fine, J.D.; Johnson, E.C.; Hansen, I.; Karcher, N.R.; Moreau, A.L.; Bondy, E.; Qu, Y.; Carter, E.B.; et al. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry 2021, 78, 64–76. [Google Scholar] [CrossRef]
- Scheyer, A.F.; Melis, M.; Trezza, V.; Manzoni, O.J.J. Consequences of Perinatal Cannabis Exposure. Trends Neurosci. 2019, 42, 871–884. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Mehmedic, Z.; Foster, S.; Gon, C.; Chandra, S.; Church, J.C. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol. Psychiatry 2016, 79, 613–619. [Google Scholar] [CrossRef]
- Chandra, S.; Radwan, M.M.; Majumdar, C.G.; Church, J.C.; Freeman, T.P.; ElSohly, M.A. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 5–15. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Chandra, S.; Radwan, M.; Majumdar, C.G.; Church, J.C. A Comprehensive Review of Cannabis Potency in the United States in the Last Decade. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2021, 6, 603–606. [Google Scholar] [CrossRef]
- Freeman, T.P.; Craft, S.; Wilson, J.; Stylianou, S.; ElSohly, M.; Di Forti, M.; Lynskey, M.T. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: Systematic review and meta-analysis. Addiction 2021, 116, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Capellini, I.; Venditti, C.; Barton, R.A. Placentation and maternal investment in mammals. Am. Nat. 2011, 177, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Neiger, R. Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J. Clin. Med. 2017, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Wardinger, J.E.; Ambati, S. Placental Insufficiency; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Panja, S.; Paria, B.C. Development of the Mouse Placenta. In Placentation in Mammals. Advances in Anatomy, Embryology and Cell Biology; Geisert, R.D., Spencer, T., Eds.; Springer: Cham, Swizterland, 2021; Volume 234, pp. 205–221. [Google Scholar]
- Soares, M.J.; Chakraborty, D.; Karim Rumi, M.; Konno, T.; Renaud, S.J. Rat placentation: An experimental model for investigating the hemochorial maternal-fetal interface. Placenta 2012, 33, 233–243. [Google Scholar] [CrossRef]
- Bardot, E.S.; Hadjantonakis, A.-K. Mouse gastrulation: Coordination of tissue patterning, specification and diversification of cell fate. Mech. Dev. 2020, 163, 103617. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, 22. [Google Scholar] [CrossRef]
- Aguilera, N.; Salas-Pérez, F.; Ortíz, M.; Álvarez, D.; Echiburú, B.; Maliqueo, M. Rodent models in placental research. Implications for fetal origins of adult disease. Anim. Reprod. 2022, 19, e20210134. [Google Scholar] [CrossRef]
- Elmore, S.A.; Cochran, R.Z.; Bolon, B.; Lubeck, B.; Mahler, B.; Sabio, D.; Ward, J.M. Histology Atlas of the Developing Mouse Placenta. Toxicol. Pathol. 2022, 50, 60–117. [Google Scholar] [CrossRef]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef] [PubMed]
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2021, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology. Advances in Experimental Medicine and Biology; Bukiya, A., Ed.; Springer: Cham, Swizterland, 2019; Volume 1162, pp. 1–12. [Google Scholar]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, P.; Nicolaou, A.; Woodward, D.F. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim. Biophys. Acta 2015, 1851, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Almada, M.; Piscitelli, F.; Fonseca, B.M.; Di Marzo, V.; Correia-Da-Silva, G.; Teixeira, N. Anandamide and decidual remodelling: COX-2 oxidative metabolism as a key regulator. Biochim. Biophys. Acta 2015, 1851, 1473–1481. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734. [Google Scholar] [CrossRef]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef]
- Garcia-Arencibia, M.; Molina-Holgado, E.; Molina-Holgado, F. Effect of endocannabinoid signalling on cell fate: Life, death, differentiation and proliferation of brain cells. Br. J. Pharmacol. 2019, 176, 1361–1369. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Habayeb, O.M.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the Endocannabinoid System in Human First Trimester Placenta and Its Role in Trophoblast Proliferation. Endocrinology 2008, 149, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; LaViolette, M.; Flamand, N. The CB(2) receptor and its role as a regulator of inflammation. Cell. Mol. life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Manduca, A.; Cacchione, C.; Vicari, S.; Trezza, V. Healing autism spectrum disorder with cannabinoids: A neuroinflammatory story. Neurosci. Biobehav. Rev. 2021, 121, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Parolaro, D. The Endocannabinoid System and Autism Spectrum Disorders: Insights from Animal Models. Int. J. Mol. Sci. 2017, 18, 1916. [Google Scholar] [CrossRef]
- Ibarra-Lecue, I.; Pilar-Cuéllar, F.; Muguruza, C.; Florensa-Zanuy, E.; Díaz, Á.; Urigüen, L.; Castro, E.; Pazos, A.; Callado, L.F. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem. Pharmacol. 2018, 157, 97–107. [Google Scholar] [CrossRef]
- Trezza, V.; Campolongo, P.; Manduca, A.; Morena, M.; Palmery, M.; Vanderschuren, L.J.; Cuomo, V. Altering endocannabinoid neurotransmission at critical developmental ages: Impact on rodent emotionality and cognitive performance. Front. Behav. Neurosci. 2012, 6, 2. [Google Scholar] [CrossRef]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Rebelo, I. Cannabis and Cannabinoids in Reproduction and Fertility: Where We Stand. Reprod. Sci. 2022, 29, 2429–2439. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and Function of the Endocannabinoid System in the Human Ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.E.; Rolland, A.D.; Rajpert-De Meyts, E.; Janfelt, C.; Jorgensen, A.; Winge, S.B.; Kristensen, D.M.; Juul, A.; Chalmel, F.; Jegou, B.; et al. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci. Rep. 2019, 9, 12866. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Amoako, A.A.; Mortlock, S.; Rogers, P.A.W.; Holdsworth-Carson, S.J.; Donoghue, J.F.; Teh, W.T.; Montgomery, G.W.; McKinnon, B. Gene expression of the endocannabinoid system in endometrium through menstrual cycle. Sci. Rep. 2022, 12, 9400. [Google Scholar] [CrossRef]
- Ding, J.; Luo, X.-T.; Yao, Y.-R.; Xiao, H.-M.; Guo, M.-Q. Investigation of changes in endocannabinoids and N-acylethanolamides in biofluids, and their correlations with female infertility. J. Chromatogr. A 2017, 1509, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gebeh, A.K.; Willets, J.M.; Marczylo, E.L.; Taylor, A.H.; Konje, J.C. Ectopic Pregnancy Is Associated with High Anandamide Levels and Aberrant Expression of FAAH and CB1 in Fallopian Tubes. J. Clin. Endocrinol. Metab. 2012, 97, 2827–2835. [Google Scholar] [CrossRef]
- Shen, X.; Duan, H.; Wang, S.; Hong, W.; Wang, Y.-Y.; Lin, S.-L. Expression of Cannabinoid Receptors in Myometrium and its Correlation With Dysmenorrhea in Adenomyosis. Reprod. Sci. 2019, 26, 1618–1625. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Taylor, A.H.; Lam, P.M.; Marczylo, T.H.; Konje, J.C.; Teixeira, N.A. Characterisation of the endocannabinoid system in rat haemochorial placenta. Reprod. Toxicol. 2012, 34, 347–356. [Google Scholar] [CrossRef]
- Maia, J.; Fonseca, B.M.; Cunha, S.C.; Braga, J.; Gonçalves, D.; Teixeira, N.; Correia-Da-Silva, G. Impact of tetrahydrocannabinol on the endocannabinoid 2-arachidonoylglycerol metabolism: ABHD6 and ABHD12 as novel players in human placenta. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 441–447. [Google Scholar] [CrossRef]
- Kremshofer, J.; Siwetz, M.; Berghold, V.M.; Lang, I.; Huppertz, B.; Gauster, M. A role for GPR55 in human placental venous endothelial cells. Histochem. 2015, 144, 49–58. [Google Scholar] [CrossRef]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Sun, X.; Kingsley, P.J.; Marnett, L.J.; Cravatt, B.F.; Dey, S.K. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins Other Lipid Mediat. 2007, 83, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010, 93, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Na Cui, N.; Wang, L.; Wang, W.; Zhang, J.; Xu, Y.; Jiang, L.; Hao, G. The correlation of anandamide with gonadotrophin and sex steroid hormones during the menstrual cycle. Iran. J. Basic Med. Sci. 2017, 20, 1268–1274. [Google Scholar] [CrossRef]
- Maccarrone, M.; De Felici, M.; Bari, M.; Klinger, F.; Siracusa, G.; Finazzi-Agrò, A. Down-regulation of anandamide hydrolase in mouse uterus by sex hormones. Eur. J. Biochem. 2000, 267, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, N.; Valensise, H.; Bari, M.; Ubaldi, F.; Battista, N.; Finazzi-Agrò, A.; Maccarrone, M. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecol. Endocrinol. 2004, 18, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Almada, M.; Silva, A.; Correia-Da-Silva, G.; Teixeira, N.; Sá, S.; Fonseca, B.M. The endocannabinoid system expression in the female reproductive tract is modulated by estrogen. J. Steroid Biochem. Mol. Biol. 2017, 174, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Habayeb, O.M.; Taylor, A.H.; Evans, M.D.; Cooke, M.S.; Taylor, D.J.; Bell, S.C.; Konje, J.C. Plasma levels of the endocannabinoid anandamide in women--a potential role in pregnancy maintenance and labor? J. Clin. Endocrinol. Metab. 2004, 89, 5482–5487. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Paria, B.C.; Deutsch, D.D.; Dey, S.K. The uterus is a potential site for anandamide synthesis and hydrolysis: Differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Mol. Reprod. Dev. 1996, 45, 183–192. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. The rat as an animal model for fetoplacental development: A reappraisal of the post-implantation period. Reprod. Biol. 2012, 12, 97–118. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Okamoto, Y.; Ueda, N.; Kingsley, P.J.; Marnett, L.J.; Schmid, H.H.; Das, S.K.; Dey, S.K. N-Acylphosphatidylethanolamine-hydrolyzing Phospholipase D Is an Important Determinant of Uterine Anandamide Levels during Implantation. J. Biol. Chem. 2005, 280, 23429–23432. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.C.; Paria, B.C.; Krebsbach, R.J.; Schmid, H.H.O.; Dey, S.K. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 1997, 94, 4188–4192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, H.; Guo, Y.; Zhang, H.; Takahashi, T.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; Cravatt, B.F.; Dey, S.K. Fatty acid amide hydrolase deficiency limits early pregnancy events. J. Clin. Investig. 2006, 116, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-Da-Silva, G.; Almada, M.; Costa, M.A.; Teixeira, N.A. The Endocannabinoid System in the Postimplantation Period: A Role during Decidualization and Placentation. Int. J. Endocrinol. 2013, 2013, 510540. [Google Scholar] [CrossRef]
- Paria, B.C.; Ma, W.; Andrenyak, D.M.; Schmid, P.C.; Schmid, H.H.; Moody, D.E.; Deng, H.; Makriyannis, A.; Dey, S.K. Effects of Cannabinoids on Preimplantation Mouse Embryo Development and Implantation are Mediated by Brain-Type Cannabinoid Receptors1. Biol. Reprod. 1998, 58, 1490–1495. [Google Scholar] [CrossRef]
- Paria, B.C.; Song, H.; Wang, X.; Schmid, P.C.; Krebsbach, R.J.; Schmid, H.H.; Bonner, T.I.; Zimmer, A.; Dey, S.K. Dysregulated Cannabinoid Signaling Disrupts Uterine Receptivity for Embryo Implantation. J. Biol. Chem. 2001, 276, 20523–20528. [Google Scholar] [CrossRef]
- Paria, B.C.; Das, S.K.; Dey, S.K. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc. Natl. Acad. Sci. USA 1995, 92, 9460–9464. [Google Scholar] [CrossRef]
- Li, Y.; Bian, F.; Sun, X.; Dey, S.K. Mice Missing Cnr1 and Cnr2 Show Implantation Defects. Endocrinology 2019, 160, 938–946. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Wang, D.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; DuBois, R.N.; Dey, S.K. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat. Med. 2004, 10, 1074–1080. [Google Scholar] [CrossRef]
- Horne, A.W.; Phillips, J.A., 3rd; Kane, N.; Lourenco, P.C.; McDonald, S.E.; Williams, A.R.; Simon, C.; Dey, S.K.; Critchley, H.O. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE 2008, 3, e3969. [Google Scholar] [CrossRef] [PubMed]
- Gebeh, A.K.; Willets, J.M.; Bari, M.; Hirst, R.A.; Marczylo, T.H.; Taylor, A.H.; Maccarrone, M.; Konje, J.C. Elevated Anandamide and Related N-Acylethanolamine Levels Occur in the Peripheral Blood of Women With Ectopic Pregnancy and Are Mirrored by Changes in Peripheral Fatty Acid Amide Hydrolase Activity. J. Clin. Endocrinol. Metab. 2013, 98, 1226–1234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bienertova-Vasku, J.; Bienert, P.; Dostalova, Z.; Chovanec, J.; Vasku, A.; Vasku, V. A common variation in the cannabinoid 1 receptor (CNR1) gene is associated with pre-eclampsia in the Central European population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, M.; Meng, C.; Shi, W.; Sun, L.; Zhang, J. Expressions of candidate molecules in the human fallopian tube and chorionic villi of tubal pregnancy exposed to levonorgestrel emergency contraception. Reprod. Biol. Endocrinol. 2013, 11, 46. [Google Scholar] [CrossRef]
- Fügedi, G.; Molnár, M.; Rigó, J., Jr.; Schönléber, J.; Kovalszky, I.; Molvarec, A. Increased placental expression of cannabinoid receptor 1 in preeclampsia: An observational study. BMC Pregnancy Childbirth 2014, 14, 395. [Google Scholar] [CrossRef]
- Abán, C.; Leguizamón, G.F.; Cella, M.; Damiano, A.; Franchi, A.M.; Farina, M.G. Differential expression of endocannabinoid system in normal and preeclamptic placentas: Effects on nitric oxide synthesis. Placenta 2013, 34, 67–74. [Google Scholar] [CrossRef]
- Lombó, M.; Giommi, C.; Paolucci, M.; Notarstefano, V.; Montik, N.; Delli Carpini, G.; Ciavattini, A.; Ragusa, A.; Maradonna, F.; Giorgini, E.; et al. Preeclampsia Correlates with an Increase in Cannabinoid Receptor 1 Levels Leading to Macromolecular Alterations in Chorionic Villi of Term Placenta. Int. J. Mol. Sci. 2022, 23, 12931. [Google Scholar] [CrossRef]
- Wang, H.; Matsumoto, H.; Guo, Y.; Paria, B.C.; Roberts, R.L.; Dey, S.K. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 14914–14919. [Google Scholar] [CrossRef]
- Xie, H.; Sun, X.; Piao, Y.; Jegga, A.G.; Handwerger, S.; Ko, M.S.; Dey, S.K. Silencing or Amplification of Endocannabinoid Signaling in Blastocysts via CB1 Compromises Trophoblast Cell Migration. J. Biol. Chem. 2012, 287, 32288–32297. [Google Scholar] [CrossRef]
- Gormley, M.; Oliverio, O.; Kapidzic, M.; Ona, K.; Hall, S.; Fisher, S.J. RNA profiling of laser microdissected human trophoblast subtypes at mid-gestation reveals a role for cannabinoid signaling in invasion. Development 2021, 148, dev199626. [Google Scholar] [CrossRef]
- Kim, Y.S.; Li, Y.; Yuan, J.; Borg, J.P.; Sun, X.; Dey, S.K. Cannabinoid and planar cell polarity signaling converges to direct placentation. Proc. Natl. Acad. Sci. USA 2021, 118, 38. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Fonseca, B.M.; Teixeira, N.; Correia-da-Silva, G. The endocannabinoids anandamide and 2-arachidonoylglycerol modulate the expression of angiogenic factors on HTR8/SVneo placental cells. Prostaglandins Leukot Essent Fat. Acids 2022, 180, 102440. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Almada, M.; Cunha, S.; Fonseca, B.M.; Amaral, C.; Piscitelli, F.; Di Marzo, V.; Correia-Da-Silva, G.; Teixeira, N. Anandamide interferes with human endometrial stromal-derived cell differentiation: An effect dependent on inhibition of cyclooxygenase-2 expression and prostaglandin E2 release. Biofactors 2016, 42, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Almada, M.; Amaral, C.; Diniz-Da-Costa, M.; Correia-Da-Silva, G.; Teixeira, N.A.; Fonseca, B.M. The endocannabinoid anandamide impairs in vitro decidualization of human cells. Reproduction 2016, 152, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. Anandamide-Induced Cell Death: Dual Effects in Primary Rat Decidual Cell Cultures. Placenta 2009, 30, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. The endocannabinoid anandamide induces apoptosis of rat decidual cells through a mechanism involving ceramide synthesis and p38 MAPK activation. Apoptosis 2013, 18, 1526–1535. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. Anandamide restricts uterine stromal differentiation and is critical for complete decidualization. Mol. Cell. Endocrinol. 2015, 411, 167–176. [Google Scholar] [CrossRef]
- Almada, M.; Fonseca, B.M.; Amaral, C.; Diniz-Da-Costa, M.; Correia-Da-Silva, G.; Teixeira, N. Anandamide oxidative metabolism-induced endoplasmic reticulum stress and apoptosis. Apoptosis Int. J. Program. Cell Death 2017, 22, 816–826. [Google Scholar] [CrossRef]
- Almada, M.; Oliveira, A.; Amaral, C.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.; Correia-Da-Silva, G.; Teixeira, N. Anandamide targets aromatase: A breakthrough on human decidualization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158512. [Google Scholar] [CrossRef]
- Das, A.; Mantena, S.R.; Kannan, A.; Evans, D.B.; Bagchi, M.K.; Bagchi, I.C. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 12542–12547. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, K.K.; Kessler, C.A.; Schroeder, J.K.; Buckley, A.R.; Brar, A.K.; Handwerger, S. Cannabinoid receptor I activation markedly inhibits human decidualization. Mol. Cell. Endocrinol. 2005, 229, 65–74. [Google Scholar] [PubMed]
- Li, Y.; Dewar, A.; Kim, Y.S.; Dey, S.K.; Sun, X. Pregnancy success in mice requires appropriate cannabinoid receptor signaling for primary decidua formation. Elife 2020, 9, e61762. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Fernandes, R.; Almada, M.; Santos, M.; Carvalho, F.; Teixeira, N.A.; Correia-Da-Silva, G. Synthetic cannabinoids and endometrial stromal cell fate: Dissimilar effects of JWH-122, UR-144 and WIN55,212-2. Toxicology 2019, 413, 40–47. [Google Scholar] [CrossRef]
- Neradugomma, N.K.; Drafton, K.; Mor, G.G.; Mao, Q. Marijuana-derived cannabinoids inhibit uterine endometrial stromal cell decidualization and compromise trophoblast-endometrium cross-talk. Reprod. Toxicol. 2019, 87, 100–107. [Google Scholar] [CrossRef]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef]
- Almada, M.; Amaral, C.; Oliveira, A.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N. Cannabidiol (CBD) but not tetrahydrocannabinol (THC) dysregulate in vitro decidualization of human endometrial stromal cells by disruption of estrogen signaling. Reprod. Toxicol. 2020, 93, 75–82. [Google Scholar] [CrossRef]
- Maia, J.; Almada, M.; Midão, L.; Fonseca, B.M.; Braga, J.; Gonçalves, D.; Teixeira, N.; Correia-Da-Silva, G. The Cannabinoid Delta-9-tetrahydrocannabinol Disrupts Estrogen Signaling in Human Placenta. Toxicol. Sci. 2020, 177, 420–430. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Taylor, A.H.; Lam, P.M.; Marczylo, T.H.; Bell, S.C.; Konje, J.C.; Teixeira, N.A. The endocannabinoid 2-arachidonoylglycerol (2-AG) and metabolizing enzymes during rat fetoplacental development: A role in uterine remodelling. Int. J. Biochem. Cell Biol. 2010, 42, 1884–1892. [Google Scholar] [CrossRef]
- Costa, M.A. Scrutinising the regulators of syncytialization and their expression in pregnancy-related conditions. Mol. Cell. Endocrinol. 2016, 420, 180–193. [Google Scholar] [CrossRef]
- Costa, M.A.; Fonseca, B.M.; Mendes, A.; Braga, J.; Teixeira, N.A.; Correia-Da-Silva, G. The endocannabinoid 2-arachidonoylglycerol dysregulates the synthesis of proteins by the human syncytiotrophoblast. Biochim. Biophys. Acta 2016, 1861, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, T.; Accialini, P.; Palligas, M.; Loureiro, F.; Saraco, N.; Martínez, N.; Farina, M. Endocannabinoid signaling impairs syncytialization: Using flow cytometry to evaluate forskolin-induced cell fusion. Placenta 2021, 103, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, H.; Yang, J.; Wang, H.; Bradshaw, H.B.; Dey, S.K. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc. Natl. Acad. Sci. USA 2010, 107, 16887–16892. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Fonseca, B.M.; Keating, E.; Teixeira, N.A.; Correia-Da-Silva, G. 2-Arachidonoylglycerol effects in cytotrophoblasts: Metabolic enzymes expression and apoptosis in BeWo cells. Reproduction 2014, 147, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Fonseca, B.M.; Teixeira, N.A.; Correia-Da-Silva, G. The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: Involvement of both mitochondrial and death receptor pathways. Placenta 2015, 36, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Almada, M.A.; Costa, L.; Fonseca, B.M.; Alves, P.; Braga, J.; Gonçalves, D.; Teixeira, N.A.; Correia-Da-Silva, G. The endocannabinoid 2-arachidonoylglycerol promotes endoplasmic reticulum stress in placental cells. Reproduction 2020, 160, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Fonseca, B.; Keating, E.; Teixeira, N.; Correia-Da-Silva, G. Transient receptor potential vanilloid 1 is expressed in human cytotrophoblasts: Induction of cell apoptosis and impairment of syncytialization. Int. J. Biochem. Cell Biol. 2014, 57, 177–185. [Google Scholar] [CrossRef]
- Alves, P.; Amaral, C.; Teixeira, N.; Correia-Da-Silva, G. Cannabidiol disrupts apoptosis, autophagy and invasion processes of placental trophoblasts. Arch. Toxicol. 2021, 95, 3393–3406. [Google Scholar] [CrossRef]
- Almada, M.; Alves, P.; Fonseca, B.M.; Carvalho, F.; Queirós, C.R.; Gaspar, H.; Amaral, C.; Teixeira, N.A.; Correia-Da-Silva, G. Synthetic cannabinoids JWH-018, JWH-122, UR-144 and the phytocannabinoid THC activate apoptosis in placental cells. Toxicol. Lett. 2020, 319, 129–137. [Google Scholar] [CrossRef]
- Almada, M.; Costa, L.; Fonseca, B.M.; Amaral, C.; Teixeira, N.; Correia-Da-Silva, G. The synthetic cannabinoid WIN-55,212 induced-apoptosis in cytotrophoblasts cells by a mechanism dependent on CB1 receptor. Toxicology 2017, 385, 67–73. [Google Scholar] [CrossRef]
- Walker, O.S.; Ragos, R.; Gurm, H.; Lapierre, M.; May, L.L.; Raha, S. Delta-9-tetrahydrocannabinol disrupts mitochondrial function and attenuates syncytialization in human placental BeWo cells. Physiol. Rep. 2020, 8, e14476. [Google Scholar] [CrossRef] [PubMed]
- Lojpur, T.; Easton, Z.; Raez-Villanueva, S.; Laviolette, S.; Holloway, A.C.; Hardy, D.B. Delta9-Tetrahydrocannabinol leads to endoplasmic reticulum stress and mitochondrial dysfunction in human BeWo trophoblasts. Reprod. Toxicol. 2019, 87, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.S.; Gurm, H.; Sharma, R.; Verma, N.; May, L.L.; Raha, S. Delta-9-tetrahydrocannabinol inhibits invasion of HTR8/SVneo human extravillous trophoblast cells and negatively impacts mitochondrial function. Sci. Rep. 2021, 11, 4029. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Gonçalves, P.; Martel, F. Effect of Cannabinoids upon the Uptake of Folic Acid by BeWo Cells. Pharmacology 2009, 83, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.T.; Composto-Wahler, G.M.; Joseph, L.B.; Wang, B.; Rosen, T.; Laskin, J.D.; Aleksunes, L.M. Anandamide down-regulates placental transporter expression through CB2 receptor-mediated inhibition of cAMP synthesis. Pharmacol. Res. 2019, 141, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.L.; Lau, D.T.T.; Allen, J.D.; Arnold, J.C. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br. J. Pharmacol. 2007, 152, 815–824. [Google Scholar] [CrossRef]
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Holcberg, G. Cannabidiol enhances xenobiotic permeability through the human placental barrier by direct inhibition of breast cancer resistance protein: An ex vivo study. Am. J. Obstet. Gynecol. 2013, 209, 573.e1–573.e15. [Google Scholar] [CrossRef]
- Anderson, L.L.; Etchart, M.G.; Bahceci, D.; Golembiewski, T.A.; Arnold, J.C. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations. Sci. Rep. 2021, 11, 14948. [Google Scholar] [CrossRef]
- Kammala, A.; Benson, M.; Ganguly, E.; Radnaa, E.; Kechichian, T.; Richardson, L.; Menon, R. Fetal Membranes Contribute to Drug Transport across the Feto-Maternal Interface Utilizing the Breast Cancer Resistance Protein (BCRP). Life 2022, 12, 166. [Google Scholar] [CrossRef]
- Natale, B.V.; Gustin, K.N.; Lee, K.; Holloway, A.C.; Laviolette, S.R.; Natale, D.R.C.; Hardy, D.B. Delta9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020, 10, 544. [Google Scholar] [CrossRef]
- Chang, X.; Li, H.; Li, Y.; He, Q.; Yao, J.; Duan, T.; Wang, K. RhoA/MLC signaling pathway is involved in Delta(9)-tetrahydrocannabinol-impaired placental angiogenesis. Toxicol. Lett. 2018, 285, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Roberts, V.H.J.; Schabel, M.C.; Boniface, E.R.; D’Mello, R.J.; Morgan, T.K.; Terrobias, J.J.D.; Graham, J.A.; Borgelt, L.M.; Grant, K.A.; Sullivan, E.L.; et al. Chronic prenatal delta-9-tetrahydrocannabinol exposure adversely impacts placental function and development in a rhesus macaque model. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.L.; Grotegut, C.A.; Howlett, A.C. Endocannabinoid System in Pregnancy Maintenance and Labor: A Mini-Review. Front. Endocrinol. 2021, 12, 699951. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V. Endocannabinoids and Immunity. Cannabis Cannabinoid Res. 2016, 1, 59–66. [Google Scholar] [CrossRef]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Bambang, K.N.; Lambert, D.G.; Lam, P.M.; Quenby, S.; Maccarrone, M.; Konje, J.C. Immunity and early pregnancy events: Are endocannabinoids the missing link? J. Reprod. Immunol. 2012, 96, 8–18. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef]
- Moore, A.R.; Vivanco Gonzalez, N.; Plummer, K.A.; Mitchel, O.R.; Kaur, H.; Rivera, M.; Collica, B.; Goldston, M.; Filiz, F.; Angelo, M.; et al. Gestationally dependent immune organization at the maternal-fetal interface. Cell Rep. 2022, 41, 111651. [Google Scholar] [CrossRef]
- Hoo, R.; Nakimuli, A.; Vento-Tormo, R. Innate Immune Mechanisms to Protect Against Infection at the Human Decidual-Placental Interface. Front. Immunol. 2020, 11, 2070. [Google Scholar] [CrossRef]
- Deshmukh, H.; Way, S.S. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu. Rev. Pathol. 2019, 14, 185–210. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020, 100, 1077–1117. [Google Scholar] [CrossRef] [PubMed]
- Parasar, P.; Guru, N.; Nayak, N.R. Contribution of macrophages to fetomaternal immunological tolerance. Hum. Immunol. 2021, 82, 325–331. [Google Scholar] [CrossRef]
- Wang, S.; Sun, F.; Han, M.; Liu, Y.; Zou, Q.; Wang, F.; Tao, Y.; Li, D.; Du, M.; Li, H.; et al. Trophoblast-derived hyaluronan promotes the regulatory phenotype of decidual macrophages. Reproduction 2019, 157, 189–198. [Google Scholar] [CrossRef]
- Lindau, R.; Vondra, S.; Spreckels, J.; Solders, M.; Svensson-Arvelund, J.; Berg, G.; Pollheimer, J.; Kaipe, H.; Jenmalm, M.C.; Ernerudh, J. Decidual stromal cells support tolerance at the human foetal-maternal interface by inducing regulatory M2 macrophages and regulatory T-cells. J. Reprod. Immunol. 2021, 146, 103330. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Sang, Y.; Li, D.-J.; Du, M. Crosstalk Between Trophoblasts and Decidual Immune Cells: The Cornerstone of Maternal-Fetal Immunotolerance. Front. Immunol. 2021, 12, 642392. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef]
- Miller, D.; Gershater, M.; Slutsky, R.; Romero, R.; Gomez-Lopez, N. Maternal and fetal T cells in term pregnancy and preterm labor. Cell. Mol. Immunol. 2020, 17, 693–704. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, J.Y.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. Th17 and Regulatory T cells in Women with Recurrent Pregnancy Loss. Am. J. Reprod. Immunol. 2012, 67, 311–318. [Google Scholar] [CrossRef]
- Sun, X.; Deng, W.; Li, Y.; Tang, S.; Leishman, E.; Bradshaw, H.B.; Dey, S.K. Sustained Endocannabinoid Signaling Compromises Decidual Function and Promotes Inflammation-induced Preterm Birth. J. Biol. Chem. 2016, 291, 8231–8240. [Google Scholar] [CrossRef]
- Maia, J.; Fonseca, B.M.; Teixeira, N.; Correia-Da-Silva, G. The fundamental role of the endocannabinoid system in endometrium and placenta: Implications in pathophysiological aspects of uterine and pregnancy disorders. Hum. Reprod. Updat. 2020, 26, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Valensise, H.; Bari, M.; Lazzarin, N.; Romanini, C.; Finazzi-Agrò, A. Progesterone Up-Regulates Anandamide Hydrolase in Human Lymphocytes: Role of Cytokines and Implications for Fertility. J. Immunol. 2001, 166, 7183–7189. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, M.T.; Chiurchiù, V.; Catanzaro, G.; Borsellino, G.; Bernardi, G.; Battistini, L.; Maccarrone, M. Anandamide Suppresses Proliferation and Cytokine Release from Primary Human T-Lymphocytes Mainly via CB2 Receptors. PLoS ONE 2010, 5, e8688. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K.; Meissler, J.J. Effects of Cannabinoids on T-cell Function and Resistance to Infection. J. Neuroimmune Pharmacol. 2015, 10, 204–216. [Google Scholar] [CrossRef]
- Gentili, M.; Ronchetti, S.; Ricci, E.; Di Paola, R.; Gugliandolo, E.; Cuzzocrea, S.; Bereshchenko, O.; Migliorati, G.; Riccardi, C. Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharmacol. Res. 2019, 141, 21–31. [Google Scholar] [CrossRef]
- Angelina, A.; Pérez-Diego, M.; López-Abente, J.; Rückert, B.; Nombela, I.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.; Palomares, O. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming. Mucosal Immunol. 2022, 15, 96–108. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Whettlock, E.M.; Von Woon, E.; Cuff, A.O.; Browne, B.; Johnson, M.R.; Male, V. Dynamic Changes in Uterine NK Cell Subset Frequency and Function Over the Menstrual Cycle and Pregnancy. Front. Immunol. 2022, 13, 35784314. [Google Scholar] [CrossRef]
- Gong, H.; Chen, Y.; Xu, J.; Xie, X.; Yu, D.; Yang, B.; Kuang, H. The regulation of ovary and conceptus on the uterine natural killer cells during early pregnancy. Reprod. Biol. Endocrinol. 2017, 15, 73. [Google Scholar] [CrossRef]
- Brosens, J.J.; Hayashi, N.; White, J.O. Progesterone Receptor Regulates Decidual Prolactin Expression in Differentiating Human Endometrial Stromal Cells1. Endocrinology 1999, 140, 4809–4820. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakai, K.; Iwashita, M. Insulin-like growth factor binding protein-1 induces decidualization of human endometrial stromal cells via alpha5beta1 integrin. Mol. Hum. Reprod. 2008, 14, 485–489. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Cunha, S.C.; Gonçalves, D.; Mendes, A.; Braga, J.; Correia-Da-Silva, G.; Teixeira, N.A. Decidual NK cell-derived conditioned medium from miscarriages affects endometrial stromal cell decidualisation: Endocannabinoid anandamide and tumour necrosis factor-α crosstalk. Hum. Reprod. 2020, 35, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, S.; Du, M. Functional regulation of decidual macrophages during pregnancy. J. Reprod. Immunol. 2020, 143, 103264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, M.-R.; Li, M.; Wang, H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell. Mol. Immunol. 2018, 15, 1027–1037. [Google Scholar] [CrossRef]
- Mezouar, S.; Katsogiannou, M.; Ben Amara, A.; Bretelle, F.; Mege, J.-L. Placental macrophages: Origin, heterogeneity, function and role in pregnancy-associated infections. Placenta 2020, 103, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, F.; Xu, Y.; Chen, L.; Chen, C.; Cui, L.; Qian, J.; Li, D.; Wang, S.; Du, M. Tim-3(+) decidual Mphis induced Th2 and Treg bias in decidual CD4(+)T cells and promoted pregnancy maintenance via CD132. Cell. Death Dis. 2022, 13, 454. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhou, W.-J.; Hou, X.-X.; Fu, Q.; Li, D.-J. Correction: Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal–fetal interface. Cell. Mol. Immunol. 2019, 16, 313. [Google Scholar] [CrossRef]
- Russell, P.; Sacks, G.; Tremellen, K.; Gee, A. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: Further observations and reference ranges. Pathology 2013, 45, 393–401. [Google Scholar] [CrossRef]
- McMaster, M.T.; Newton, R.C.; Dey, S.K.; Andrews, G.K. Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J. Immunol. 1992, 148, 1699–1705. [Google Scholar] [CrossRef]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; Silva, P.M.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the signaling: Role of cAMP for the resolution of inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, F.; Li, M.; Qian, J.; Chen, C.; Wang, M.; Zang, X.; Li, D.; Yu, M.; Du, M. The appropriate frequency and function of decidual Tim-3(+)CTLA-4(+)CD8(+) T cells are important in maintaining normal pregnancy. Cell Death Dis. 2019, 10, 407. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, D.; Hong, X.; Tao, Y.; Wang, S.; Xu, Y.; Piao, H.; Yin, W.; Yu, M.; et al. Tim-3 signaling in peripheral NK cells promotes maternal-fetal immune tolerance and alleviates pregnancy loss. Sci. Signal. 2017, 10, eaah4323. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, E.; Maldonado, R.; Robledo, P. CB2 cannabinoid receptors modulate HIF-1α and TIM-3 expression in a hypoxia-ischemia mouse model. Eur. Neuropsychopharmacol. 2016, 26, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Lee, B.; Komori, K.; Lee, M.J.; Lee, B.G.; Kim, K.; Park, S. Regulation of TIM-3 expression in a human T cell line by tumor-conditioned media and cyclic AMP-dependent signaling. Mol. Immunol. 2019, 105, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Seol, T.-K.; Lee, W.; Park, S.; Kim, K.N.; Kim, T.Y.; Na Oh, Y.; Jun, J.H. Effect of palmitoylethanolamide on inflammatory and neuropathic pain in rats. Korean J. Anesthesiol. 2017, 70, 561–566. [Google Scholar] [CrossRef]
- Scipioni, L.; Ciaramellano, F.; Carnicelli, V.; Leuti, A.; Lizzi, A.R.; De Dominicis, N.; Oddi, S.; Maccarrone, M. Microglial Endocannabinoid Signalling in AD. Cells 2022, 11, 1237. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. The Role of Nitric Oxide on Male and Female Reproduction. Malays. J. Med. Sci. 2022, 29, 18–30. [Google Scholar]
- Melford, S.E.; Taylor, A.H.; Konje, J.C. Nitric oxide positively affects endometrial receptivity via FAAH and NAPE-PLD in vitro. Reprod. Fertil. 2021, 2, 107–116. [Google Scholar] [CrossRef]
- Sordelli, M.S.; Beltrame, J.S.; Burdet, J.; Zotta, E.; Pardo, R.; Cella, M.; Franchi, A.M.; Ribeiro, M.L. The Effect of Anandamide on Uterine Nitric Oxide Synthase Activity Depends on the Presence of the Blastocyst. PLoS ONE 2011, 6, e18368. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric Oxide Production and Signaling in Inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.A.; Aisemberg, J.; Billi, S.; Wolfson, M.L.; Franchi, A.M. Endocannabinoid System and Nitric Oxide are Involved in the Deleterious Effects of Lipopolysaccharide on Murine Decidua. Placenta 2009, 30, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.; Leng, Y.; Romero, R.; Xu, Y.; Panaitescu, B.; Miller, D.; Arif, A.; Mumuni, S.; Qureshi, F.; Hsu, C.; et al. The immunophenotype of decidual macrophages in acute atherosis. Am. J. Reprod. Immunol. 2019, 81, e13098. [Google Scholar] [CrossRef]
- Osborn, B.H.; Haney, A.F.; Misukonis, M.A.; Weinberg, J.B. Inducible nitric oxide synthase expression by peritoneal macrophages in endometriosis-associated infertility. Fertil. Steril. 2002, 77, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, F.; Kuang, L.; Tang, W.; Li, Y.; Chen, D. eNOS/iNOS and endoplasmic reticulum stress-induced apoptosis in the placentas of patients with preeclampsia. J. Hum. Hypertens. 2017, 31, 49–55. [Google Scholar] [CrossRef]
- Ogando, D.G.; Paz, D.; Cella, M.; Franchi, A.M. The fundamental role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction 2003, 125, 95–110. [Google Scholar] [CrossRef]
- Aisemberg, J.; Vercelli, C.; Billi, S.; Ribeiro, M.L.; Ogando, D.; Meiss, R.; McCann, S.M.; Rettori, V.; Franchi, A.M. Nitric oxide mediates prostaglandins’ deleterious effect on lipopolysaccharide-triggered murine fetal resorption. Proc. Natl. Acad. Sci. USA 2007, 104, 7534–7539. [Google Scholar] [CrossRef] [PubMed]
- Aisemberg, J.; Vercelli, C.A.; Bariani, M.V.; Billi, S.C.; Wolfson, M.L.; Franchi, A.M. Progesterone Is Essential for Protecting against LPS-Induced Pregnancy Loss. LIF as a Potential Mediator of the Anti-inflammatory Effect of Progesterone. PLoS ONE 2013, 8, e56161. [Google Scholar] [CrossRef]
- Wolfson, M.L.; Correa, F.; Leishman, E.; Vercelli, C.; Cymeryng, C.; Blanco, J.; Bradshaw, H.B.; Franchi, A.M. Lipopolysaccharide-induced murine embryonic resorption involves changes in endocannabinoid profiling and alters progesterone secretion and inflammatory response by a CB1-mediated fashion. Mol. Cell. Endocrinol. 2015, 411, 214–222. [Google Scholar] [CrossRef]
- Maccarrone, M.; De Petrocellis, L.; Bari, M.; Fezza, F.; Salvati, S.; Di Marzo, V.; Finazzi-Agrò, A. Lipopolysaccharide Downregulates Fatty Acid Amide Hydrolase Expression and Increases Anandamide Levels in Human Peripheral Lymphocytes. Arch. Biochem. Biophys. 2001, 393, 321–328. [Google Scholar] [CrossRef]
- Csabai, T.; Pallinger, E.; Kovacs, A.F.; Miko, E.; Bognar, Z.; Szekeres-Bartho, J. Altered Immune Response and Implantation Failure in Progesterone-Induced Blocking Factor-Deficient Mice. Front. Immunol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.A.; Aisemberg, J.; Billi, S.; Cervini, M.L.; Ribeiro, M.; Farina, M.A.; Franchi, A. Anandamide regulates lipopolysaccharide-induced nitric oxide synthesis and tissue damage in the murine uterus. Reprod. Biomed. Online 2009, 18, 824–831. [Google Scholar] [CrossRef]
- Bariani, M.V.; Correa, F.; Leishman, E.; Dominguez Rubio, A.P.; Arias, A.; Stern, A.; Bradshaw, H.B.; Franchi, A.M. Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth. Mol. Hum. Reprod. 2017, 23, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Abán, C.E.; Accialini, P.L.; Etcheverry, T.; Leguizamón, G.F.; Martinez, N.A.; Farina, M.G. Crosstalk Between Nitric Oxide and Endocannabinoid Signaling Pathways in Normal and Pathological Placentation. Front. Physiol. 2018, 9, 1699. [Google Scholar] [CrossRef] [PubMed]
- Asghari-Roodsari, A.; Lesani, A.; Javadi-Paydar, M.; Tabatabaeefar, L.; Tavangar, S.M.; Norouzi, A.; Dehpour, A.R. Tocolytic effect of delta9-tetrahydrocannabinol in mice model of lipopolysaccharide—Induced preterm delivery: Role of nitric oxide. Reprod. Sci. 2010, 17, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.K.; Rosales, C.B.; Center, K.E.; Nuñez, A.; Gibson, S.J.; Christ, C.; Ehiri, J.E. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open 2016, 6, e009986. [Google Scholar] [CrossRef]
- Haight, S.C.; King, B.A.; Bombard, J.M.; Coy, K.C.; Ferré, C.D.; Grant, A.M.; Ko, J.Y. Frequency of cannabis use during pregnancy and adverse infant outcomes, by cigarette smoking status—8 PRAMS states, 2017. Drug Alcohol Depend. 2021, 220, 108507. [Google Scholar] [CrossRef]
- Gabrhelík, R.; Mahic, M.; Lund, I.O.; Bramness, J.; Selmer, R.; Skovlund, E.; Handal, M.; Skurtveit, S. Cannabis Use during Pregnancy and Risk of Adverse Birth Outcomes: A Longitudinal Cohort Study. Eur. Addict. Res. 2021, 27, 131–141. [Google Scholar] [CrossRef]
- Marchand, G.; Masoud, A.T.; Govindan, M.; Ware, K.; King, A.; Ruther, S.; Brazil, G.; Ulibarri, H.; Parise, J.; Arroyo, A.; et al. Birth Outcomes of Neonates Exposed to Marijuana in Utero: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2145653. [Google Scholar] [CrossRef]
- Grant, K.S.; Petroff, R.; Isoherranen, N.; Stella, N.; Burbacher, T.M. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol. Ther. 2018, 182, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Pinky, P.D.; Bloemer, J.; Smith, W.D.; Moore, T.; Hong, H.; Suppiramaniam, V.; Reed, M.N. Prenatal cannabinoid exposure and altered neurotransmission. Neuropharmacology 2019, 149, 181–194. [Google Scholar] [CrossRef]
- Bara, A.; Ferland, J.N.; Rompala, G.; Szutorisz, H.; Hurd, Y.L. Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 2021, 22, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Cinquina, V. Physiological Rules of Endocannabinoid Action During Fetal and Neonatal Brain Development. Cannabis Cannabinoid Res. 2021, 6, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yan, Y.; Li, Q.; Ye, J.; Pei, L. Endocannabinoid System Unlocks the Puzzle of Autism Treatment via Microglia. Front. Psychiatry 2021, 12, 734837. [Google Scholar] [CrossRef]
- Scheyer, A.; Yasmin, F.; Naskar, S.; Patel, S. Endocannabinoids at the synapse and beyond: Implications for neuropsychiatric disease pathophysiology and treatment. Neuropsychopharmacology 2023, 48, 37–53. [Google Scholar] [CrossRef]
- Beiersdorf, J.; Hevesi, Z.; Calvigioni, D.; Pyszkowski, J.; Romanov, R.; Szodorai, E.; Lubec, G.; Shirran, S.; Botting, C.H.; Kasper, S.; et al. Adverse effects of Delta9-tetrahydrocannabinol on neuronal bioenergetics during postnatal development. JCI Insight 2020, 5, 23. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef]
- Lee, H.L.; Jung, K.M.; Fotio, Y.; Squire, E.; Palese, F.; Lin, L.; Torrens, A.; Ahmed, F.; Mabou Tagne, A.; Ramirez, J.; et al. Frequent Low-Dose Delta(9)-Tetrahydrocannabinol in Adolescence Disrupts Microglia Homeostasis and Disables Responses to Microbial Infection and Social Stress in Young Adulthood. Biol. Psychiatry 2022, 92, 845–860. [Google Scholar] [CrossRef]
- Newsom, R.J.; Kelly, S.J. Perinatal delta-9-tetrahydrocannabinol exposure disrupts social and open field behavior in adult male rats. Neurotoxicol. Teratol. 2008, 30, 213–219. [Google Scholar] [CrossRef]
- Trezza, V.; Campolongo, P.; Cassano, T.; Macheda, T.; Dipasquale, P.; Carratù, M.R.; Gaetani, S.; Cuomo, V. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology 2008, 198, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, P.; Trezza, V.; Ratano, P.; Palmery, M.; Cuomo, V. Developmental consequences of perinatal cannabis exposure: Behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 2010, 214, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Hawkey, A.B.; Hall, B.J.; Cauley, M.; Slade, S.; Yazdani, E.; Kenou, B.; White, H.; Wells, C.; Rezvani, A.H.; et al. Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol. 2019, 74, 106806. [Google Scholar] [CrossRef] [PubMed]
- Holloway, Z.R.; Hawkey, A.B.; Pippin, E.; White, H.; Wells, C.; Kenou, B.; Rezvani, A.H.; Murphy, S.K.; Levin, E.D. Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology 2020, 78, 57–63. [Google Scholar] [CrossRef]
- Manduca, A.; Servadio, M.; Melancia, F.; Schiavi, S.; Manzoni, O.J.; Trezza, V. Sex-specific behavioural deficits induced at early life by prenatal exposure to the cannabinoid receptor agonist WIN55, 212-2 depend on mGlu5 receptor signalling. Br. J. Pharmacol. 2020, 177, 449–463. [Google Scholar] [CrossRef]
- Weimar, H.V.; Wright, H.R.; Warrick, C.R.; Brown, A.M.; Lugo, J.M.; Freels, T.G.; McLaughlin, R.J. Long-term effects of maternal cannabis vapor exposure on emotional reactivity, social behavior, and behavioral flexibility in offspring. Neuropharmacology 2020, 179, 108288. [Google Scholar] [CrossRef]
- Sagheddu, C.; Traccis, F.; Serra, V.; Congiu, M.; Frau, R.; Cheer, J.F.; Melis, M. Mesolimbic dopamine dysregulation as a signature of information processing deficits imposed by prenatal THC exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110128. [Google Scholar] [CrossRef]
- Breit, K.R.; Rodriguez, C.G.; Lei, A.; Hussain, S.; Thomas, J.D. Effects of prenatal alcohol and delta-9-tetrahydrocannabinol exposure via electronic cigarettes on motor development. Alcohol. Clin. Exp. Res. 2022, 46, 1408–1422. [Google Scholar] [CrossRef]
- Ellis, R.J.; Bara, A.; Vargas, C.A.; Frick, A.L.; Loh, E.; Landry, J.; Uzamere, T.O.; Callens, J.E.; Martin, Q.; Rajarajan, P.; et al. Prenatal Delta(9)-Tetrahydrocannabinol Exposure in Males Leads to Motivational Disturbances Related to Striatal Epigenetic Dysregulation. Biol. Psychiatry 2022, 92, 127–138. [Google Scholar] [CrossRef]
- Hussain, S.; Breit, K.R.; Thomas, J.D. The effects of prenatal nicotine and THC E-cigarette exposure on motor development in rats. Psychopharmacology 2022, 239, 1579–1591. [Google Scholar] [CrossRef]
- Sarikahya, M.H.; Cousineau, S.; De Felice, M.; Lee, K.; Wong, K.K.; DeVuono, M.V.; Jung, T.; Rodríguez-Ruiz, M.; Ng, T.H.J.; Gummerson, D.; et al. Prenatal THC Exposure Induces Sex-Dependent Neuropsychiatric Endophenotypes in Offspring and Long-Term Disruptions in Fatty-Acid Signaling Pathways Directly in the Mesolimbic Circuitry. Eneuro 2022, 9. [Google Scholar] [CrossRef]
- Frau, R.; Miczán, V.; Traccis, F.; Aroni, S.; Pongor, C.; Saba, P.; Serra, V.; Sagheddu, C.; Fanni, S.; Congiu, M.; et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nat. Neurosci. 2019, 22, 1975–1985. [Google Scholar] [CrossRef]
- Bara, A.; Manduca, A.; Bernabeu, A.; Borsoi, M.; Serviado, M.; Lassalle, O.; Murphy, M.; Wager-Miller, J.; Mackie, K.; Pelissier-Alicot, A.-L.; et al. Sex-dependent effects of in utero cannabinoid exposure on cortical function. Elife 2018, 7, e36234. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.; Nomura, Y.; Hurd, Y.L. Maternal cannabis use is associated with suppression of immune gene networks in placenta and increased anxiety phenotypes in offspring. Proc. Natl. Acad. Sci. USA 2021, 118, 47. [Google Scholar] [CrossRef] [PubMed]
- Szutorisz, H.; Hurd, Y.L. High times for cannabis: Epigenetic imprint and its legacy on brain and behavior. Neurosci. Biobehav. Rev. 2018, 85, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.K.; Itchon-Ramos, N.; Visco, Z.; Huang, Z.; Grenier, C.; Schrott, R.; Acharya, K.; Boudreau, M.H.; Price, T.M.; Raburn, D.J.; et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018, 13, 1208–1221. [Google Scholar] [CrossRef]
- Schrott, R.; Modliszewski, J.L.; Hawkey, A.B.; Grenier, C.; Holloway, Z.; Evans, J.; Pippen, E.; Corcoran, D.L.; Levin, E.D.; Murphy, S.K. Sperm DNA methylation alterations from cannabis extract exposure are evident in offspring. Epigenetics Chromatin 2022, 15, 1–15. [Google Scholar] [CrossRef]
- Schrott, R.; Acharya, K.; Itchon-Ramos, N.; Hawkey, A.B.; Pippen, E.; Mitchell, J.T.; Kollins, S.H.; Levin, E.D.; Murphy, S.K. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 2020, 15, 161–173. [Google Scholar] [CrossRef]
- Schrott, R.; Rajavel, M.; Acharya, K.; Huang, Z.; Acharya, C.; Hawkey, A.; Pippen, E.; Lyerly, H.K.; Levin, E.D.; Murphy, S.K. Sperm DNA methylation altered by THC and nicotine: Vulnerability of neurodevelopmental genes with bivalent chromatin. Sci. Rep. 2020, 10, 16022. [Google Scholar] [CrossRef]
- Ibn Lahmar Andaloussi, Z.; Taghzouti, K.; Abboussi, O. Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2019, 72, 48–54. [Google Scholar] [CrossRef]
- Watson, C.T.; Szutorisz, H.; Garg, P.; Martin, Q.; Landry, J.A.; Sharp, A.J.; Hurd, Y.L. Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated With Cross-Generational Effects of Adolescent THC Exposure. Neuropsychopharmacology Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 2993–3005. [Google Scholar] [CrossRef]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal Cannabis Use Alters Ventral Striatal Dopamine D2 Gene Regulation in the Offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Stark, T.; Maurel, O.M.; Iannotti, F.A.; Kuchar, M.; Ruda-Kucerova, J.; Piscitelli, F.; Laudani, S.; Pekarik, V.; Salomone, S.; et al. Crosstalk between the transcriptional regulation of dopamine D2 and cannabinoid CB1 receptors in schizophrenia: Analyses in patients and in perinatal Delta9-tetrahydrocannabinol-exposed rats. Pharmacol. Res. 2021, 164, 105357. [Google Scholar] [CrossRef] [PubMed]
- Wanner, N.M.; Colwell, M.; Drown, C.; Faulk, C. Developmental cannabidiol exposure increases anxiety and modifies genome-wide brain DNA methylation in adult female mice. Clin. Epigenetics 2021, 13, 4. [Google Scholar] [CrossRef]
- Innocenzi, E.; De Domenico, E.; Ciccarone, F.; Zampieri, M.; Rossi, G.; Cicconi, R.; Bernardini, R.; Mattei, M.; Grimaldi, P. Paternal activation of CB2 cannabinoid receptor impairs placental and embryonic growth via an epigenetic mechanism. Sci. Rep. 2019, 9, 17034. [Google Scholar] [CrossRef]
- Schrott, R.; Murphy, S.K.; Modliszewski, J.L.; E King, D.; Hill, B.; Itchon-Ramos, N.; Raburn, D.; Price, T.; Levin, E.D.; Vandrey, R.; et al. Refraining from use diminishes cannabis-associated epigenetic changes in human sperm. Environ. Epigenetics 2021, 7, dvab009. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Rossi, G.; Oddi, S.; Di Nisio, V.; Maccarrone, M. Role of Major Endocannabinoid-Binding Receptors during Mouse Oocyte Maturation. Int. J. Mol. Sci. 2019, 20, 2866. [Google Scholar] [CrossRef] [PubMed]
- Totorikaguena, L.; Olabarrieta, E.; Lolicato, F.; Romero-Aguirregomezcorta, J.; Smitz, J.; Agirregoitia, N.; Agirregoitia, E. The endocannabinoid system modulates the ovarian physiology and its activation can improve in vitro oocyte maturation. J. Cell. Physiol. 2020, 235, 7580–7591. [Google Scholar] [CrossRef]
- Totorikaguena, L.; Olabarrieta, E.; Lopez-Cardona, A.P.; Agirregoitia, N.; Agirregoitia, E. Tetrahydrocannabinol Modulates in Vitro Maturation of Oocytes and Improves the Blastocyst Rates after in Vitro Fertilization. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 53, 439–452. [Google Scholar] [CrossRef]
- Fuchs Weizman, N.; Wyse, B.A.; Szaraz, P.; Defer, M.; Jahangiri, S.; Librach, C.L. Cannabis alters epigenetic integrity and endocannabinoid signalling in the human follicular niche. Hum. Reprod. 2021, 36, 1922–1931. [Google Scholar] [CrossRef]
- Fuchs Weizman, N.; Wyse, B.A.; Montbriand, J.; Jahangiri, S.; Librach, C.L. Cannabis significantly alters DNA methylation of the human ovarian follicle in a concentration-dependent manner. Mol. Hum. Reprod. 2022, 28, gaac022. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rava, A.; Trezza, V. Emerging Roles of Endocannabinoids as Key Lipid Mediators for a Successful Pregnancy. Int. J. Mol. Sci. 2023, 24, 5220. https://doi.org/10.3390/ijms24065220
Rava A, Trezza V. Emerging Roles of Endocannabinoids as Key Lipid Mediators for a Successful Pregnancy. International Journal of Molecular Sciences. 2023; 24(6):5220. https://doi.org/10.3390/ijms24065220
Chicago/Turabian StyleRava, Alessandro, and Viviana Trezza. 2023. "Emerging Roles of Endocannabinoids as Key Lipid Mediators for a Successful Pregnancy" International Journal of Molecular Sciences 24, no. 6: 5220. https://doi.org/10.3390/ijms24065220
APA StyleRava, A., & Trezza, V. (2023). Emerging Roles of Endocannabinoids as Key Lipid Mediators for a Successful Pregnancy. International Journal of Molecular Sciences, 24(6), 5220. https://doi.org/10.3390/ijms24065220





