Radiological Assessment in Idiopathic Pulmonary Fibrosis (IPF) Patients According to MUC5B Polymorphism
Abstract
1. Introduction
2. Results
2.1. Clinical and Functional Evaluation at Baseline and during the First Year of Follow Up
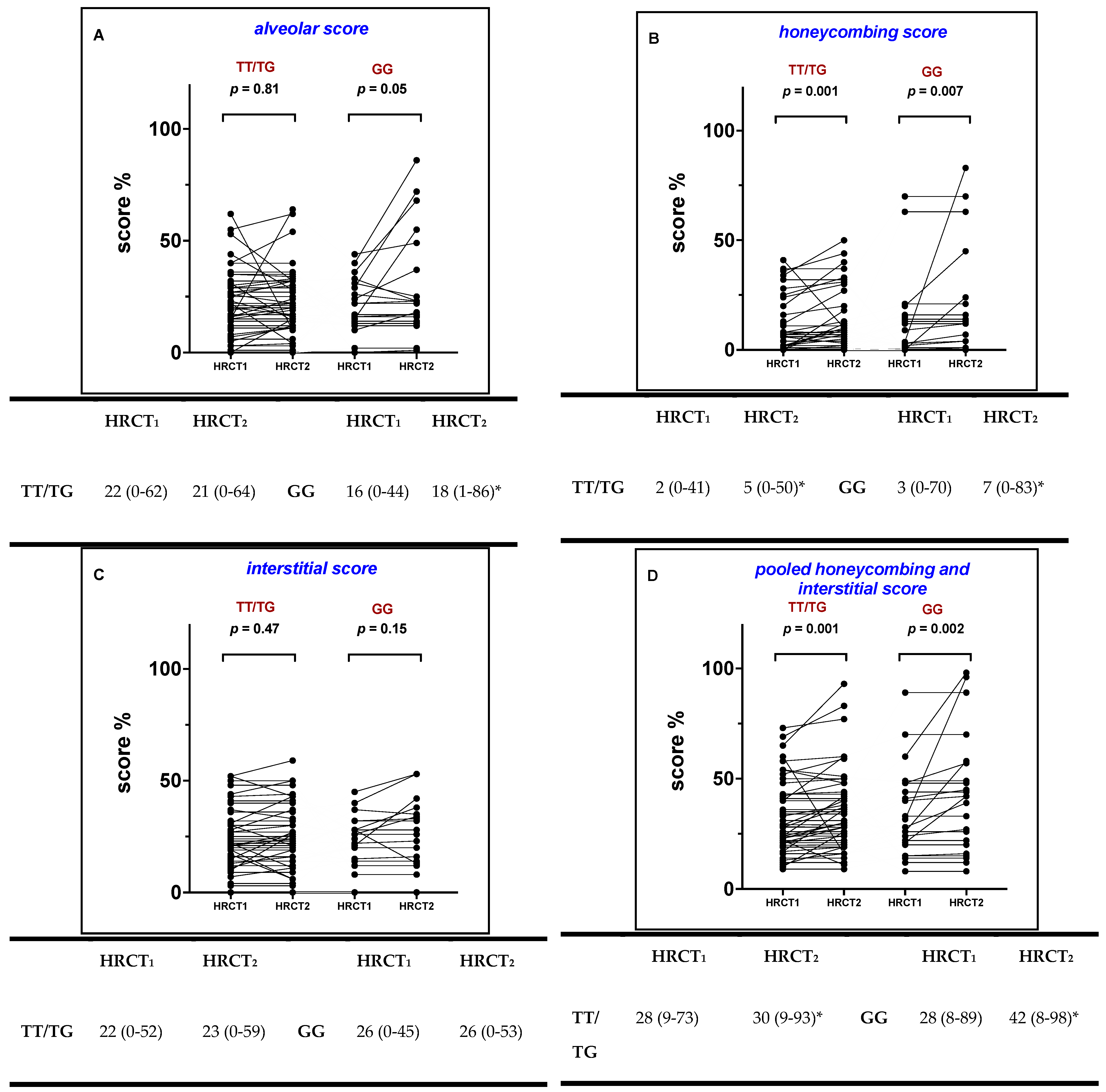
2.2. Radiological Score at Baseline
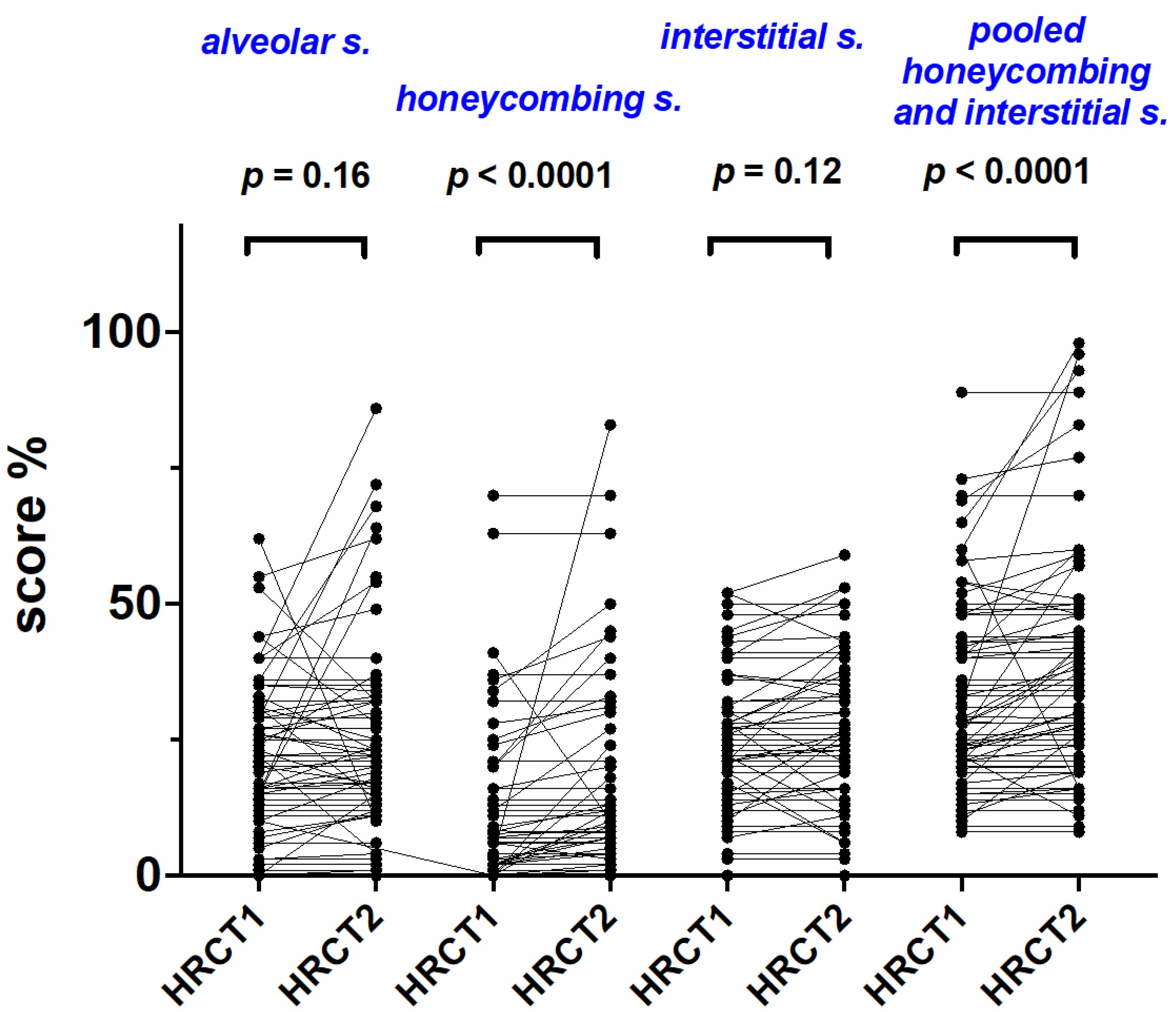
2.3. Radiological Scoring during 1-st Year Follow Up
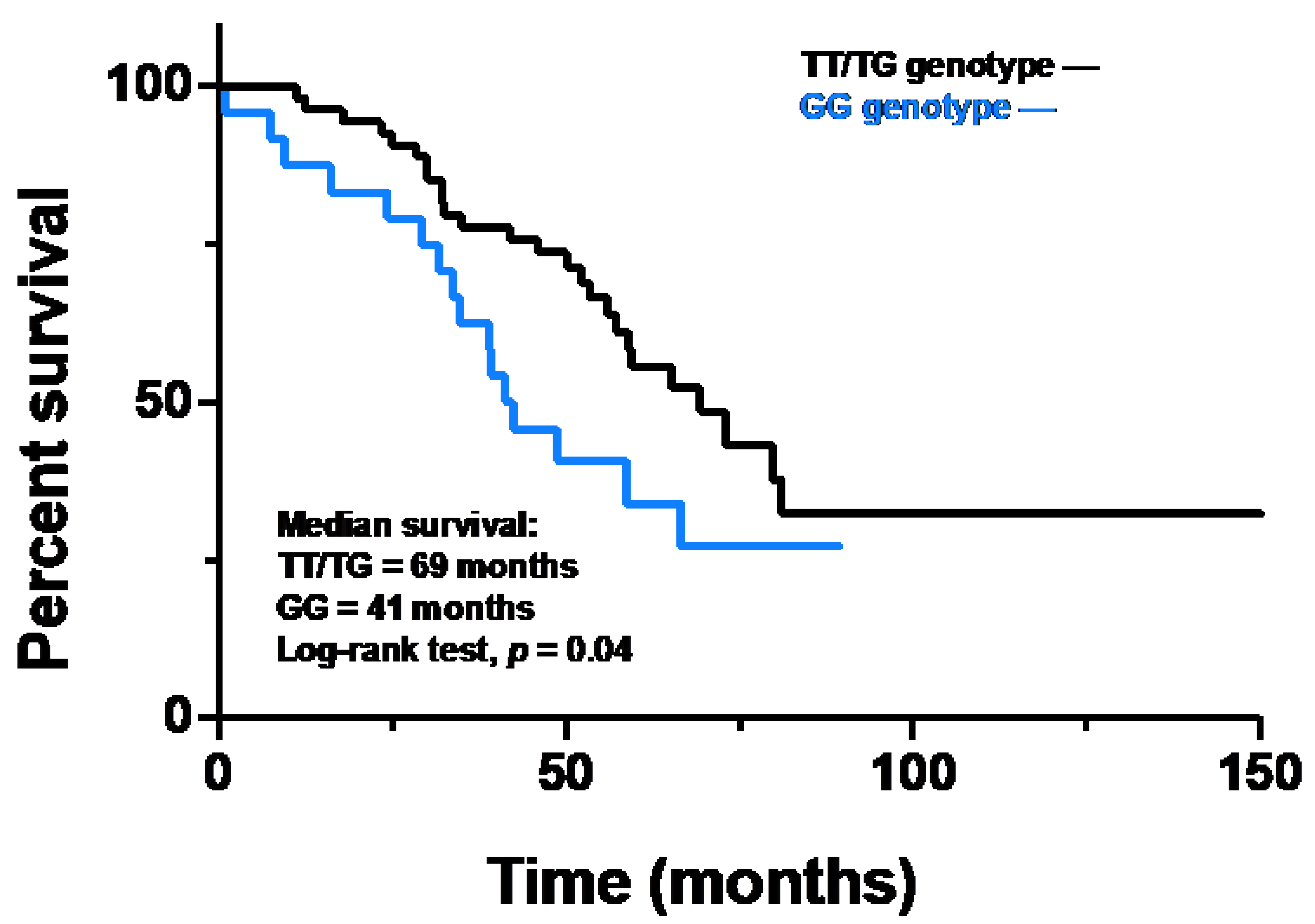
2.4. Survival Analysis and Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population and Study Design
4.2. Radiological Scoring
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Kropski, J.A.; Jones, M.G.; Lee, J.S.; Rossi, G.; Karampitsakos, T.; Maher, T.M.; Tzouvelekis, A.; Ryerson, C.J. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol. Ther. 2021, 222, 107798. [Google Scholar] [CrossRef] [PubMed]
- Adegunsoye, A.; Vij, R.; Noth, I. Integrating Genomics Into Management of Fibrotic Interstitial Lung Disease. Chest 2019, 155, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A Common MUC5B Promoter Polymorphism and Pulmonary Fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.J.; Sato, H.; Fonseca, C.; Banya, W.A.S.; Molyneaux, P.L.; Adamali, H.; Russell, A.-M.; Denton, C.P.; Abraham, D.J.; Hansell, D.M.; et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax 2013, 68, 436–441. [Google Scholar] [CrossRef]
- Noth, I.; Zhang, Y.; Ma, S.-F.; Flores, C.; Barber, M.; Huang, Y.; Broderick, S.M.; Wade, M.S.; Hysi, P.; Scuirba, J.; et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: A genome-wide association study. Lancet Respir. Med. 2013, 1, 309–317. [Google Scholar] [CrossRef]
- Borie, R.; Crestani, B.; Dieude, P.; Nunes, H.; Allanore, Y.; Kannengiesser, C.; Airo, P.; Matucci-Cerinic, M.; Wallaert, B.; Israel-Biet, D.; et al. The MUC5B Variant Is Associated with Idiopathic Pulmonary Fibrosis but Not with Systemic Sclerosis Interstitial Lung Disease in the European Caucasian Population. PLoS ONE 2013, 8, e70621. [Google Scholar] [CrossRef]
- Peljto, A.L.; Zhang, Y.; Fingerlin, T.E.; Ma, S.-F.; Garcia, J.G.N.; Richards, T.J.; Silveira, L.J.; Lindell, K.O.; Steele, M.P.; Loyd, J.; et al. Association Between the MUC5B Promoter Polymorphism and Survival in Patients With Idiopathic Pulmonary Fibrosis. JAMA 2013, 309, 2232–2239. [Google Scholar] [CrossRef]
- Sterclova, M.; Kishore, A.; Sikorova, K.; Skibova, J.; Petrek, M.; Vasakova, M. Effect of genotype on the disease course in idiopathic pulmonary fibrosis despite antifibrotic treatment. Biomed. Rep. 2021, 15, 1–7. [Google Scholar] [CrossRef]
- Bonella, F.; Campo, I.; Zorzetto, M.; Boerner, E.; Ohshimo, S.; Theegarten, D.; Taube, C.; Costabel, U. Potential clinical utility of MUC5B und TOLLIP single nucleotide polymorphisms (SNPs) in the management of patients with IPF. Orphanet J. Rare Dis. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Mota, P.C.; Soares, M.L.; Vasconcelos, C.D.; Ferreira, A.C.; Lima, B.A.; Manduchi, E.; Moore, J.H.; Melo, N.; Novais-Bastos, H.; Pereira, J.M.; et al. Predictive value of common genetic variants in idiopathic pulmonary fibrosis survival. J. Mol. Med. 2022, 100, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Dhooria, S.; Bal, A.; Sehgal, I.S.; Prasad, K.T.; Kashyap, D.; Sharma, R.; Muthu, V.; Agarwal, R.; Aggarwal, A.N. MUC5B Promoter Polymorphism and Survival in Indian Patients With Idiopathic Pulmonary Fibrosis. Chest 2022, 162, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Biondini, D.; Cocconcelli, E.; Bernardinello, N.; Lorenzoni, G.; Rigobello, C.; Lococo, S.; Castelli, G.; Baraldo, S.; Cosio, M.G.; Gregori, D.; et al. Prognostic role of MUC5B rs35705950 genotype in patients with idiopathic pulmonary fibrosis (IPF) on antifibrotic treatment. Respir. Res. 2021, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peljto, A.L.; Selman, M.; Kim, D.S.; Murphy, E.; Tucker, L.; Pardo, A.; Lee, J.S.; Ji, W.; Schwarz, M.I.; Yang, I.V.; et al. The MUC5B Promoter Polymorphism Is Associated With Idiopathic Pulmonary Fibrosis in a Mexican Cohort but Is Rare Among Asian Ancestries. Chest 2015, 147, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.J.; Conti, C.; Montero-Fernandez, Á.; Caramori, G.; Molyneaux, P.L.; George, P.M.; Kokosi, M.; Kouranos, V.; Maher, T.M.; Chua, F.; et al. Interaction between the promoter MUC5B polymorphism and mucin expression: Is there a difference according to ILD subtype? Thorax 2020, 75, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.A.; Zhang, D.; Oldham, J.M.; Kozlitina, J.; Ma, S.-F.; Martinez, F.J.; Raghu, G.; Noth, I.; Garcia, C.K. Telomere Length and Use of Immunosuppressive Medications in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 336–347. [Google Scholar] [CrossRef]
- Livraghi-Butrico, A.; Grubb, B.R.; Wilkinson, K.J.; Volmer, A.S.; Burns, K.A.; Evans, C.; O’Neal, W.K.; Boucher, R.C. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017, 10, 395–407. [Google Scholar] [CrossRef]
- Michalski, J.E.; Schwartz, D.A. Genetic Risk Factors for Idiopathic Pulmonary Fibrosis: Insights into Immunopathogenesis. J. Inflamm. Res. 2020, 13, 1305–1318. [Google Scholar] [CrossRef]
- Boucher, R.C. Idiopathic Pulmonary Fibrosis—A Sticky Business. N. Engl. J. Med. 2011, 364, 1560–1561. [Google Scholar] [CrossRef]
- Schwartz, D.A. Idiopathic Pulmonary Fibrosis Is a Genetic Disease Involving Mucus and the Peripheral Airways. Ann. Am. Thorac. Soc. 2018, 15, S192–S197. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Peljto, A.L.; Chawla, A.; Talbert, J.L.; McKean, D.F.; Rho, B.-H.; Fingerlin, T.E.; Schwarz, M.I.; Schwartz, D.A.; Lynch, D.A. CT Imaging Phenotypes of Pulmonary Fibrosis in the MUC5B Promoter Site Polymorphism. Chest 2016, 149, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhuang, Y.; Peng, H.; Cao, M.; Li, Y.; Xu, Q.; Xin, X.; Zhou, K.; Liang, G.; Cai, H.; et al. The relationship between MUC5B promoter, TERT polymorphisms and telomere lengths with radiographic extent and survival in a Chinese IPF cohort. Sci. Rep. 2019, 9, 15307. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Biondini, D.; Balestro, E.; Lacedonia, D.; Cerri, S.; Milaneschi, R.; Luppi, F.; Cocconcelli, E.; Bazzan, E.; Clini, E.; Barbaro, M.P.F.; et al. Pretreatment rate of decay in forced vital capacity predicts long-term response to pirfenidone in patients with idiopathic pulmonary fibrosis. Sci. Rep. 2018, 8, 5961. [Google Scholar] [CrossRef]
- Cocconcelli, E.; Balestro, E.; Biondini, D.; Barbiero, G.; Polverosi, R.; Calabrese, F.; Pezzuto, F.; Lacedonia, D.; Rea, F.; Schiavon, M.; et al. High-Resolution Computed Tomography (HRCT) Reflects Disease Progression in Patients with Idiopathic Pulmonary Fibrosis (IPF): Relationship with Lung Pathology. J. Clin. Med. 2019, 8, 399. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 379, 1811–1823. [Google Scholar] [CrossRef]
- Balestro, E.; Cocconcelli, E.; Giraudo, C.; Polverosi, R.; Biondini, D.; Lacedonia, D.; Bazzan, E.; Mazzai, L.; Rizzon, G.; Lococo, S.; et al. High-Resolution CT Change over Time in Patients with Idiopathic Pulmonary Fibrosis on Antifibrotic Treatment. J. Clin. Med. 2019, 8, 1469. [Google Scholar] [CrossRef]
- Lynch, D.A.; Godwin, J.D.; Safrin, S.; Starko, K.M.; Hormel, P.; Brown, K.K.; Raghu, G.; King, T.E., Jr.; Bradford, W.Z.; Schwartz, D.A.; et al. High-Resolution Computed Tomography in Idiopathic Pulmonary Fibrosis: Diagnosis and Prognosis. Am. J. Respir. Crit. Care Med. 2005, 172, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Fell, C.D.; Martinez, F.J.; Liu, L.X.; Murray, S.; Han, M.K.; Kazerooni, E.A.; Gross, B.H.; Myers, J.; Travis, W.D.; Colby, T.V.; et al. Clinical Predictors of a Diagnosis of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2010, 181, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991. [Google Scholar]
| HRCT1 | HRCT2 | p Value | HRCT1 | HRCT2 | p Value | ||
|---|---|---|---|---|---|---|---|
| AS | 20 (0–62) | 20 (0–64) | 0.16 | IS | 22 (0–52) | 23 (0–59) | 0.12 |
| HC | 2 (0–70) | 6 (0–83) | <0.0001 | HC + IS | 28 (8–89) | 33 (8–98) | <0.0001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% IC) | p | HR (95% IC) | p | |
| Alveolar score in HRCT1 (%) | 1.01 (0.99–1.03) | 0.11 | - | - |
| Honeycombing in HRCT1 (%) | 1.00 (0.98–1.02) | 0.52 | - | - |
| Interstitial score in HRCT1 (%) | 1.03 (1.00–1.05) | 0.01 | 1.08 (0.99–1.17) | 0.07 |
| Interstitial s. and honeycombing in HRCT1 (%) | 1.01 (1.00–1.03) | 0.02 | 0.97 (0.93–1.01) | 0.15 |
| Alveolar score in HRCT2 (%) | 1.02 (1.00–1.04) | 0.008 | 1.01 (0.99–1.04) | 0.15 |
| Honeycombing in HRCT2 (%) | 1.01 (0.99–1.03) | 0.16 | - | - |
| Interstitial score in HRCT2 (%) | 1.03 (1.00–1.05) | 0.009 | 0.94 (0.88–1.01) | 0.14 |
| Interstitial s. and honeycombing in HRCT2 (%) | 1.02 (1.00–1.03) | 0.003 | 1.02 (1.00–1.03) | 0.01 |
| Change in Alveolar score (%) | 1.00 (0.99–1.05) | 0.98 | - | - |
| Change in Interstitial score (%) | 1.02 (0.94–1.10) | 0.44 | - | - |
| Change in Honeycombing (%) | 1.03 (1.01–1.06) | 0.03 | 1.05 (0.97–1.13) | 0.18 |
| Change in Interstitial s. and honeycombing (%) | 1.02 (0.99–1.05) | 0.058 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocconcelli, E.; Bernardinello, N.; Giraudo, C.; Castelli, G.; Greco, C.; Polverosi, R.; Saetta, M.; Spagnolo, P.; Balestro, E. Radiological Assessment in Idiopathic Pulmonary Fibrosis (IPF) Patients According to MUC5B Polymorphism. Int. J. Mol. Sci. 2022, 23, 15890. https://doi.org/10.3390/ijms232415890
Cocconcelli E, Bernardinello N, Giraudo C, Castelli G, Greco C, Polverosi R, Saetta M, Spagnolo P, Balestro E. Radiological Assessment in Idiopathic Pulmonary Fibrosis (IPF) Patients According to MUC5B Polymorphism. International Journal of Molecular Sciences. 2022; 23(24):15890. https://doi.org/10.3390/ijms232415890
Chicago/Turabian StyleCocconcelli, Elisabetta, Nicol Bernardinello, Chiara Giraudo, Gioele Castelli, Clorinda Greco, Roberta Polverosi, Marina Saetta, Paolo Spagnolo, and Elisabetta Balestro. 2022. "Radiological Assessment in Idiopathic Pulmonary Fibrosis (IPF) Patients According to MUC5B Polymorphism" International Journal of Molecular Sciences 23, no. 24: 15890. https://doi.org/10.3390/ijms232415890
APA StyleCocconcelli, E., Bernardinello, N., Giraudo, C., Castelli, G., Greco, C., Polverosi, R., Saetta, M., Spagnolo, P., & Balestro, E. (2022). Radiological Assessment in Idiopathic Pulmonary Fibrosis (IPF) Patients According to MUC5B Polymorphism. International Journal of Molecular Sciences, 23(24), 15890. https://doi.org/10.3390/ijms232415890






