Candida Administration in 5/6 Nephrectomized Mice Enhanced Fibrosis in Internal Organs: An Impact of Lipopolysaccharide and (1→3)-β-D-Glucan from Leaky Gut
Abstract
:1. Introduction
2. Results
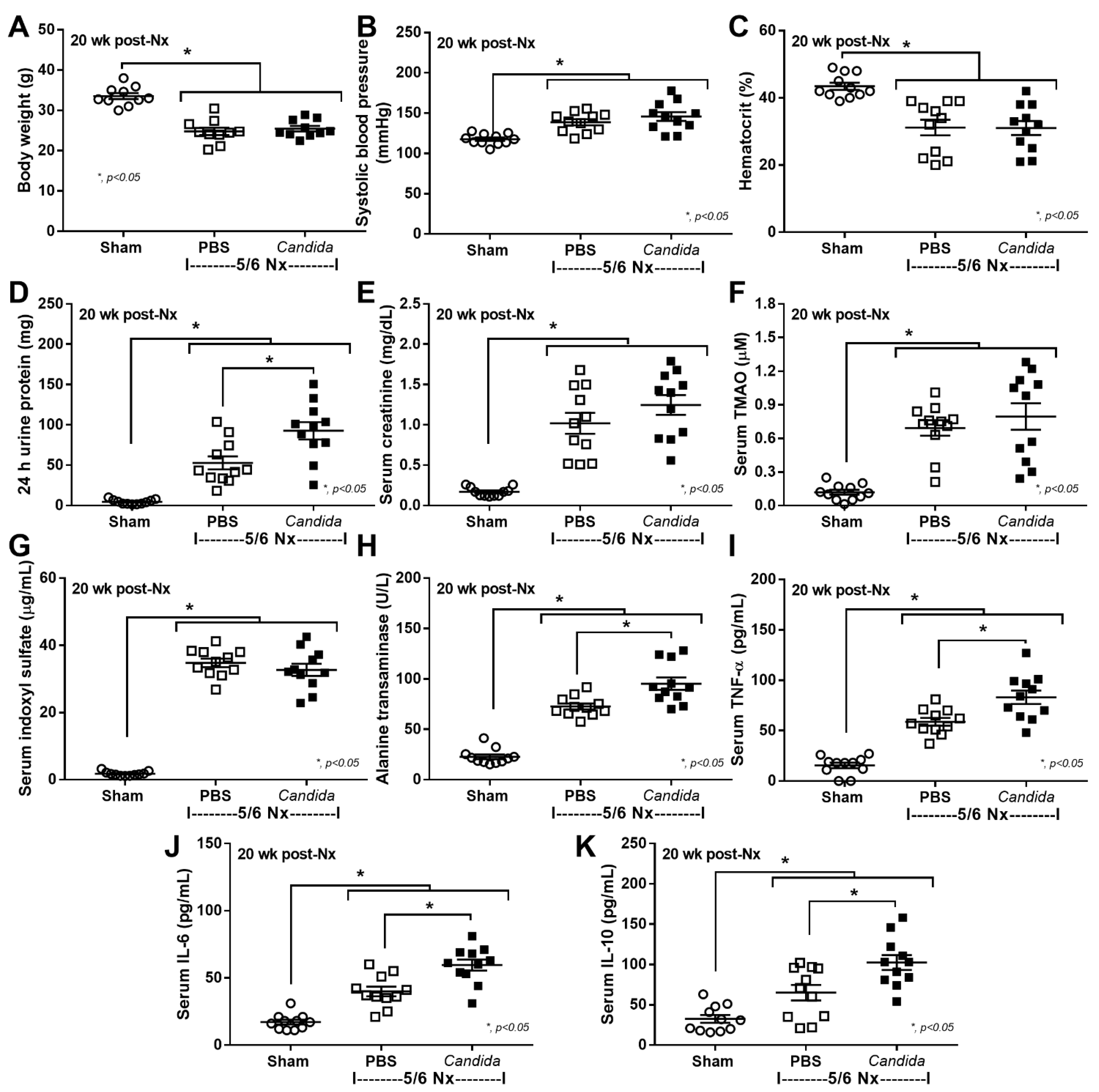
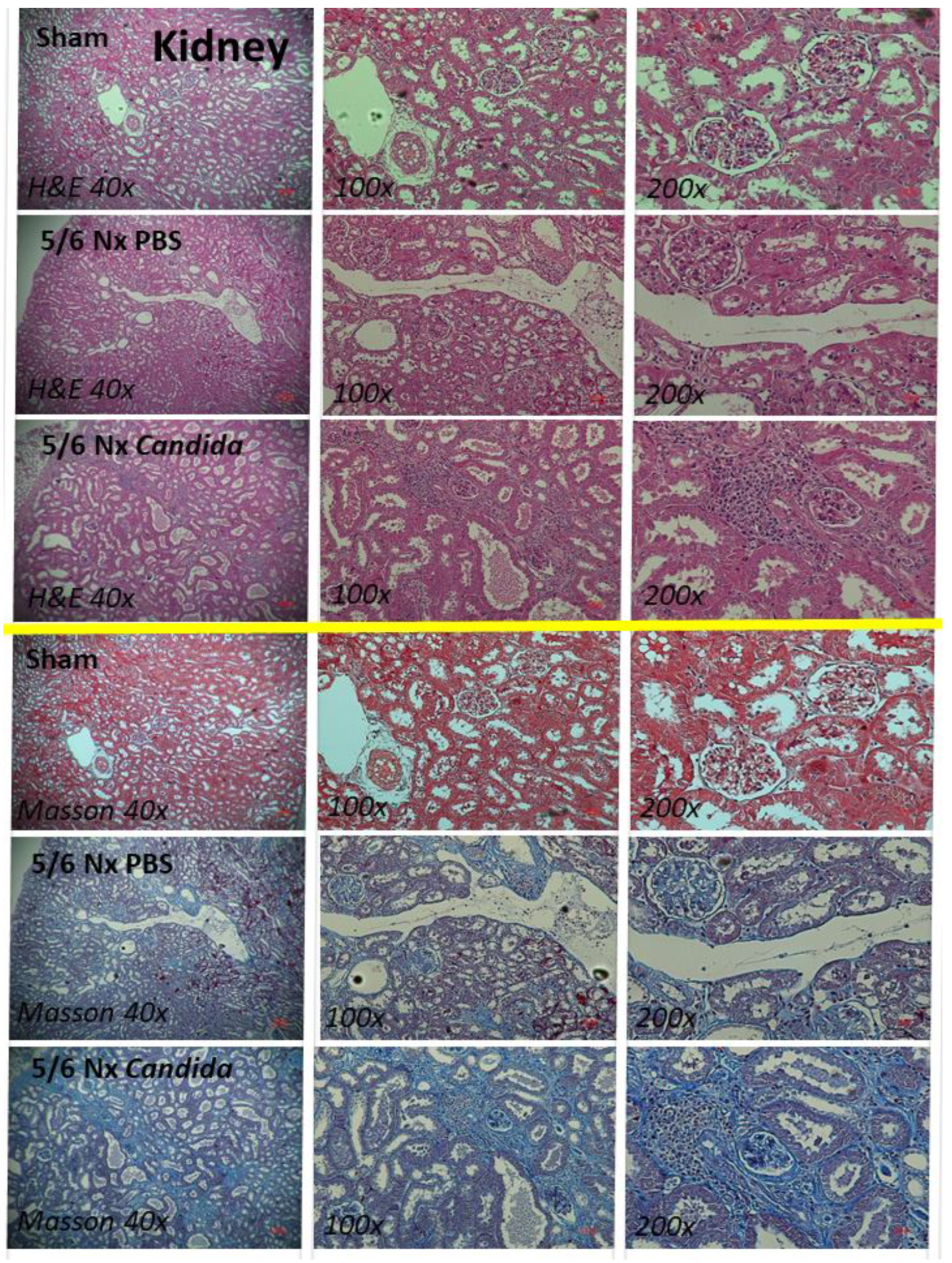
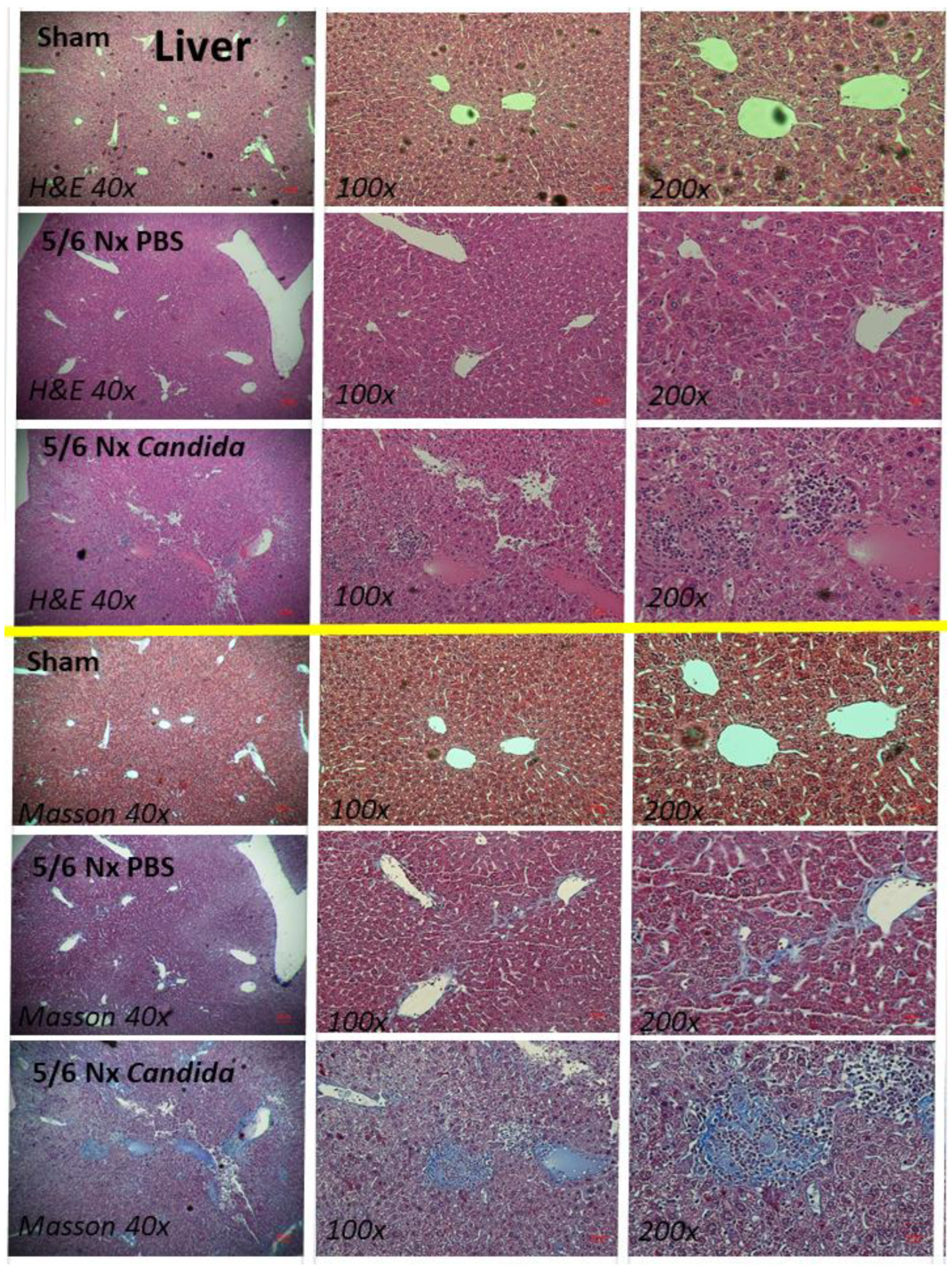
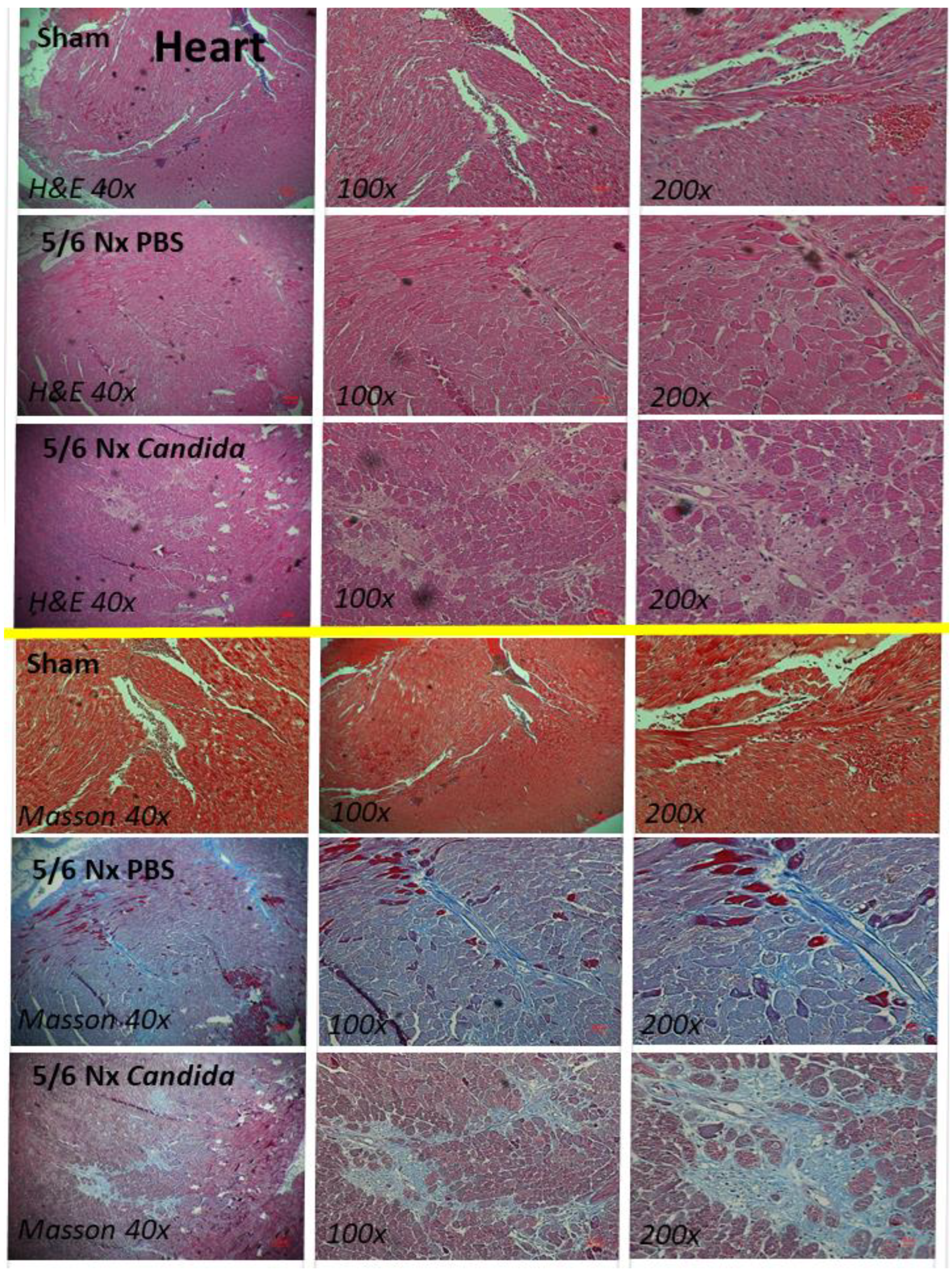
2.1. The Presence of Candida in the Uremic Gut Enhanced Injury in Internal Organs (Kidney, Liver, and Heart) through Systemic Inflammation
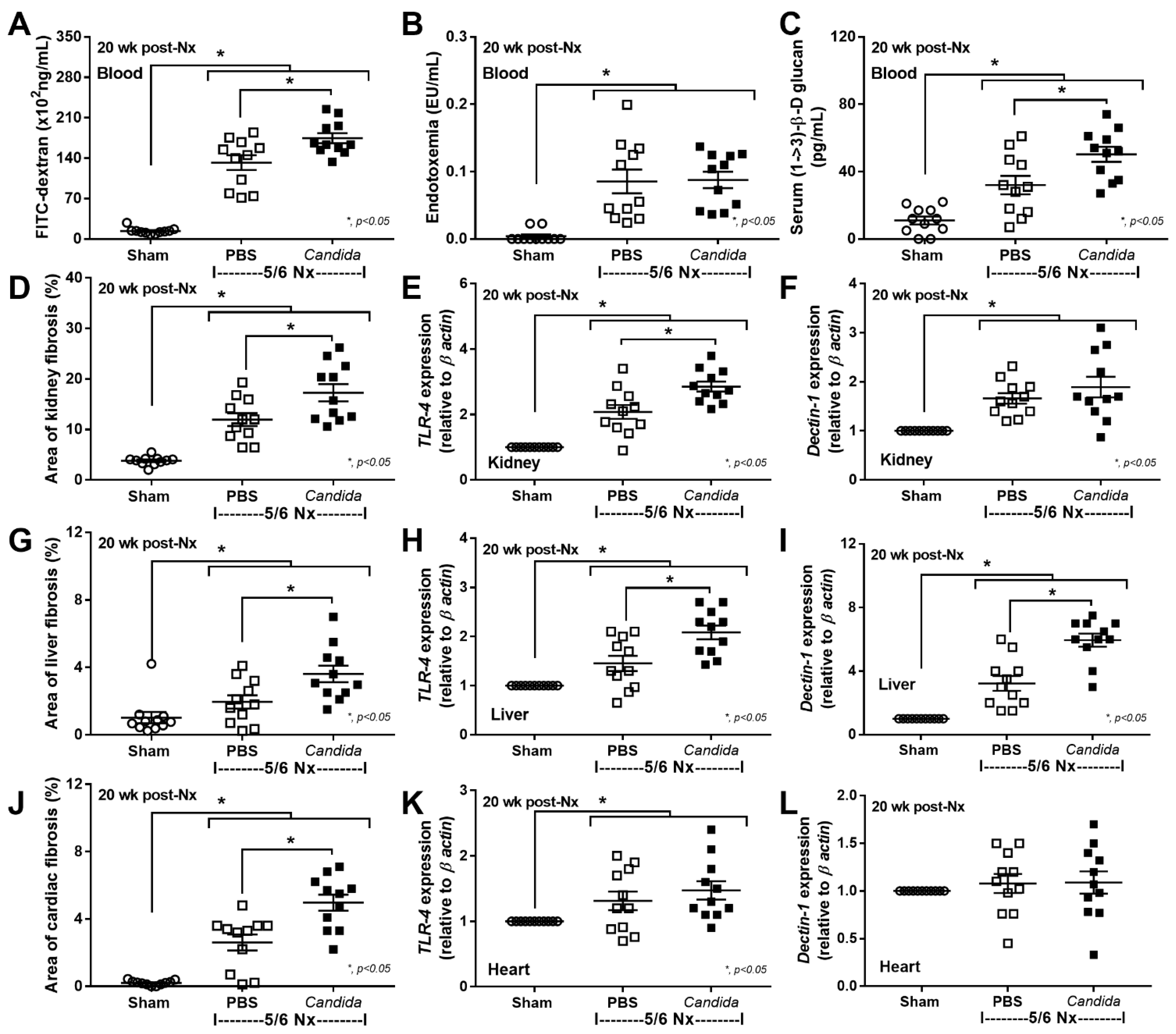
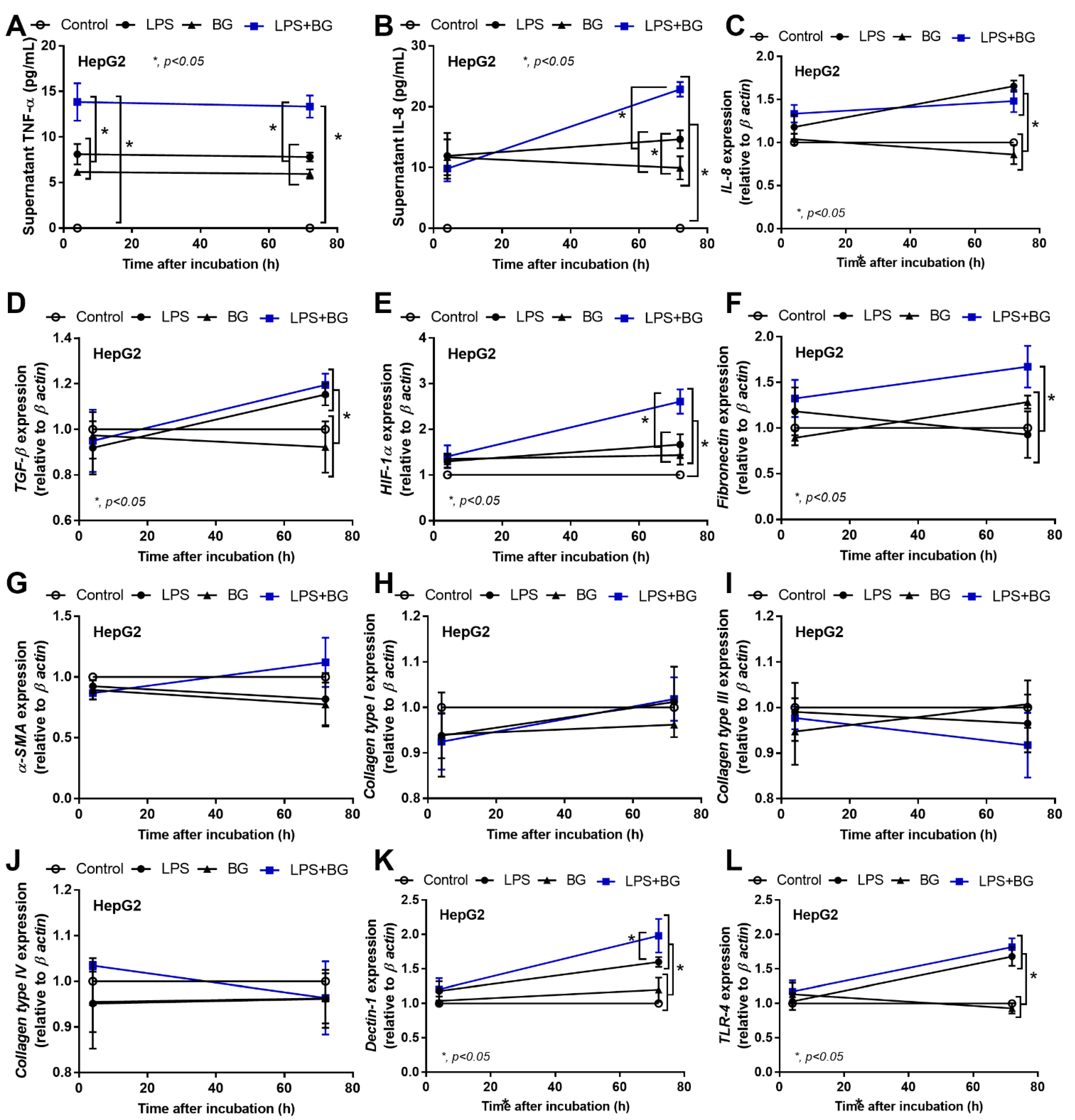
2.2. An Additive Effect of Lipopolysaccharide (LPS) and (BG) on Inflammation-Induced Fibrosis
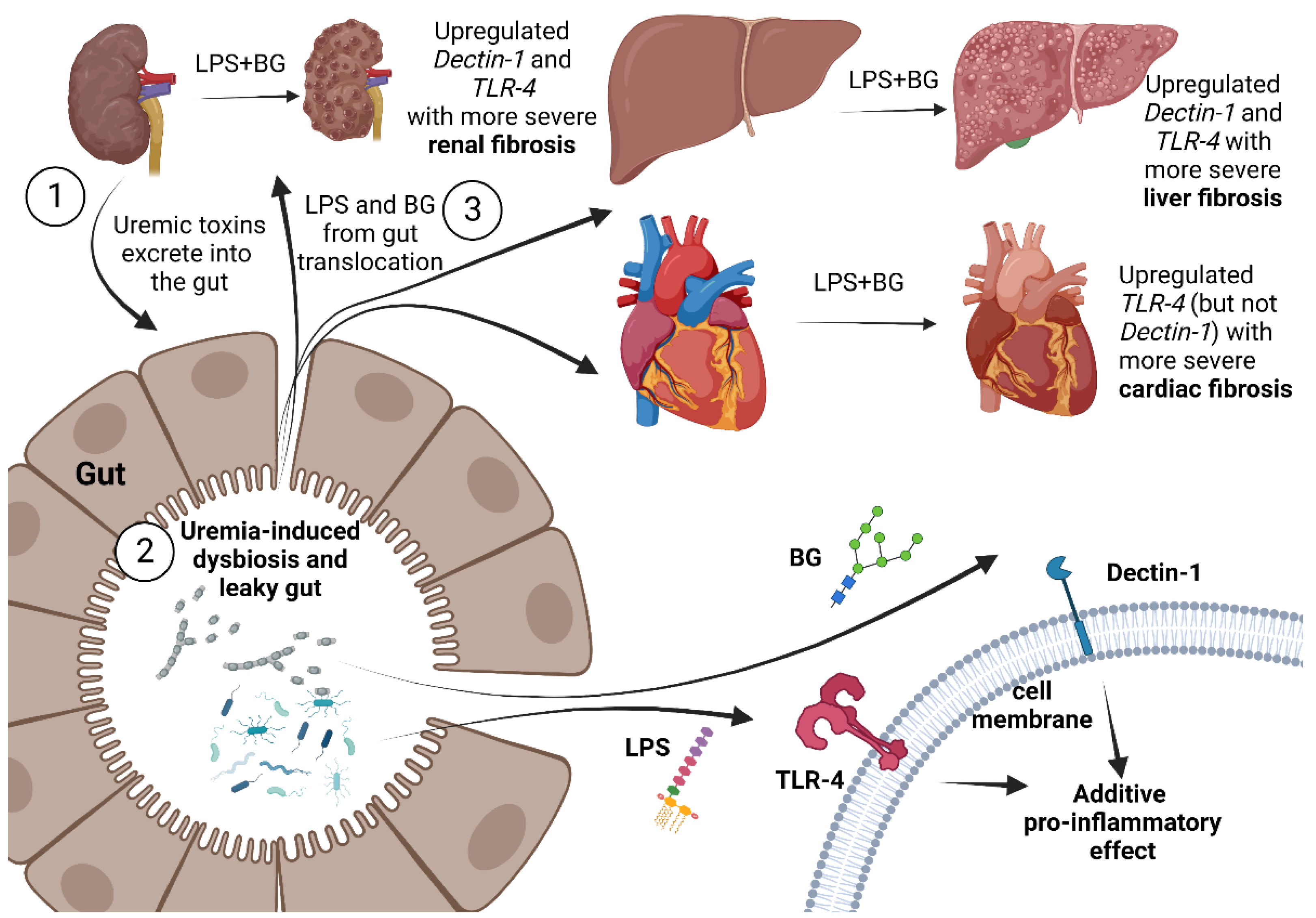
3. Discussion
3.1. Gut Candida Enhanced Systemic Inflammation and Organ Fibrosis in Chronic Kidney Disease (CKD) of 5/6 Nx Mice through Gut Translocation of Glucans
3.2. The Additive Inflammatory Effect of LPS Plus BG, a Key Pro-Inflammatory Factor in Leaky Gut
3.3. Clinical Aspect and Future Experiments
4. Materials and Methods
4.1. Animals and Animal Model
4.1.1. Candida-Administered Chronic Kidney Disease Model
4.1.2. Mouse Sample Analysis
4.1.3. Gut Permeability Determination
4.2. The In Vitro Experiments
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronov, P.A.; Luo, F.J.; Plummer, N.S.; Quan, Z.; Holmes, S.; Hostetter, T.H.; Meyer, T.W. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol. 2011, 22, 1769–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panpetch, W.; Kullapanich, C.; Dang, C.P.; Visitchanakun, P.; Saisorn, W.; Wongphoom, J.; Wannigama, D.L.; Thim-Uam, A.; Patarakul, K.; Somboonna, N.; et al. Candida Administration Worsens Uremia-Induced Gut Leakage in Bilateral Nephrectomy Mice, an Impact of Gut Fungi and Organismal Molecules in Uremia. Msystems 2021, 6, e01187-20. [Google Scholar] [CrossRef]
- McIntyre, C.W.; Harrison, L.E.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Ellis, R.J.; Small, D.M.; Ng, K.L.; Vesey, D.A.; Vitetta, L.; Francis, R.S.; Gobe, G.C.; Morais, C. Indoxyl Sulfate Induces Apoptosis and Hypertrophy in Human Kidney Proximal Tubular Cells. Toxicol. Pathol. 2018, 46, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhou, J.; Wang, S.; Xiong, J.; Chen, Y.; Liu, Y.; Xiao, T.; Li, Y.; He, T.; Li, Y.; et al. Indoxyl sulfate induces intestinal barrier injury through IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics 2020, 10, 7384–7400. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Amornphimoltham, P.; Yuen, P.S.T.; Star, R.A.; Leelahavanichkul, A. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig. Dis. Sci. 2019, 64, 2416–2428. [Google Scholar] [CrossRef]
- Koh, A.Y. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot. Cell 2013, 12, 1416–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, F.M.; de Paula, T.O.; Sarmiento, M.R.A.; de Oliveira, M.G.; Pereira, M.L.M.; Toledo, I.V.; Nascimento, T.C.; Ferreira-Machado, A.B.; Silva, V.L.; Diniz, C.G. Fungal Diversity of Human Gut Microbiota Among Eutrophic, Overweight, and Obese Individuals Based on Aerobic Culture-Dependent Approach. Curr. Microbiol. 2018, 75, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Panpetch, W.; Phuengmaung, P.; Hiengrach, P.; Issara-Amphorn, J.; Cheibchalard, T.; Somboonna, N.; Tumwasorn, S.; Leelahavanichkul, A. Candida Worsens Klebsiella pneumoniae Induced-Sepsis in a Mouse Model with Low Dose Dextran Sulfate Solution through Gut Dysbiosis and Enhanced Inflammation. Int. J. Mol. Sci. 2022, 23, 7050. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Kamolratanakul, S.; Ariyanon, W.; Thanachartwet, V.; Phumratanaprapin, W.; Wilairatana, P.; Leelahavanichkul, A. Abnormal Blood Bacteriome, Gut Dysbiosis, and Progression to Severe Dengue Disease. Front. Cell. Infect. Microbiol. 2022, 12, 890817. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Panpetch, W.; Chindamporn, A.; Leelahavanichkul, A. Macrophage depletion alters bacterial gut microbiota partly through fungal overgrowth in feces that worsens cecal ligation and puncture sepsis mice. Sci. Rep. 2022, 12, 9345. [Google Scholar] [CrossRef] [PubMed]
- Saithong, S.; Worasilchai, N.; Saisorn, W.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Chindamporn, A.; Tovichayathamrong, P.; Torvorapanit, P.; Chiewchengchol, D.; Chancharoenthana, W.; et al. Neutrophil Extracellular Traps in Severe SARS-CoV-2 Infection: A Possible Impact of LPS and (1→3)-beta-D-glucan in Blood from Gut Translocation. Cells 2022, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Tungsanga, S.; Panpetch, W.; Bhunyakarnjanarat, T.; Udompornpitak, K.; Katavetin, P.; Chancharoenthana, W.; Chatthanathon, P.; Somboonna, N.; Tungsanga, K.; Tumwasorn, S.; et al. Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (13)-beta-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34. Int. J. Mol. Sci. 2022, 23, 2511. [Google Scholar] [CrossRef]
- Thim-Uam, A.; Makjaroen, J.; Issara-Amphorn, J.; Saisorn, W.; Wannigama, D.L.; Chancharoenthana, W.; Leelahavanichkul, A. Enhanced Bacteremia in Dextran Sulfate-Induced Colitis in Splenectomy Mice Correlates with Gut Dysbiosis and LPS Tolerance. Int. J. Mol. Sci. 2022, 23, 1676. [Google Scholar] [CrossRef]
- Kaewduangduen, W.; Visitchanakun, P.; Saisorn, W.; Phawadee, A.; Manonitnantawat, C.; Chutimaskul, C.; Susantitaphong, P.; Ritprajak, P.; Somboonna, N.; Cheibchalard, T.; et al. Blood Bacteria-Free DNA in Septic Mice Enhances LPS-Induced Inflammation in Mice through Macrophage Response. Int. J. Mol. Sci. 2022, 23, 1907. [Google Scholar] [CrossRef]
- Liu, G.L.; Chi, Z.; Wang, G.Y.; Wang, Z.P.; Li, Y.; Chi, Z.M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Geertinger, P.; Bodenhoff, J.; Helweg-Larsen, K.; Lund, A. Endogenous alcohol production by intestinal fermentation in sudden infant death. Z. Rechtsmed. 1982, 89, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Asanuma, Y.; Yahara, O.; Shibue, H.; Hisamura, M.; Saito, N.; Kawakami, Y.; Murao, M. Intragastrointestinal alcohol fermentation syndrome: Report of two cases and review of the literature. J. Forensic. Sci. Soc. 1984, 24, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.A.; Kainz, K.; Carmona-Gutierrez, D.; Madeo, F. Microbial wars: Competition in ecological niches and within the microbiome. Microb. Cell 2018, 5, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Panpetch, W.; Worasilchai, N.; Chindamporn, A.; Tumwasorn, S.; Jaroonwitchawan, T.; Wilantho, A.; Chatthanathon, P.; Somboonna, N.; Leelahavanichkul, A. Administration of Candida Albicans to Dextran Sulfate Solution Treated Mice Causes Intestinal Dysbiosis, Emergence and Dissemination of Intestinal Pseudomonas Aeruginosa and Lethal Sepsis. Shock 2020, 53, 189–198. [Google Scholar] [CrossRef]
- Charoensappakit, A.; Sae-Khow, K.; Leelahavanichkul, A. Gut Barrier Damage and Gut Translocation of Pathogen Molecules in Lupus, an Impact of Innate Immunity (Macrophages and Neutrophils) in Autoimmune Disease. Int. J. Mol. Sci. 2022, 23, 8223. [Google Scholar] [CrossRef]
- Panpetch, W.; Somboonna, N.; Palasuk, M.; Hiengrach, P.; Finkelman, M.; Tumwasorn, S.; Leelahavanichkul, A. Oral Candida administration in a Clostridium difficile mouse model worsens disease severity but is attenuated by Bifidobacterium. PLoS ONE 2019, 14, e0210798. [Google Scholar] [CrossRef]
- Panpetch, W.; Hiengrach, P.; Nilgate, S.; Tumwasorn, S.; Somboonna, N.; Wilantho, A.; Chatthanathon, P.; Prueksapanich, P.; Leelahavanichkul, A. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020, 11, 465–480. [Google Scholar] [CrossRef]
- Panpetch, W.; Sawaswong, V.; Chanchaem, P.; Ondee, T.; Dang, C.P.; Payungporn, S.; Tumwasorn, S.; Leelahavanichkul, A. Corrigendum: Candida Administration Worsens Cecal Ligation and Puncture-Induced Sepsis in Obese Mice Through Gut Dysbiosis Enhanced Systemic Inflammation, Impact of Pathogen-Associated Molecules From Gut Translocation and Saturated Fatty Acid. Front. Immunol. 2020, 11, 613095. [Google Scholar] [CrossRef]
- Panpetch, W.; Somboonna, N.; Bulan, D.E.; Issara-Amphorn, J.; Finkelman, M.; Worasilchai, N.; Chindamporn, A.; Palaga, T.; Tumwasorn, S.; Leelahavanichkul, A. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1→3)-beta-D-glucan. PLoS ONE 2017, 12, e0181439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panpetch, W.; Somboonna, N.; Bulan, D.E.; Issara-Amphorn, J.; Worasilchai, N.; Finkelman, M.; Chindamporn, A.; Palaga, T.; Tumwasorn, S.; Leelahavanichkul, A. Gastrointestinal Colonization of Candida Albicans Increases Serum (1→3)-beta-D-Glucan, without Candidemia, and Worsens Cecal Ligation and Puncture Sepsis in Murine Model. Shock 2018, 49, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.M.; Inamine, T.; Hochrath, K.; Chen, P.; Wang, L.; Llorente, C.; Bluemel, S.; Hartmann, P.; Xu, J.; Koyama, Y.; et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Investig. 2017, 127, 2829–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, L.; Deutsch, M.; Alothman, S.; Alqunaibit, D.; Werba, G.; Pansari, M.; Pergamo, M.; Ochi, A.; Torres-Hernandez, A.; Levie, E.; et al. Dectin-1 Regulates Hepatic Fibrosis and Hepatocarcinogenesis by Suppressing TLR4 Signaling Pathways. Cell. Rep. 2015, 13, 1909–1921. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bian, Y.; Pang, P.; Yu, S.; Wang, X.; Gao, Y.; Liu, K.; Liu, Q.; Yuan, Y.; Du, W. Inhibition of Dectin-1 in mice ameliorates cardiac remodeling by suppressing NF-kappaB/NLRP3 signaling after myocardial infarction. Int. Immunopharmacol. 2020, 80, 106116. [Google Scholar] [CrossRef]
- Calabrese, D.R.; Wang, P.; Chong, T.; Hoover, J.; Singer, J.P.; Torgerson, D.; Hays, S.R.; Golden, J.A.; Kukreja, J.; Dugger, D.; et al. Dectin-1 genetic deficiency predicts chronic lung allograft dysfunction and death. JCI Insight 2019, 4, 133083. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [Green Version]
- Chancharoenthana, W.; Leelahavanichkul, A.; Taratummarat, S.; Wongphom, J.; Tiranathanagul, K.; Eiam-Ong, S. Cilostazol attenuates intimal hyperplasia in a mouse model of chronic kidney disease. PLoS ONE 2017, 12, e0187872. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transpl. 2011, 26, 938–947. [Google Scholar] [CrossRef]
- Panpetch, W.; Chancharoenthana, W.; Bootdee, K.; Nilgate, S.; Finkelman, M.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut. Infect. Immun. 2018, 86, e00700-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungsanga, S.; Katavetin, P.; Panpetch, W.; Udompornpitak, K.; Saisorn, W.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules. Nephrol. Dial. Transpl. 2022, 37, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Forkosh, E.; Ilan, Y. The heart-gut axis: New target for atherosclerosis and congestive heart failure therapy. Open. Heart 2019, 6, e000993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obert, L.A.; Elmore, S.A.; Ennulat, D.; Frazier, K.S. A Review of Specific Biomarkers of Chronic Renal Injury and Their Potential Application in Nonclinical Safety Assessment Studies. Toxicol. Pathol. 2021, 49, 996–1023. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, M.; Grander, C.; Grabherr, F.; Griesmacher, A.; Ploner, T.; Hartig, F.; Bellmann-Weiler, R.; Joannidis, M.; Zoller, H.; Weiss, G.; et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig. Liver. Dis. 2021, 53, 158–165. [Google Scholar] [CrossRef]
- Trimarchi, H.; Muryan, A.; Dicugno, M.; Young, P.; Forrester, M.; Lombi, F.; Pomeranz, V.; Iriarte, R.; Rana, M.S.; Alonso, M. Proteinuria: An ignored marker of inflammation and cardiovascular disease in chronic hemodialysis. Int. J. Nephrol. Renovasc. Dis. 2012, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ge, S.; Peng, Y.; Chen, X. Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis. Burns. Trauma 2013, 1, 109–121. [Google Scholar]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Camara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.A.; Elbastawisy, Y.M.; Elsaed, W.M. Attenuation of lipopolysaccharide-induced lung inflammation by ascorbic acid in rats: Histopathological and ultrastructural study. SAGE Open Med. 2019, 7, 2050312119828260. [Google Scholar] [CrossRef]
- Heisel, T.; Montassier, E.; Johnson, A.; Al-Ghalith, G.; Lin, Y.W.; Wei, L.N.; Knights, D.; Gale, C.A. High-Fat Diet Changes Fungal Microbiomes and Interkingdom Relationships in the Murine Gut. Msphere 2017, 2, e00351-17. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Liu, Y.; Xu, J.; Liu, Y. Gut-Liver Axis: Liver Sinusoidal Endothelial Cells Function as the Hepatic Barrier in Colitis-Induced Liver Injury. Front. Cell. Dev. Biol. 2021, 9, 702890. [Google Scholar] [CrossRef] [PubMed]
- Prytz, H.; Holst-Christensen, J.; Korner, B.; Liehr, H. Portal venous and systemic endotoxaemia in patients without liver disease and systemic endotoxaemia in patients with cirrhosis. Scand. J. Gastroenterol. 1976, 11, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issara-Amphorn, J.; Chancharoenthana, W.; Visitchanakun, P.; Leelahavanichkul, A. Syk Inhibitor Attenuates Polymicrobial Sepsis in FcgRIIb-Deficient Lupus Mouse Model, the Impact of Lupus Characteristics in Sepsis. J. Innate Immun. 2020, 12, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Issara-Amphorn, J.; Dang, C.P.; Saisorn, W.; Limbutara, K.; Leelahavanichkul, A. Candida Administration in Bilateral Nephrectomy Mice Elevates Serum (1→3)-beta-D-glucan That Enhances Systemic Inflammation Through Energy Augmentation in Macrophages. Int. J. Mol. Sci. 2021, 22, 5031. [Google Scholar] [CrossRef] [PubMed]
- Issara-Amphorn, J.; Somboonna, N.; Pisitkun, P.; Hirankarn, N.; Leelahavanichkul, A. Syk inhibitor attenuates inflammation in lupus mice from FcgRIIb deficiency but not in pristane induction: The influence of lupus pathogenesis on the therapeutic effect. Lupus 2020, 29, 1248–1262. [Google Scholar] [CrossRef]
- Issara-Amphorn, J.; Surawut, S.; Worasilchai, N.; Thim-Uam, A.; Finkelman, M.; Chindamporn, A.; Palaga, T.; Hirankarn, N.; Pisitkun, P.; Leelahavanichkul, A. The Synergy of Endotoxin and (1→3)-beta-D-Glucan, from Gut Translocation, Worsens Sepsis Severity in a Lupus Model of Fc Gamma Receptor IIb-Deficient Mice. J. Innate Immun. 2018, 10, 189–201. [Google Scholar] [CrossRef]
- Sae-Khow, K.; Charoensappakit, A.; Visitchanakun, P.; Saisorn, W.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Pathogen-Associated Molecules from Gut Translocation Enhance Severity of Cecal Ligation and Puncture Sepsis in Iron-Overload beta-Thalassemia Mice. J. Inflamm. Res. 2020, 13, 719–735. [Google Scholar] [CrossRef]
- Saithong, S.; Saisorn, W.; Dang, C.P.; Visitchanakun, P.; Chiewchengchol, D.; Leelahavanichkul, A. Candida Administration Worsens Neutrophil Extracellular Traps in Renal Ischemia Reperfusion Injury Mice: An Impact of Gut Fungi on Acute Kidney Injury. J. Innate Immun. 2022, 14, 502–517. [Google Scholar] [CrossRef]
- Saithong, S.; Saisorn, W.; Visitchanakun, P.; Sae-Khow, K.; Chiewchengchol, D.; Leelahavanichkul, A. A Synergy Between Endotoxin and (1→3)-Beta-D-Glucan Enhanced Neutrophil Extracellular Traps in Candida Administered Dextran Sulfate Solution Induced Colitis in FcGRIIB-/- Lupus Mice, an Impact of Intestinal Fungi in Lupus. J. Inflamm. Res. 2021, 14, 2333–2352. [Google Scholar] [CrossRef]
- Thim-Uam, A.; Surawut, S.; Issara-Amphorn, J.; Jaroonwitchawan, T.; Hiengrach, P.; Chatthanathon, P.; Wilantho, A.; Somboonna, N.; Palaga, T.; Pisitkun, P.; et al. Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus. Sci. Rep. 2020, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Ferwerda, G.; Meyer-Wentrup, F.; Kullberg, B.J.; Netea, M.G.; Adema, G.J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 2008, 10, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Benjaskulluecha, S.; Boonmee, A.; Pattarakankul, T.; Wongprom, B.; Klomsing, J.; Palaga, T. Screening of compounds to identify novel epigenetic regulatory factors that affect innate immune memory in macrophages. Sci. Rep. 2022, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Susanti, H.E.; Rommele, C.; Grobmayr, R.; Gunthner, R.; Anders, H.J. Quantitative expression of C-type lectin receptors in humans and mice. Int. J. Mol. Sci. 2012, 13, 10113–10131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Wang, J.; Shanmukhappa, S.K.; Gandhi, C.R. Toll-Like Receptor 4-Independent Carbon Tetrachloride-Induced Fibrosis and Lipopolysaccharide-Induced Acute Liver Injury in Mice: Role of Hepatic Stellate Cells. Am. J. Pathol. 2017, 187, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wen, Y.; Kang, J.; Wei, C.; Wang, M.; Zheng, Z.; Peng, J. Regulation of TLR4 expression mediates the attenuating effect of erythropoietin on inflammation and myocardial fibrosis in rat heart. Int. J. Mol. Med. 2018, 42, 1436–1444. [Google Scholar] [CrossRef] [Green Version]
- Skuginna, V.; Lech, M.; Allam, R.; Ryu, M.; Clauss, S.; Susanti, H.E.; Rommele, C.; Garlanda, C.; Mantovani, A.; Anders, H.J. Toll-like receptor signaling and SIGIRR in renal fibrosis upon unilateral ureteral obstruction. PLoS ONE 2011, 6, e19204. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Feng, Z. The Role of Toll-Like Receptor Signaling in the Progression of Heart Failure. Mediat. Inflamm. 2018, 2018, 9874109. [Google Scholar] [CrossRef] [Green Version]
- Pradere, J.P.; Troeger, J.S.; Dapito, D.H.; Mencin, A.A.; Schwabe, R.F. Toll-like receptor 4 and hepatic fibrogenesis. Semin. Liver. Dis. 2010, 30, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Pulskens, W.P.; Rampanelli, E.; Teske, G.J.; Butter, L.M.; Claessen, N.; Luirink, I.K.; van der Poll, T.; Florquin, S.; Leemans, J.C. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J. Am. Soc. Nephrol. 2010, 21, 1299–1308. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Zhao, Y.; Liang, Y.; He, Y. Silencing of TLR4 Inhibits Atrial Fibrosis and Susceptibility to Atrial Fibrillation via Downregulation of NLRP3-TGF-beta in Spontaneously Hypertensive Rats. Dis. Markers 2022, 2022, 2466150. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Liu, R.X.; Hou, F.; Cui, L.J.; Li, C.Y.; Chi, C.; Yi, E.; Wen, Y.; Yin, C.H. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol. Med. Rep. 2016, 14, 3669–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; He, Y.; Zhao, H.; Xu, X. Hypoxia inducible factor-1 promotes liver fibrosis in nonalcoholic fatty liver disease by activating PTEN/p65 signaling pathway. J. Cell. Biochem. 2019, 120, 14735–14744. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Y.; Cao, R.; Xu, H. MicroRNA-363-3p Inhibits the Expression of Renal Fibrosis Markers in TGF-beta1-Treated HK-2 Cells by Targeting TGF-beta2. Biochem. Genet. 2021, 59, 1033–1048. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Leelahavanichkul, A.; Ariyanon, W.; Vadcharavivad, S.; Phatcharophaswattanakul, S.; Kamolratanakul, S.; Leaungwutiwong, P.; Phumratanaprapin, W.; Wilairatana, P. Leaky Gut Syndrome Is Associated with Endotoxemia and Serum (1→3)-beta-D-Glucan in Severe Dengue Infection. Microorganisms 2021, 9, 2390. [Google Scholar] [CrossRef]
- National Research Council (US). Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Leelahavanichkul, A.; Yan, Q.; Hu, X.; Eisner, C.; Huang, Y.; Chen, R.; Mizel, D.; Zhou, H.; Wright, E.C.; Kopp, J.B.; et al. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 2010, 78, 1136–1153. [Google Scholar] [CrossRef] [Green Version]
- Seujange, Y.; Leelahavanichkul, A.; Yisarakun, W.; Khawsuk, W.; Meepool, A.; Phamonleatmongkol, P.; Saechau, W.; Onlamul, W.; Tantiwarattanatikul, P.; Oonsook, W.; et al. Hibiscus sabdariffa Linnaeus aqueous extracts attenuate the progression of renal injury in 5/6 nephrectomy rats. Ren. Fail. 2013, 35, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Boonhai, S.; Bootdee, K.; Saisorn, W.; Takkavatakarn, K.; Sitticharoenchai, P.; Tungsanga, S.; Tiranathanagul, K.; Leelahavanichkul, A. TMAO reductase, a biomarker for gut permeability defect induced inflammation, in mouse model of chronic kidney disease and dextran sulfate solution-induced mucositis. Asian Pac. J. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Udompornpitak, K.; Charoensappakit, A.; Sae-Khow, K.; Bhunyakarnjanarat, T.; Dang, C.P.; Saisorn, W.; Visitchanakun, P.; Phuengmaung, P.; Palaga, T.; Ritprajak, P.; et al. Obesity Exacerbates Lupus Activity in Fc Gamma Receptor IIb Deficient Lupus Mice Partly through Saturated Fatty Acid-Induced Gut Barrier Defect and Systemic Inflammation. J. Innate Immun. 2022, 11, 1–22. [Google Scholar] [CrossRef]
- Hiengrach, P.; Visitchanakun, P.; Tongchairawewat, P.; Tangsirisatian, P.; Jungteerapanich, T.; Ritprajak, P.; Wannigama, D.L.; Tangtanatakul, P.; Leelahavanichkul, A. Sepsis Encephalopathy Is Partly Mediated by miR370-3p-Induced Mitochondrial Injury but Attenuated by BAM15 in Cecal Ligation and Puncture Sepsis Male Mice. Int. J. Mol. Sci. 2022, 23, 5445. [Google Scholar] [CrossRef]
- Panpetch, W.; Phuengmaung, P.; Cheibchalard, T.; Somboonna, N.; Leelahavanichkul, A.; Tumwasorn, S. Lacticaseibacillus casei Strain T21 Attenuates Clostridioides difficile Infection in a Murine Model Through Reduction of Inflammation and Gut Dysbiosis With Decreased Toxin Lethality and Enhanced Mucin Production. Front. Microbiol. 2021, 12, 745299. [Google Scholar] [CrossRef] [PubMed]
- Visitchanakun, P.; Tangtanatakul, P.; Trithiphen, O.; Soonthornchai, W.; Wongphoom, J.; Tachaboon, S.; Srisawat, N.; Leelahavanichkul, A. Plasma miR-370-3P as a Biomarker of Sepsis-Associated Encephalopathy, the Transcriptomic Profiling Analysis of Microrna-Arrays From Mouse Brains. Shock 2020, 54, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Visitchanakun, P.; Saisorn, W.; Wongphoom, J.; Chatthanathon, P.; Somboonna, N.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Gut leakage enhances sepsis susceptibility in iron-overloaded beta-thalassemia mice through macrophage hyperinflammatory responses. Am. J. Physiol. Gastrointest. Liver. Physiol. 2020, 318, G966–G979. [Google Scholar] [CrossRef] [PubMed]
- Bhunyakarnjanarat, T.; Udompornpitak, K.; Saisorn, W.; Chantraprapawat, B.; Visitchanakun, P.; Dang, C.P.; Issara-Amphorn, J.; Leelahavanichkul, A. Prominent Indomethacin-Induced Enteropathy in Fcgriib Defi-cient lupus Mice: An Impact of Macrophage Responses and Immune Deposition in Gut. Int. J. Mol. Sci. 2021, 22, 1377. [Google Scholar] [CrossRef]

| Name | Forward Primer | Reverse Primer |
|---|---|---|
| α-Smooth muscle actin (α-SMA) (human) | 5′-ACTGAGCGTGGCTATTCCTCCGTT-3′ | 5′-GCAGTGGCCATCTCATTTTCA-3′ |
| Collagen type I (Col I) (human) | 5′-CGATGGATTCCAGTTCGAGT-3′ | 5′-TTTTGAGGGGGTTCAGTTTG-3′ |
| Collagen type III (Col III) (human) | 5′-GTCCTATTGGTCCTCCTGGC-3′ | 5′-ACCAGGGAAACCAGCAGG-3′ |
| Collagen type IV (Col IV) (human) | 5′-ATGGGGCCCCGGCTCAGC-3′ | 5′-ATCCTCTTTCACCTTTCAATAGC-3′ |
| Dectin-1 (human) | 5′-CGCCTCATTGCTGTAATTTTGG-3′ | 5′-ATCCATCCTCCCAGAGCCA-3′ |
| Fibronectin I (human) | 5′-CCGTGGGCAACTCTGTC-3′ | 5′-TGCGGCAGTTGTCACAG-3′ |
| Hypoxia-inducible factor (HIF-1α) (human) | 5′-TTCACCTGAGCCTAATAGTCC-3′ | 5′-CAAGTCTAAATCTGTGTCCTG-3′ |
| Interleukin-8 (IL-8) (human) | 5′-GAGAGTGATTGAGAGTGGACCAC-3′ | 5′-CACAACCCTCTGCACCCAGTTT-3′ |
| Toll-like receptor-4 (TLR-4) (human) | 5′-CACAGACTTGCGGGTTCTAC-3′ | 5′-AGGACCGACACACCAATGATG -3′ |
| Transforming growth factor β (TGF-β) (human) | 5′-CAGAGCTGCGCTTGCAGAG-3′ | 5′-GTCAGCAGCCGGTTACCAAG-3′ |
| β-actin (human) | 5′-CCTGGCACCCAGCACAAT-3′ | 5′-GCCGATCCACACGGAGTACT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungsanga, S.; Udompornpitak, K.; Worasilchai, J.; Ratana-aneckchai, T.; Wannigama, D.L.; Katavetin, P.; Leelahavanichkul, A. Candida Administration in 5/6 Nephrectomized Mice Enhanced Fibrosis in Internal Organs: An Impact of Lipopolysaccharide and (1→3)-β-D-Glucan from Leaky Gut. Int. J. Mol. Sci. 2022, 23, 15987. https://doi.org/10.3390/ijms232415987
Tungsanga S, Udompornpitak K, Worasilchai J, Ratana-aneckchai T, Wannigama DL, Katavetin P, Leelahavanichkul A. Candida Administration in 5/6 Nephrectomized Mice Enhanced Fibrosis in Internal Organs: An Impact of Lipopolysaccharide and (1→3)-β-D-Glucan from Leaky Gut. International Journal of Molecular Sciences. 2022; 23(24):15987. https://doi.org/10.3390/ijms232415987
Chicago/Turabian StyleTungsanga, Somkanya, Kanyarat Udompornpitak, Jesadakorn Worasilchai, Tharit Ratana-aneckchai, Dhammika Leshan Wannigama, Pisut Katavetin, and Asada Leelahavanichkul. 2022. "Candida Administration in 5/6 Nephrectomized Mice Enhanced Fibrosis in Internal Organs: An Impact of Lipopolysaccharide and (1→3)-β-D-Glucan from Leaky Gut" International Journal of Molecular Sciences 23, no. 24: 15987. https://doi.org/10.3390/ijms232415987






