A Pan-Cancer Landscape of ABCG2 across Human Cancers: Friend or Foe?
Abstract
1. Introduction
2. Results
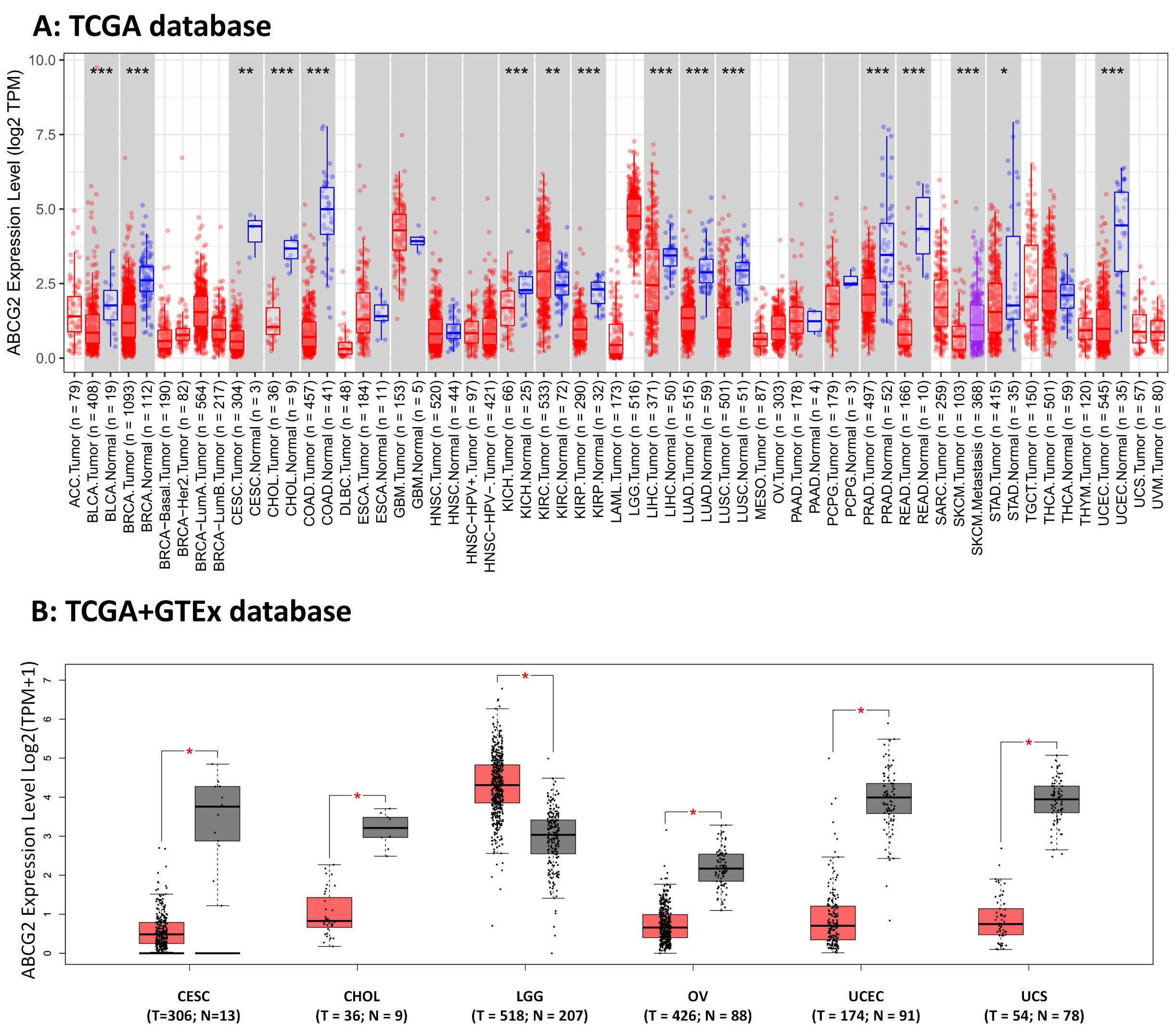
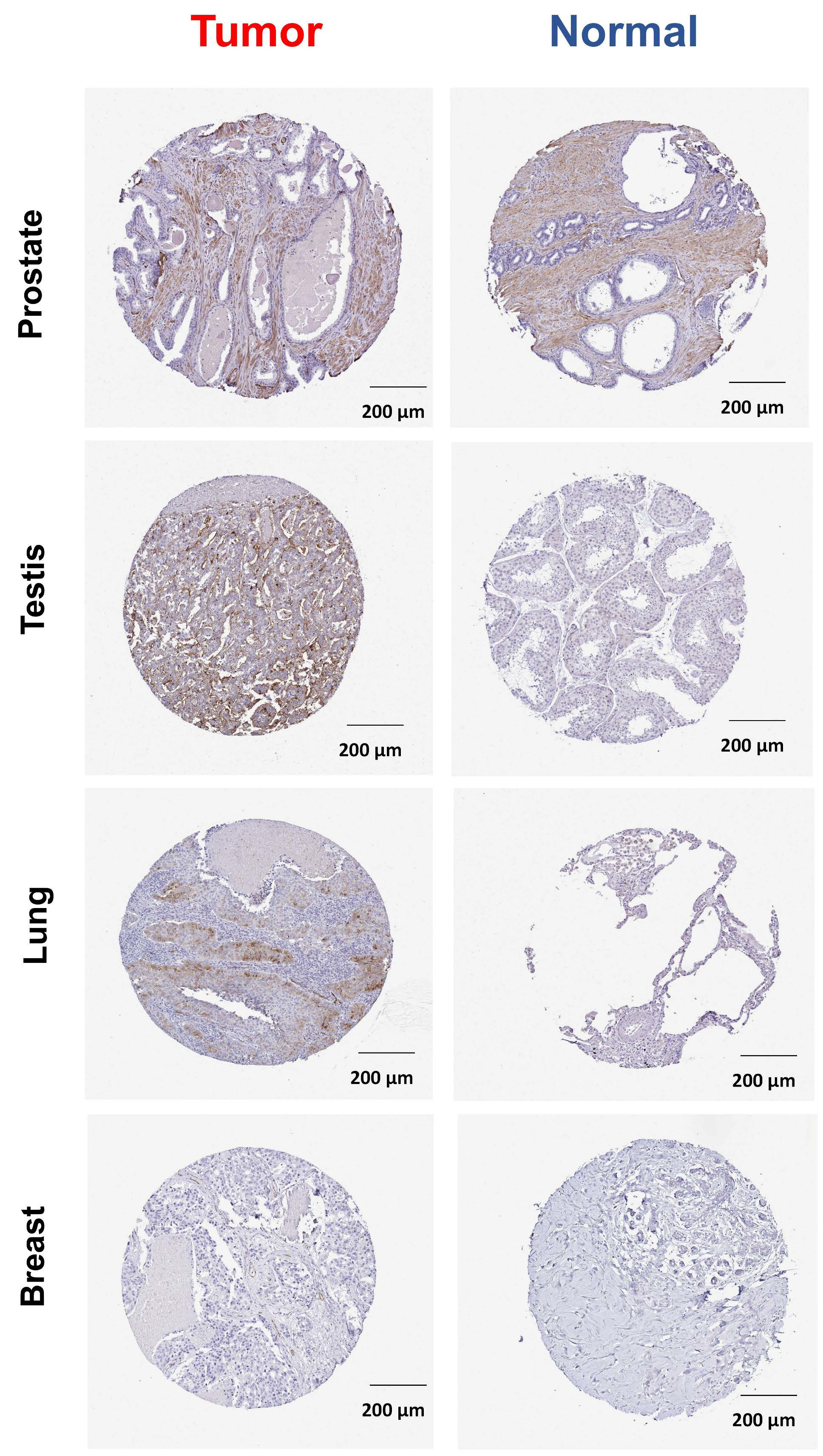
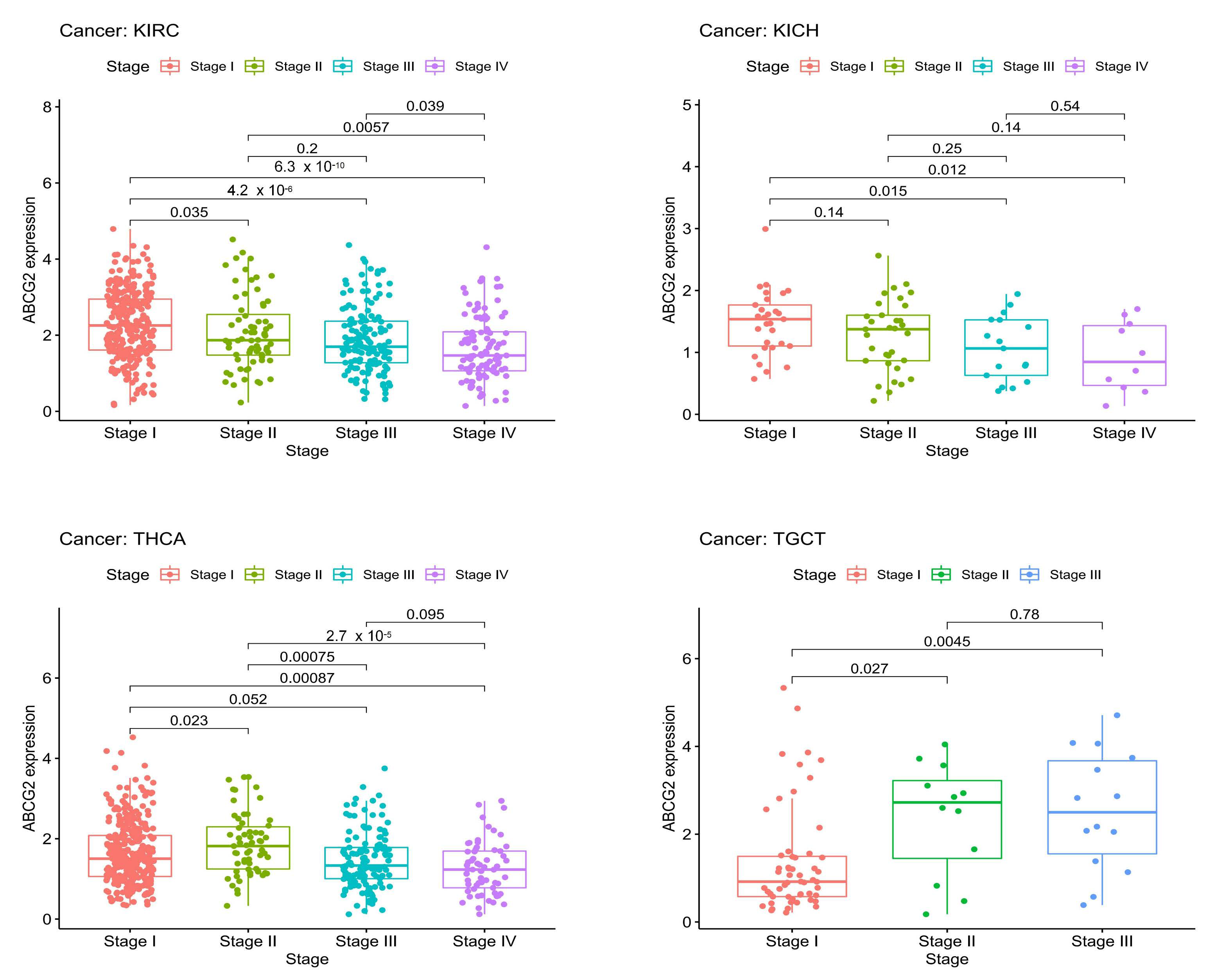
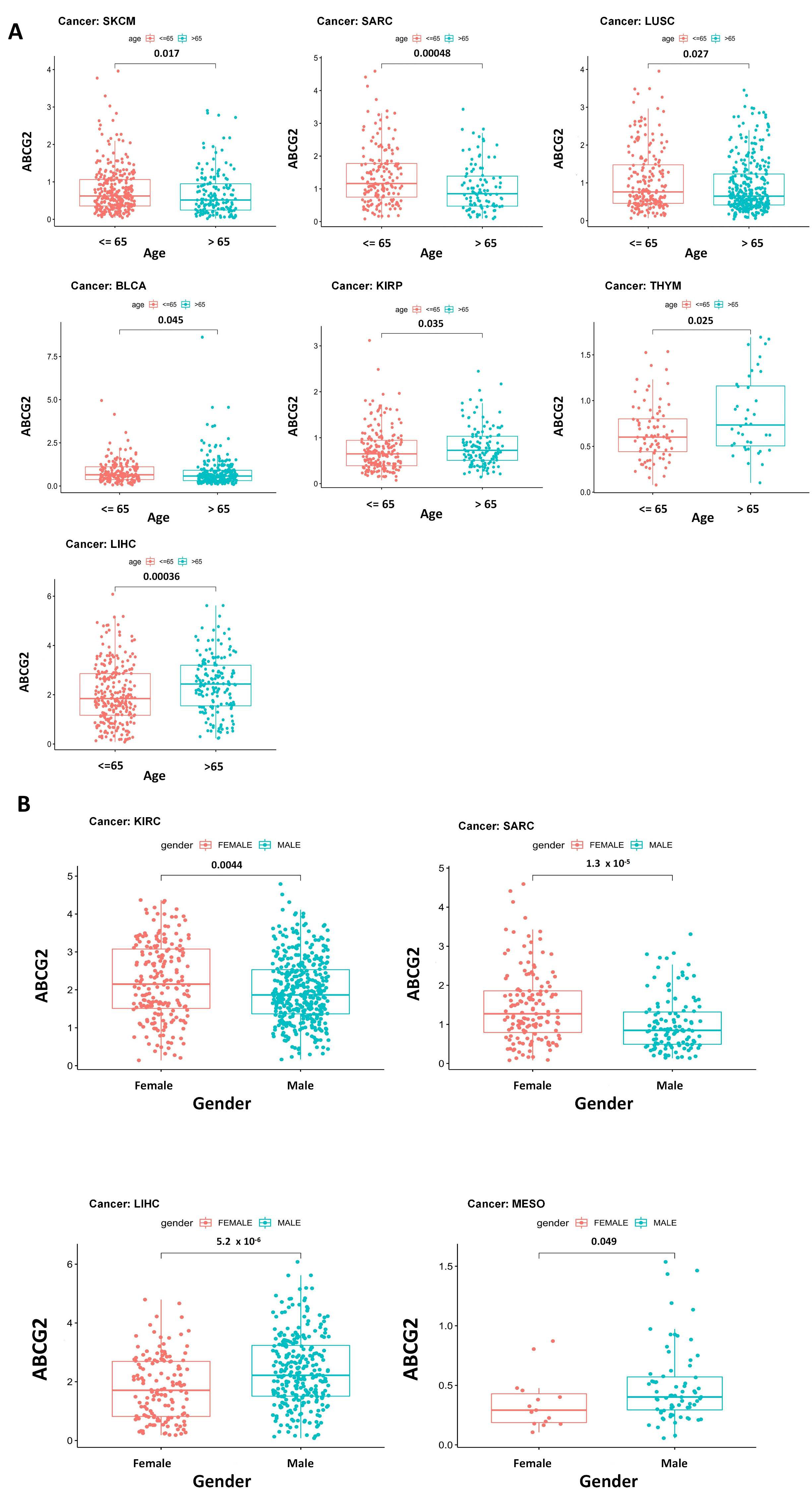
2.1. ABCG2 Expression in Human Cancers
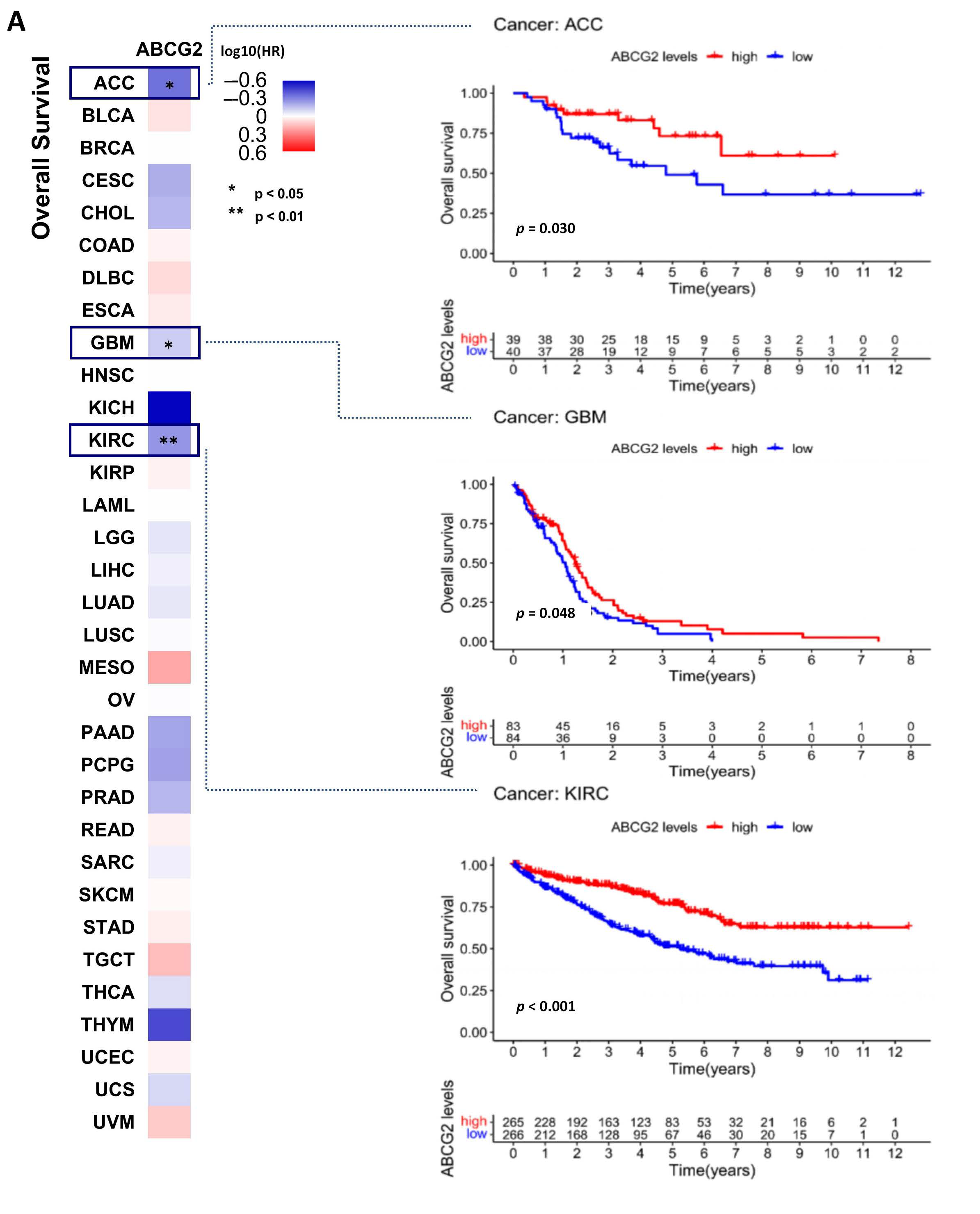
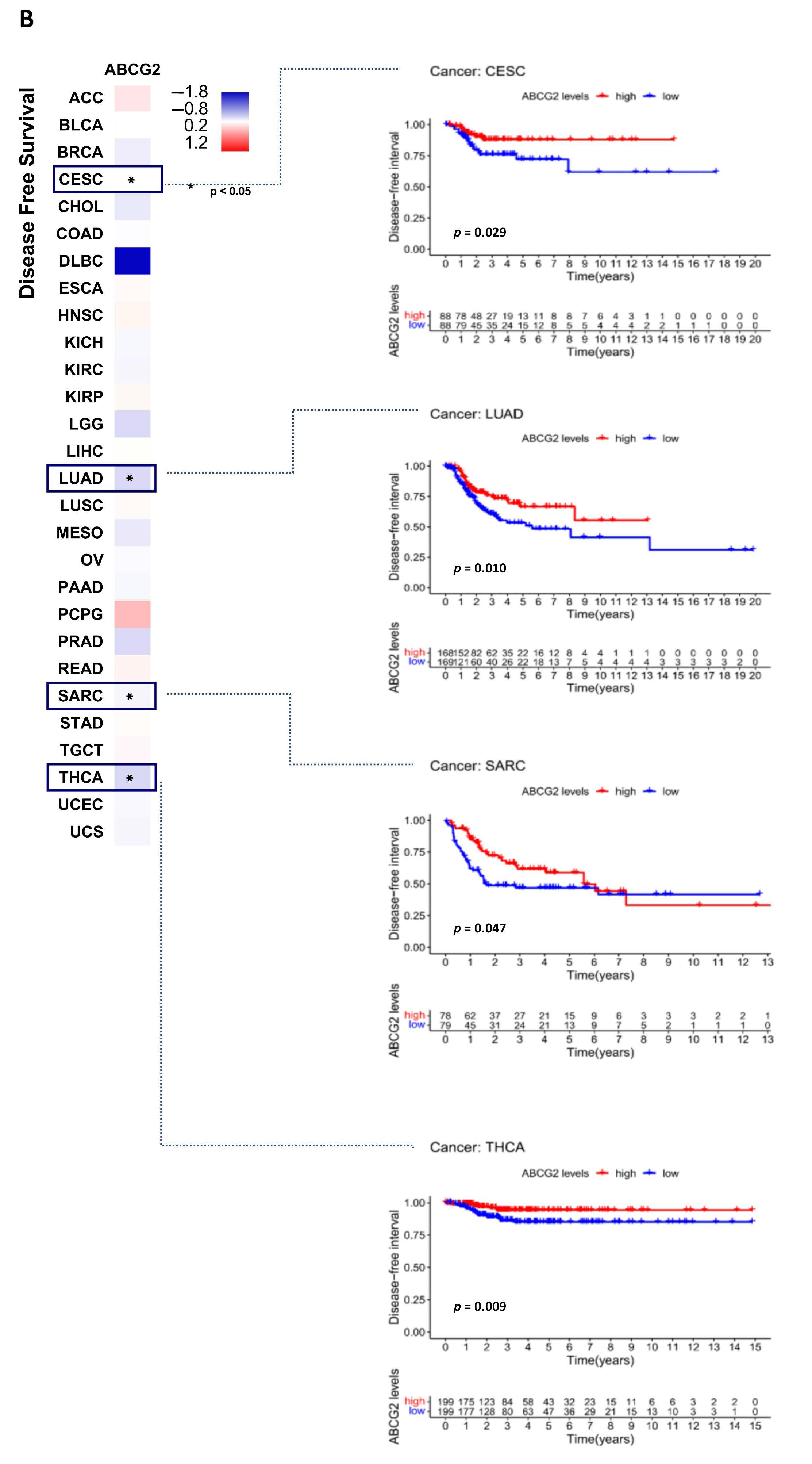
2.2. ABCG2 as a Prognostic Factor in Human Cancers
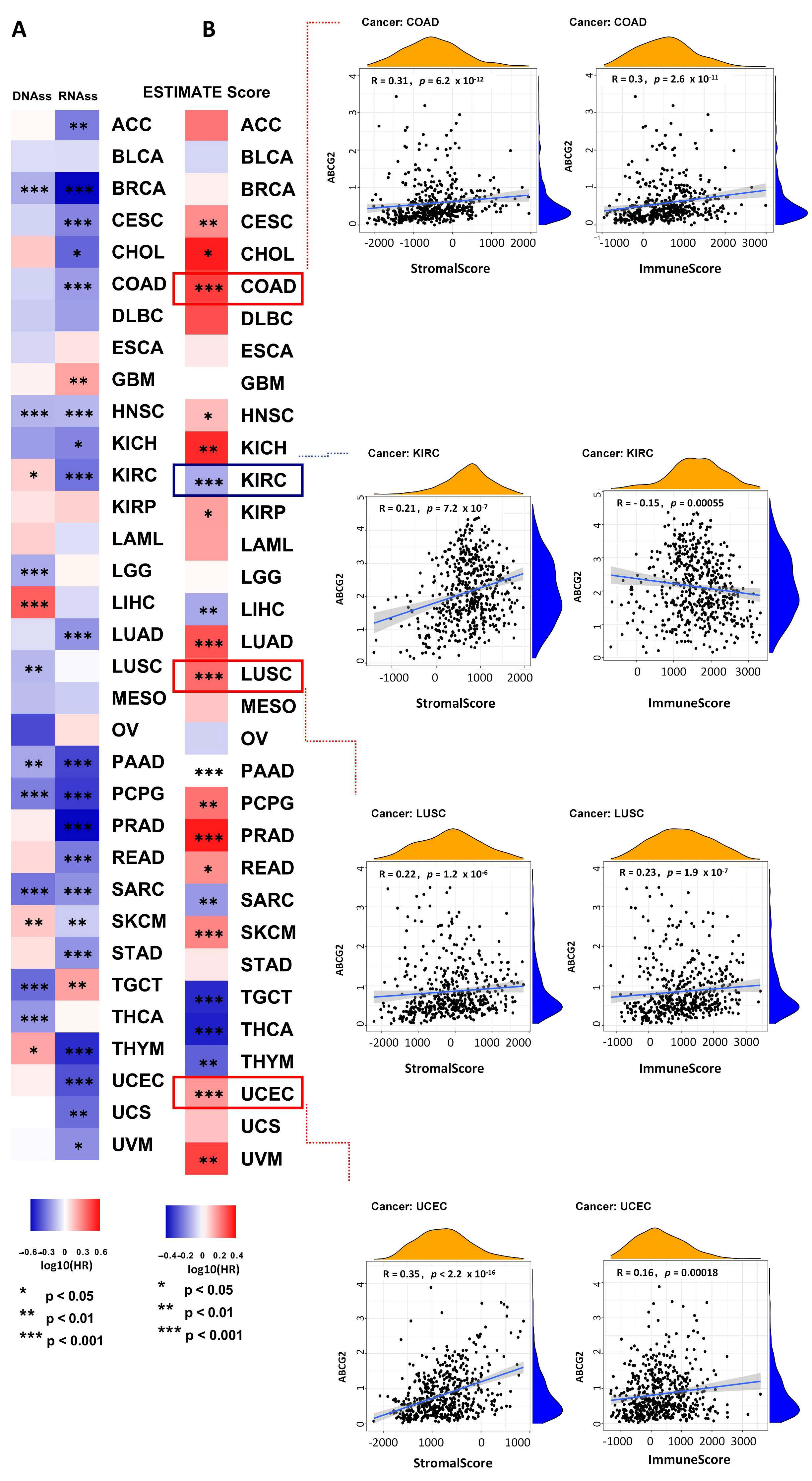
2.3. The Correlation between ABCG2 Expression and RNAss, DNAss, ImmuneScore, and StromalScore
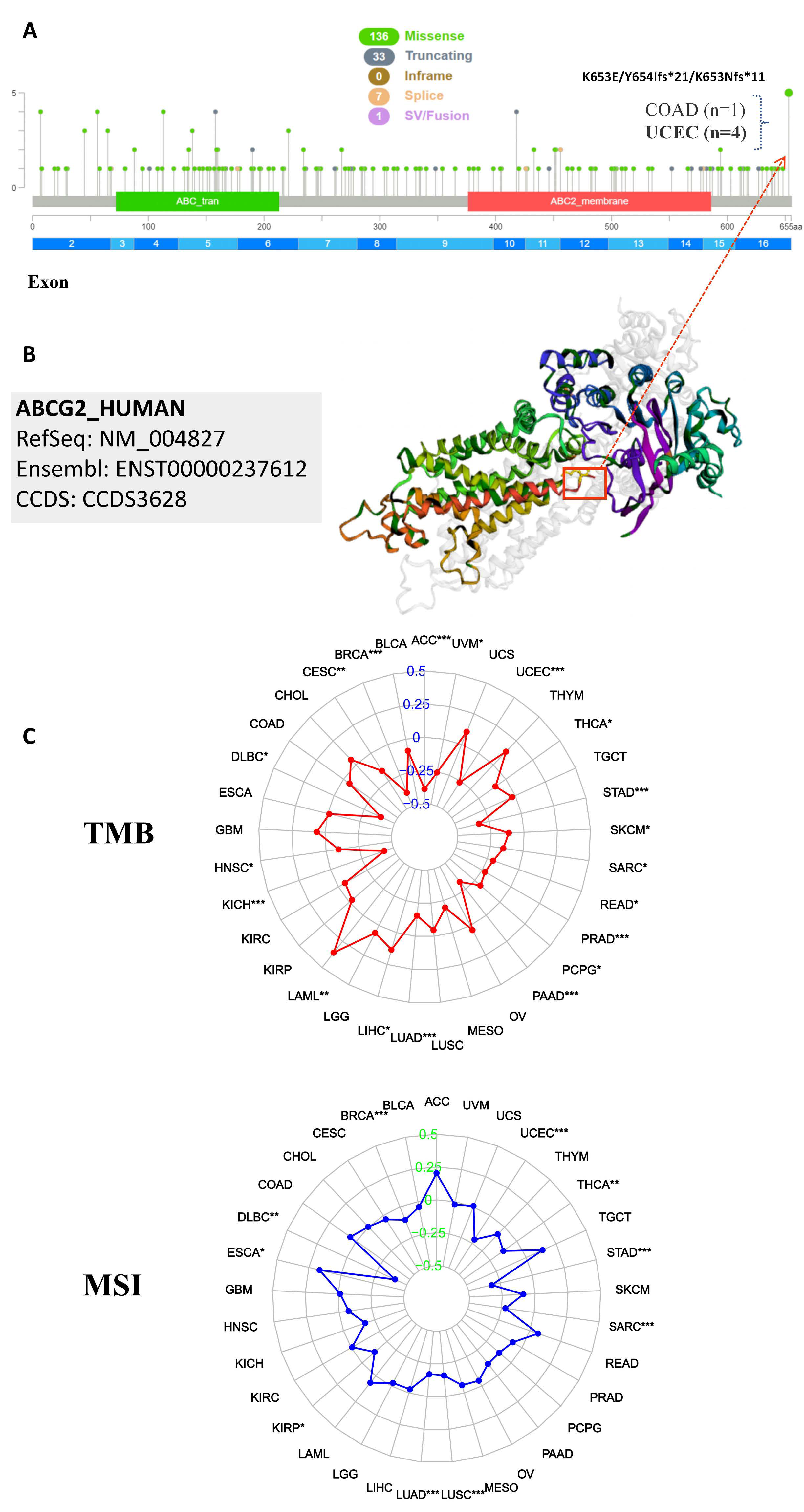
2.4. The Correlation between ABCG2 Expression and TMB, MSI, Genetic Alteration Analysis
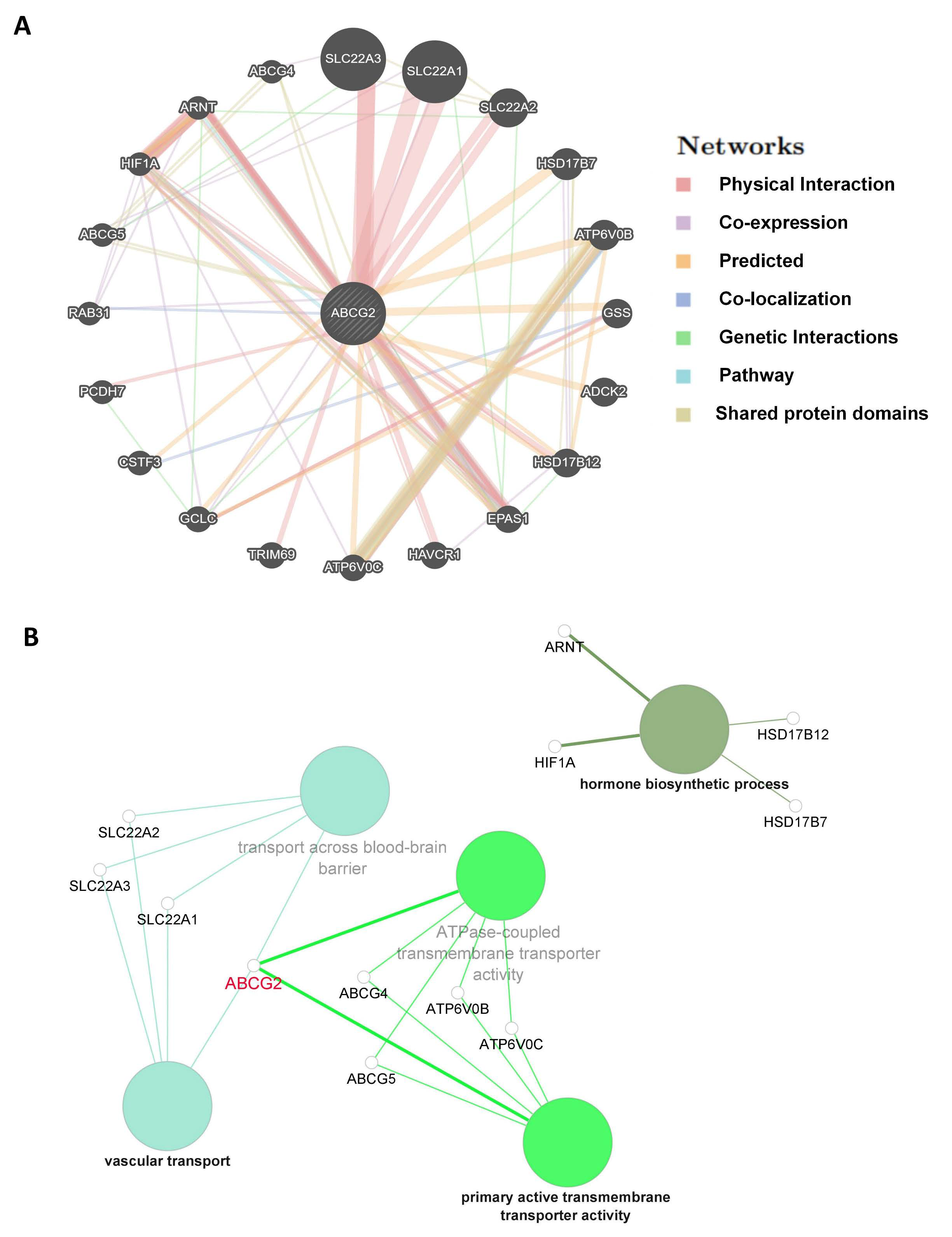
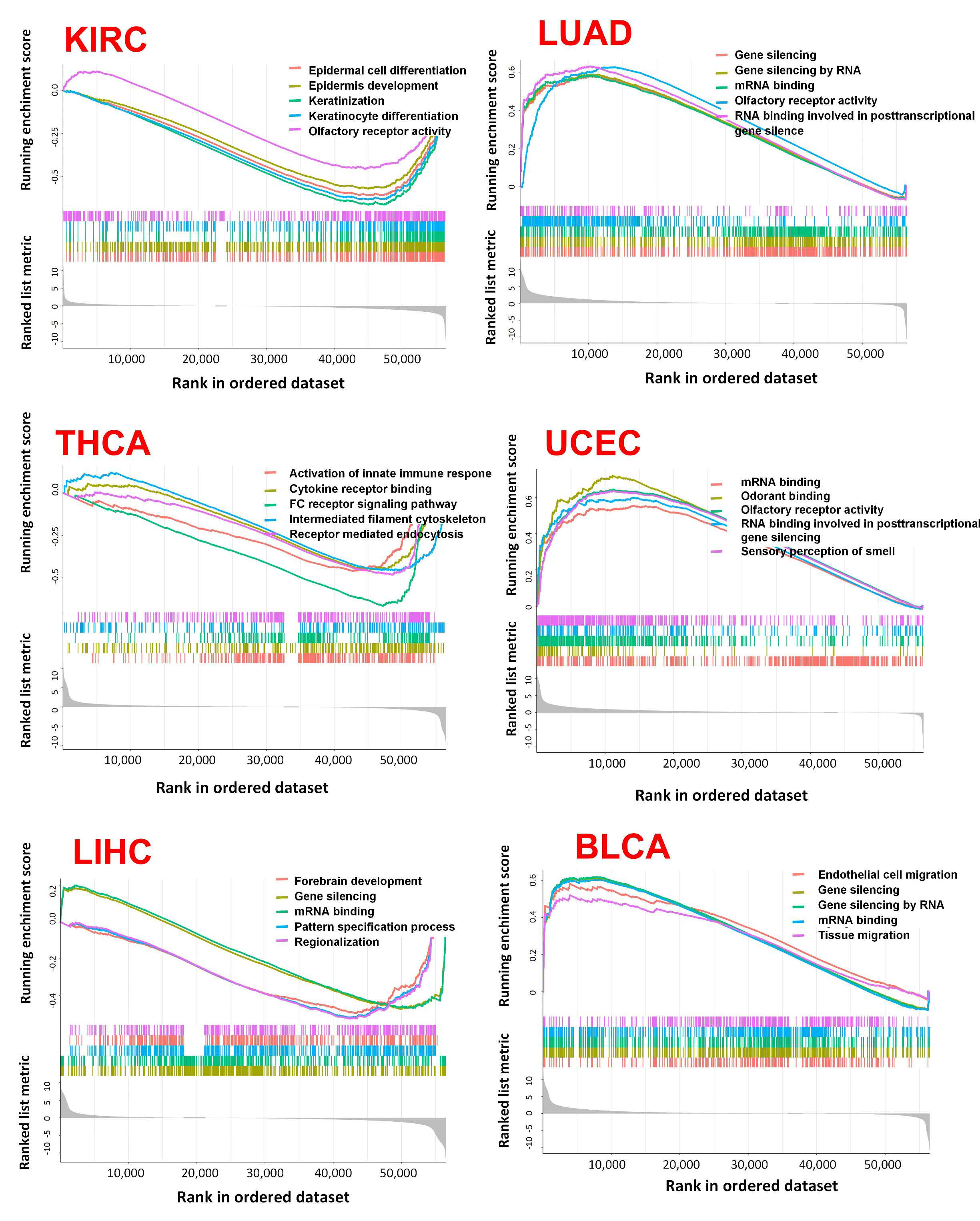
2.5. Analysis of ABCG2-Related Genes and the Correlation between ABCG2 Expression, GSEA, and Checkpoint Gene Expression
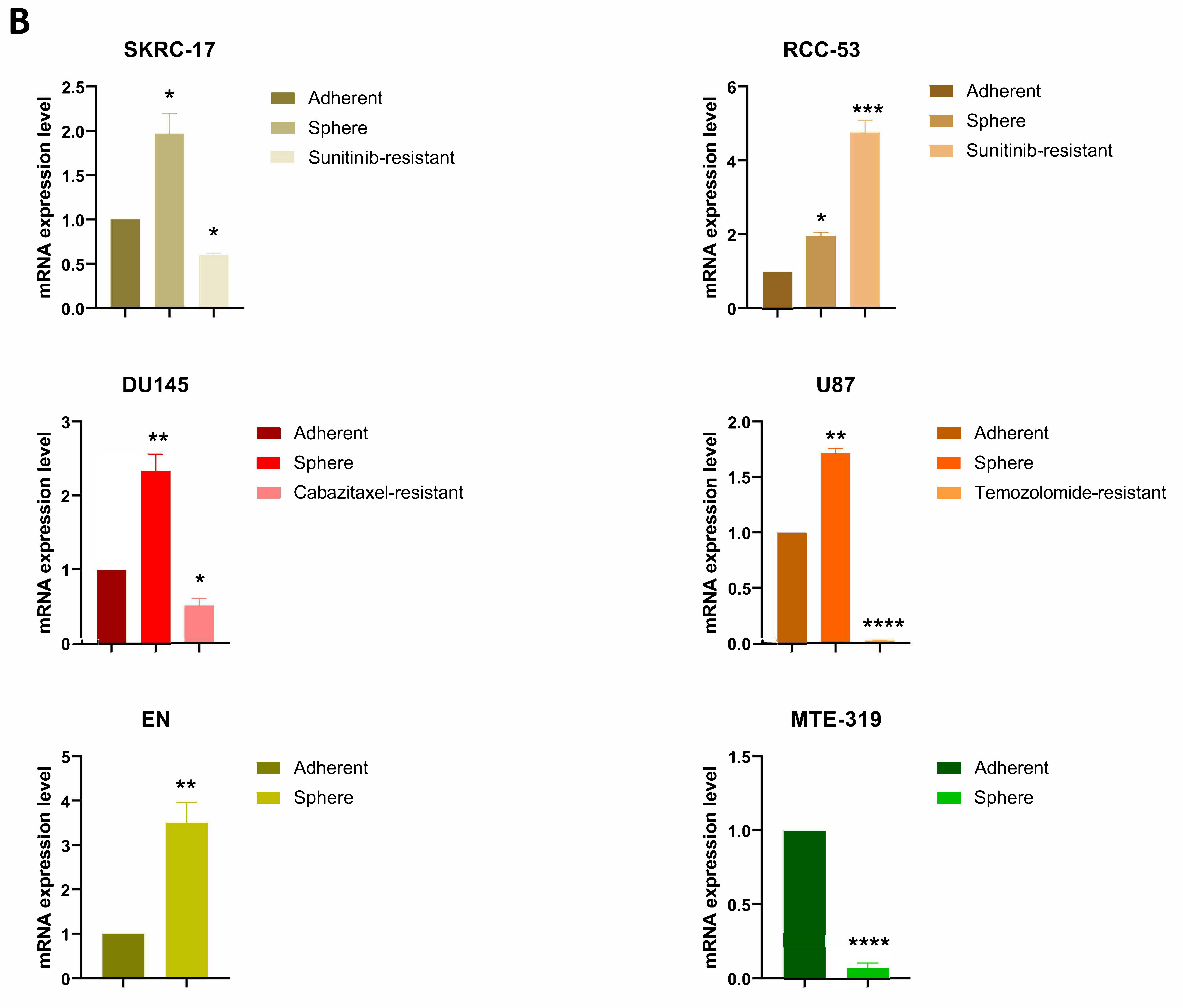
2.6. ABCG2 mRNA Expression among Six Types of Cancer in Cancer Cells, Cancer Stem Cells, and Corresponding Drug Resistant Cells
3. Discussion
4. Materials and Methods
4.1. Gene Expression Analysis
4.2. Prognosis Analysis
4.3. Tumor Stemness Indices and Tumor Microenvironment Scores
4.4. Genetic Alteration Analysis
4.5. Co-Expression of ABCG2 with Immune-Related Genes, ABCG2-Related Genes, and Pathways in Tumors
4.6. Cell Culture
4.7. Sphere Formation Assay
4.8. RNA Extraction, Reverse Transcription, and Sequencing
4.9. Quantitative Reverse Transcription PCR (RT-qPCR)
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fetsch, P.A.; Abati, A.; Litman, T.; Morisaki, K.; Honjo, Y.; Mittal, K.; Bates, S.E. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006, 235, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharm. Pers. Med. 2014, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer, M.C.; de Vries, N.A.; Buckle, T.; Buil, L.C.; Beijnen, J.H.; Boogerd, W.; van Tellingen, O.J.N. Improved brain penetration and antitumor efficacy of temozolomide by inhibition of ABCB1 and ABCG2. Neoplasia 2018, 20, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Polgar, O.; Robey, R.W.; Bates, S.E. ABCG2: Structure, function and role in drug response. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1–15. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Benndorf, R.A. 3D structure of the transporter ABCG2—What’s new? Br. J. Pharmacol. 2020, 177, 1485–1496. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, J.-T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1. [Google Scholar]
- Orlando, B.J.; Liao, M. ABCG2 transports anticancer drugs via a closed-to-open switch. Nat. Commun. 2020, 11, 2264. [Google Scholar] [CrossRef]
- Zámbó, B.; Bartos, Z.; Mózner, O.; Szabó, E.; Várady, G.; Poór, G.; Pálinkás, M.; Andrikovics, H.; Hegedűs, T.; Homolya, L. Clinically relevant mutations in the ABCG2 transporter uncovered by genetic analysis linked to erythrocyte membrane protein expression. Sci. Rep. 2018, 8, 7487. [Google Scholar] [CrossRef]
- Hira, D.; Terada, T. BCRP/ABCG2 and high-alert medications: Biochemical, pharmacokinetic, pharmacogenetic, and clinical implications. Biochem. Pharmacol. 2018, 147, 201–210. [Google Scholar] [CrossRef]
- Khunweeraphong, N.; Stockner, T.; Kuchler, K. The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion. Sci. Rep. 2017, 7, 13767. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, N.; van den Heuvel, J.J.; Koenderink, J.B.; Kidron, H. Transmembrane domain single-nucleotide polymorphisms impair expression and transport activity of ABC transporter ABCG2. Pharm. Res. 2017, 34, 1626–1636. [Google Scholar] [CrossRef]
- Wang, H.; Luo, F.; Zhu, Z.; Xu, Z.; Huang, X.; Ma, R.; He, H.; Zhu, Y.; Shao, K.; Zhao, J. ABCG2 is a potential prognostic marker of overall survival in patients with clear cell renal cell carcinoma. BMC Cancer 2017, 17, 222. [Google Scholar] [CrossRef][Green Version]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Wu, Z.; Zeng, F.; Song, B.; Zhang, Y.; Li, J.; Lui, S.; Wu, M. Tumor Mutational Burden Predicting the Efficacy of Immune Checkpoint Inhibitors in Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 751407. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Kurian, P.; Mobley, B.; Burks, T.; Beg, M.S.; Ross, J.S.; Ali, S.M.; Bowles, D.W. Relationship of anaplastic thyroid cancer high tumor mutation burden and MSI-H status with response to anti-PD1 monotherapy. J. Clin. Oncol. 2018, 36 (Suppl. 15), e18114. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef]
- Gassenmaier, M.; Chen, D.; Buchner, A.; Henkel, L.; Schiemann, M.; Mack, B.; Schendel, D.J.; Zimmermann, W.; Pohla, H. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem Cells 2013, 31, 1467–1476. [Google Scholar] [CrossRef]
- Mickey, D.; Stone, K.; Wunderli, H.; Mickey, G.; Paulson, D. Characterization of a human prostate adenocarcinoma cell line (DU 145) as a monolayer culture and as a solid tumor in athymic mice. Prog. Clin. Biol. Res. 1980, 37, 67–84. [Google Scholar]
- Wang, L.; Stadlbauer, B.; Lyu, C.; Buchner, A.; Pohla, H. Shikonin enhances the antitumor effect of cabazitaxel in prostate cancer stem cells and reverses cabazitaxel resistance by inhibiting ABCG2 and ALDH3A1. Am. J. Cancer Res. 2020, 10, 3784. [Google Scholar]
- Isaka, K.; Nishi, H.; Sagawa, Y.; Nakada, T.; Osakabe, Y.; Serizawa, H.; Ebihara, Y.; Takayama, M. Establishment of a new human cell line (EN) with TP53 mutation derived from endometrial carcinoma. Cancer Genet. Cytogenet. 2003, 141, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, R.; Hawighorst, T.; Hild, F.; Schulz, K.-D. Establishment of new epithelial carcinoma cell lines by blocking monolayer formation. J. Cancer Res. Clin. Oncol. 1997, 123, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Kukal, S.; Guin, D.; Rawat, C.; Bora, S.; Mishra, M.K.; Sharma, P.; Paul, P.R.; Kanojia, N.; Grewal, G.K.; Kukreti, S. Multidrug efflux transporter ABCG2: Expression and regulation. Cell. Mol. Life Sci. 2021, 78, 6887–6939. [Google Scholar] [CrossRef]
- Hoque, K.M.; Dixon, E.E.; Lewis, R.M.; Allan, J.; Gamble, G.D.; Phipps-Green, A.J.; Kuhns, V.L.H.; Horne, A.M.; Stamp, L.K.; Merriman, T.R. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat. Commun. 2020, 11, 2767. [Google Scholar] [CrossRef]
- Fujita, K.; Ichida, K. ABCG2 as a therapeutic target candidate for gout. Expert Opin. Ther. Targets 2018, 22, 123–129. [Google Scholar] [CrossRef]
- Sorf, A.; Sucha, S.; Morell, A.; Novotna, E.; Staud, F.; Zavrelova, A.; Visek, B.; Wsol, V.; Ceckova, M. Targeting Pharmacokinetic Drug Resistance in Acute Myeloid Leukemia Cells with CDK4/6 Inhibitors. Cancers 2020, 12, 1596. [Google Scholar] [CrossRef]
- Megias-Vericat, J.E.; Martinez-Cuadron, D.; Herrero, M.J.; Alino, S.F.; Poveda, J.L.; Sanz, M.A.; Montesinos, P. Pharmacogenetics of metabolic genes of anthracyclines in acute myeloid leukemia. Curr. Drug Metab. 2018, 19, 55–74. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, S.; Zhang, W.; Shi, Z. Polymorphisms of ABCG2 and its impact on clinical relevance. Biochem. Biophys. Res. Commun. 2018, 503, 408–413. [Google Scholar] [CrossRef]
- Okubo, H.; Ando, H.; Ishizuka, K.; Morishige, J.-i.; Ikejima, K.; Shiina, S.; Nagahara, A. Impact of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of lenvatinib in patients with hepatocellular carcinoma. J. Pharmacol. Sci. 2022, 148, 6–13. [Google Scholar] [CrossRef]
- Robey, R.W.; To, K.K.; Polgar, O.; Dohse, M.; Fetsch, P.; Dean, M.; Bates, S.E. ABCG2: A perspective. Adv. Drug Deliv. Rev. 2009, 61, 3–13. [Google Scholar] [CrossRef]
- Reustle, A.; Fisel, P.; Renner, O.; Büttner, F.; Winter, S.; Rausch, S.; Kruck, S.; Nies, A.T.; Hennenlotter, J.; Scharpf, M. Characterization of the breast cancer resistance protein (BCRP/ABCG2) in clear cell renal cell carcinoma. Int. J. Cancer 2018, 143, 3181–3193. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-W.; Wu, J.-H.; Jiang, C.-P. ABCG2: A potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010, 86, 631–637. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Ongkeko, W.M. ABCG2: The key to chemoresistance in cancer stem cells? Expert Opin. Drug Metab. Toxicol. 2009, 5, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kamińska, B.; Huelsken, J.; Omberg, L.; Gevaert, O. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 2018, 173, 338–354.e15. [Google Scholar] [CrossRef]
- Howley, R.; Mansi, M.; Shinde, J.; Restrepo, J.; Chen, B. Evaluation of aminolevulinic acid-mediated protoporphyrin IX fluorescence and enhancement by ABCG2 inhibitors in renal cell carcinoma cells. J. Photochem. Photobiol. B Biol. 2020, 211, 112017. [Google Scholar] [CrossRef]
- Poller, B.; Iusuf, D.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Differential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokinetics. Drug Metab. Dispos. 2011, 39, 729–735. [Google Scholar] [CrossRef]
- Li, J.; Cusatis, G.; Brahmer, J.; Sparreboom, A.; Robey, R.W.; Bates, S.E.; Hidalgo, M.; Baker, S. Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol. Ther. 2007, 6, 432–438. [Google Scholar] [CrossRef]
- Elmeliegy, M.A.; Carcaboso, A.M.; Tagen, M.; Bai, F.; Stewart, C.F. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin. Cancer Res. 2011, 17, 89–99. [Google Scholar] [CrossRef]
- Brendel, C.; Scharenberg, C.; Dohse, M.; Robey, R.; Bates, S.; Shukla, S.; Ambudkar, S.; Wang, Y.; Wennemuth, G.; Burchert, A. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 2007, 21, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kitamura, Y.; Maeda, K.; Sugiyama, Y. Quantitative analysis of the ABCG2 c. 421C> A polymorphism effect on in vivo transport activity of breast cancer resistance protein (BCRP) using an intestinal absorption model. J. Pharm. Sci. 2015, 104, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Lakhani, N.J.; Venitz, J.; Figg, W.D.; Sparreboom, A. Pharmacogenetics of estrogen metabolism and transport in relation to cancer. Curr. Drug Metab. 2003, 4, 505–513. [Google Scholar] [CrossRef]
- Haqqani, M.; Fox, H. Adenosquamous carcinoma of the endometrium. J. Clin. Pathol. 1976, 29, 959–966. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2. 0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Edwards, N.J.; Oberti, M.; Thangudu, R.R.; Cai, S.; McGarvey, P.B.; Jacob, S.; Madhavan, S.; Ketchum, K.A. The CPTAC data portal: A resource for cancer proteomics research. J. Proteome Res. 2015, 14, 2707–2713. [Google Scholar] [CrossRef]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. J. Pathol. Soc. Great Br. Reland 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. In Data Mining in Proteomics; Springer: Berlin/Heidelberg, Germany, 2011; pp. 291–303. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]




| Transcript | Primer | Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| GAPDH | GAPDH-F | CATGGGTGTGAACCATGA | 104 |
| GAPDH-R | TGTCATGGATGACCTTGG | ||
| ACTB | ACTB-F | CTGCCCTGAGGCACTC | 197 |
| ACTB-R | GTGCCAGGGCAGTGAT | ||
| ABCG2 | ABCG2-f | CATCAACTTTCCGGGGGTGA | 266 |
| ABCG2-r | CACTGGTTGGTCGTCAGGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, C.; Wang, L.; Stadlbauer, B.; Buchner, A.; Pohla, H. A Pan-Cancer Landscape of ABCG2 across Human Cancers: Friend or Foe? Int. J. Mol. Sci. 2022, 23, 15955. https://doi.org/10.3390/ijms232415955
Lyu C, Wang L, Stadlbauer B, Buchner A, Pohla H. A Pan-Cancer Landscape of ABCG2 across Human Cancers: Friend or Foe? International Journal of Molecular Sciences. 2022; 23(24):15955. https://doi.org/10.3390/ijms232415955
Chicago/Turabian StyleLyu, Chen, Lili Wang, Birgit Stadlbauer, Alexander Buchner, and Heike Pohla. 2022. "A Pan-Cancer Landscape of ABCG2 across Human Cancers: Friend or Foe?" International Journal of Molecular Sciences 23, no. 24: 15955. https://doi.org/10.3390/ijms232415955
APA StyleLyu, C., Wang, L., Stadlbauer, B., Buchner, A., & Pohla, H. (2022). A Pan-Cancer Landscape of ABCG2 across Human Cancers: Friend or Foe? International Journal of Molecular Sciences, 23(24), 15955. https://doi.org/10.3390/ijms232415955





