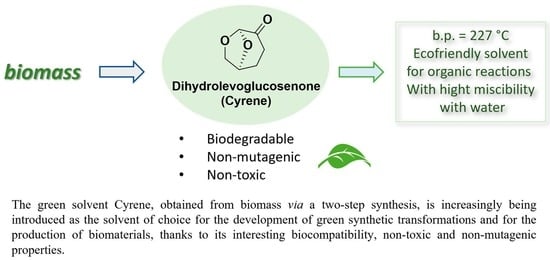
Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials
Abstract
:1. Introduction
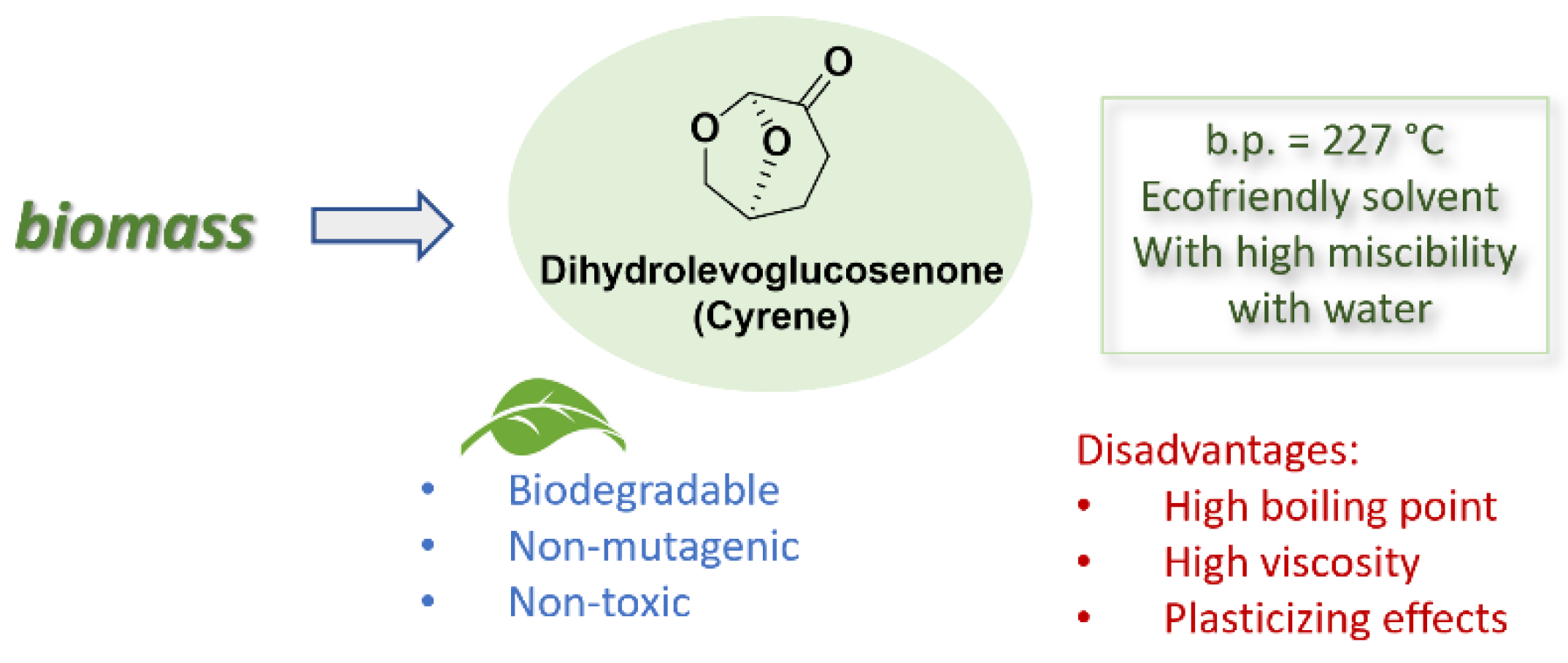
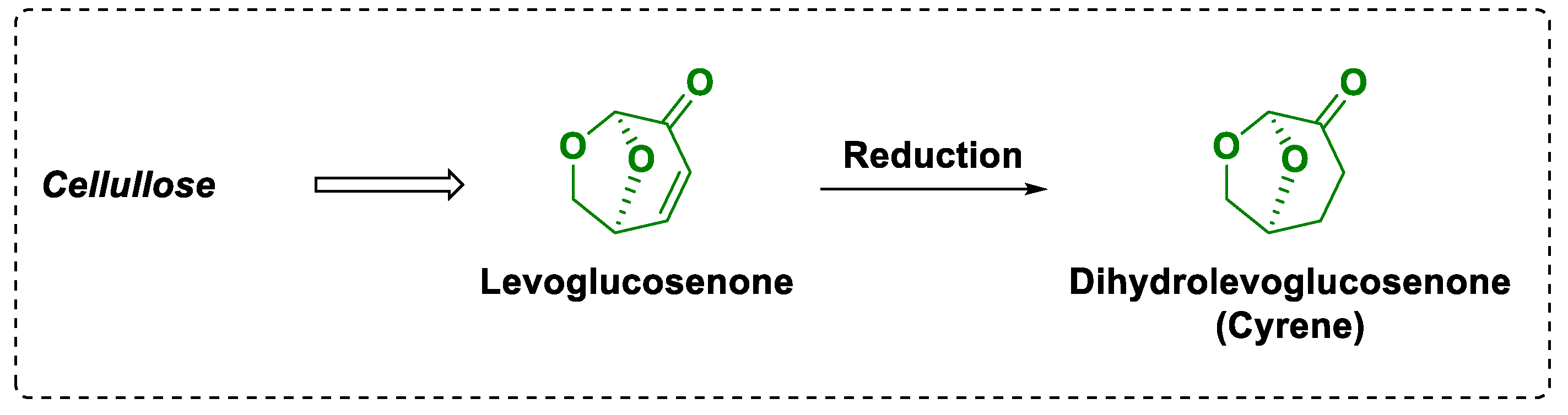
2. Cyrene: Chemical-Physical Properties, Synthesis and Reactivity
3. Cyrene as a Solvent for Organic Synthesis Transformations
3.1. Amide Synthesis

3.2. Ureas Synthesis
3.3. Isothiocyanates Synthesis
3.4. Carbon–Carbon Bond Couplings
3.5. Introduction of Fluorinated Functionalities
3.6. N-Alkylation Reactions
3.7. Formylation of Amines
3.8. Microwave-Assisted Multicomponent Reactions
3.9. Biocatalyzed Reduction of α-Ketoesters to α-Hydroxyesters
4. Cyrene as an Innovative Solvent for the Preparation of Nanomaterials with Advanced Applications
4.1. Nanoparticles as Drug Delivery Systems
4.2. Graphene-Based Nanomaterials
4.3. Nano–Sized Metal Organic Frameworks (MOFs)
4.4. Lignin-Based Nanomaterials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sherwood, J.; De bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.E.; Nyamini, S.B.; Scott, F.J. Cyrene™ is a green alternative to DMSO as a solvent for antibacterial drug discovery against ESKAPE pathogens. RSC Med. Chem. 2020, 11, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kisanthia, R.; Hunt, A.J.; Sherwood, J.; Somsakeesit, L.-o.; Phaosiri, C. Impact of Conventional and Sustainable Solvents on the Yield, Selectivity, and Recovery of Curcuminoids from Turmeric. ACS Sustain. Chem. Eng. 2022, 10, 104–114. [Google Scholar] [CrossRef]
- Waaijers-van der Loop, S.L.; Dang, Z.; Rorije, E.; Janssen, N. Toxicity screening of potential bio-based Polar Aprotic Solvents (PAS). Methodology 2018, 5, 1. [Google Scholar]
- Camp, J.E. Bio-available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Kong, D.; Dolzhenko, A.V. Cyrene: A bio-based sustainable solvent for organic synthesis. Sustain. Chem. Pharm. 2022, 25, 100591. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef]
- Halpern, Y.; Riffer, R.; Broido, A. Levoglucosenone (1,6-anhydro-3,4-dideoxy-.DELTA.3-.beta.-D-pyranosen-2-one). Major product of the acid-catalyzed pyrolysis of cellulose and related carbohydrates. J. Org. Chem. 1973, 38, 204–209. [Google Scholar] [CrossRef]
- Bousfield, T.W.; Pearce, K.P.R.; Nyamini, S.B.; Angelis-Dimakis, A.; Camp, J.E. Synthesis of amides from acid chlorides and amines in the bio-based solvent Cyrene™. Green Chem. 2019, 21, 3675–3681. [Google Scholar] [CrossRef]
- Alves Costa Pacheco, A.; Sherwood, J.; Zhenova, A.; McElroy, C.R.; Hunt, A.J.; Parker, H.L.; Farmer, T.J.; Constantinou, A.; De Bruyn, M.; Whitwood, A.C.; et al. Intelligent Approach to Solvent Substitution: The Identification of a New Class of Levoglucosenone Derivatives. ChemSusChem 2016, 9, 3503–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhifthi, A.; Harris, B.L.; Goerigk, L.; White, J.M.; Williams, S.J. Structure-reactivity correlations of the abnormal Beckmann reaction of dihydrolevoglucosenone oxime. Org. Biomol. Chem. 2017, 15, 10105–10115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Sun, P.; Wei, W.; Liu, F.; Zhang, H.; Wang, H. Copper-Catalyzed Regioselective Cleavage of C-X and C-H Bonds: A Strategy for Sulfur Dioxide Fixation. Chemistry 2018, 24, 4423–4427. [Google Scholar] [CrossRef] [PubMed]
- Hohol, R.E.; Arcure, H.; Witczak, Z.J.; Bielski, R.; Kirschbaum, K.; Andreana, P.; Mencer, D.E. One-pot synthesis of carbohydrate exo-cyclic enones and hemiketals with 6,8-dioxabicyclo-[3.2.1]octane moieties. Serendipitous formation of a spironolactone when 2-pyridinecarboxaldehyde is used as the reactant. Part II. Tetrahedron 2018, 74, 7303–7309. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Bielski, R.; Mencer, D.E. Concise and efficient synthesis of E-stereoisomers of exo-cyclic carbohydrate enones. Aldol condensation of dihydrolevoglucosenone with five-membered aromatic aldehydes1 Part 1. Tetrahedron Lett. 2017, 58, 4069–4072. [Google Scholar] [CrossRef]
- Sener, C.; Petrolini, D.D.; McClelland, D.J.; He, J.; Ball, M.R.; Liu, Y.; Martins, L.; Dumesic, J.A.; Huber, G.W.; Weckhuysen, B.M. Catalytic hydrogenation of dihydrolevoglucosenone to levoglucosanol with a hydrotalcite/mixed oxide copper catalyst. Green Chem. 2019, 21, 5000–5007. [Google Scholar]
- Bonneau, G.; Peru, A.A.; Flourat, A.L.; Allais, F. Organic solvent-and catalyst-free Baeyer–Villiger oxidation of levoglucosenone and dihydrolevoglucosenone (Cyrene®): A sustainable route to (S)-γ-hydroxymethyl-α, β-butenolide and (S)-γ-hydroxymethyl-γ-butyrolactone. Green Chem. 2018, 20, 2455–2458. [Google Scholar] [CrossRef]
- Wilson, K.L.; Murray, J.; Jamieson, C.; Watson, A.J. Cyrene as a bio-based solvent for HATU mediated amide coupling. Org. Biomol. Chem. 2018, 16, 2851–2854. [Google Scholar] [CrossRef] [Green Version]
- Ferrazzano, L.; Corbisiero, D.; Martelli, G.; Tolomelli, A.; Viola, A.; Ricci, A.; Cabri, W. Green Solvent Mixtures for Solid-Phase Peptide Synthesis: A Dimethylformamide-Free Highly Efficient Synthesis of Pharmaceutical-Grade Peptides. ACS Sustain. Chem. Eng. 2019, 7, 12867–12877. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Urea derivatives in modern drug discovery and medicinal chemistry. J. Med. Chem. 2019, 63, 2751–2788. [Google Scholar] [CrossRef] [PubMed]
- Mistry, L.; Mapesa, K.; Bousfield, T.W.; Camp, J.E. Synthesis of ureas in the bio-alternative solvent Cyrene. Green Chem. 2017, 19, 2123–2128. [Google Scholar] [CrossRef]
- Nickisch, R.; Conen, P.; Gabrielsen, S.M.; Meier, M.A.R. A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv. 2021, 11, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Kennedy, A.R.; Murray, J.; Greatrex, B.; Jamieson, C.; Watson, A.J. Scope and limitations of a DMF bio-alternative within Sonogashira cross-coupling and Cacchi-type annulation. Beilstein J. Org. Chem. 2016, 12, 2005–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, K.L.; Murray, J.; Jamieson, C.; Watson, A.J. Cyrene as a bio-based solvent for the Suzuki–Miyaura cross-coupling. Synlett 2018, 29, 650–654. [Google Scholar]
- Sangon, S.; Supanchaiyamat, N.; Sherwood, J.; McElroy, C.R.; Hunt, A.J. Direct comparison of safer or sustainable alternative dipolar aprotic solvents for use in carbon–carbon bond formation. React. Chem. Eng. 2020, 5, 1798–1804. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [Green Version]
- Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. Difluoromethyl bioisostere: Examining the “lipophilic hydrogen bond donor” concept. J. Med. Chem. 2017, 60, 797–804. [Google Scholar] [CrossRef]
- Miele, M.; Citarella, A.; Micale, N.; Holzer, W.; Pace, V. Direct and chemoselective synthesis of tertiary difluoroketones via weinreb amide homologation with a CHF2-carbene equivalent. Org. Lett. 2019, 21, 8261–8265. [Google Scholar] [CrossRef]
- Citarella, A.; Gentile, D.; Rescifina, A.; Piperno, A.; Mognetti, B.; Gribaudo, G.; Sciortino, M.T.; Holzer, W.; Pace, V.; Micale, N. Pseudo-dipeptide bearing α, α-difluoromethyl ketone moiety as electrophilic warhead with activity against coronaviruses. Int. J. Mol. Sci. 2021, 22, 1398. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Ielo, L.; Stagno, C.; Cristani, M.; Muscarà, C.; Pace, V.; Micale, N. Synthesis, computational investigation and biological evaluation of α, α-difluoromethyl ketones embodying pyrazole and isoxazole nuclei as COX inhibitors. Org. Biomol. Chem. 2022, 20, 8293–8304. [Google Scholar] [CrossRef] [PubMed]
- Veerabagu, U.; Jaikumar, G.; Lu, F.; Quero, F. High yield and greener C–H difluoromethylation reactions using copper iodide nanoparticles/boron nitride nanosheets as a versatile and recyclable heterogeneous catalyst. React. Chem. Eng. 2021, 6, 1900–1910. [Google Scholar] [CrossRef]
- Andrew, O.B.; Sherwood, J.; Hurst, G.A. A greener synthesis of the antidepressant bupropion hydrochloride. J. Chem. Educ. 2022, 99, 3277–3282. [Google Scholar] [CrossRef]
- Yu, H.; Yu, D.; Xue, Z.; Zhang, B.; Mu, T. Dihydrolevoglucosenone as a bio-based catalytic solvent for efficient reductive-transformation of CO2 with amines into formamides and benzothiazoles. Chem. Eng. J. 2022, 431, 133397. [Google Scholar] [CrossRef]
- Tamargo, R.J.I.; Rubio, P.Y.M.; Mohandoss, S.; Shim, J.J.; Lee, Y.R. Cyrene™ as a Neoteric Bio-Based Solvent for Catalyst-Free Microwave-Assisted Construction of Diverse Bipyridine Analogues for Heavy-Metal Sensing. ChemSusChem 2021, 14, 2133–2140. [Google Scholar] [CrossRef]
- De Gonzalo, G. Biocatalysed reductions of α-ketoesters employing CyreneTM as cosolvent. Biocat. Biotransform. 2022, 40, 252–257. [Google Scholar] [CrossRef]
- Guajardo, N.; de María, P.D. Assessing biocatalysis using dihydrolevoglucosenone (Cyrene™) as versatile bio-based (co) solvent. Mol. Cat. 2020, 485, 110813. [Google Scholar] [CrossRef]
- Lanctôt, A.G.; Attard, T.M.; Sherwood, J.; McElroy, C.R.; Hunt, A.J. Synthesis of cholesterol-reducing sterol esters by enzymatic catalysis in bio-based solvents or solvent-free. RSC Adv. 2016, 6, 48753–48756. [Google Scholar] [CrossRef] [Green Version]
- Iemhoff, A.; Sherwood, J.; McElroy, C.R.; Hunt, A.J. Towards sustainable kinetic resolution, a combination of bio-catalysis, flow chemistry and bio-based solvents. Green Chem. 2018, 20, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grizić, D.; Lamprecht, A. Microparticle preparation by a propylene carbonate emulsification-extraction method. Int. J. Pharm. 2018, 544, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Grune, C.; Thamm, J.; Werz, O.; Fischer, D. Cyrene™ as an Alternative Sustainable Solvent for the Preparation of Poly(lactic-co-glycolic acid) Nanoparticles. J. Pharm. Sci. 2021, 110, 959–964. [Google Scholar] [CrossRef]
- Czapka, A.; Grune, C.; Schädel, P.; Bachmann, V.; Scheuer, K.; Dirauf, M.; Weber, C.; Skaltsounis, A.-L.; Jandt, K.D.; Schubert, U.S. Drug delivery of 6-bromoindirubin-3′-glycerol-oxime ether employing poly (D, L-lactide-co-glycolide)-based nanoencapsulation techniques with sustainable solvents. J. Nanobiotechnol. 2022, 20, 5. [Google Scholar] [CrossRef]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Sadatlu, M.A.A.; Ghasemi, A.; Abbaspour, S.; Akbarian, M.; Farjadian, F.; Karimi, M. Applications of graphene and graphene oxide in smart drug/gene delivery: Is the world still flat? Int. J. Nanomed. 2020, 15, 9469. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Sherwood, J.; Budarin, V.; Ellis, G.; Clark, J.; Shuttleworth, P. Identification of high performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550–2560. [Google Scholar] [CrossRef] [Green Version]
- Pan, K.; Fan, Y.; Leng, T.; Li, J.; Xin, Z.; Zhang, J.; Hao, L.; Gallop, J.; Novoselov, K.S.; Hu, Z. Sustainable production of highly conductive multilayer graphene ink for wireless connectivity and IoT applications. Nature Commun. 2018, 9, 5197. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Zhang, J.; White, G.B.; Ryan, M.D.; Hunt, A.J.; Katz, M.J. Dihydrolevoglucosenone (Cyrene) As a Green Alternative to N,N-Dimethylformamide (DMF) in MOF Synthesis. ACS Sustain. Chem. Eng. 2016, 4, 7186–7192. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for nano-and microscaled carrier systems: Applications, trends, and challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef]
- Meng, X.; Pu, Y.; Li, M.; Ragauskas, A.J. A biomass pretreatment using cellulose-derived solvent Cyrene. Green Chem. 2020, 22, 2862–2872. [Google Scholar] [CrossRef]
- Duval, A.; Avérous, L. Dihydrolevoglucosenone (Cyrene™) as a versatile biobased solvent for lignin fractionation, processing, and chemistry. Green Chem. 2022, 24, 338–349. [Google Scholar] [CrossRef]





















| Solvent | Density (g/mL) | Boiling Point | Miscibility with Water | π* |
|---|---|---|---|---|
| Cyrene | 1.25 | 227 °C | High | 0.93 |
| NMP | 1.03 | 202 °C | High | 0.90 |
| DMF | 0.95 | 153 °C | High | 0.88 |
| DMSO | 1.1 | 189 °C | High | 1.00 |
| Reaction | Functional Group | Temperature | Reference |
|---|---|---|---|
| Amide coupling |  | RT | Scheme 2 and Scheme 3 |
| Urea synthesis |  | RT | Scheme 4 |
| Isothiocyanates formation |  | 40 °C | Scheme 5 |
| Sonogashira |  | RT | Scheme 6 |
| Suzuki–Miyaura | C–C bond | 50 °C | Scheme 7 |
| Heck |  | 100 °C | Scheme 8 |
| Baylis-Hillman | C–C bond | RT | Scheme 9 |
| Difluoromethylation |  | RT | Scheme 10 |
| Aryl Fluorination |  | 90 °C | Scheme 11 |
| N-alkylation |  | 50–60 °C | Scheme 12 and Scheme 13 |
| Formylation |  | 80 °C | Scheme 14 |
| Multicomponent reaction | 130 °C | Scheme 15 | |
| Biocatalyzed reduction | 30 °C | Scheme 16 | |
| Biocatalyzed esterification | 60 °C | Scheme 17 |
| MOFs | Solvents | BET SAs |
|---|---|---|
| HKUST-1 | ||
| Entry 1 | DMF/EtOH/H2O (1:1:1) | 1400 |
| Entry 2 | Cyrene/EtOH/H2O (1:1:1) | 1100 |
| Entry 3 | Cyrene/EtOH (1:1) | 1500 |
| MOFs | BET SA in DMF | BET SA in Cyrene |
|---|---|---|
| HKUST-1 | 1400 | 1500 |
| UiO-66 | 1300 | 500 |
| ZIF-8 | 1700 | 600 |
| Zn2(BCD)2(DABCO) | 1950 | 1300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. https://doi.org/10.3390/ijms232415960
Citarella A, Amenta A, Passarella D, Micale N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. International Journal of Molecular Sciences. 2022; 23(24):15960. https://doi.org/10.3390/ijms232415960
Chicago/Turabian StyleCitarella, Andrea, Arianna Amenta, Daniele Passarella, and Nicola Micale. 2022. "Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials" International Journal of Molecular Sciences 23, no. 24: 15960. https://doi.org/10.3390/ijms232415960