The Application of Microwaves, Ultrasounds, and Their Combination in the Synthesis of Nitrogen-Containing Bicyclic Heterocycles
Abstract
1. Introduction
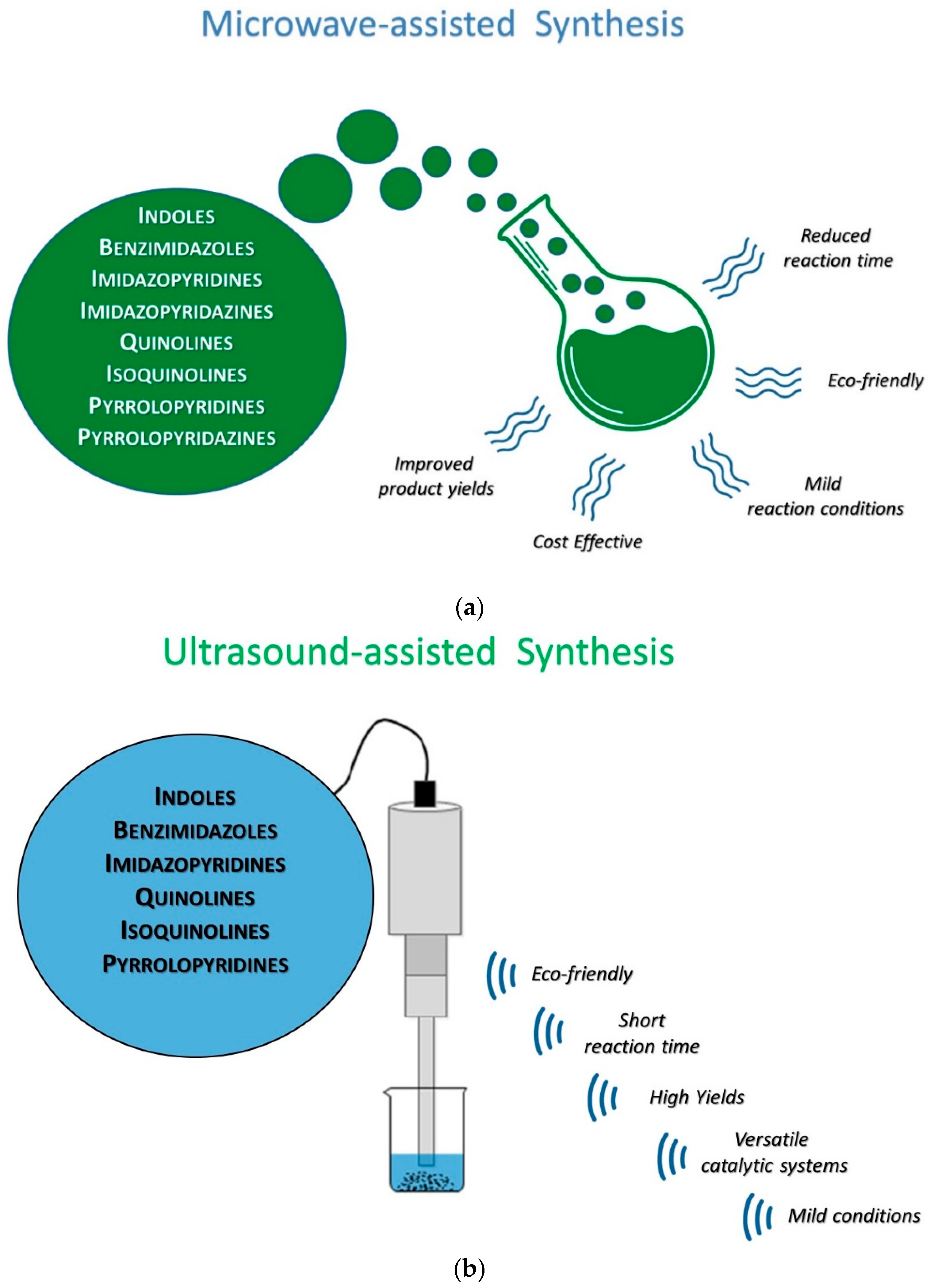
1.1. The Use of Microwaves in Medicinal Chemistry
1.2. Ultrasounds in Medicinal Chemistry
1.3. Combined Use of Microwaves and Sonochemistry
2. Indole
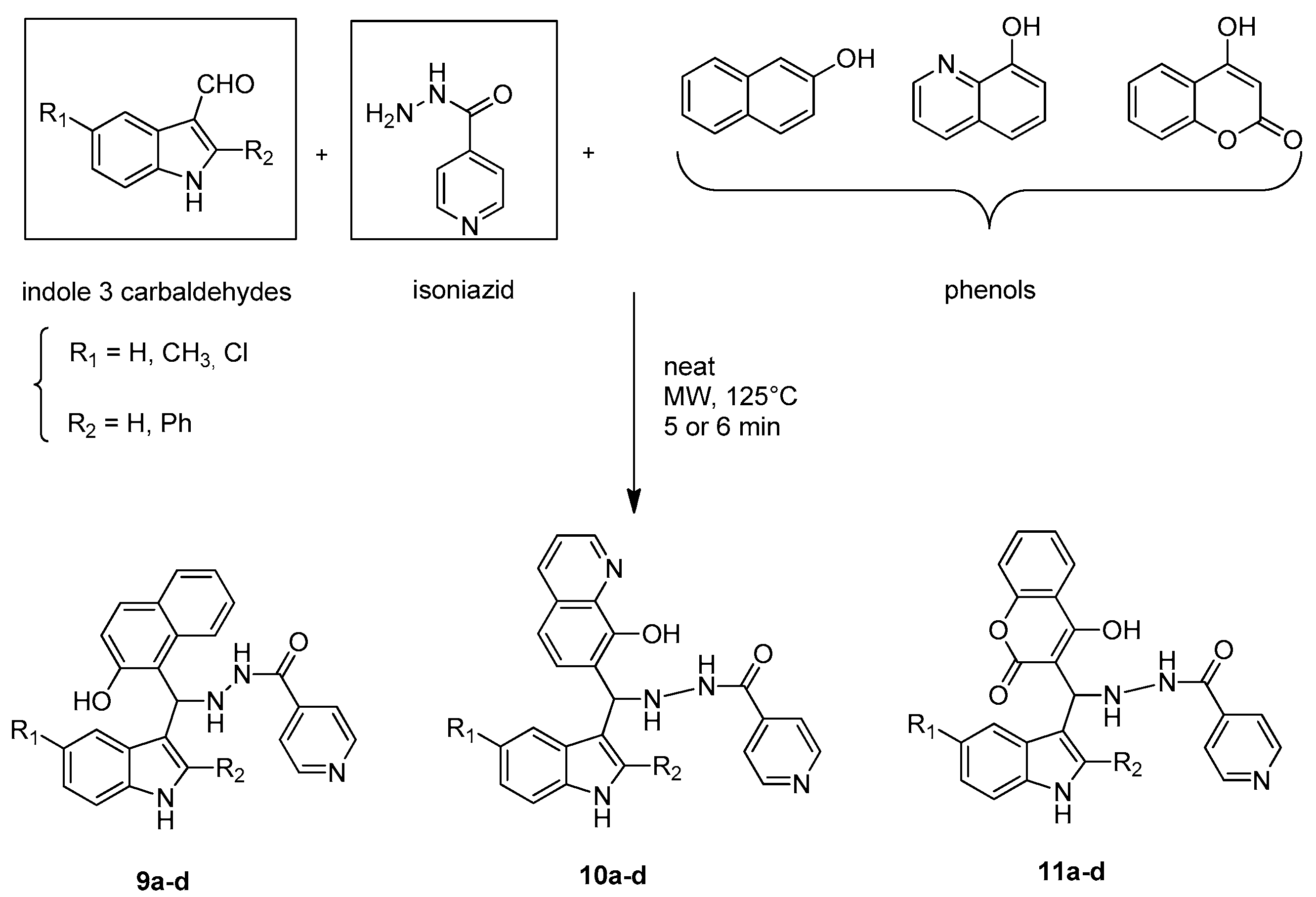
2.1. Microwave Assisted Synthesis of Indole Derivatives
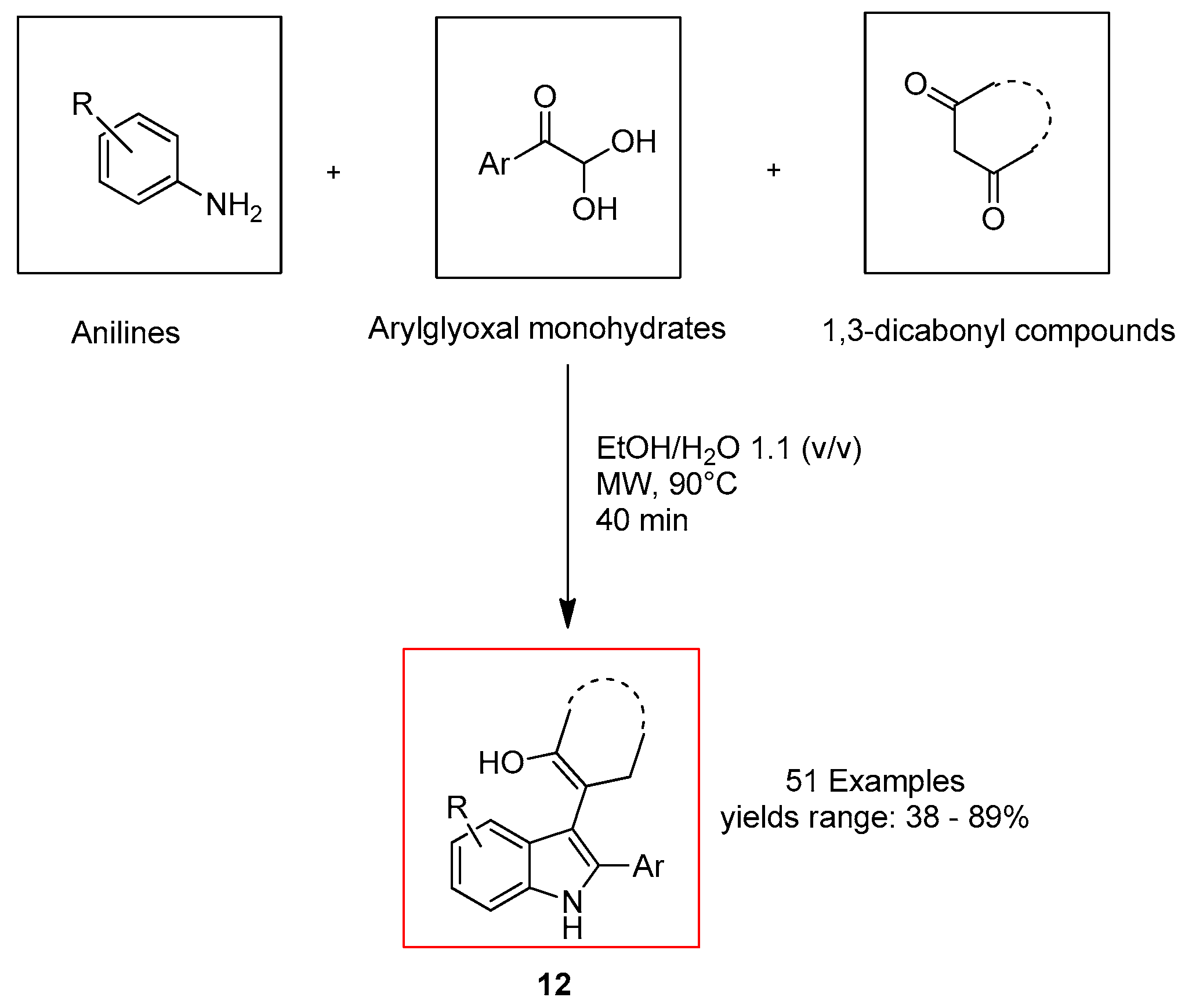
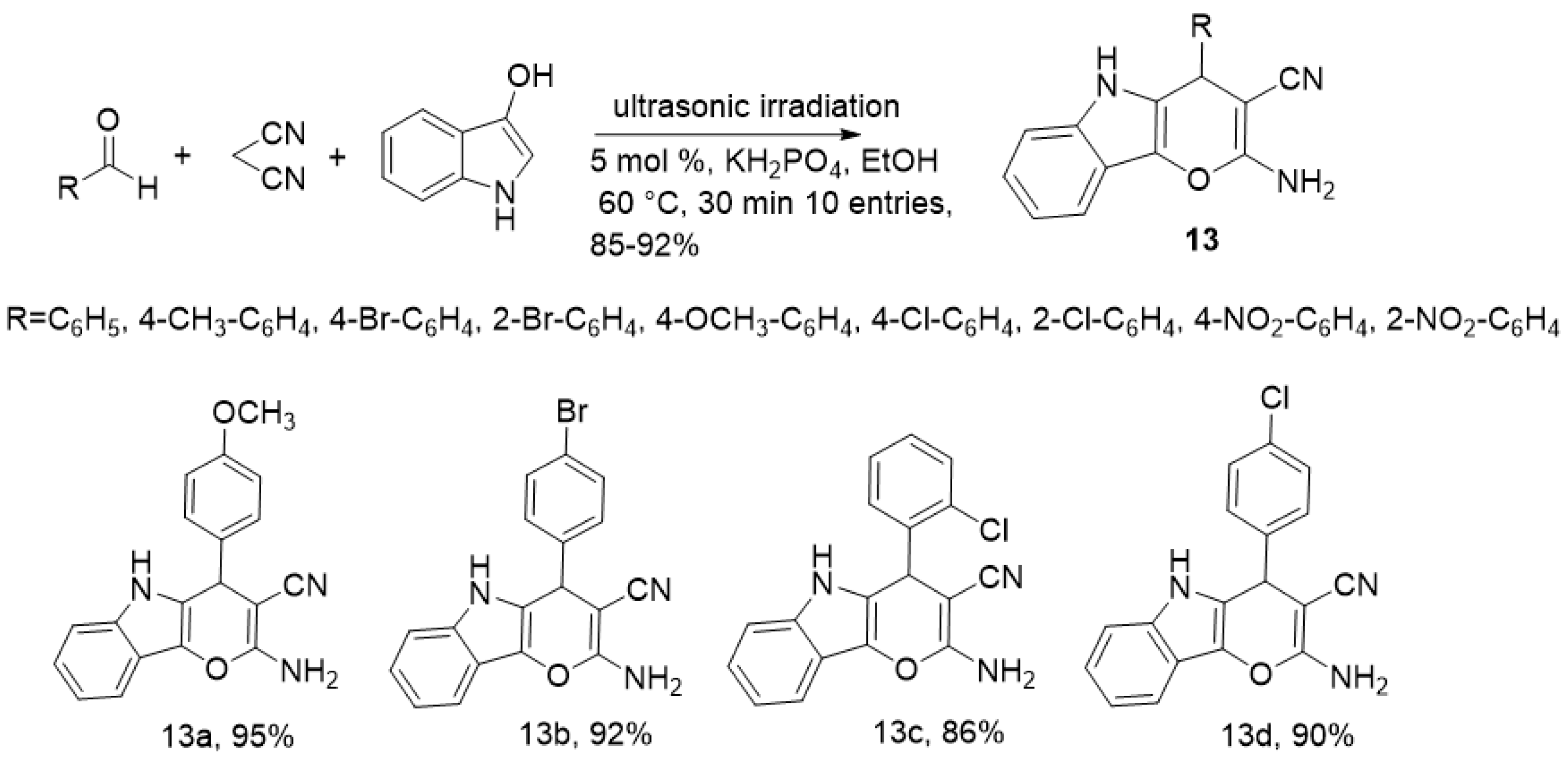
2.2. Ultrasound-Assisted Synthesis of Indole Derivatives
3. Benzimidazole
3.1. Microwave-Assisted Synthesis of Benzimidazole Derivatives
3.2. Ultrasound Assisted Synthesis of Benzimidazole Derivatives
4. Imidazo[1,2-a]pyridines
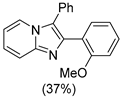
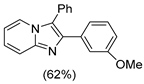
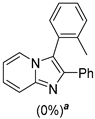
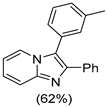
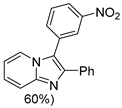
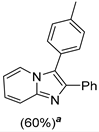
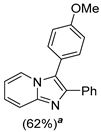
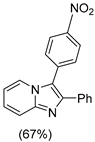
4.1. Microwave-Assisted Synthesis of Imidazo[1,2-a]pyridine Derivatives
4.2. Ultrasound-Assisted Synthesis of Imidazo[1,2-a]pyridine Derivatives
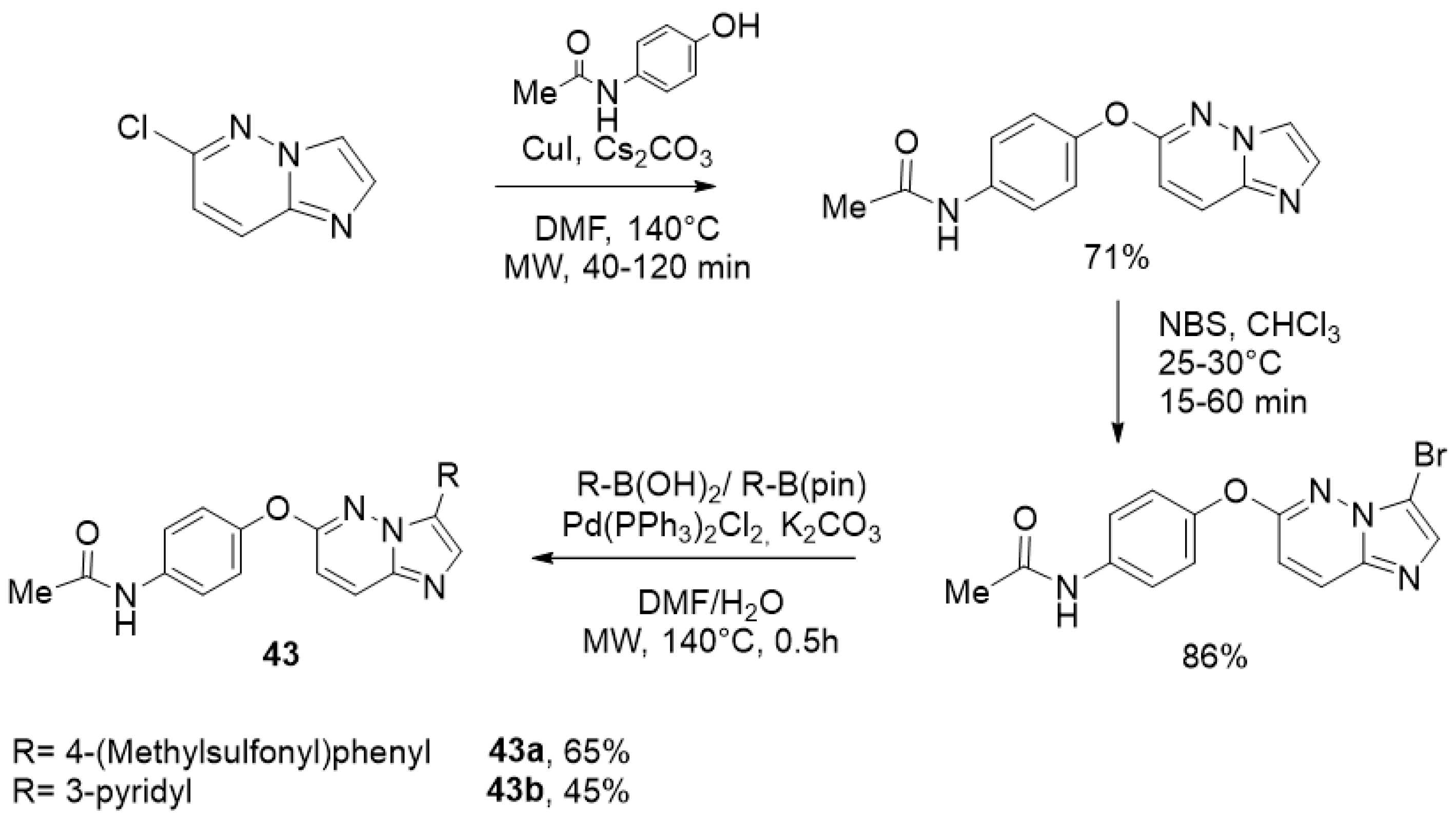
5. Imidazo[1,2-b]pyridazine
Microwave-Assisted Synthesis of Imidazo[[1,2-b]]pyridazine Derivatives
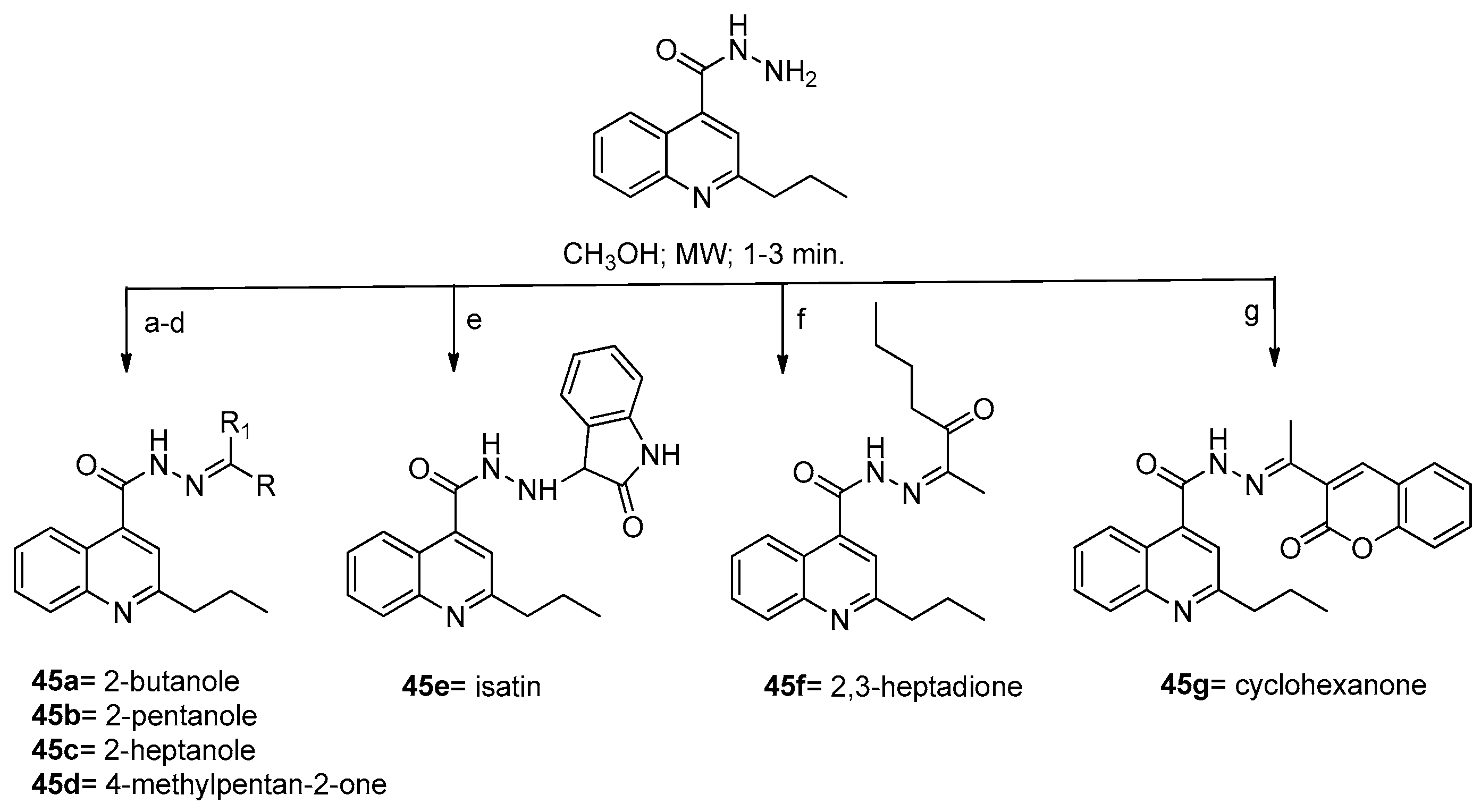
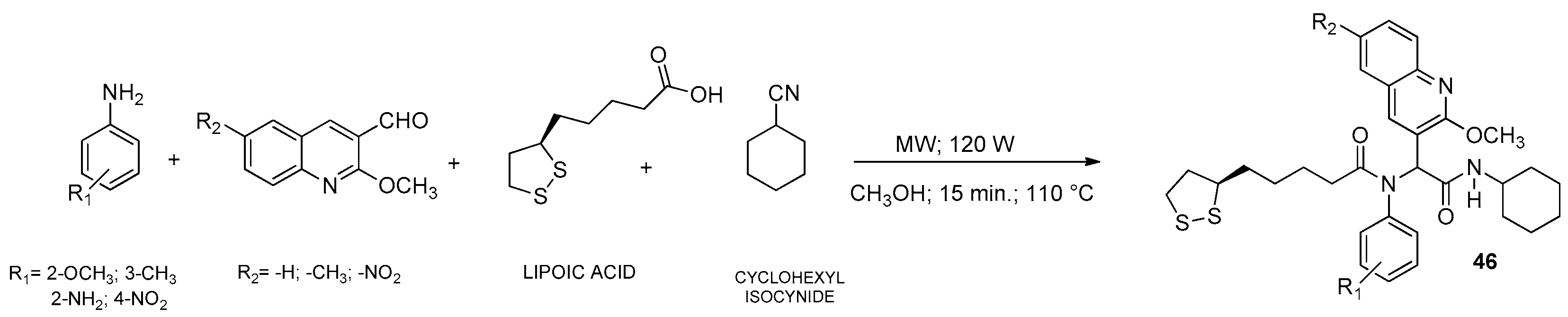
6. Quinoline
6.1. Microwave-Assisted Synthesis Synthesis of Quinoline Derivatives
6.2. Ultrasound-Assisted Synthesis of Quinoline Derivatives
7. Isoquinoline
7.1. Microwave-Assisted Synthesis of Isoquinoline Derivatives
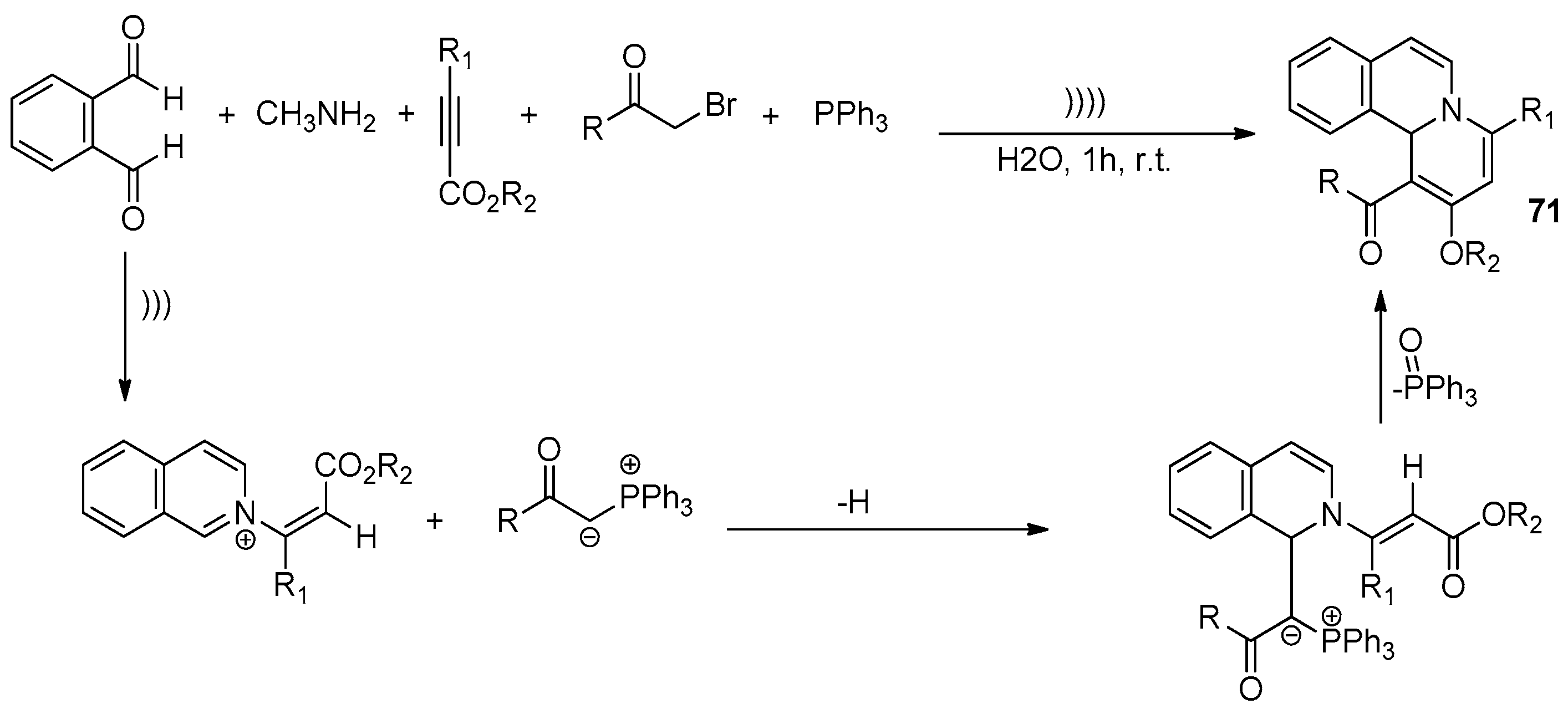
7.2. Ultrasound-Assisted Synthesis of Isoquinoline Derivatives
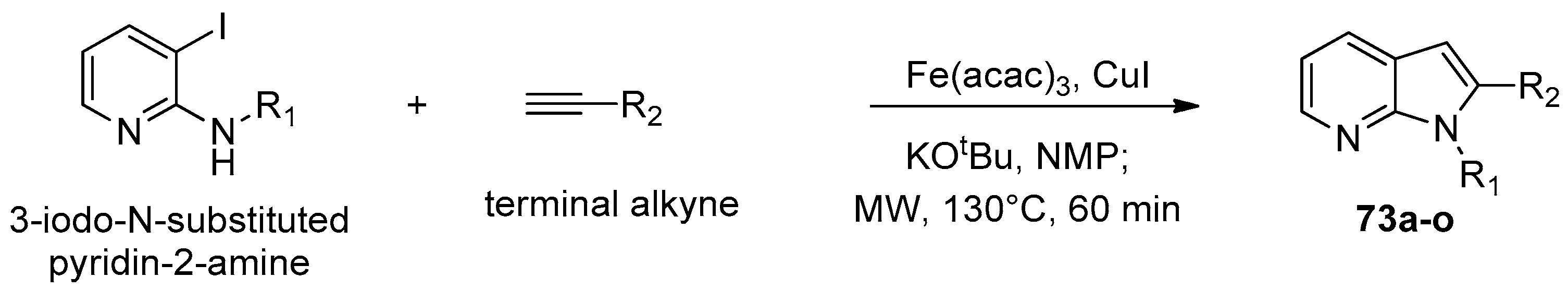
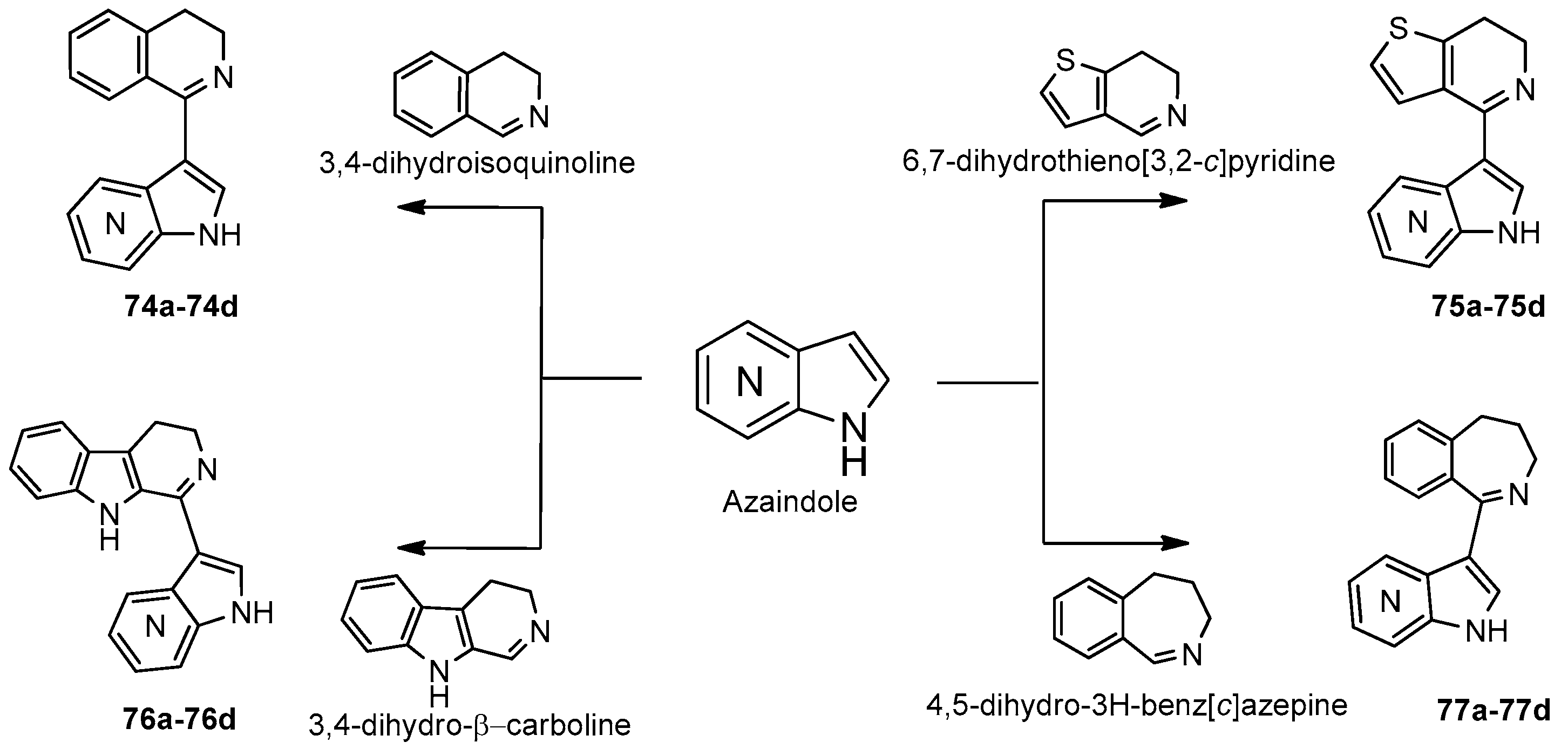
8. Pyrrolopyridine
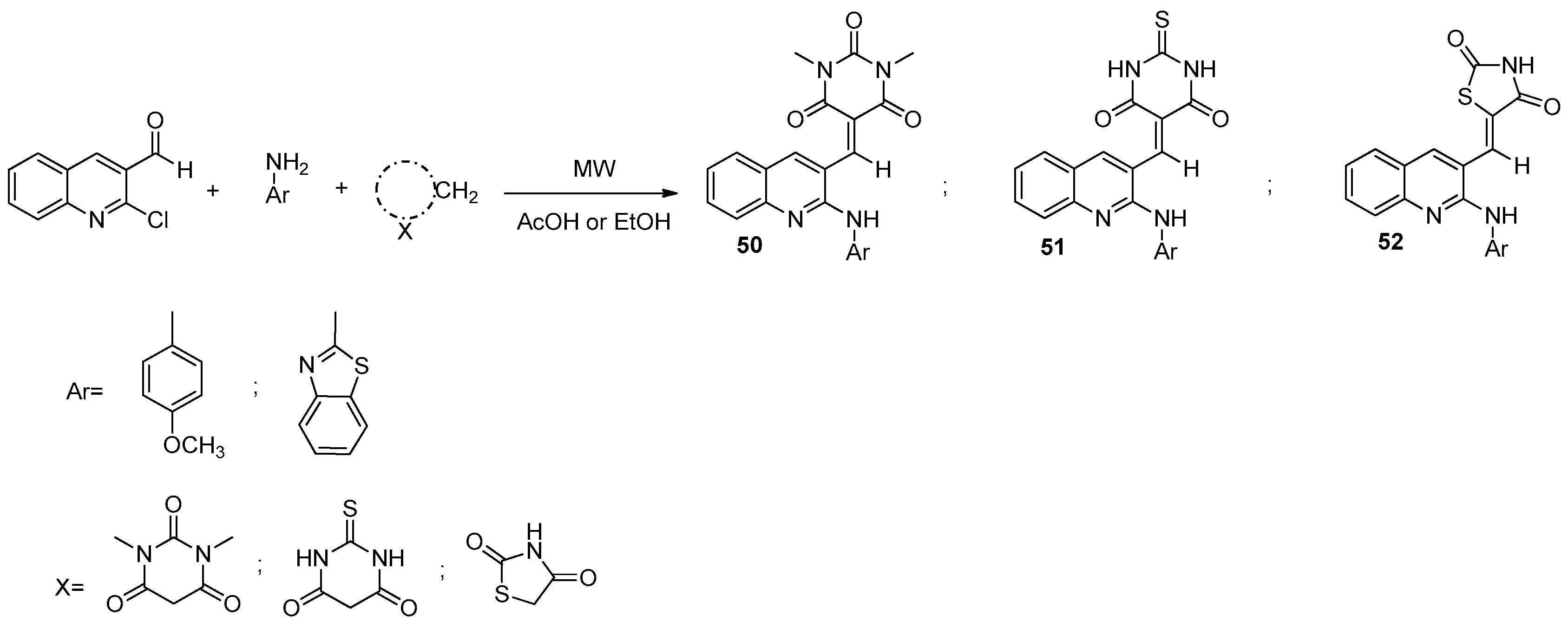
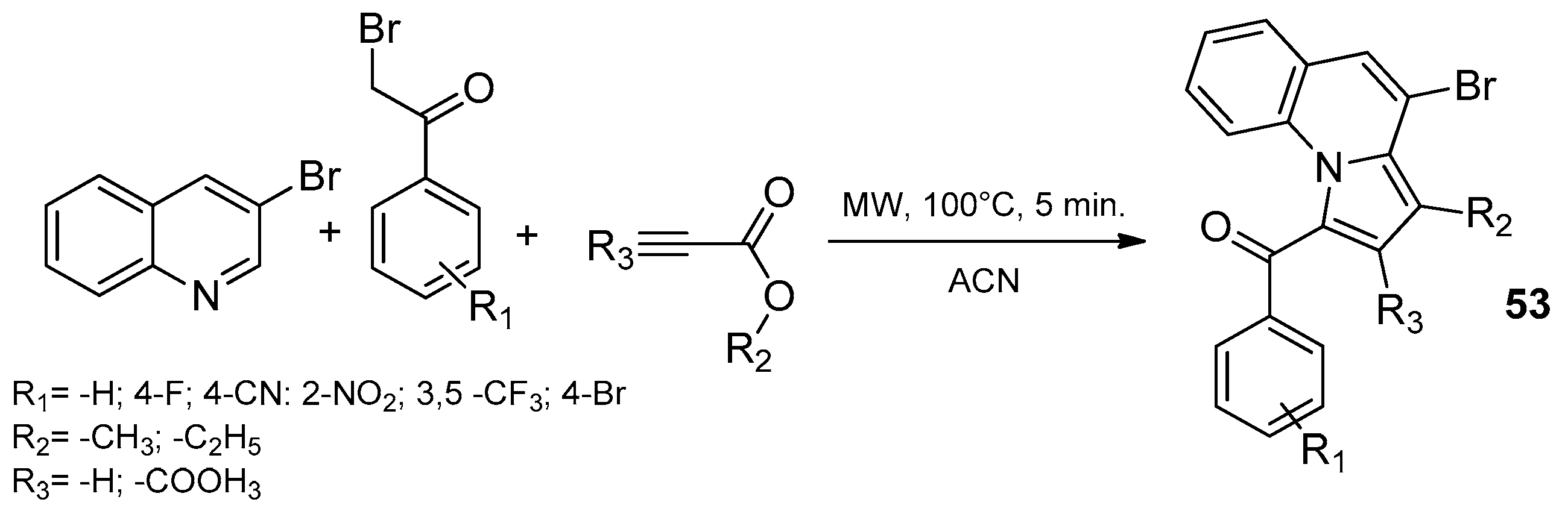
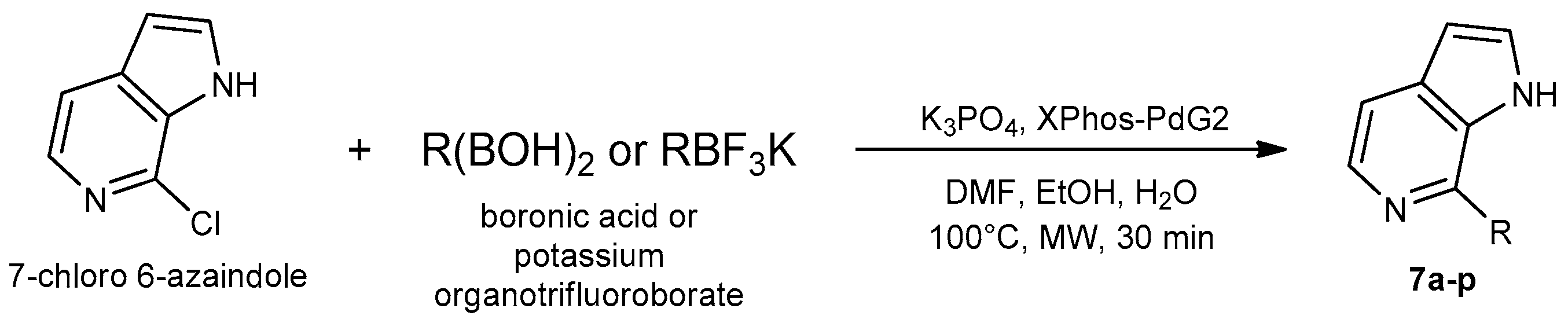
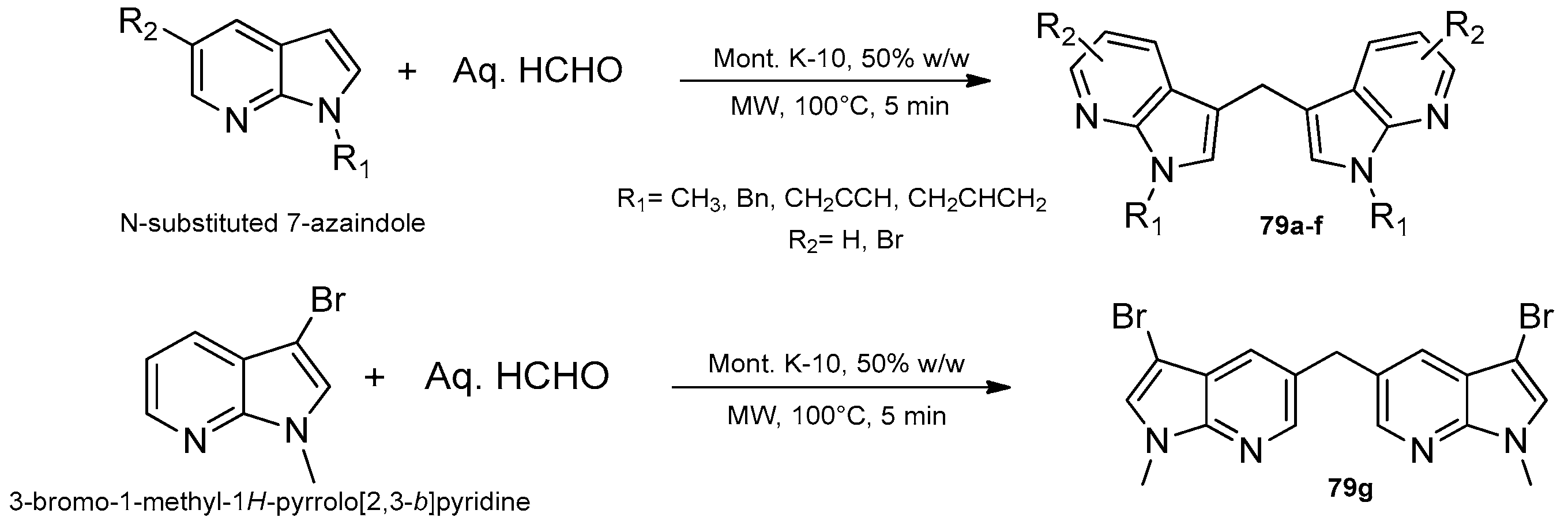
8.1. Microwave-Assisted Synthesis of Pyrrolopyridine Derivatives
8.2. Ultrasound-Assisted Synthesis of Pyrrolopyridine Derivatives
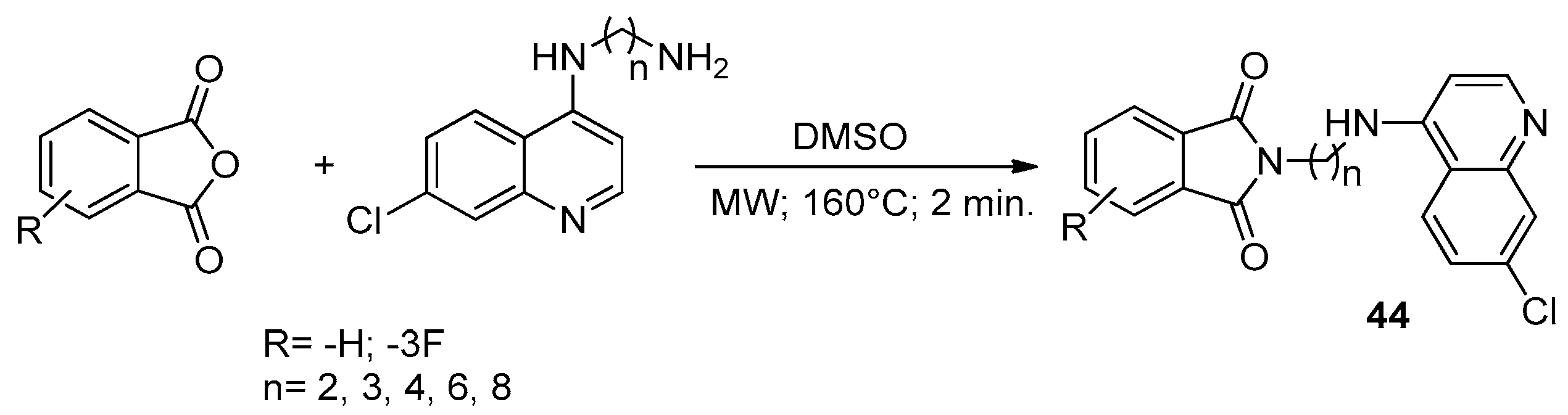
9. Pyrrolopyridazine
Microwave-Assisted Synthesis of Pyrrolopyridazine Derivatives
10. Hybrid Microwave–Ultrasound Irradiation: Selected Applications
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krasnodębski, M. Lost Green Chemistries: History of Forgotten Environmental Trajectories. Centaurus 2022, 64, 509–536. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; ISBN 0198502346. [Google Scholar]
- Roschangar, F.; Colberg, J.; Dunn, P.J.; Gallou, F.; Hayler, J.D.; Koenig, S.G.; Kopach, M.E.; Leahy, D.K.; Mergelsberg, I.; Tucker, J.L.; et al. A Deeper Shade of Green: Inspiring Sustainable Drug Manufacturing. Green Chem. 2017, 19, 281–285. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Uzzaman, M. A Review on Biological and Medicinal Impact of Heterocyclic Compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Rajput, A.P.; Kankhare, A.R. Synthetic Utility of Aza Heterocyclics: A Short Review. Int. J. Pharm. Sci. Invent. 2017, 6, 19–25. [Google Scholar]
- Majumder, A.; Gupta, R.; Jain, A. Microwave-assisted Synthesis of Nitrogen-containing Heterocycles. Green Chem. Lett. Rev. 2013, 6, 151–182. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and Applications of Microwave-Assisted Synthesis of Nitrogen Containing Heterocycles in Medicinal Chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef]
- Singh, R. A Brief Review on Recent Developments in Ultrasound Assisted Synthesis of Heterocycles. Indian J. Sci. Res. 2021, 11, 77–84. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Barge, A.; Cravotto, G. Combined Microwaves/Ultrasound, a Hybrid Technology. Top. Curr. Chem. 2016, 374, 79–101. [Google Scholar] [CrossRef]
- Kappe, O.C. Microwave Dielectric Heating in Synthetic Organic Chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A. A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199. [Google Scholar] [CrossRef]
- Santagada, V.; Perissutti, E.; Caliendo, G. The Application of Microwave Irradiation as New Convenient Synthetic Procedure in Drug Discovery. Curr. Med. Chem. 2002, 9, 1251–1283. [Google Scholar] [CrossRef]
- Leadbeatera, N.E.; Torenius, H.M.; Tye, H. Microwave-Promoted Organic Synthesis Using Ionic Liquids: A Mini Review. Comb. Chem. High Throughput Screen. 2004, 7, 511–528. [Google Scholar] [CrossRef]
- Loupy, A. Solvent-Free Microwave Organic Synthesis as an Efficient Procedure for Green Chemistry. Comptes Rendus Chim. 2004, 7, 103–112. [Google Scholar] [CrossRef]
- Martínez-Palou, R. Microwave-assisted Synthesis Using Ionic Liquids. Mol. Divers. 2010, 14, 3–25. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Cavalieri, F.; Chemat, F.; Okitsu, K.; Sambandam, A.; Yasui, K.; Zisu, B. Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2018; ISBN 9789812872784. [Google Scholar]
- Cravotto, G.; Cintas, P. Power Ultrasound in Organic Synthesis: Moving Cavitational Chemistry from Academia to Innovative and Large-scale Applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef]
- Gogate, P.R.; Patil, P.N. Sonochemical Reactors. Top. Curr. Chem. 2016, 374, 255–281. [Google Scholar] [CrossRef]
- Draye, M.; Chatel, G.; Duwald, R. Ultrasound for Drug Synthesis: A Green Approach. Pharmaceuticals 2020, 13, 23. [Google Scholar] [CrossRef]
- Lagha, A.; Chemat, S.; Bartels, P.V.; Chemat, F. Microwave–Ultrasound Combined Reactor Suitable for Atmospheric Sample Preparation Procedure of Biological and Chemical Products. Analusis 1999, 27, 452–457. [Google Scholar] [CrossRef]
- Leonelli, C.; Mason, T.J. Microwave and Ultrasonic Processing: Now a Realistic Option for Industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Chemat, S.; Lagha, A.; Amar, H.A.; Chemat, F. Ultrasound Assisted Microwave Digestion. Ultrason. Sonochemistry 2004, 11, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Beggiato, M.; Penoni, A.; Palmisano, G.; Tollari, S.; Lévêque, J.M.; Bonrath, W. High-Intensity Ultrasound and Microwave, Alone or Combined, Promote Pd/C-Catalyzed Aryl–Aryl Couplings. Tetrahedron Lett. 2005, 46, 2267–2271. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. The Combined Use of Microwaves and Ultrasound: Improved Tools in Process Chemistry and Organic Synthesis. Chem.—Eur. J. 2007, 13, 1902–1909. [Google Scholar] [CrossRef]
- Cravotto, G.; Di Carlo, S.; Curini, M.; Tumiatti, V.; Roggero, C. A New Flow Reactor for the Treatment of Polluted Water with Microwave and Ultrasound. J. Chem. Technol. Biotechnol. 2007, 82, 205–208. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Chadha, N.; Silakari, O. Indoles as Therapeutics of Interest in Medicinal Chemistry: Bird’s Eye View. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal Chemistry of Indole Derivatives: Current to Future Therapeutic Prospectives. Bioorganic Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Bellavita, R.; Casertano, M.; Grasso, N.; Gillick-Healy, M.; Kelly, B.G.; Adamo, M.F.A.; Menna, M.; Merlino, F.; Grieco, P. Microwave-Assisted Synthesis of 2-Methyl-1H-indole-3-carboxylate Derivatives via Pd-Catalyzed Heterocyclization. Symmetry 2022, 14, 435. [Google Scholar] [CrossRef]
- Rathod, A.S.; Reddy, P.V.; Biradar, J.S. Microwave-Assisted Synthesis of Some Indole and Isoniazid Derivatives as Antitubercular Agents and Molecular Docking Study. Russ. J. Org. Chem. 2020, 56, 662–670. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, Y.X.; Xun, Z.; Huang, Z.B.; Shi, D.Q. Microwave-Assisted Regioselective Synthesis of 3-Functionalized Indole Derivatives via Three-Component Domino Reaction. ACS Comb. Sci. 2017, 19, 708–713. [Google Scholar] [CrossRef]
- Damavandi, S.; Sandaroos, R. KHPO4/Ultrasonic Irradiation Catalyzed Multicomponent Synthesis of Aminocyanopyrano [3,2-b]indole. Res. Chem. Intermed. 2013, 39, 1251–1256. [Google Scholar] [CrossRef]
- Dandia, A.; Singh, R.; Joshi, J.; Maheshwari, S.; Soni, P. Ultrasound Promoted Catalyst-Free and Selective Synthesis of Spiro[indole-3,4′-pyrazolo[3,4-e][1,4]thiazepines] in Aqueous Media and Evaluation of Their Anti-hyperglycemic Activity. RSC Adv. 2013, 3, 18992. [Google Scholar] [CrossRef]
- Vieira, B.M.; Thurow, S.; da Costa, M.; Casaril, A.M.; Domingues, M.; Schumacher, R.F.; Lenardão, E.J. Ultrasound-Assisted Synthesis and Antioxidant Activity of 3-Selanyl-1-H-indole and 3-selanylimidazo[1,2-a]pyridine Derivatives. Asian J. Org. Chem. 2017, 6, 1635–1646. [Google Scholar] [CrossRef]
- Jaiswal, P.K.; Sharma, V.; Prikhodko, J.; Mashevskaya, I.V.; Chaudhary, S. “On water” Ultrasound-Assisted One Pot Efficient Synthesis of Functionalized 2-oxo-benzo[1,4]oxazines: First Application to the Synthesis of Anticancer Indole Alkaloid, Cephalandole A. Tetrahedron Lett. 2017, 58, 2077–2083. [Google Scholar] [CrossRef]
- Pogaku, V.; Krishna, V.S.; Sriram, D.; Rangan, K.; Basavoju, S. Ultrasonication-Ionic Liquid Synergy for the Synthesis of New Potent Anti-Tuberculosis 1,2,4-triazol-1-yl-pyrazole based Spirooxindolopyrrolizidines. Bioorg. Med. Chem. Lett. 2019, 29, 1682–1687. [Google Scholar] [CrossRef]
- Tahlan, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Narasimhan, B. 4-(2-(1H-Benzo [d] imidazol-2-ylthio)acetamido)-N-(substituted phenyl) Benzamides: Design, Synthesis and Biological Evaluation. BMC Chem. 2019, 13, 12. [Google Scholar] [CrossRef]
- Achar, K.C.; Hosamani, K.M.; Seetharamareddy, H.R. In-Vivo Analgesic and Anti-Inflammatory Activities of Newly Synthesized Benzimidazole Derivatives. Eur. J. Med. Chem. 2010, 45, 2048–2054. [Google Scholar] [CrossRef]
- Starčević, K.; Kralj, M.; Ester, K.; Sabol, I.; Grce, M.; Pavelić, K.; Karminski-Zamola, G. Synthesis, Antiviral and Antitumor Activity of 2-substituted-5-amidino-benzimidazoles. Bioorg. Med. Chem. 2007, 15, 4419–4426. [Google Scholar]
- Vausselin, T.; Séron, K.; Lavie, M.; Mesalam, A.A.; Lemasson, M.; Belouzard, S.; Dubuisson, J. Identification of a New Benzimidazole Derivative as an Antiviral Against Hepatitis C Virus. J. Virol. 2016, 90, 8422–8434. [Google Scholar] [CrossRef]
- Camacho, J.; Barazarte, A.; Gamboa, N.; Rodrigues, J.; Rojas, R.; Vaisberg, A.; Charris, J. Synthesis and Biological Evaluation of Benzimidazole-5-carbohydrazide Derivatives as Antimalarial, Cytotoxic and Antitubercular Agents. Bioorg. Med. Chem. 2011, 19, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Bougrin, K.; Soufiaoui, M. Nouvelle Voie de Synthèse des Arylimidazole Sous Irradiation Micro-ondes en Milieu Sec. Tetrahedron Lett. 1995, 36, 3683–3686. [Google Scholar] [CrossRef]
- Song, G.T.; Xu, Z.G.; Tang, D.Y.; Li, S.Q.; Xie, Z.G.; Zhong, H.L.; Yang, Z.W.; Zhu, J.; Zhang, J.; Chen, Z.Z. Facile Microwave-Assisted Synthesis of Benzimidazole Scaffolds via Ugi-type Three-Component Condensation (3CC) Reactions. Mol. Divers. 2016, 20, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Karlık, Ö.; Gençer, N.; Karataş, M.O.; Ergün, A.; Çıkrıkcı, K.; Arslan, O.; Alıcı, B.; Kılıç-Cıkla, I.; Özdemir, N. Microwave-Assisted Synthesis of 1-Substituted-1H-Benzimidazolium Salts: Non-Competitive Inhibition of Human Carbonic Anhydrase I and II. Arch. Pharm. 2019, 352, e1800325. [Google Scholar] [CrossRef] [PubMed]
- Mah, W.L.; Jun Tan, X.; Choo, K.B.; Lee, S.X.; Tan, K.W.; Yeong, K.Y.; Lee, S.M.; Cheow, Y.L. Microwave-Assisted Synthesis of Bioactive Pyridine-Functionalized N-Alkyl-Substituted (Benz) Imidazolium Salts. ChemistrySelect 2022, 7, e202203864. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Herrera Cano, N.; Oliverio, M.; Procopio, A. The Highly Efficient Synthesis of 1,2-Disubstituted Benzimidazoles Using Microwave Irradiation. Molecules 2022, 27, 1751. [Google Scholar] [CrossRef]
- Karami, C.; Ghodrati, K.; Izadi, M.; Farrokh, A.; Jafari, S.; Mahmoudiyani, M.; Haghnazari, N. A Fast Procedure for the Preparation of Benzimidazole Derivatives using Polymer-Supported with Trifluoromethanesulfonic Acid as Novel and Reusable Catalyst. J. Chil. Chem. Soc. 2013, 58, 1914–1917. [Google Scholar] [CrossRef]
- Nile, S.H.; Kumar, B.; Park, S.W. Chemo Selective Onepot Synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles using Amberlite IR-120. Arab. J. Chem. 2015, 8, 685–691. [Google Scholar] [CrossRef]
- Chen, L.H.; Chung, T.W.; Narhe, B.D.; Sun, C.M. A Novel Mechanistic Study on Ultrasound-assisted, One-pot Synthesis of Functionalized Benzimidaz[2,1-b]quinazolin-1(1H)-ones. ACS Comb. Sci. 2016, 18, 162–169. [Google Scholar] [CrossRef]
- Naeimi, H.; Babaei, Z. MnO2 Nanoparticles as Efficient Oxidant for Ultrasound-Assisted Synthesis of 2-substituted Benzimidazoles under Mild Conditions. Polycycl. Aromat. Compd. 2016, 36, 490–505. [Google Scholar] [CrossRef]
- Ziarati, A.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ganjali, M.R.; Badiei, A. Sonication Method Synergism with Rare Earth Based Nanocatalyst: Preparation of NiFe2–xEuxO4 Nanostructures and its Catalytic Applications for the Synthesis of B enzimidazoles, Benzoxazoles, and Benzothiazoles under Ultrasonic Irradiation. J. Rare Earths 2017, 35, 374–381. [Google Scholar] [CrossRef]
- Sapkal, B.M.; Labhane, P.K.; Satam, J.R. In Water—ultrasound-promoted Synthesis of Tetraketones and 2-substituted-1H-benzimidazoles Catalyzed by BiOCl Nanoparticles. Res. Chem. Intermed. 2017, 43, 4967–4979. [Google Scholar] [CrossRef]
- Kishbaugh, L.S.T. Pyridines and Imidazopyridines with medicinal significance. Curr. Top. Med. Chem. 2016, 16, 3274–3302. [Google Scholar] [CrossRef]
- Hiebel, M.A.; Fall, Y.; Scherrmann, M.C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2, 3-Diarylimidazo [1, 2-a] pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Wagare, D.S.; Farooqui, M.; Keche, T.D.; Durrani, A. Efficient and Green Microwave-assisted One-pot Synthesis of Azaindolizines in PEG-400 and Water. Synth. Commun. 2016, 46, 1741–1746. [Google Scholar] [CrossRef]
- Karamthulla, S.; Khan, M.N.; Choudhury, L.H. Microwave-assisted Synthesis of Novel 2,3-disubstituted imidazo[1, 2-a]pyridines via One-pot Three Component Reactions. RSC Adv. 2015, 5, 19724–19733. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.Y.; Park, A.; Yum, E.K. Diversification of Imidazo [1, 2-a] pyridines under Microwave-Assisted Palladium-Catalyzed Suzuki Reaction. J. Korean Chem. Soc. 2017, 61, 299–303. [Google Scholar]
- Rao, R.N.; Mm, B.; Maiti, B.; Thakuria, R.; Chanda, K. Efficient Access to Imidazo[1, 2-a]pyridines/pyrazines/pyrimidines via Catalyst-free Annulation Reaction under Microwave Irradiation in Green Solvent. ACS Comb. Sci. 2018, 20, 164–171. [Google Scholar] [CrossRef]
- Zarate-Hernández, C.; Basavanag, U.; Gámez-Montaño, R. Synthesis of Imidazo [1, 2-a] pyridine-Chromones via Microwave-Assisted Groebke-Blackburn-Bienaymé Reaction. Multidiscip. Digit. Publ. Inst. Proc. 2019, 41, 68. [Google Scholar]
- Rodríguez, J.C.; Maldonado, R.A.; Ramírez-García, G.; Diaz Cervantes, E.; de la Cruz, F.N. Microwave-assisted Synthesis and Luminescent Activity of Imidazo[1, 2-a]pyridine Derivatives. J. Heterocycl. Chem. 2020, 57, 2279–2287. [Google Scholar] [CrossRef]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound Assisted Green Synthesis of Bound type Bis-heterocyclic Carbazolyl Imidazo[1, 2-a]pyridines via Groebke-Blackburn-Bienayme Reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]
- Vieira, B.M.; Padilha, N.; Nascimento, N.M.; Perin, G.; Alves, D.; Schumacher, R.F.; Lenardão, E.J. Ultrasound-assisted Synthesis of Imidazo[1,2-a]pyridines and Sequential One-pot Preparation of 3-selanyl-imidazo[1,2-a]pyridine Derivatives. Arkivoc 2019, 2, 6. [Google Scholar] [CrossRef]
- Paengphua, P.; Wichai, U.; Chancharunee, S. An Efficient Synthesis of Imidazo[1,2-a]pyridines. Naresuan Univ. J. Sci. Technol. 2019, 27, 43–54. [Google Scholar]
- Yang, H.; Huang, N.; Wang, N.; Shen, H.; Teng, F.; Liu, X.; Jiang, H.; Tan, M.C.; Gui, Q.W. Ultrasound-Assisted Iodination of Imidazo[1,2-α]pyridines via C–H Functionalization Mediated by tert-Butyl Hydroperoxide. ACS Omega 2021, 6, 25940–25949. [Google Scholar] [CrossRef]
- Ramarao, S.; Pothireddy, M.; Venkateshwarlu, R.; Moturu, K.M.V.; Siddaiah, V.; Kapavarapu, R.; Rambabu, D.; Pal, M. Sonochemical Synthesis and In Silico Evaluation of Imidazo[1,2-a]Pyridine Derivatives as Potential Inhibitors of Sirtuins. Polycycl. Aromat. Compd. 2022, 27, 2077774. [Google Scholar] [CrossRef]
- Garrido, A.; Vera, G.; Delaye, P.O.; Enguehard-Gueiffier, C. Imidazo [1, 2-b] pyridazine as privileged scaffold in medicinal chemistry: An extensive review. Eur. J. Med. Chem. 2021, 226, 113867. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M. Microwave-assisted Synthesis in Water: First One-pot Synthesis of a Novel Class of Polysubstituted benzo[4,5]imidazo [1, 2-b]pyridazines via Intramolecular SN Ar. RSC Adv. 2015, 5, 89226–89237. [Google Scholar] [CrossRef]
- Moine, E.; Dimier-Poisson, I.; Enguehard-Gueiffier, C.; Logé, C.; Pénichon, M.; Moiré, N.; Delehouzé, C.; Foll-Josselin, B.; Ruchaud, S.; Bach, S.; et al. Development of New Highly Potent Imidazo[[1,2-b]]pyridazines targeting Toxoplasma Gondii Calcium-dependent Protein Kinase 1. Eur. J. Med. Chem. 2015, 105, 80–105. [Google Scholar] [CrossRef]
- Pandit, S.S.; Kulkarni, M.R.; Ghosh, U.; Pandit, Y.B.; Lad, N.P. Synthesis and Biological evaluation of Imidazo[[1,2-b]]pyridazines as inhibitors of TNF-α production. Mol. Divers. 2018, 22, 545–560. [Google Scholar] [CrossRef]
- Tople, M.S.; Patel, N.B.; Patel, P.P. Microwave Irradiation for the Synthesis of Quinoline Scaffolds: A Review. J. Iran. Chem. Soc. 2023, 20, 1–28. [Google Scholar] [CrossRef]
- Rani, A.; Singh, A.; Gut, J.; Rosenthal, P.J.; Kumar, V. Microwave Promoted Facile Access to 4-Aminoquinoline-Phthalimides: Synthesis and Anti-Plasmodial Evaluation. Eur. J. Med. Chem. 2018, 143, 150–156. [Google Scholar] [CrossRef]
- Ajani, O.O.; Iyaye, K.T.; Audu, O.Y.; Olorunshola, S.J.; Kuye, A.O.; Olanrewaju, I.O. Microwave Assisted Synthesis and Antimicrobial Potential of Quinoline-Based 4-Hydrazide-Hydrazone Derivatives. J. Heterocycl. Chem. 2018, 55, 302–312. [Google Scholar] [CrossRef]
- Thangaraj, M.; Gengan, R.M.; Ranjan, B.; Muthusamy, R. Synthesis, Molecular Docking, Antimicrobial, Antioxidant and Toxicity Assessment of Quinoline Peptides. J. Photochem. Photobiol. 2018, B 178, 287–295. [Google Scholar] [CrossRef]
- Khumalo, M.R.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. A Multicomponent, Facile and Catalyst-Free Microwave-Assisted Protocol for the Synthesis of Pyrazolo-[3, 4-b]-Quinolines under Green Conditions. RSC Adv. 2019, 9, 30768–30772. [Google Scholar] [CrossRef]
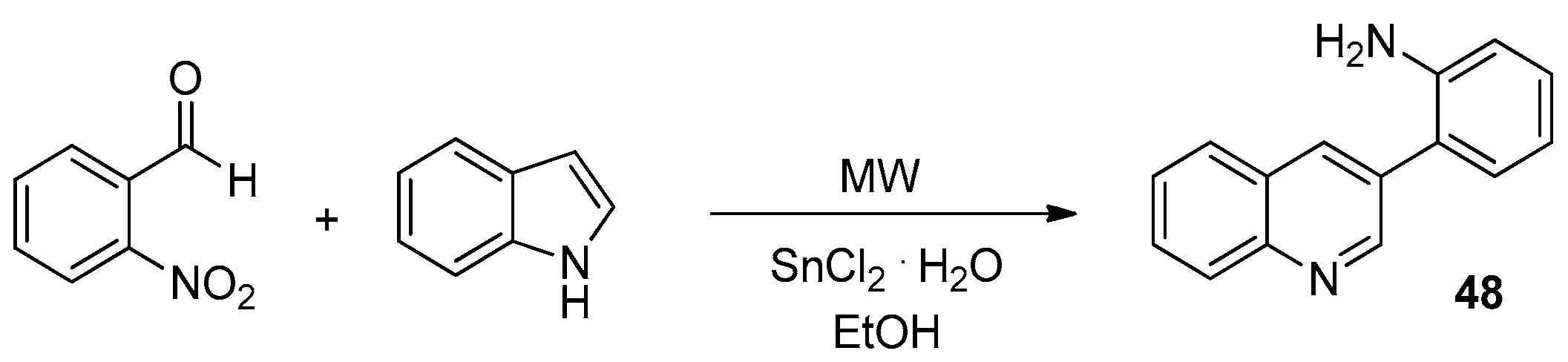
- Rao, M.S.; Sarkar, S.; Hussain, S. Microwave-Assisted Synthesis of 3-Aminoarylquinolines From 2-Nitrobenzaldehyde and Indole via SnCl2-Mediated Reduction and Facile Indole Ring Opening. Tetrahedron Lett. 2019, 60, 1221–1225. [Google Scholar] [CrossRef]
- Devi, K.R.; Ashok, D.; Patnaik, K.S.K.R.; Bathula, R.; Rani, S.S.; Bhakshi, V. Synthesis of Quinoline Derivatives by Microwave Irradiation Method and Evaluation for Their Anti-helminthic Activity. IOSR J. Pharm. Biol. Sci. 2019, 14, 16–22. [Google Scholar]
- El-Naggar, A.M.; Ramadan, S.K. Efficient Synthesis of Some Pyrimidine and Thiazolidine Derivatives Bearing Quinoline Scaffold under Microwave Irradiation. Synth. Commun. 2020, 50, 2188–2198. [Google Scholar] [CrossRef]
- Uppar, V.; Mudnakudu-Nagaraju, K.K.; Basarikatti, A.I.; Chougala, M.; Chandrashekharappa, S.; Mohan, M.K.; Banuprakash, G.; Venugopala, K.N.; Ningegowda, R.; Padmashali, B. Microwave Induced Synthesis, and Pharmacological Properties of Novel 1-Benzoyl-4-Bromopyrrolo [1, 2-a] Quinoline-3-Carboxylate Analogues. Chem. Data Collect. 2020, 25, 100316. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.; Chen, X.L.; Lu, X.Y.; Liang, X.X.; Zhu, S.S.; Wei, C.W.; Qu, L.B.; Yu, B. 6π-Electrocyclization in Water: Microwave-Assisted Synthesis of Polyheterocyclic-Fused Quinoline-2-Thiones. Green Chem. 2020, 22, 4445–4449. [Google Scholar] [CrossRef]
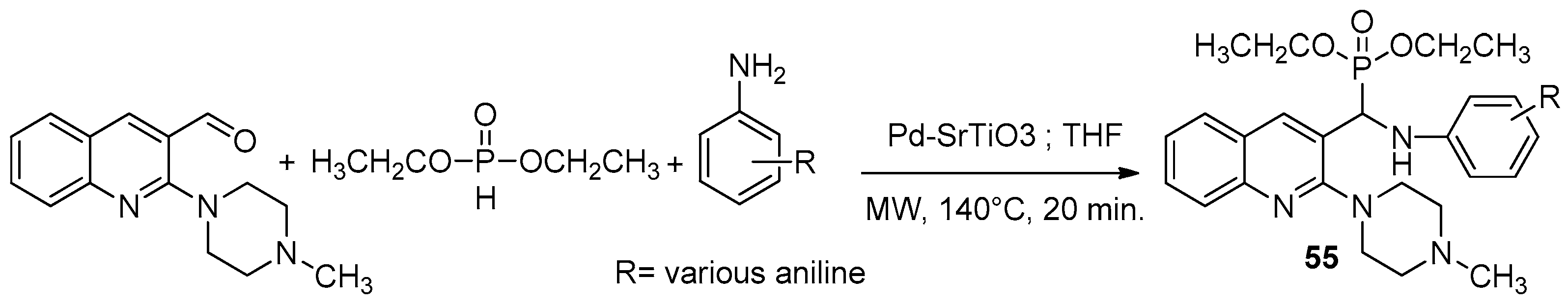
- Rajkoomar, N.; Murugesan, A.; Prabu, S.; Gengan, R.M. Synthesis of Methyl Piperazinyl-Quinolinyl A-Aminophosphonates Derivatives under Microwave Irradiation with Pd–SrTiO3 Catalyst and Their Antibacterial and Antioxidant Activities. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 1031–1038. [Google Scholar]
- Patel, D.B.; Rajani, D.P.; Rajani, S.D.; Patel, H.D. A Green Synthesis of Quinoline-4-Carboxylic Derivatives Using p-Toluenesulfonic Acid as an Efficient Organocatalyst Under Microwave Irradiation and Their Docking, Molecular Dynamics, ADME-Tox and Biological Evaluation. J. Heterocycl. Chem. 2020, 57, 1524–1544. [Google Scholar] [CrossRef]
- Tasqeeruddin, S.; Asiri, Y.; Alsherhri, J.A. An Efficient and Green Microwave-Assisted Synthesis of Quinoline Derivatives via Knoevengal Condensation. Lett. Org. Chem. 2020, 17, 157–163. [Google Scholar] [CrossRef]
- Pradeep, M.; Vishnuvardhan, M.; Thalari, G. A Simple and Efficient Microwave Assisted Synthesis of Pyrrolidinyl-Quinoline Based Pyrazoline Derivatives and Their Antimicrobial Activity. Chem. Data Collect. 2021, 32, 100666. [Google Scholar] [CrossRef]
- Seliem, I.A.; Panda, S.S.; Girgis, A.S.; Moatasim, Y.; Kandeil, A.; Mostafa, A.; Ali, M.A.; Nossier, E.S.; Rasslan, F.; Srour, A.M.; et al. New Quinoline-Triazole Conjugates: Synthesis, and Antiviral Properties against SARS-CoV-2. Bioorg. Chem. 2021, 114, 105117. [Google Scholar] [CrossRef] [PubMed]
- Sacchelli, B.A.L.; Rocha, B.C.; Andrade, L.H. Cascade Reactions Assisted by Microwave Irradiation: Ultrafast Construction of 2-Quinolinone-Fused γ-Lactones from N-(O-Ethynylaryl)Acrylamides and Formamide. Org. Lett. 2021, 23, 5071–5075. [Google Scholar] [CrossRef]
- Bhuyan, D. Microwave Assisted Catalyst and Solvent Free Efficient Synthesis of Quinoline Derivatives by Three Component One Pot Aza-Diels-Alder Reaction Strategy. Structure 2021, 9, 15. [Google Scholar] [CrossRef]
- Attia, Y.A.; Abdel-Hafez, S.H. Nano-Co3O4-Catalyzed Microwave Assisted One-Pot Synthesis of Some Seleno [2,3-B]-Pyridine/Quinoline Derivatives. Res. Chem. Intermed. 2021, 47, 3719–3732. [Google Scholar] [CrossRef]
- Marganakop, S.B.; Kamble, R.R.; Nesaragi, A.R.; Bayannavar, P.K.; Joshi, S.D.; Kattimani, P.P.; Sudha, B.S. Microwave Assisted Synthesis of Quinoline Fused Benzodiazepines as Anxiolytic and Antimicrobial Agents. Asian J. Chem. 2021, 33, 1107–1114. [Google Scholar] [CrossRef]
- Nesaragi, A.R.; Kamble, R.R.; Bayannavar, P.K.; Shaikh, S.K.J.; Hoolageri, S.R.; Kodasi, B.; Joshi, S.D.; Kumbar, V.M. Microwave Assisted Regioselective Synthesis of Quinoline Appended Triazoles as Potent Anti-Tubercular and Antifungal Agents via Copper (I) Catalyzed Cycloaddition. Bioorg. Med. Chem. Lett. 2021, 41, 127984. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Ai, Y.; Ren, Y.; Bai, X.; Chen, H.; Zhou, Y.; Li, W.; Wang, C.; Liu, Y. Microwave-Assisted and Solvent-Free Synthesis of Quinoline Derivatives and Their Fluorescence Properties. Heterocycles 2021, 102, 647–658. [Google Scholar]
- Babaei, P.; Safaei-Ghomi, J. Engineered N-Doped Graphene Quantum Dots/CoFe2O4 Spherical Composites as a Robust and Retrievable Catalyst: Fabrication, Characterization, and Catalytic Performance Investigation in Microwave-Assisted Synthesis of Quinoline-3-Carbonitrile Derivatives. RSC Adv. 2021, 11, 34724. [Google Scholar] [CrossRef]
- Prasad, A.S.G.; Bhaskara Rao, T.; Rambabu, D.; Basaveswara Rao, M.V.; Pal, M. Ultrasound Assisted Synthesis of Quinoline Derivatives in the Presence of SnCl2·2H2O as a Precatalyst in Water: Evaluation of their Antibacterial Activities. Mini-Rev. Med. Chem. 2018, 18, 895–903. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Luo, Y.; Wang, H.; Wei, H.; Guo, T.; Tan, H.; Yuan, L.; Zhang, X.B. A Novel Ratiometric and Reversible Fluorescence Probe with a Large Stokes Shift for Cu2+ Based on a New Clamp-On Unit. Anal. Chim. Acta 2019, 1065, 134–141. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Li, Y.; Guo, T.; Tang, Z.; Xie, W.; Zhao, G. Selective Synthesis of Pyrazolo[5,1-A]Isoquinolines via 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Lett. 2016, 57, 2257–2261. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Abuelizz, H.A.; EI Dib, R.; Marzouk, M. Antimicrobial Activity of New 2-Thioxo-benzo[g]quinazolin-4(3H)-one Derivatives. Med. Chem. 2017, 13, 85–92. [Google Scholar] [CrossRef]
- Mortier, J.; Frederick, R.; Ganef, C.; Remouchamps, C.; Talaga, P.; Pochet, L.; Wouters, J.; Piette, J.; Dejardin, E.; Masereel, B. Pyrazolo[4,3-c]Isoquinolines as Potential Inhibitors of NF-Kb Activation. Biochem. Pharmacol. 2010, 79, 1462–1472. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Luo, M.; Tang, Z.; Cao, C.; Deng, K. Synthesis of Isoquinolines Derivatives from o-Alkynyl Aldehydes. Chin. J. Org. Chem. 2016, 36, 2504–2509. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Al-Salahi, R.; Al-Asri, J.; Mortier, J.; Marzouk, M.; Ezzeldin, E.; Ali, A.A.; Khalil, M.G.; Wolber, G.; Ghabbour, H.A.; et al. Synthesis, Crystallographic Characterization, Molecular Docking and Biological Activity of Isoquinoline Derivatives. Chem. Cent. J. 2017, 11, 103. [Google Scholar] [CrossRef]
- Luo, C.; Ampomah-Wireko, M.; Wang, H.; Wu, C.; Wang, Q.; Zhang, H.; Cao, Y. Isoquinolines: Important Cores in Many Marketed and Clinical Drugs. Anti-Cancer Agents Med. Chem. 2021, 21, 811–824. [Google Scholar] [CrossRef]
- Luk, L.Y.P.; Bunn, S.; Liscombe, D.K.; Facchini, P.J.; Tanner, M.E. Mechanistic Studies on Norcoclaurine Synthase of Benzylisoquinoline Alkaloid Biosynthesis: An Enzymatic Pictet-Spengler Reaction. Biochemistry 2007, 46, 10153–10161. [Google Scholar] [CrossRef]
- Kametani, T.; Okubo, K.; Takano, S. Syntheses of 1-Substituted Isoquinoline and 1,4-Benzoxazepine derivatives by Pomeranz-Fritsch Reaction (Studies on the Syntheses of Heterocyclic Compounds CCCXX). Yakugaku Zasshi 1969, 89, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Chen, H.; Xu, Y.; Yu, H.; Yu, L.; Li, W.; Xie, Q.; Shao, L. Microwave-Assisted Synthesis of HydroxylContaining Isoquinolines by Metal-Free Radical Cyclization of Vinyl Isocyanides with Alcohols. Org. Biomol. Chem. 2017, 15, 10044–10052. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, D.S.; Gangwar, N.; Bhanage, B.M. Rapid and Atom Economic Synthesis of Isoquinolines and Isoquinolinones by C–H/N–N Activation Using a Homogeneous Recyclable Ruthenium Catalyst in PEG Media. Eur. J. Org. Chem. 2019, 18, 2919–2927. [Google Scholar] [CrossRef]
- Deshmukh, D.S.; Bhanage, B.M. Ruthenium-Catalyzed Annulation of N-Cbz Hydrazones via C–H/N–N Bond Activation for the Rapid Synthesis of Isoquinolines. Synthesis 2019, 51, 2506–2514. [Google Scholar] [CrossRef]
- Xu, X.; Feng, H.; Van der Eycken, E.V. Microwave-Assisted Palladium-Catalyzed Reductive Cyclization/Ring-Opening/Aromatization Cascade of Oxazolidines to Isoquinolines. Org. Lett. 2021, 23, 6578–6582. [Google Scholar] [CrossRef]
- Sharafian, S.; Hossaini, Z.; Rostami-Charati, F.; Kalilzadeh, M.A. Ultrasound-promoted Green Synthesis of pyrido[2,1-a]isoquinoline Derivatives and Studies on Their Antioxidant Activity. Comb. Chem. High Throughput Screen 2021, 24, 118–128. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z. Azaindoles: Suitable Ligands of Cytotoxic Transition Metal Complexes. J. Inorg. Biochem. 2019, 197, 110695. [Google Scholar] [CrossRef]
- Motati, D.R.; Amaradhi, R.; Ganesh, T. Azaindole Therapeutic Agents. Bioorganic Med. Chem. 2020, 28, 115830. [Google Scholar] [CrossRef]
- 2-Phenyl-3-Pyridinyl Substituted 7-Azaindoles as Inhibitors of Mitogen-Activated Protein Kinase. Expert Opin. Ther. Pat. 1998, 8, 1517–1523. [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The Importance of Indole and Azaindole Scaffold in the Development of Antitumor Agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Sreenivasachary, N.; Kroth, H.; Benderitter, P.; Hamel, A.; Varisco, Y.; Hickman, D.T.; Froestl, W.; Pfeifer, A.; Muhs, A. Discovery and Characterization of Novel Indole and 7-Azaindole Derivatives as Inhibitors of β-Amyloid-42 Aggregation for the Treatment of Alzheimer’s Disease. Bioorganic Med. Chem. Lett. 2017, 27, 1405–1411. [Google Scholar] [CrossRef]
- Liu, Y.A.; Jin, Q.; Zou, Y.; Ding, Q.; Yan, S.; Wang, Z.; Hao, X.; Nguyen, B.; Zhang, X.; Pan, J. Selective DYRK1A Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative GNF2133. J. Med. Chem. 2020, 63, 2958–2973. [Google Scholar] [CrossRef]
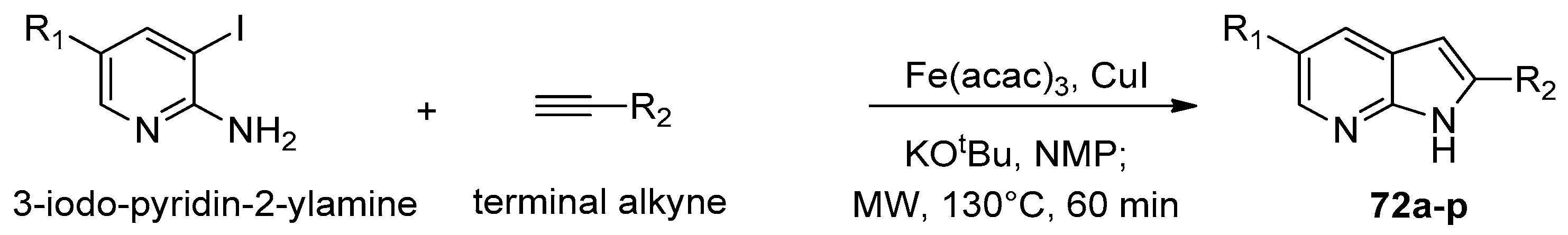
- Le, Y.; Yang, Z.; Chen, Y.; Chen, D.; Yan, L.; Wang, Z.; Ouyang, G. Microwave-Assisted Synthesis of 7-Azaindoles via Iron-Catalyzed Cyclization of an o-Haloaromatic Amine with Terminal Alkynes. RSC Adv. 2019, 9, 39684–39688. [Google Scholar] [CrossRef]
- Belasri, K.; Fülöp, F.; Szatmári, I. Solvent-Free C-3 Coupling of Azaindoles with Cyclic Imines. Molecules 2019, 24, 3578. [Google Scholar] [CrossRef]
- Savitha, B.; Reddy, E.K.; Parthasarathi, D.; Pakkath, R.; Karuvalam, R.P.; Ananda Kumar, C.; Haridas, K.; Syed Ali Padusha, M.; Sajith, A.M. A Highly Efficient Precatalytic System (Xphos-Pdg2) for the Suzuki–Miyaura Cross-Coupling of 7-Chloro-1 H-Pyrrolo [2, 3-C] Pyridine Employing Low Catalyst Loading. Mol. Divers. 2019, 23, 697–707. [Google Scholar] [CrossRef]
- Pavithra, E.; Kannadasan, S.; Shanmugam, P. Synthesis of 5-Aryl-3, 3′-Bis-Indolyl and Bis-7-Aza-Indolyl Methanone Derivatives from 5-Bromo-7-Azaindoles via Sequential Methylenation Using Microwave Irradiation, CAN Oxidation, and Suzuki Coupling Reactions. RSC Adv. 2022, 12, 30712–30721. [Google Scholar] [CrossRef]
- Kour, J.; Khajuria, P.; Sharma, A.; Sawant, S.D. TBAX/Oxone-Mediated Halogenation of Pyrazoles and Other Heterocycles: An Entry to Important Cross-Coupling Reactions. Chem.–Asian J. 2022, 17, e202200778. [Google Scholar] [CrossRef]
- Venkateshwarlu, R.; Singh, S.N.; Siddaiah, V.; Ramamohan, H.; Dandela, R.; Pal, M. Ultrasound Assisted One-Pot Synthesis of 1,2-Diaryl Azaindoles via Pd/C-Cu Catalysis: Identification of Potential Cytotoxic Agents. Tetrahedron Lett. 2019, 60, 151326. [Google Scholar] [CrossRef]
- Popov, L.; Amarandi, R.M.; Mangalagiu, I.I.; Mangalagiu, V.; Danac, R. Synthesis, Molecular Modelling and Anticancer Evaluation of New Pyrrolo[[1,2-b]] Pyridazine and Pyrrolo[2,1-A]Phthalazine Derivatives. J. Enzym. Inhib. Med. Chem. 2019, 34, 230–243. [Google Scholar]
- Xiang, H.Y.; Chen, J.Y.; Huan, X.J.; Chen, Y.; Gao, Z.B.; Ding, J.; Miao, Z.H.; Yang, C.H. Identification of 2-Substituted Pyrrolo[[1,2-b]]Pyridazine Derivatives as New PARP-1 Inhibitors. Bioorganic Med. Chem. Lett. 2021, 31, 127710. [Google Scholar] [CrossRef]
- Moldoveanu, c.; Amariucai-Mantu, D.; Mangalagiu, V.; Antoci, V.; Maftei, D.; Mangalagiu, I.I.; Zbancioc, C. Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules 2019, 24, 3760. [Google Scholar] [CrossRef] [PubMed]
- Streit, A.D.; Zoll, A.J.; Hoang, G.L.; Ellman, J.A. Annulation of Hydrazones and Alkynes via Rhodium(III)-Catalyzed Dual C−H Activation: Synthesis of Pyrrolopyridazines and Azolopyridazines. Org. Lett. 2020, 22, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Garella, D.; Calcio Gaudino, E.; Lévêque, J.M. Microwaves–Ultrasound Coupling: A tool for Process Intensification in Organic Synthesis. Chim. Oggi-Chem. Today 2008, 26, 39–41. [Google Scholar]
- Maeda, M.; Amemiya, H. Chemical Effects under Simultaneous Irradiation by Microwaves and Ultrasound. New J. Chem. 1995, 19, 1023–1028. [Google Scholar]
- Penga, Y.; Song, G. Simultaneous Microwave and Ultrasound Irradiation: A Rapid Synthesis of Hydrazides. Green Chem. 2001, 3, 302–304. [Google Scholar] [CrossRef]
- Karkare, Y.Y.; Rathod, W.R.; Sathe, V.S.; Chavan, A.R. Combined Ultrasound-Microwave Assisted Synthesis of Aripiprazole: Process Optimization Using RSM-ANN. Chem. Eng. Process. Process Intensif. 2023, 183, 109250. [Google Scholar] [CrossRef]
- Mokariya, J.A.; Kalola, A.G.; Prasad, P. Simultaneous Ultrasound- And Microwave-Assisted One-Pot ‘Click’ Synthesis of 3-Formyl-Indole Clubbed 1,2,3-Triazole Derivatives and Their Biological Evaluation. Mol. Divers. 2022, 26, 963–979. [Google Scholar] [CrossRef]

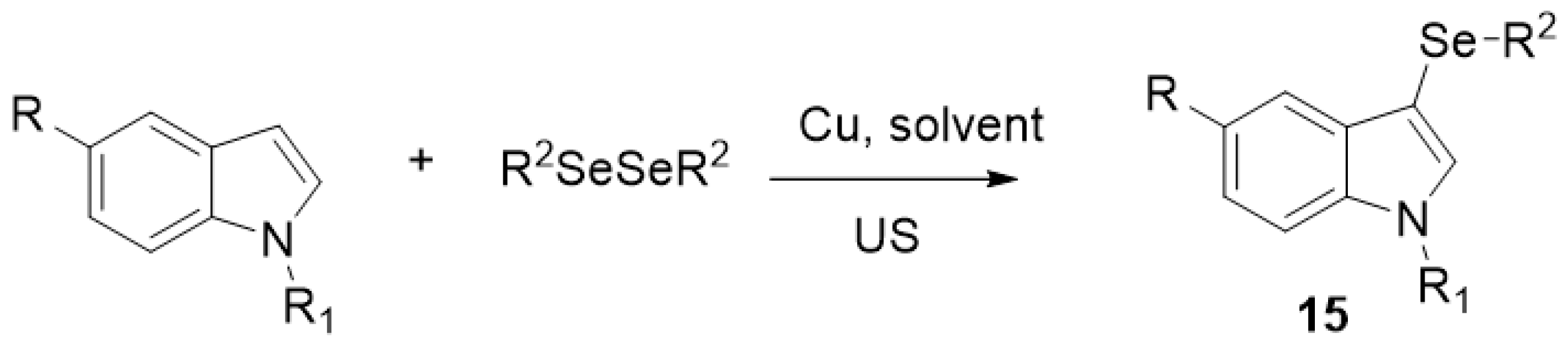

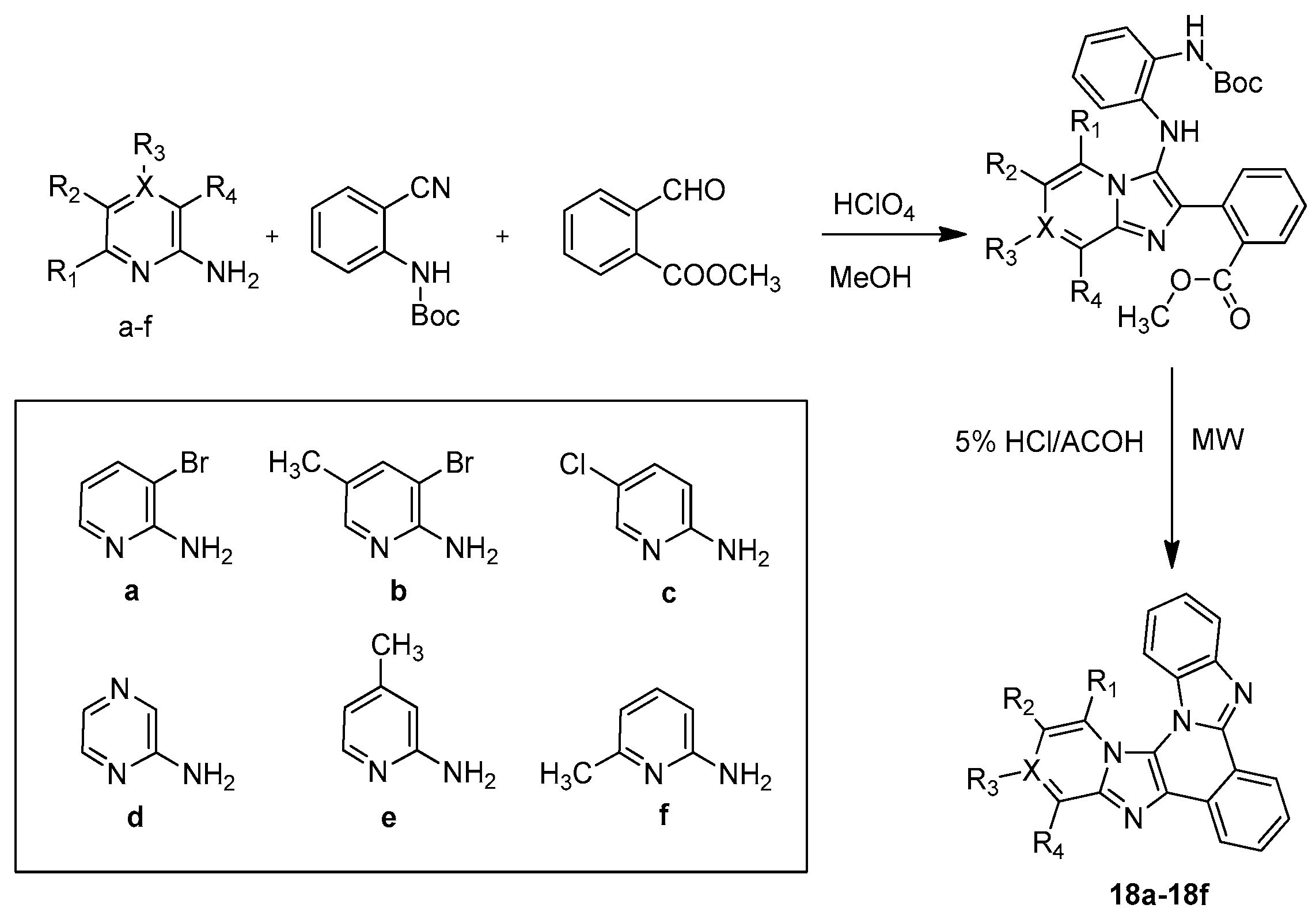

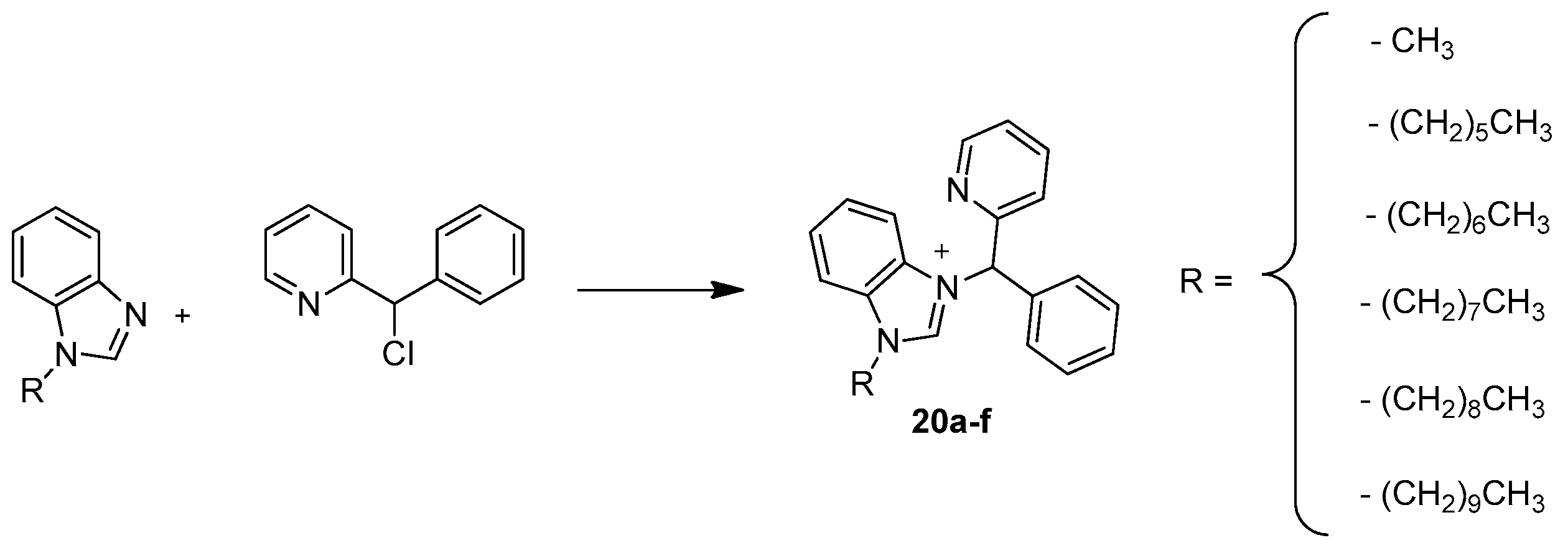


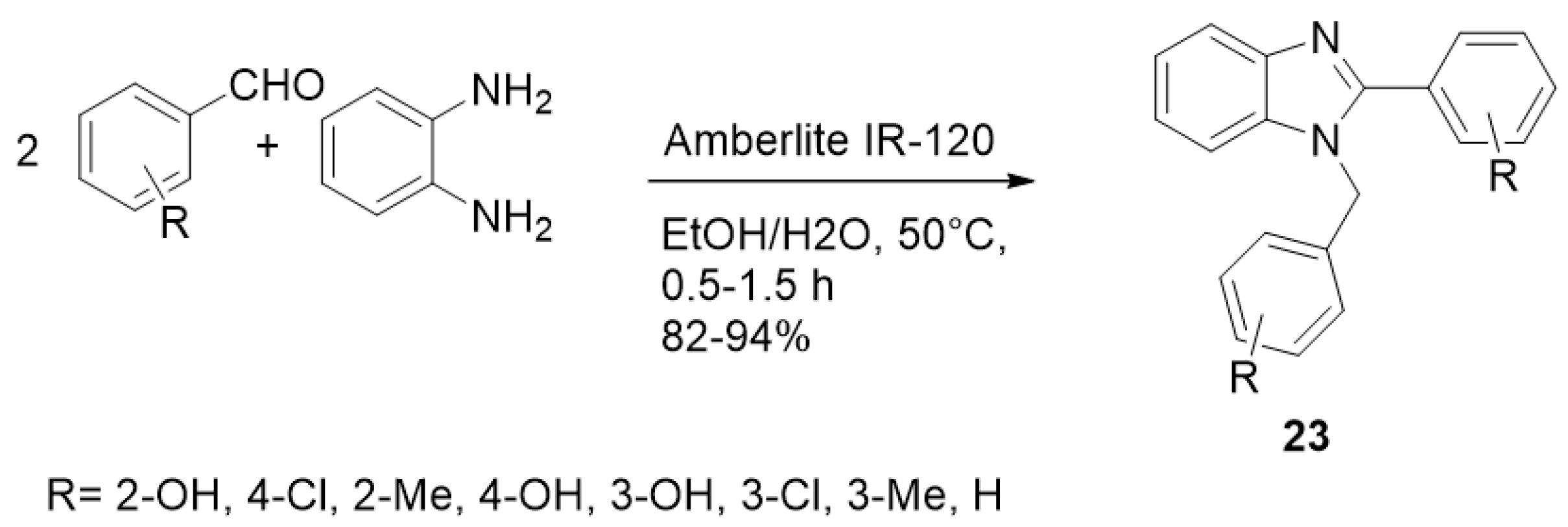
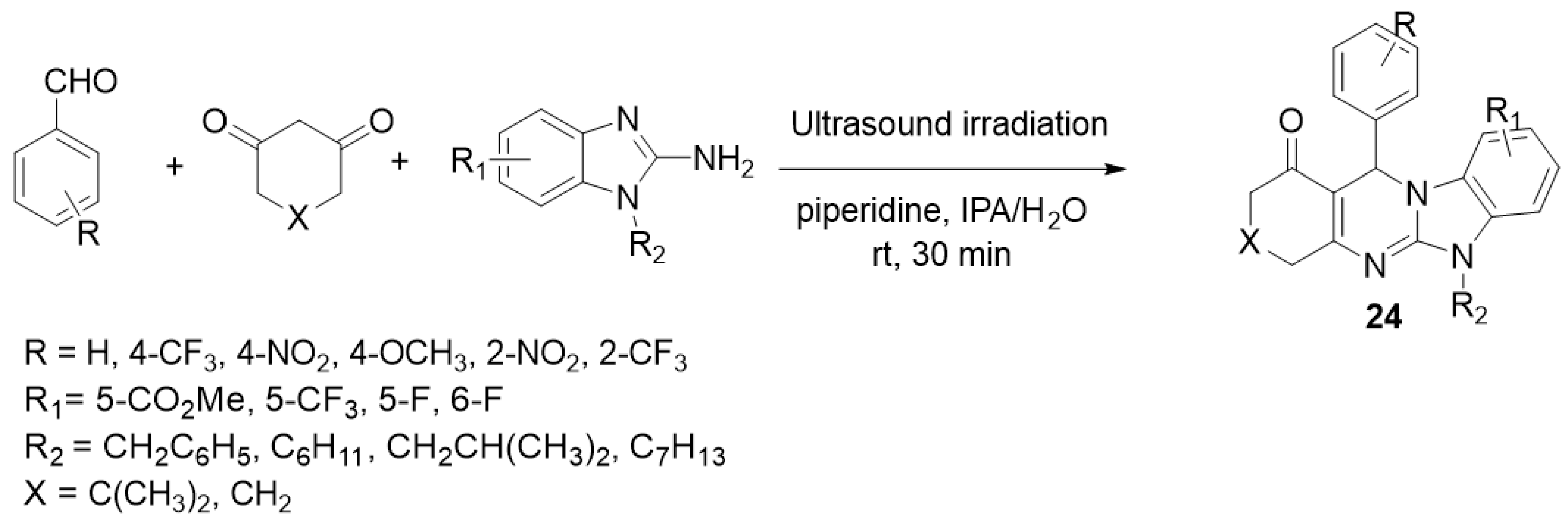
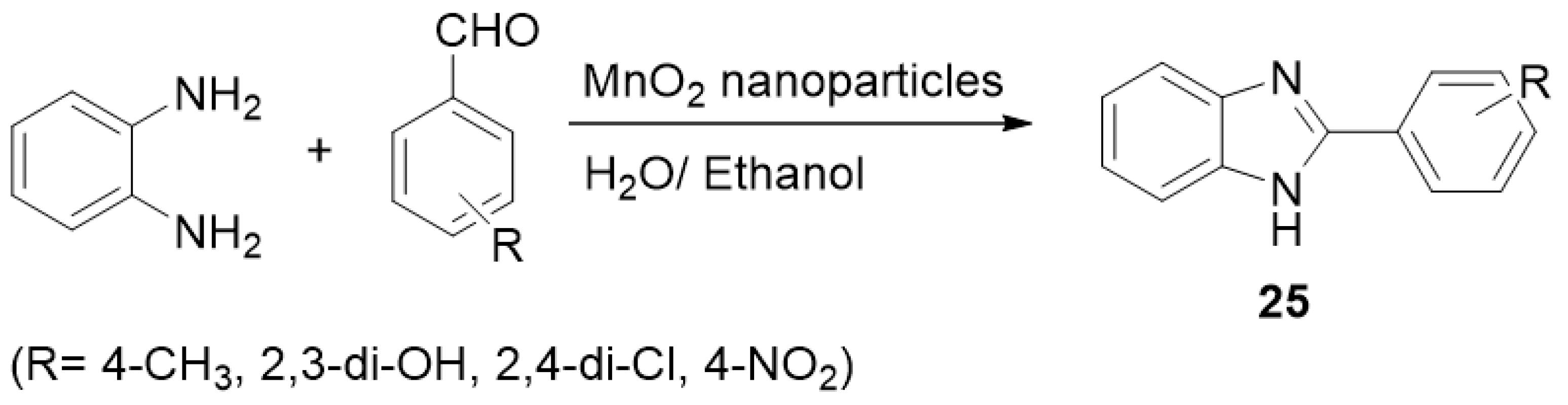
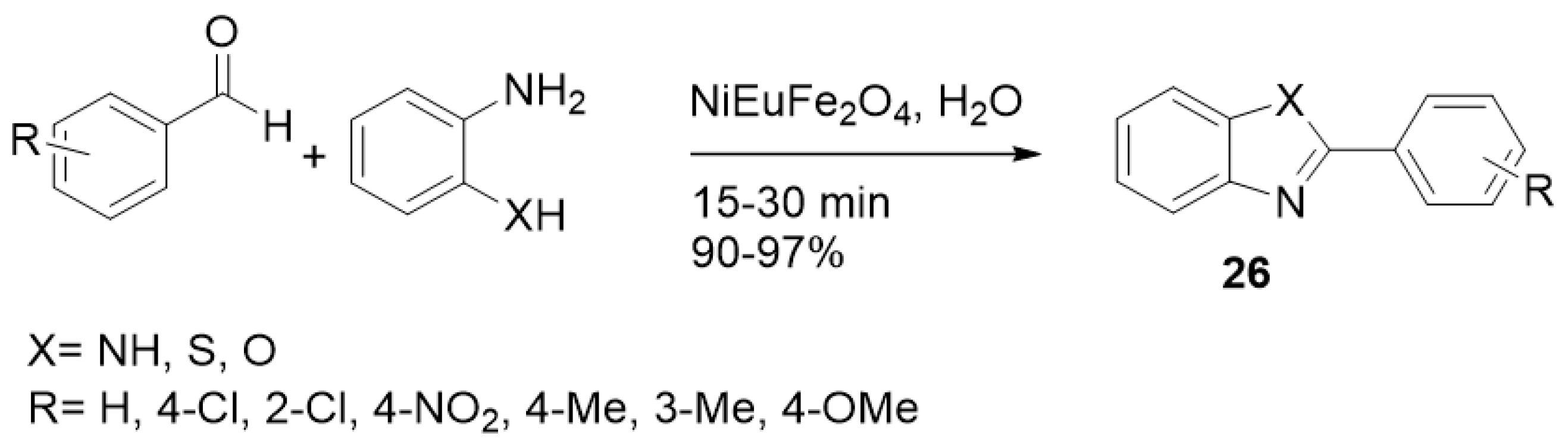
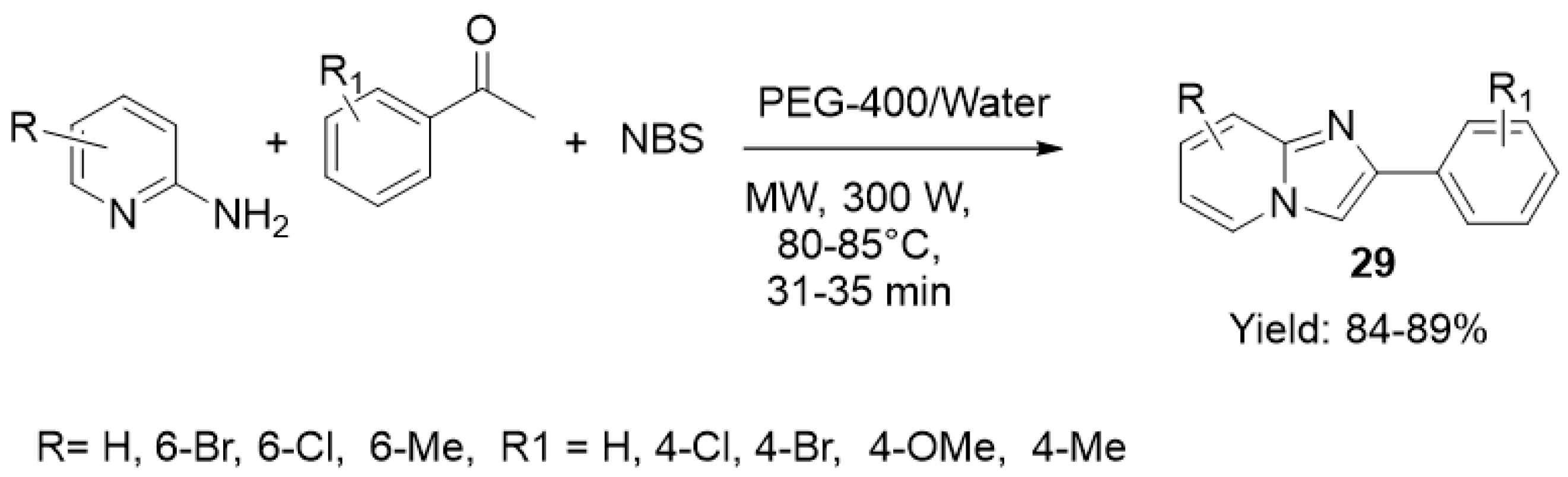
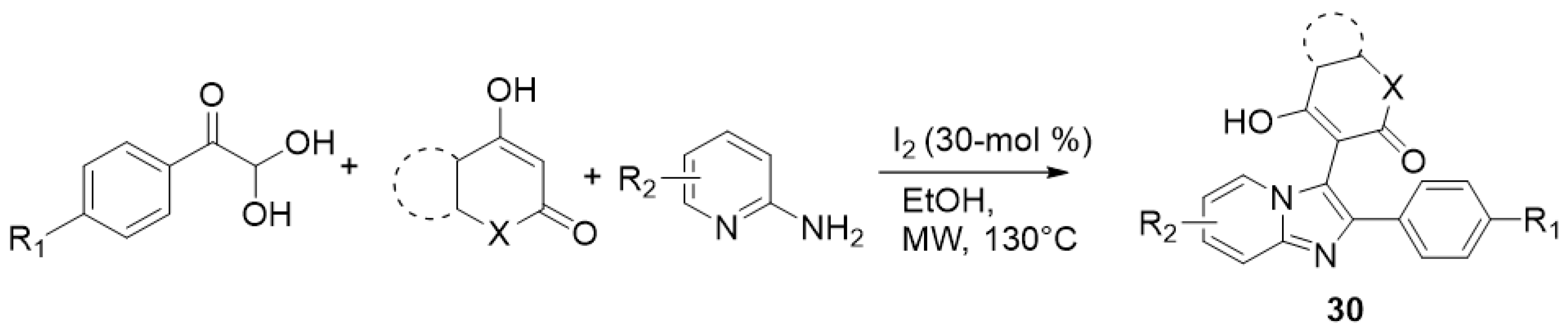
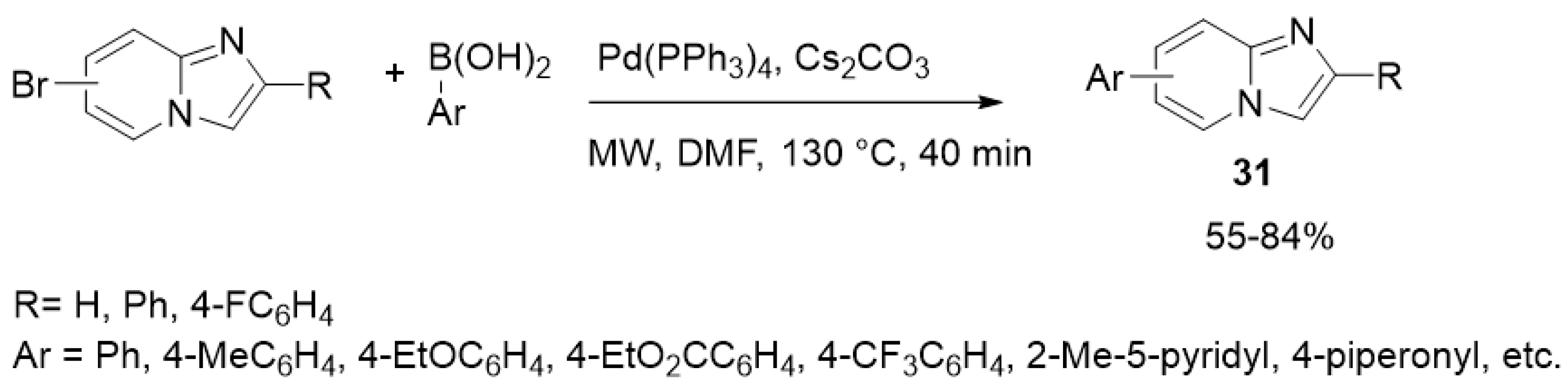


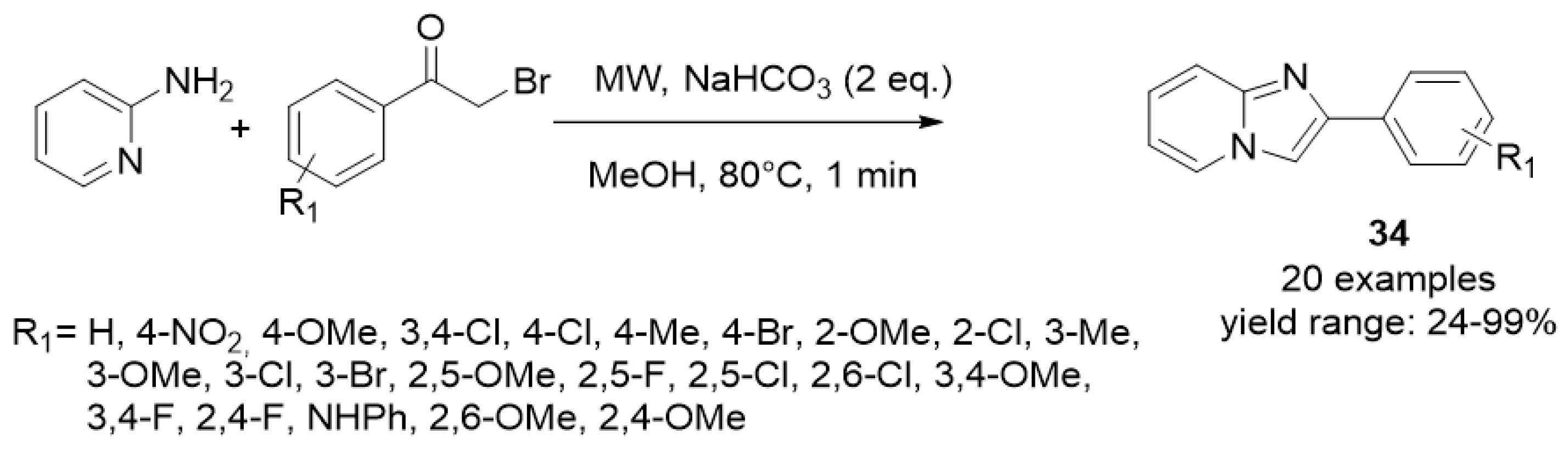




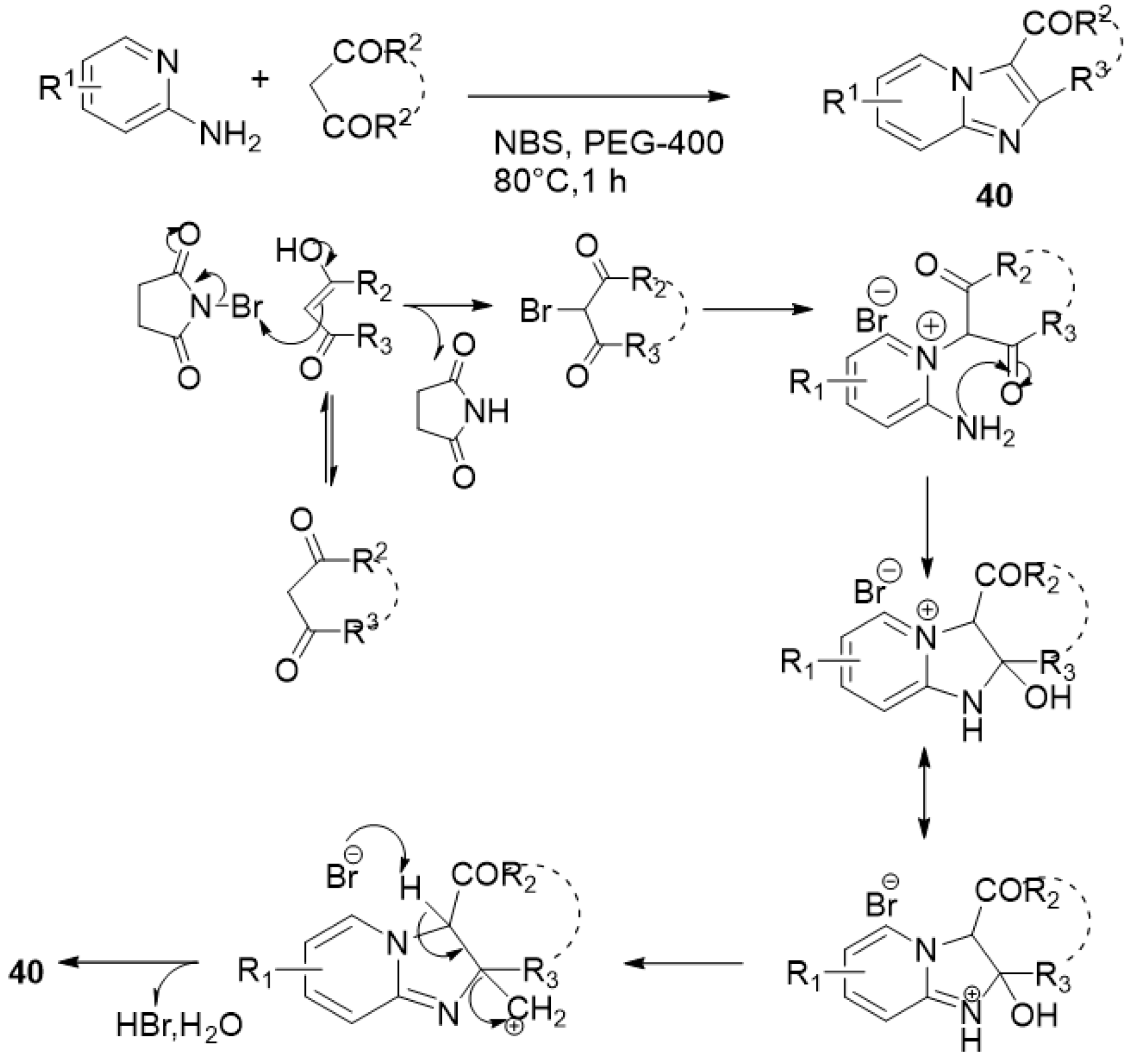




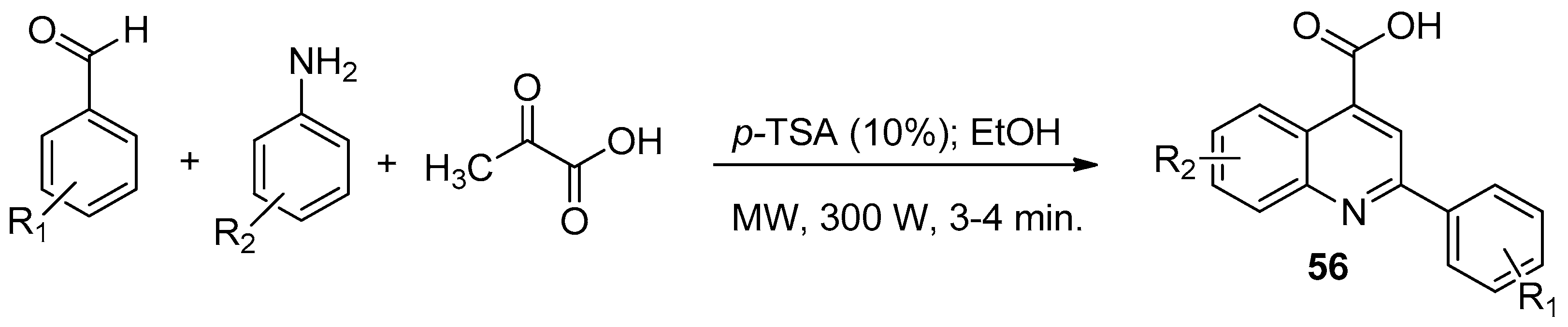

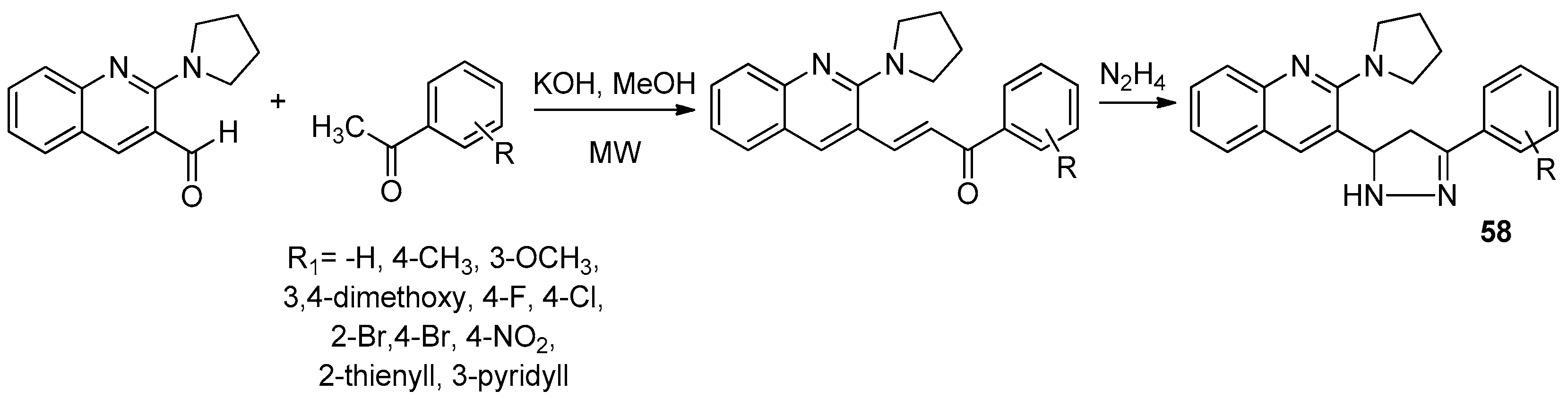




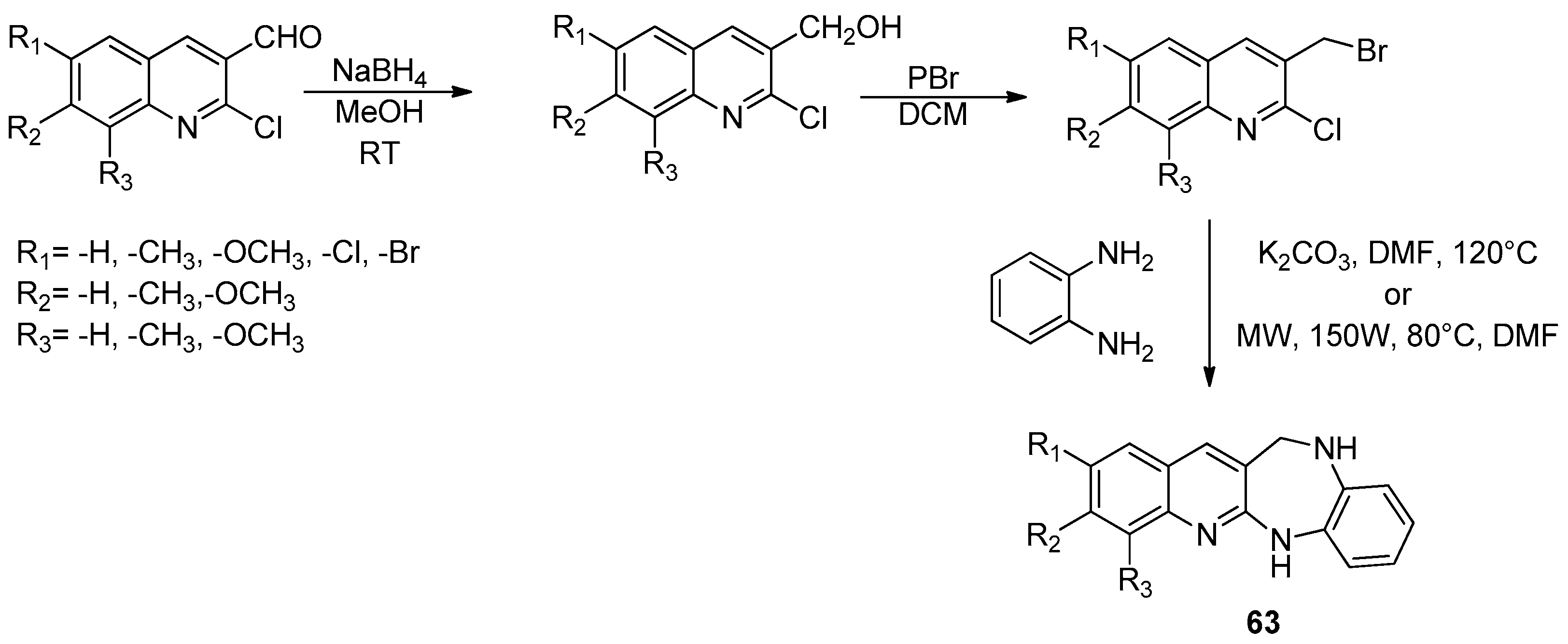


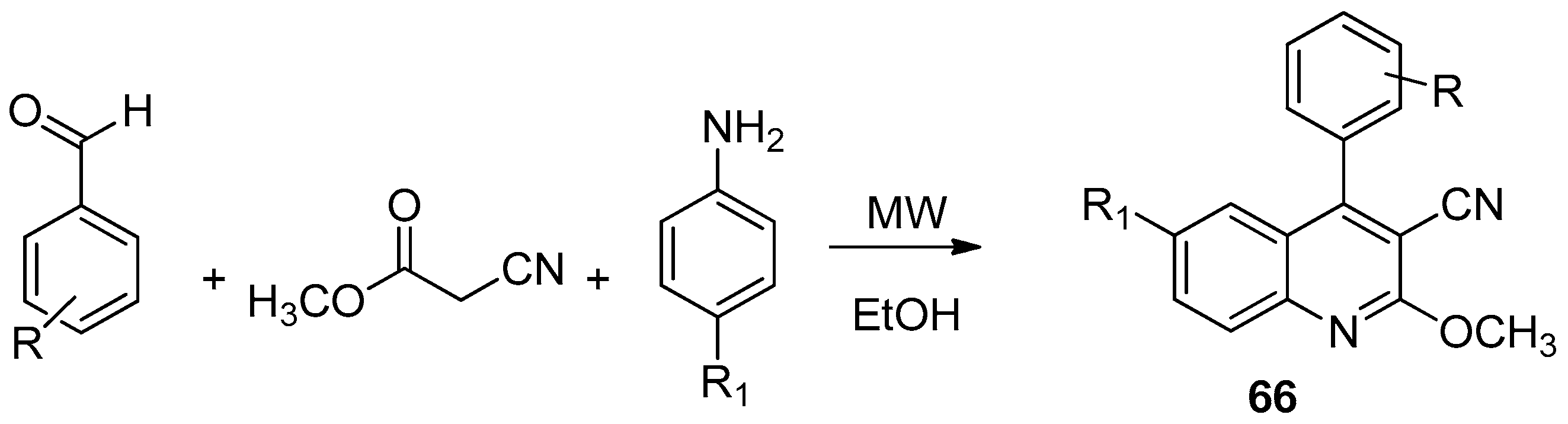
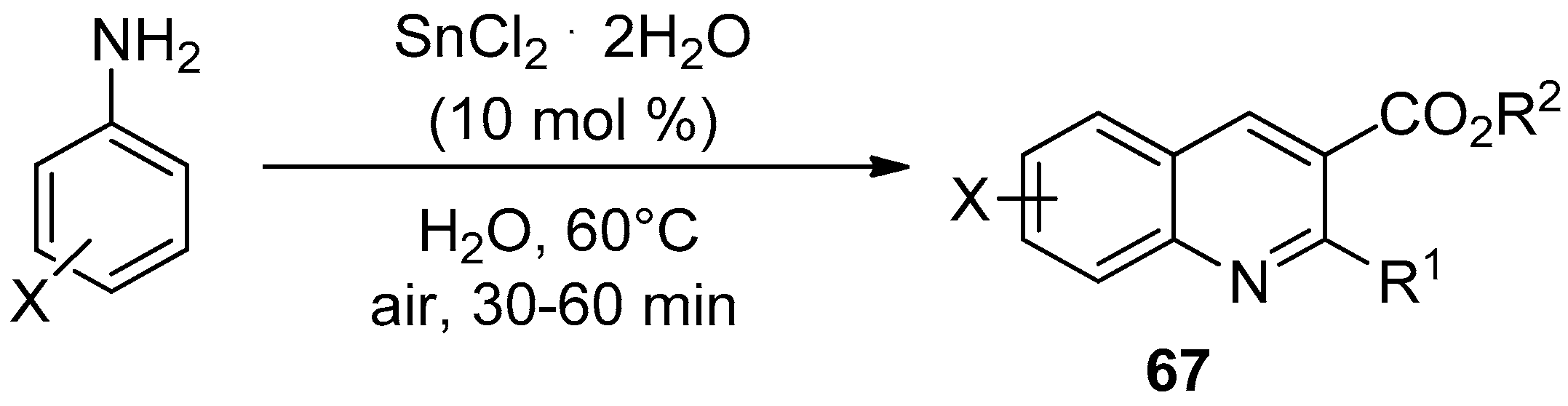


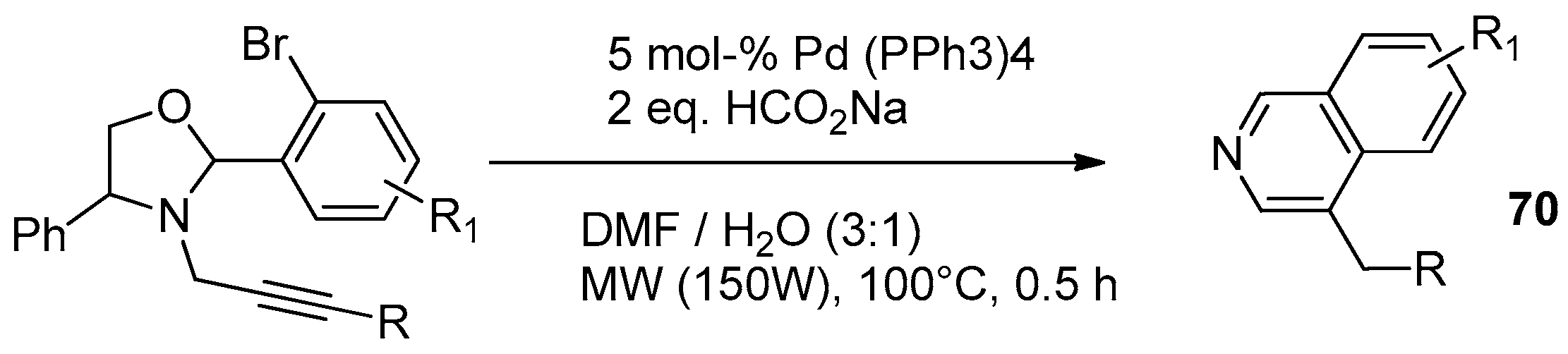

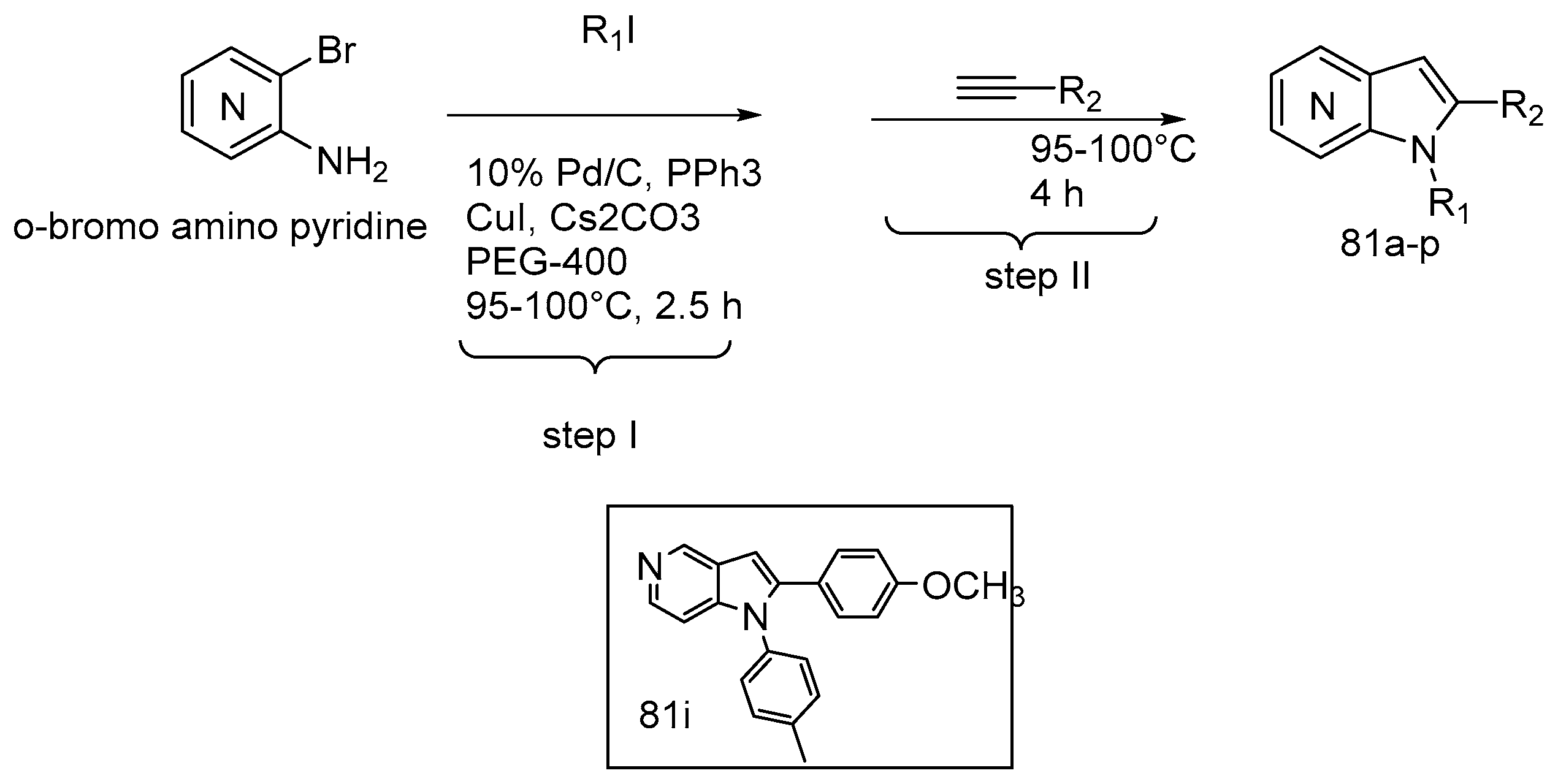

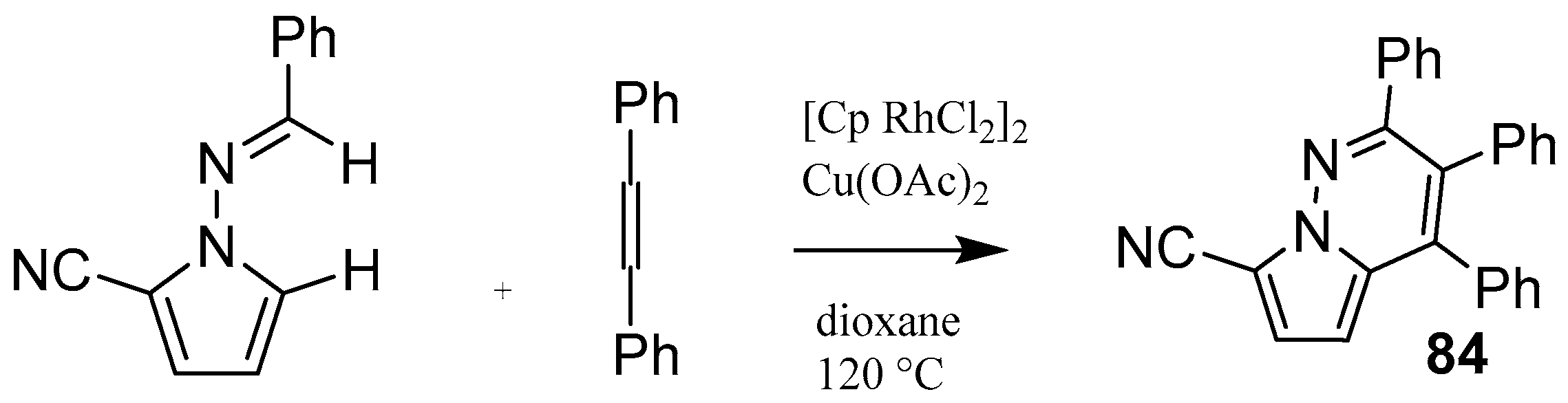
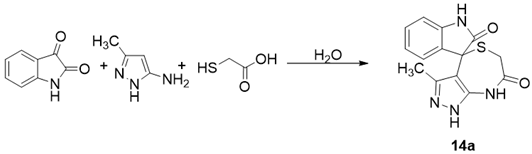 | ||||
|---|---|---|---|---|
| Solvent | Conditions | Catalyst | Time (Min) | Yield a |
| H2O | US radiation | AcOH | 30 | 72 |
| H2O | US radiation | p-TSA | 30 | 75 |
| H2O | US radiation | InCl3 | 30 | 74 |
| H2O | US radiation | CTABr | 30 | 78 |
| H2O | US radiation | - | 20 | 92 |
| Hexane | US radiation | - | 60 | 57 |
| DCM | US radiation | - | 60 | 48 |
| DMF | US radiation | - | 60 | 65 |
| Ethanol | US radiation | - | 60 | 69 |
| H2O | Stirring | - | 20 | No reaction |
| H2O | Stirring | - | 60 | Mixture of products |
| H2O | Reflux | - | 20 | Traces |
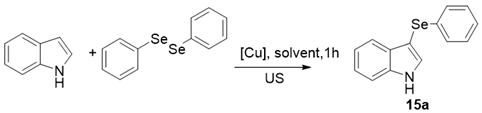 | ||
|---|---|---|
| Catalyst (Mol %) | Solvent | 15a Conv. % a |
| CuO NPs (10) | DMSO | - |
| CuBr (10) | DMSO | 18 |
| CuCl (10) | DMSO | 34 |
| CuI (10) | DMSO | 82 |
| CuI (15) | DMSO | 75 |
| CuI (20) | DMSO | 97 (69) b |
| CuI (20) | DMSO | 81 c |
| CuI (20) | H2O | 1.5 |
| CuI (20) | Glycerol | 1 |
| CuI (20) | DMF | 30 |
| CuI (20) | Toluene | No Reaction |
| CuI (20) | THF | No Reaction |
| FeCl3 (20) | DMSO | 20 |
| NiCl2·6H2O | DMSO | 6 |
| Catalyst | Solvent | Conditions | Time (Min) | Yield (%) |
|---|---|---|---|---|
| Nano-CeO2 | H2O | r.t | 20–30 | 82–98 |
| TSAMNP a | H2O | Reflux | 3–10 h | 84–97 |
| NiEuFe2O4 | H2O | Reflux | 45–60 | 80–87 |
| NiEuFe2O4 | H2O | US | 15–30 | 90–97 |
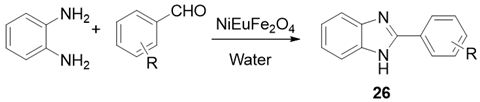 | ||||
|---|---|---|---|---|
| Conventional Method | Sonication Method | |||
| R | Time (Min) | Isolated Yield % | Time (Min) | Isolated Yield % |
| H | 50 | 84 | 20 | 95 |
| 4-Cl | 45 | 87 | 15 | 97 |
| 2-Cl | 60 | 81 | 30 | 91 |
| 4-NO2 | 50 | 86 | 15 | 96 |
| 4-CH3 | 50 | 82 | 20 | 93 |
| 3-CH3 | 60 | 80 | 30 | 90 |
| 4-OCH3 | 55 | 82 | 20 | 93 |
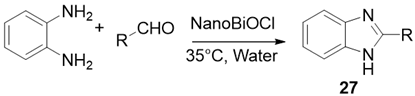 | ||
|---|---|---|
| R | Time (Min) | Yield % |
| C6H5 | 12 | 88 |
| 4-NO2C6H4 | 8 | 93 |
| 3-NO2C6H4 | 5 | 91 |
| 2-NO2C6H4 | 10 | 93 |
| 4-CH3OC6H4 | 15 | 89 |
| 4-ClC6H4 | 7 | 95 |
| 4-CNC6H4 | 7 | 93 |
| 4-(Me2N)C6H4 | 15 | 94 |
| 4-CH3C6H4 | 12 | 70 |
| 2-Pyridyl | 15 | 67 |
| 2-Furyl | 17 | 60 |
| 4-FC6H4 | 17 | 90 |
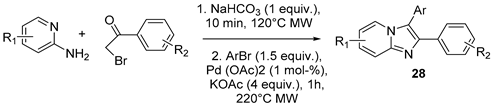 | ||
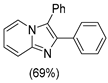 |  |  |
 |  |  |
 |  |  |
 |  |  |
 | 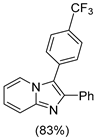 | 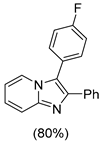 |
 | 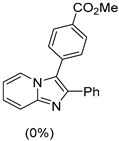 | 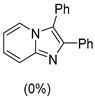 |
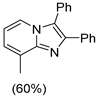 | 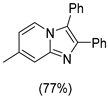 | 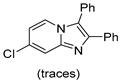 |
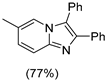 | 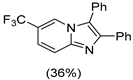 | 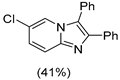 |
 | ||
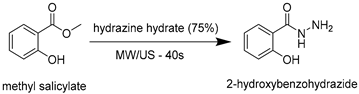 | ||
|---|---|---|
| Conditions | Time (Min) | Yield (%) |
| Reflux | 540 | 73 |
| US (50 W) + reflux | 90 | 79 |
| MW (200 W) | 18 | 80 |
| US (50 W) + MW (200 W) | 0.67 (40 s) | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frecentese, F.; Sodano, F.; Corvino, A.; Schiano, M.E.; Magli, E.; Albrizio, S.; Sparaco, R.; Andreozzi, G.; Nieddu, M.; Rimoli, M.G. The Application of Microwaves, Ultrasounds, and Their Combination in the Synthesis of Nitrogen-Containing Bicyclic Heterocycles. Int. J. Mol. Sci. 2023, 24, 10722. https://doi.org/10.3390/ijms241310722
Frecentese F, Sodano F, Corvino A, Schiano ME, Magli E, Albrizio S, Sparaco R, Andreozzi G, Nieddu M, Rimoli MG. The Application of Microwaves, Ultrasounds, and Their Combination in the Synthesis of Nitrogen-Containing Bicyclic Heterocycles. International Journal of Molecular Sciences. 2023; 24(13):10722. https://doi.org/10.3390/ijms241310722
Chicago/Turabian StyleFrecentese, Francesco, Federica Sodano, Angela Corvino, Marica Erminia Schiano, Elisa Magli, Stefania Albrizio, Rosa Sparaco, Giorgia Andreozzi, Maria Nieddu, and Maria Grazia Rimoli. 2023. "The Application of Microwaves, Ultrasounds, and Their Combination in the Synthesis of Nitrogen-Containing Bicyclic Heterocycles" International Journal of Molecular Sciences 24, no. 13: 10722. https://doi.org/10.3390/ijms241310722
APA StyleFrecentese, F., Sodano, F., Corvino, A., Schiano, M. E., Magli, E., Albrizio, S., Sparaco, R., Andreozzi, G., Nieddu, M., & Rimoli, M. G. (2023). The Application of Microwaves, Ultrasounds, and Their Combination in the Synthesis of Nitrogen-Containing Bicyclic Heterocycles. International Journal of Molecular Sciences, 24(13), 10722. https://doi.org/10.3390/ijms241310722













