Markers of Oxidative Stress, Inflammation and Endothelial Function following High-Dose Intravenous Iron in Patients with Non-Dialysis-Dependent Chronic Kidney Disease—A Pooled Analysis
Abstract
1. Introduction
2. Results
2.1. Baseline Data
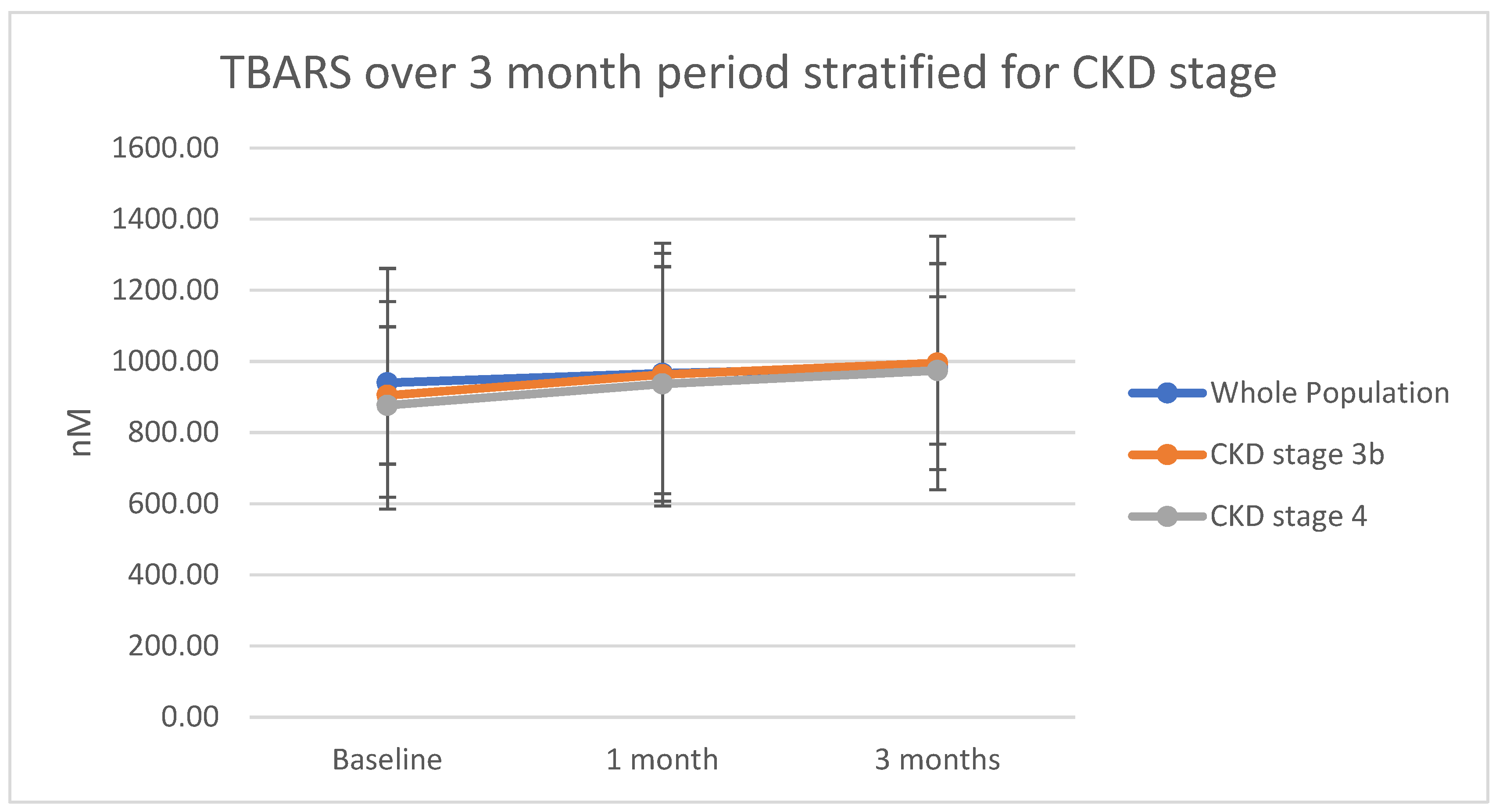
2.2. Oxidative Stress: Thiobarbituric Acid Reactive Substances (TBARS)
2.3. Inflammation: C-reactive Protein, Interleukin-6 and Interleukin-10
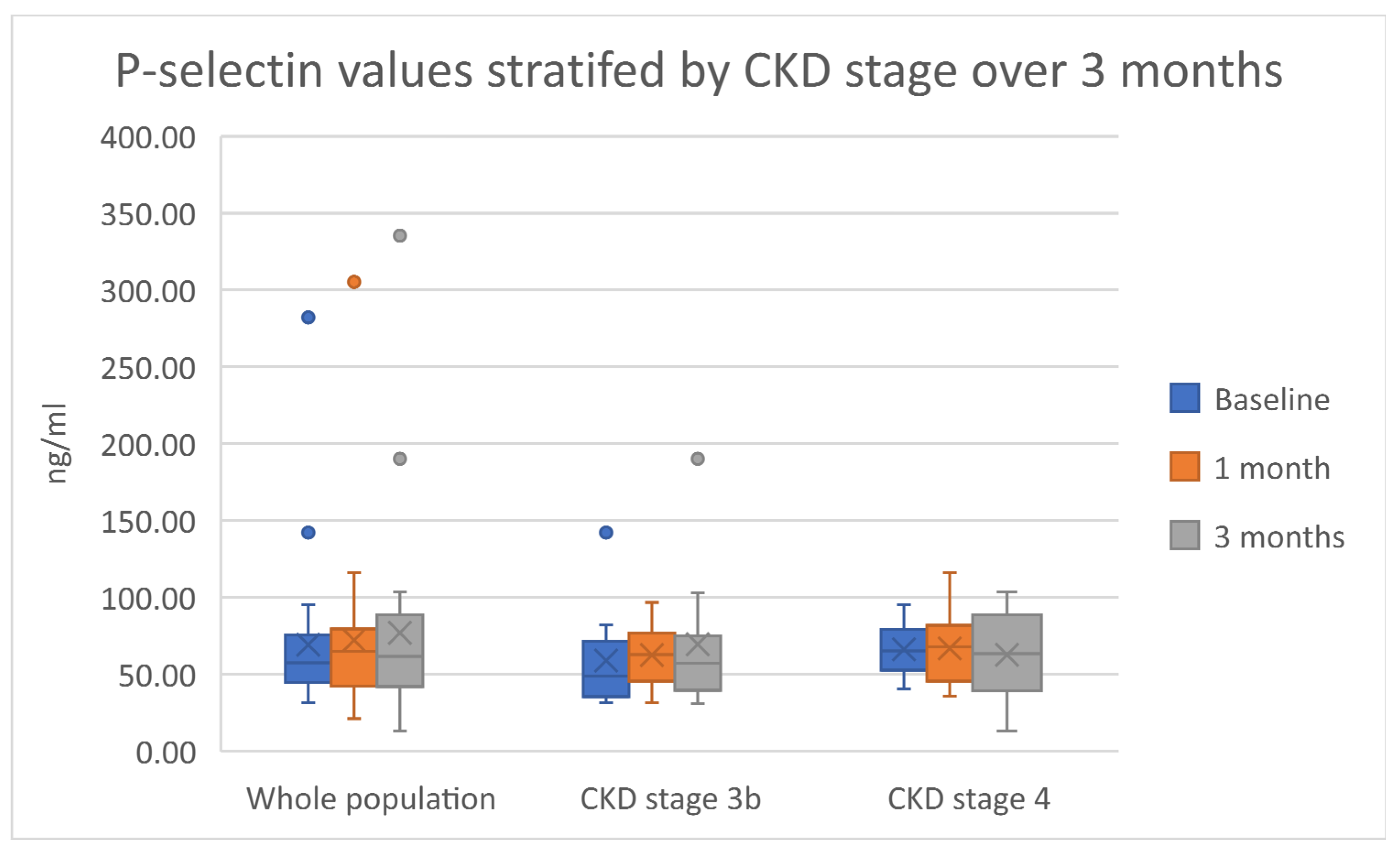
2.4. Endothelial Function and Vascular Response: E-selectin, P-selectin and Pulse Wave Velocity
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2018, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M.; Stratakis, D.; Fischer, R. Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol. Dial. Transplant. 2001, 16, 1863–1869. [Google Scholar] [CrossRef]
- Ward, R.A.; McLeish, K.R. Hemodialysis with Cellulose Membranes Primes the Neutrophil Oxidative Burst. Artif. Organs 1995, 19, 801–807. [Google Scholar] [CrossRef]
- Fishbane, S.; Spinowitz, B. Update on Anemia in ESRD and Earlier Stages of CKD: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 423–435. [Google Scholar] [CrossRef]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef]
- Baccin, A.C.; Lazzaretti, L.L.; Brandao, V.D.M.; Manfredini, V.; Peralba, M.C.R.; Benfato, M.S. Oxidative stress in older patients with iron deficiency anaemia. J. Nutr. Health Aging 2009, 13, 666–670. [Google Scholar] [CrossRef]
- Bhandari, S.; Pereira, D.I.A.; Chappell, H.F.; Drakesmith, H. Intravenous Irons: From Basic Science to Clinical Practice. Pharmaceuticals 2018, 11, 82. [Google Scholar] [CrossRef]
- Kassianides, X.; Hazara, A.M.; Bhandari, S. Improving the safety of intravenous iron treatments for patients with chronic kidney disease. Expert Opin. Drug Saf. 2021, 20, 23–35. [Google Scholar] [CrossRef]
- Del Vecchio, L.; Ekart, R.; Ferro, C.J.; Malyszko, J.; Mark, P.B.; Ortiz, A.; Sarafidis, P.; Valdivielso, J.M.; Mallamaci, F. Intravenous iron therapy and the cardiovascular system: Risks and benefits. Clin. Kidney J. 2021, 14, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Kohli, H.S.; Khullar, M.; Gupta, K.L.; Jha, V.; Sakhuja, V. Lipid Peroxidation Products Formation with Various Intravenous Iron Preparations in Chronic Kidney Disease. Ren. Fail. 2009, 31, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S. Impact of intravenous iron on cardiac and skeletal oxidative stress and cardiac mitochondrial function in experimental uraemia chronic kidney disease. Front. Biosci. 2021, 26, 442–464. [Google Scholar] [CrossRef]
- Bhandari, S.; Allgar, V.; Lamplugh, A.; Macdougall, I.; Kalra, P.A. A multicentre prospective double blinded randomised controlled trial of intravenous iron (ferric Derisomaltose (FDI)) in Iron deficient but not anaemic patients with chronic kidney disease on functional status. BMC Nephrol. 2021, 22, 115. [Google Scholar] [CrossRef] [PubMed]
- Kassianides, X.; Gordon, A.; Sturmey, R.; Bhandari, S. The comparative effects of intravenous iron on oxidative stress and inflammation in patients with chronic kidney disease and iron deficiency: A randomized controlled pilot study. Kidney Res. Clin. Pract. 2021, 40, 89–98. [Google Scholar] [CrossRef]
- Jahn, M.R.; Andreasen, H.B.; Fütterer, S.; Nawroth, T.; Schünemann, V.; Kolb, U.; Hofmeister, W.; Muñoz, M.; Bock, K.; Meldal, M.; et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011, 78, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Martin-Malo, A.; Merino, A.; Carracedo, J.; Alvarez-Lara, M.A.; Ojeda, R.; Soriano, S.; Crespo, R.; Ramirez, R.; Aljama, P. Effects of intravenous iron on mononuclear cells during the haemodialysis session. Nephrol. Dial. Transplant. 2012, 27, 2465–2471. [Google Scholar] [CrossRef]
- Malindretos, P.; Sarafidis, P.A.; Rudenco, I.; Raptis, V.; Makedou, K.; Makedou, A.; Grekas, D.M. Slow Intravenous Iron Administration Does Not Aggravate Oxidative Stress and Inflammatory Biomarkers during Hemodialysis: A Comparative Study between Iron Sucrose and Iron Dextran. Am. J. Nephrol. 2007, 27, 572–579. [Google Scholar] [CrossRef]
- De Souza, L.V.; Hoffmann, A.; Fischer, C.; Petzer, V.; Asshoff, M.; Theurl, I.; Tymoszuk, P.; Seifert, M.; Brigo, N.; Hilbe, R.; et al. Comparative analysis of oral and intravenous iron therapy in rat models of inflammatory anemia and iron deficiency. Haematologica 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Fukao, W.; Hasuike, Y.; Yamakawa, T.; Toyoda, K.; Aichi, M.; Masachika, S.; Kantou, M.; Takahishi, S.; Iwasaki, T.; Yahiro, M.; et al. Oral Versus Intravenous Iron Supplementation for the Treatment of Iron Deficiency Anemia in Patients on Maintenance Hemodialysis—Effect on Fibroblast Growth Factor-23 Metabolism. J. Ren. Nutr. 2018, 28, 270–277. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.-L.; Hung, S.-C.; Lee, T.-S.; Tarng, D.-C. Iron Sucrose Accelerates Early Atherogenesis by Increasing Superoxide Production and Upregulating Adhesion Molecules in CKD. J. Am. Soc. Nephrol. 2014, 25, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.; Witko-Sarsat, V.; Massy, Z.; Descamps-Latscha, B.; Guerin, A.P.; Marchais, S.J.; Gausson, V.; London, G.M. Iron Therapy, Advanced Oxidation Protein Products, and Carotid Artery Intima-Media Thickness in End-Stage Renal Disease. Circulation 2002, 106, 2212–2217. [Google Scholar] [CrossRef]
- Kulnigg-Dabsch, S.; Schmid, W.; Howaldt, S.; Stein, J.; Mickisch, O.; Waldhör, T.; Evstatiev, R.; Kamali, H.; Volf, I.; Gasche, C. Iron Deficiency Generates Secondary Thrombocytosis and Platelet Activation in IBD: The Randomized, Controlled ThromboVIT Trial. Inflamm. Bowel Dis. 2013, 19, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Elstrott, B.K.; Lakshmanan, H.H.; Melrose, A.R.; Jordan, K.R.; Martens, K.L.; Yang, C.; Peterson, D.F.; McMurry, H.S.; Lavasseur, C.; Lo, J.O.; et al. Platelet reactivity and platelet count in women with iron deficiency treated with intravenous iron. Res. Pract. Thromb. Haemost. 2022, 6, e12692. [Google Scholar] [CrossRef]
- Baaten, C.C.F.M.J.; Sternkopf, M.; Henning, T.; Marx, N.; Jankowski, J.; Noels, H. Platelet Function in CKD: A Systematic Review and Meta-Analysis. J. Am. Soc. Nephrol. 2021, 32, 1583–1598. [Google Scholar] [CrossRef]
- van Bladel, E.R.; de Jager, R.L.; Walter, D.; Cornelissen, L.; Gaillard, C.A.; Boven, L.A.; Roest, M.; Fijnheer, R. Platelets of patients with chronic kidney disease demonstrate deficient platelet reactivity in vitro. BMC Nephrol. 2012, 13, 127. [Google Scholar] [CrossRef]
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017, 18, 345. [Google Scholar] [CrossRef]
- Kalra, P.A.; Bhandari, S.; Spyridon, M.; Davison, R.; Lawman, S.; Mikhail, A.; Reaich, D.; Pritchard, N.; McCafferty, K.; Moore, J. NIMO-CKD-UK: A real-world, observational study of iron isomaltoside in patients with iron deficiency anaemia and chronic kidney disease. BMC Nephrol. 2020, 21, 539. [Google Scholar] [CrossRef]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.; Murray, H.; Tomson, C.R.; Wheeler, D.C.; et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N. Engl. J. Med. 2019, 380, 447–458. [Google Scholar] [CrossRef]
- Bhandari, S.; Kalra, P.A.; Berkowitz, M.; Belo, D.; Thomsen, L.L.; Wolf, M. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: The FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol. Dial. Transplant. 2020, 36, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kassianides, X.; Bhandari, S. Hypophosphataemia, fibroblast growth factor 23 and third-generation intravenous iron compounds: A narrative review. Drugs Context 2021, 10, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, S.; Piednoir, P.; Couffignal, C.; Rineau, E.; Dufour, G.; Lefebvre, T.; Puy, H.; Duval, X.; Driss, F.; Schilte, C. Does IV Iron Induce Plasma Oxidative Stress in Critically Ill Patients? A Comparison With Healthy Volunteers. Crit. Care Med. 2016, 44, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peiró, M.; Martín-Ontiyuelo, C.; Rodó-Pi, A.; Piccari, L.; Admetlló, M.; Durán, X.; Rodríguez-Chiaradía, D.A.; Barreiro, E. Iron Replacement and Redox Balance in Non-Anemic and Mildly Anemic Iron Deficiency COPD Patients: Insights from a Clinical Trial. Biomedicines 2021, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Allgar, V.; Lamplugh, A.; MacDougall, I.C.; Kalra, P.A. Protocol and Baseline Data of a Multicentre Prospective Double-Blinded Randomized Study of Intravenous Iron on Functional Status in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2020, 51, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ziedan, A.; Bhandari, S. Protocol and baseline data for a prospective open-label explorative randomized single-center comparative study to determine the effects of various intravenous iron preparations on markers of oxidative stress and kidney injury in chronic kidney disease (IRON-CKD). Trials 2019, 20, 194. [Google Scholar] [CrossRef]
- Wang, N. Cell Adhesion Molecules (CAMs). Encycl. Neurol. Sci. 2014, 628–629. [Google Scholar] [CrossRef]

| Continuous Data | ||
|---|---|---|
| Variable | Mean (SD) | Median (IQR) |
| Age/years | 60.94 (12.27) | 62.00 (15.00) |
| Hemoglobin/g/L | 127.67 (11.24) | 130.50 (10.25) |
| Serum Ferritin/μg/L | 65.58 (38.57) | 55.00 (36.50) |
| Transferrin saturation/% | 21.19 (7.90) | 19.00 (9.25) |
| Creatinine/μmol/L | 185.78 (57.43) | 172.00 (71.75) |
| Estimated glomerular filtration rate/mL/min/1.73 m2 | 32.77 (10.87) | 32.63 (18.58) |
| Thiobarbituric acid reactive substances/nM | 939.64 (321.49) | 958.21 (304.45) |
| Interleukin-6/pg/mL | 18.50 (31.23) | 8.03 (2.04) |
| Interleukin-10/pg/mL | 52.96 (75.24) | 15.26 (31.48) |
| C-reactive protein/mg/L | 6.52 (6.37) | 4.00 (6.80) |
| E-selectin/ng/mL | 56.74 (24.27) | 54.86 (29.76) |
| P-selectin/ng/mL | 69.26 (49.67) | 57.50 (29.19) |
| Pulse wave velocity/m/sec | 7.90 (2.98) | 7.55 (2.15) |
| Augmentation index/% | 23.97 (12.10) | 25.00 (15.00) |
| Categorical data | ||
| Variable | Number | Percentage |
| Gender | ||
| Male | 18 | 50.0 |
| Female | 17 | 47.2 |
| Unknown (not speciified) | 1 | 2.8 |
| CKD Stage | ||
| 3a | 3 | 8.3 |
| 3b | 16 | 44.4 |
| 4 | 16 | 44.4 |
| Unknown | 1 | 2.8 |
| Absolute iron deficiency | ||
| Yes | 18 | 50.0 |
| No | 18 | 50.0 |
| Anemia | ||
| Yes | 10 | 27.8 |
| No | 25 | 69.8 |
| Unknown | 1 | 2.8 |
| Primary cause of kidney disease | ||
| Hypertension | 8 | 22.2 |
| Diabetes mellitus | 8 | 22.2 |
| IgA nephropathy | 6 | 16.7 |
| Congenital anomaly | 1 | 2.8 |
| Glomerulonephritis | 1 | 2.8 |
| HIV associated glomerulonephritis | 1 | 2.8 |
| Polycystic kidney disease | 3 | 8.3 |
| Chronic pyelonephritis | 2 | 5.6 |
| Tubular interstitial nephritis | 1 | 2.8 |
| Other/non-clear | 5 | 13.9 |
| Whole Population (n = 36) | ||||
|---|---|---|---|---|
| Baseline | 1 Month | 3 Months | p-Value | |
| C-reactive protein/mg/L | 6.52 (6.37) | 5.85 (7.05) | 9.21 (13.19) | 0.27 |
| Interleukin-6/pg/mL | 18.50 (31.23) | 22.02 (34.85) | 22.35 (36.86) | 0.74 |
| Interleukin-10/pg/mL | 52.96 (75.24) | 52.79 (74.43) | 51.04 (75.58) | 0.93 |
| CKD stage 3b (n = 16) | ||||
| C-reactive protein/mg/L | 4.28 (4.24) | 3.25 (1.84) | 9.78 (8.97) | 0.03 |
| Interleukin-6/pg/mL | 29.88 (45.38) | 39.24 (51.86) | 37.48 (51.38) | 0.76 |
| Interleukin-10/pg/mL | 49.17 (70.66) | 54.38 (82.61) | 50.51 (76.59) | 0.97 |
| CKD stage 4 (n = 16) | ||||
| C-reactive protein/mg/L | 8.54 (7.96) | 8.14 (9.51) | 9.67 (16.7) | 0.79 |
| Intereukin-6/pg/mL | 9.31 (6.10) | 11.09 (9.82) | 9.29 (5.77) | 0.95 |
| Interleukin-10/pg/mL | 54.46 (93.98) | 46.55 (73.87) | 43.59 (79.43) | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassianides, X.; White, S.; Bhandari, S. Markers of Oxidative Stress, Inflammation and Endothelial Function following High-Dose Intravenous Iron in Patients with Non-Dialysis-Dependent Chronic Kidney Disease—A Pooled Analysis. Int. J. Mol. Sci. 2022, 23, 16016. https://doi.org/10.3390/ijms232416016
Kassianides X, White S, Bhandari S. Markers of Oxidative Stress, Inflammation and Endothelial Function following High-Dose Intravenous Iron in Patients with Non-Dialysis-Dependent Chronic Kidney Disease—A Pooled Analysis. International Journal of Molecular Sciences. 2022; 23(24):16016. https://doi.org/10.3390/ijms232416016
Chicago/Turabian StyleKassianides, Xenophon, Steven White, and Sunil Bhandari. 2022. "Markers of Oxidative Stress, Inflammation and Endothelial Function following High-Dose Intravenous Iron in Patients with Non-Dialysis-Dependent Chronic Kidney Disease—A Pooled Analysis" International Journal of Molecular Sciences 23, no. 24: 16016. https://doi.org/10.3390/ijms232416016
APA StyleKassianides, X., White, S., & Bhandari, S. (2022). Markers of Oxidative Stress, Inflammation and Endothelial Function following High-Dose Intravenous Iron in Patients with Non-Dialysis-Dependent Chronic Kidney Disease—A Pooled Analysis. International Journal of Molecular Sciences, 23(24), 16016. https://doi.org/10.3390/ijms232416016







