Influence of Metal Salts Addition on Physical and Electrochemical Properties of Ethyl and Propylammonium Nitrate
Abstract
1. Introduction
2. Results
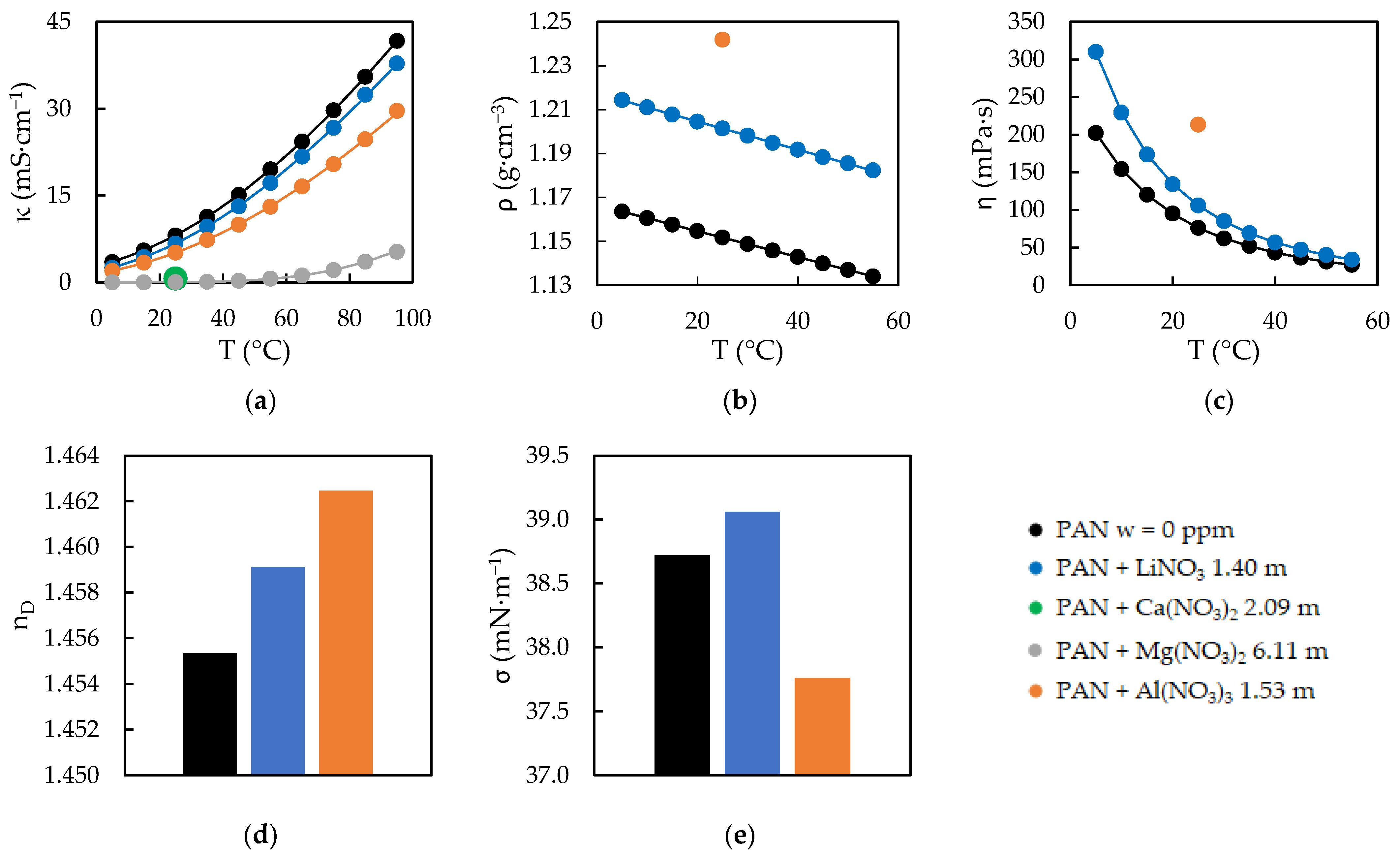
2.1. Physical Properties of the PILs Samples
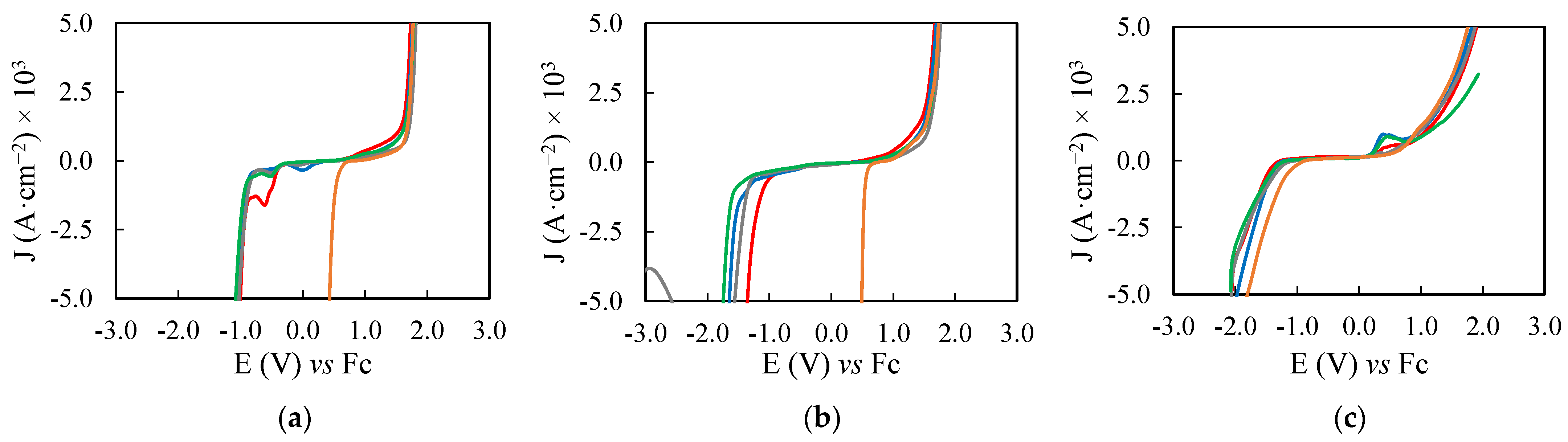
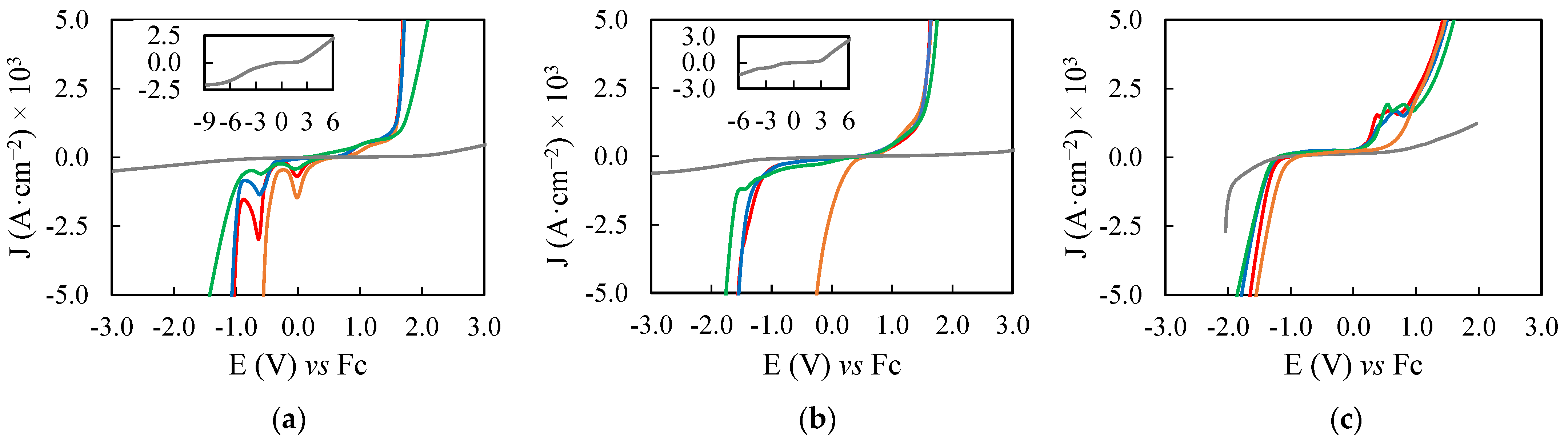
2.2. Electrochemical Potential Windows of the PILs Samples
3. Discussion
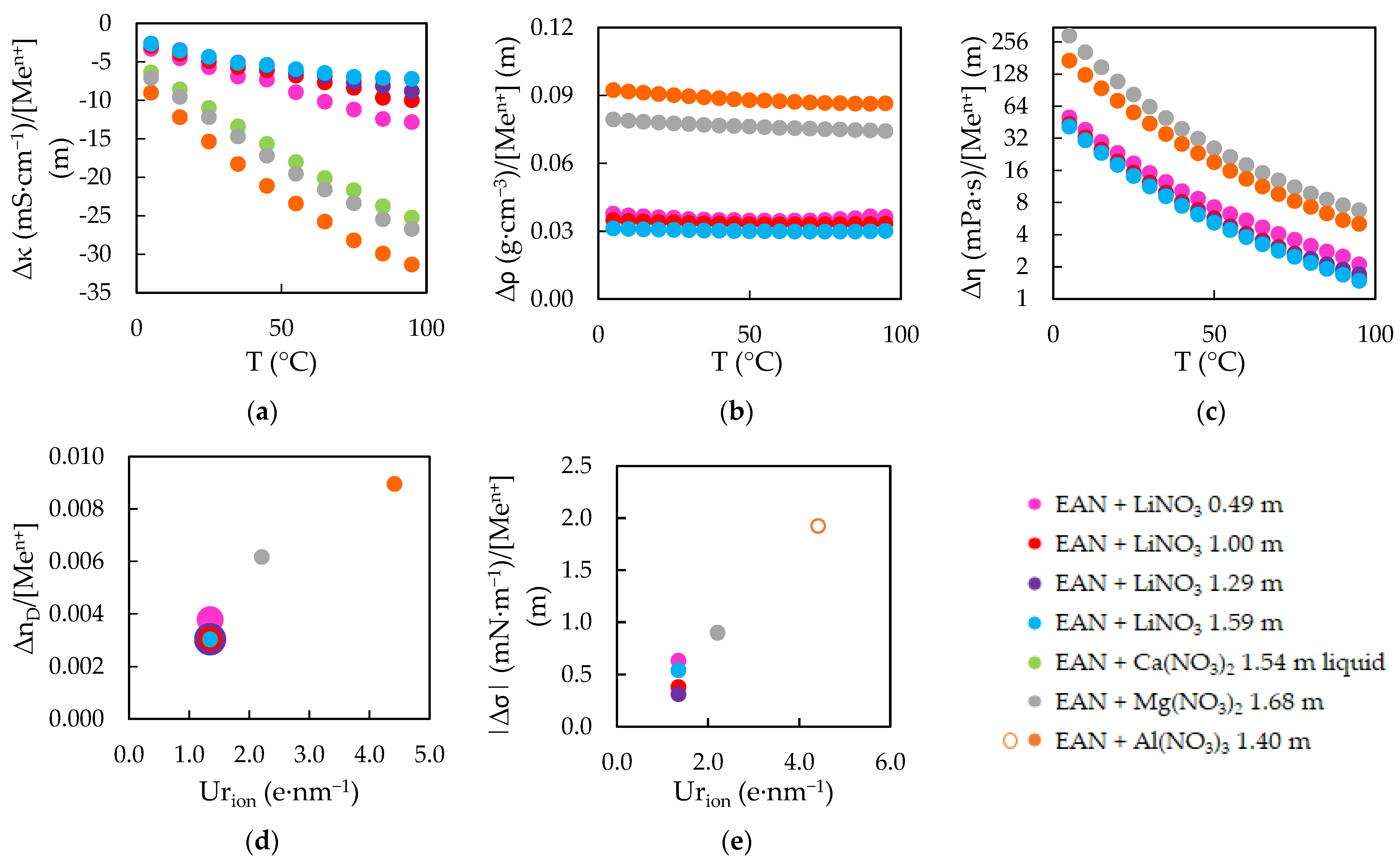
3.1. Physical Properties as a Function of Temperature
3.2. Physical Properties as a Function of Salt Doping
3.3. Electrochemical Study
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Electrical Conductivity
4.3. Density and Viscosity
4.4. Refractive Index
4.5. Surface Tension
4.6. Electrochemical Potential Windows
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Holbrey, J.; Seddon, K. Ionic Liquids. Clean Prod. Process. 1999, 1, 223–236. [Google Scholar] [CrossRef]
- Angell, C.A.; Ansari, Y.; Zhao, Z. Ionic Liquids: Past, present and future. Faraday Discuss. 2012, 154, 9–27. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic liquids for advanced materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Koo, Y.-M. (Eds.) Application of Ionic Liquids in Biotechnology; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Heng, H.; Deng, Q.; Yang, Y.; Wang, F. Recent Research Progress of Ionic Liquid Dissolving Silks for Biomedicine and Tissue Engineering Applications. Int. J. Mol. Sci. 2022, 23, 8706. [Google Scholar] [CrossRef]
- Qader, I.B.; Prasad, K. Recent Developments on Ionic Liquids and Deep Eutectic Solvents for Drug Delivery Applications. Pharm. Res. 2022, 39, 2367–2377. [Google Scholar] [CrossRef]
- Gomes, A.; Aguiar, L.; Ferraz, R.; Teixeira, C.; Gomes, P. The Emerging Role of Ionic Liquid-Based Approaches for Enhanced Skin Permeation of Bioactive Molecules: A Snapshot of the Past Couple of Years. Int. J. Mol. Sci. 2021, 22, 11991. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Sousa, Â.; Pedro, A.Q.; Romão, M.J.; Queiroz, J.A.; Gallardo, E.; Passarinha, L.A. Advances in Membrane-Bound Catechol-O-Methyltransferase Stability Achieved Using a New Ionic Liquid-Based Storage Formulation. Int. J. Mol. Sci. 2022, 23, 7264. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Almeida, C.; Sousa, A.C.A.; Neves, M.C.; Freire, M.G. High Performance of Ionic-Liquid-Based Materials to Remove Insecticides. Int. J. Mol. Sci. 2022, 23, 2989. [Google Scholar] [CrossRef] [PubMed]
- Filimon, A.; Dobos, A.M.; Dumbrava, O.; Doroftei, F.; Lupa, L. Green Blends Based on Ionic Liquids with Improved Performance for Membrane Technology: Perspectives for Environmental Applications. Int. J. Mol. Sci. 2022, 23, 7961. [Google Scholar] [CrossRef] [PubMed]
- Barrulas, R.V.; López-Iglesias, C.; Zanatta, M.; Casimiro, T.; Mármol, G.; Carrott, M.R.; García-González, C.A.; Corvo, M.C. The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture. Int. J. Mol. Sci. 2022, 23, 200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wu, C.; Gao, W.; Li, H.; Ma, Y.; Liu, S.; Yang, D. CO2 Absorption Mechanism by the Deep Eutectic Solvents Formed by Monoethanolamine-Based Protic Ionic Liquid and Ethylene Glycol. Int. J. Mol. Sci. 2022, 23, 1893. [Google Scholar] [CrossRef]
- Zhang, R.; Ke, Q.; Zhang, Z.; Zhou, B.; Cui, G.; Lu, H. Tuning Functionalized Ionic Liquids for CO2 Capture. Int. J. Mol. Sci. 2022, 23, 11401. [Google Scholar] [CrossRef] [PubMed]
- Cocheci, L.; Lupa, L.; Tolea, N.S.; Lazău, R.; Pode, R. IL-Functionalized Mg3Al-LDH as New Efficient Adsorbent for Pd Recovery from Aqueous Solutions. Int. J. Mol. Sci. 2022, 23, 9107. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Ginés, P.; Pico, J.M.; Franjo, C.; Jiménez, E.; Varela, L.M.; Cabeza, O. Temperature dependence of the electrical conductivity in EMIM−based ionic liquids: Evidence of Vogel–Tamman–Fulcher behaviour. Fluid Phase Equilib. 2006, 242, 141–146. [Google Scholar] [CrossRef]
- Rilo, E.; Dominguez−Perez, M.; Vila, J.; Segade, L.; Garcia, M.; Varela, L.M.; Cabeza, O. Easy prediction of the refractive index for binary mixtures of ionic liquids with water or ethanol. J. Chem. Thermodyn. 2012, 47, 219–222. [Google Scholar] [CrossRef]
- Cabeza, O.; Varela, L.M.; Rilo, E.; Segade, L.; Dominguez−Perez, M.; Ausin, D.; de Pedro, I.; Rodriguez Fernandez, J.; Gonzalez, J.; Vazquez−Tato, M.P.; et al. Synthesis, microstructure and volumetry of novel metal thiocyanate ionic liquids with [BMIM] cation. J. Mol. Liq. 2019, 283, 638–651. [Google Scholar] [CrossRef]
- Portela, D.; Segade, L.; Arosa, Y.; López Lago, E.; Varela, L.M.; Tojo, E.; Cabeza, O. Experimental device to measure the ionic conductivity anisotropy in liquid crystal hydrogel based in [EMIM] alkyl sulfate Ionic Liquids. Fluid Phase Equilib. 2022, 555, 113353. [Google Scholar] [CrossRef]
- Segade, L.; Cabanas, M.; Domínguez−Pérez, M.; Rilo, E.; García−Garabal, S.; Turmine, M.; Varela, L.M.; Gómez−González, V.; Docampo−Álvarez, B.; Cabeza, O. Surface and bulk characterisation of mixtures containing alkylammonium nitrates and water or ethanol: Experimental and simulated properties at 298.15 K. J. Mol. Liq. 2016, 222, 663–670. [Google Scholar] [CrossRef]
- Ausín, D.; Parajó, J.J.; Trenzado, J.L.; Varela, L.M.; Cabeza, O.; Segade, L. Influence of Small Quantities of Water on the Physical Properties of Alkylammonium Nitrate Ionic Liquids. Int. J. Mol. Sci. 2021, 22, 7334. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Ahmad, A. Phosphorylated chitosan/poly(vinyl alcohol) based proton exchange membranes modified with propylammonium nitrate ionic liquid and silica filler for fuel cell applications. Int. J. Hydrogen Energy 2022, 47, 19217–19236. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, B.; Dou, H.; Yang, N.; Xiao, X.; Tantai, X.; Sun, Y.; Zhang, L. Double-salt ionic liquid derived facilitated transport membranes for ethylene/ethane separation. J. Membr. Sci. 2021, 639, 119773. [Google Scholar] [CrossRef]
- Dou, H.; Xu, M.; Wang, B.; Zhang, Z.; Luo, D.; Shi, B.; Wen, G.; Mousavi, M.; Yu, A.; Bai, Z.; et al. Analogous Mixed Matrix Membranes with Self-Assembled Interface Pathways. Angew. Chem. Int. 2021, 60, 5864–5870. [Google Scholar] [CrossRef]
- Goh, J.T.E.; Abdul Rahim, A.R.; Masdar, M.S.; Shyuan, L.K. Enhanced Performance of Polymer Electrolyte Membranes via Modification with Ionic Liquids for Fuel Cell Applications. Membranes 2021, 11, 395. [Google Scholar] [CrossRef]
- Ciftcioglu, G.A.; Frank, C.W. Effect of Increased Ionic Liquid Uptake via Thermal Annealing on Mechanical Properties of Polyimide-Poly(ethylene glycol) Segmented Block Copolymer Membranes. Molecules 2021, 26, 2143. [Google Scholar] [CrossRef]
- Greaves, T.L.; Dharmadana, D.; Yalcin, D.; Clarke-Hannaford, J.; Christofferson, J.; Murdoch, B.J.; Han, Q.; Brown, S.J.; Weber, C.C.; Spencer, M.J.S.; et al. Electrochemical Stability of Zinc and Copper Surfaces in Protic Ionic Liquids. Langmuir 2022, 38, 4633–4644. [Google Scholar] [CrossRef]
- Zhai, J.; Sarkar, S.; Tran, N.; Pandiancherri, S.; Greaves, T.L.; Drummond, C.J. Tuning Nanostructured Lyotropic Liquid Crystalline Mesophases in Lipid Nanoparticles with Protic Ionic Liquids. J. Phys. Chem. Lett. 2021, 12, 399–404. [Google Scholar] [CrossRef]
- Qi, P.; Li, X.; Huang, Z.; Liu, Y.; Song, A.; Hao, J. G-quadruplex-based ionogels with controllable chirality for circularly polarized luminiscence. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127411. [Google Scholar] [CrossRef]
- Han, Q.; Smith, K.M.; Darmanin, C.; Ryan, T.M.; Drummond, C.J.; Greaves, T.L. Lysozyme conformational changes with ionic liquids: Spectroscopic, small angle x-ray and crystallographic study. J. Colloid Interface Sci. 2021, 585, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.S.; Kumari, P.; Kolagatla, S.; Sakai, V.G.; Rudic, S.; Rodriguez, B.J.; Rubini, M.; Tych, K.M.; Benedetto, A. Controlling Amyloid Fibril Properties Via Ionic Liquids: The Representative Case of Ethylammonium Nitrate and Tetramethylguanidinium Acetate on the Amyloidogenesis of Lysozyme. J. Phys. Chem. Lett. 2022, 13, 7058–7064. [Google Scholar] [CrossRef] [PubMed]
- Ciftcioglu, G.A.; Frank, C.W. Influence of Mixed Imide Composition and Thermal Annealing on Ionic Liquid Uptake and Conductivity of Polyimide-Poly(ethylene glycol) Segmented Block Copolymer Membranes. Molecules 2021, 26, 7450. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Onghena, B.; Li, X.; Binnemans, K. Ethylammonium nitrate enhances the extraction of transition metal nitrates by tri-n-butyl phosphate (TBP). AIChE J. 2021, 67, e17213. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kulshrestha, A.; Sharma, S.; Kumar, A. Room temperature depolymerization of lignin using a protic and metal based ionic liquid system: An efficient method of catalytic conversion and value addition. Green Chem. 2021, 23, 1240–1247. [Google Scholar] [CrossRef]
- Demirelli, M.; Peyre, V.; Sirieix-Plénet, J.; Malikova, N.; Fresnais, J. Influence of polycation/cation competition on the aggregation threshold of magnetic nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125876. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kumar, A. Green and Efficient Processing of Cinnamomum cassia Bark by Using Ionic Liquids: Extraction of Essential Oil and Construction of UV-Resistant Composite Films from Residual Biomass. Chem. Asian J. 2017, 12, 3150–3155. [Google Scholar] [CrossRef]
- Abu Khalifeh, H.; Al Nashef, I.M.; Zhuman, B.; Zuburtikudis, I. Double Layer Graphene Oxide Loaded with Propylammonium Nitrate for Selective Adsorption of Inorganic Salts. In Proceedings of the 7th World Congress on Recent Advances in Nanotechnology (RAN’22), Virtual Conference, 4–6 April 2022. ICNNFC 147. [Google Scholar] [CrossRef]
- Gómez-González, V.; Docampo-Álvarez, B.; Montes-Campos, A.; Otero, J.C.; López Lago, E.; Cabeza, O.; Gallego, L.J.; Varela, L.M. Solvation of Al3+ cations in bulk and confined protic ionic liquids: A computational study. Phys. Chem. Chem. Phys. 2018, 20, 19071–19081. [Google Scholar] [CrossRef]
- Vogel, H. The law of the relationship between viscosity of liquids and the temperature. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Tammann, G.; Hesse, W. Die Abhängigkeit der viskosität von der temperatur bie unterkühlten flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1952, 8, 339–360. [Google Scholar] [CrossRef]
- Cabeza, O.; García-Garabal, S.; Segade, L.; Domínguez-Pérez, M.; Rilo, E.; Varela, L.M. Physical Properties of Binary Mixtures of ILs with Water and Ethanol. A Review. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 111–136. [Google Scholar]
- O’Mahony, A.M.; Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. Effect of Water on the Electrochemical Window and Potential Limits of Room-Temperature Ionic Liquids. J. Chem. Eng. Data 2008, 53, 2884–2891. [Google Scholar] [CrossRef]
- Evans, D.F.; Chen, S.-H. Thermodynamics of Solution of Nonpolar Gases in a Fused Salt. “Hydrophobic Bonding” Behavior in a Nonaqueous System. J. Am. Chem. Soc. 1981, 103, 481–482. [Google Scholar] [CrossRef]
- Atkin, R.; Warr, G.G. The Smallest Amphiphiles: Nanostructure in Protic Room-Temperature Ionic Liquids with Short Alkyl Groups. J. Phys. Chem. B 2008, 112, 4164–4166. [Google Scholar] [CrossRef]
- Hayes, R.; Imberti, S.; Warr, G.G.; Atkin, R. Amphiphilicity determines nanostructure in protic ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Bodo, E.; Mangialardo, S.; Ramondo, F.; Ceccacci, F.; Postorino, P. Unravelling the Structure of Protic Ionic Liquids with Theoretical and Experimental Methods: Ethyl-, Propyl- and Butylammonium Nitrate Explored by Raman Spectroscopy and DFT Calculations. J. Phys. Chem. B 2012, 116, 13878–13888. [Google Scholar] [CrossRef] [PubMed]
- Bouzón Capelo, S.; Méndez-Morales, T.; Carrete, J.; López Lago, E.; Vila, J.; Cabeza, O.; Rodríguez, J.R.; Turmine, M.; Varela, L.M. Effect of Temperature and Cationic Chain Length on the Physical Properties of Ammonium Nitrate-Based Protic Ionic Liquids. J. Phys. Chem. B 2012, 116, 11302–11312. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hamano, H.; Minofar, B.; Kanzaki, R.; Fujii, K.; Kameda, Y.; Kohara, S.; Watanabe, M.; Ishiguro, S.; Umebayashi, Y. Structural Heterogeneity and Unique Distorted Hydrogen Bonding in Primary Ammonium Nitrate Ionic Liquids Studied by High-Energy X-ray Diffraction Experiments and MD Simulations. J. Phys. Chem. B 2012, 116, 2801–2813. [Google Scholar] [CrossRef]
- Smith, J.A.; Webber, G.B.; Warr, G.G.; Atkin, R. Rheology of Protic Ionic Liquids and Their Mixtures. J. Phys. Chem. B 2013, 117, 13930–13935. [Google Scholar] [CrossRef]
- Smith, J.A.; Webber, G.B.; Warr, G.G.; Zimmer, A.; Atkin, R.; Werzer, O. Shear dependent viscosity of poly(ethylene oxide) in two protic ionic liquids. J. Colloid Interface Sci. 2014, 430, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Nakama, K.; Hayashi, R.; Aono, M.; Takekiyo, T.; Yoshimura, Y.; Saihara, K.; Shimizu, A. Electrochemical anomalies of protic ionic liquid—Water systems: A case study using ethylammonium nitrate—Water system. Chem. Phys. 2016, 475, 119–125. [Google Scholar] [CrossRef]
- Perron, G.; Hardy, A.; Justice, J.-C.; Desnoyers, J.E. Model System for Concentrated Electrolyte Solutions: Thermodynamic and Transport Properties of Ethylammonium Nitrate in Acetonitrile and in Water. J. Solut. Chem. 1993, 22, 1159–1178. [Google Scholar] [CrossRef]
- Greaves, T.L.; Kennedy, D.F.; Weerawardena, A.; Tse, N.M.K.; Kirby, N.; Drummond, C.J. Nanostructured Protic Ionic Liquids Retain Nanoscale Features in Aqueous Solution While Precursor Brønsted Acids and Bases Exhibit Different Behavior. J. Phys. Chem. B 2011, 115, 2055–2066. [Google Scholar] [CrossRef]
- Porcedda, S.; Marongiu, B.; Schirru, M.; Falconieri, D.; Piras, A. Excess enthalpy and excess volume for binary systems of two ionic liquids + water. J. Therm. Anal. Calorim. 2011, 103, 29–33. [Google Scholar] [CrossRef]
- Hayes, R.; Imberti, S.; Warr, G.G.; Atkin, R. How Water Dissolves in Protic Ionic Liquids. Angew. Chem. Int. Ed. 2012, 51, 7468–7471. [Google Scholar] [CrossRef]
- Anaredy, R.S.; Lucio, A.J.; Shaw, S.K. Adventitious Water Sorption in a Hydrophilic and a Hydrophobic Ionic Liquid: Analysis and Implications. ACS Omega 2016, 1, 407–416. [Google Scholar] [CrossRef]
- Abe, H.; Aono, M.; Takekiyo, T.; Yoshimura, Y.; Shimizu, A. Phase behavior of water-mediated protic ionic liquid: Ethylammonium nitrate. J. Mol. Liq. 2017, 241, 301–307. [Google Scholar] [CrossRef]
- Gómez-González, V.; Docampo-Álvarez, B.; Cabeza, O.; Fedorov, M.; Lynden-Bell, R.M.; Gallego, L.J.; Varela, L.M. Molecular dynamics simulations of the structure and single-particle dynamics of mixtures of divalent salts and ionic liquids. J. Chem. Phys. 2015, 143, 124507. [Google Scholar] [CrossRef]
- Gómez-González, V.; Docampo-Álvarez, B.; Otero-Mato, J.M.; Cabeza, O.; Gallego, L.J.; Varela, L.M. Molecular dynamics simulations of the structure of mixtures of protic ionic liquids and monovalent and divalent salts at the electrochemical interface. Phys. Chem. Chem. Phys. 2018, 20, 12767–12776. [Google Scholar] [CrossRef]
- Russina, O.; Caminiti, R.; Méndez-Morales, T.; Carrete, J.; Cabeza, O.; Gallego, L.J.; Varela, L.M.; Triolo, A. How does lithium nitrate dissolve in a protic ionic liquid? J. Mol. Liq. 2015, 205, 16–21. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Cabeza, O.; Varela, L.M. Liquid range of ionic liquid—Metal salt mixtures for electrochemical applications. J. Chem. Thermodyn. 2019, 134, 164–174. [Google Scholar] [CrossRef]
- Hayes, R.; Bernard, S.A.; Imberti, S.; Warr, G.G.; Atkin, R. Solvation of Inorganic Nitrate Salts in Protic Ionic Liquids. J. Phys. Chem. C 2014, 118, 21215–21225. [Google Scholar] [CrossRef]
- Méndez-Morales, T.; Carrete, J.; Cabeza, O.; Russina, O.; Triolo, A.; Gallego, L.J.; Varela, L.M. Solvation of Lithium Salts in Protic Ionic Liquids: A Molecular Dynamics Study. J. Phys. Chem. B 2014, 118, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Morales, T.; Carrete, J.; Rodríguez, J.R.; Cabeza, O.; Gallego, L.J.; Russina, O.; Varela, L.M. Nanostructure of mixtures of protic ionic liquids and lithium salts: Effect of alkyl chain length. Phys. Chem. Chem. Phys. 2015, 17, 5298–5307. [Google Scholar] [CrossRef]
- Hjalmarsson, N.; Atkin, R.; Rutland, M.W. Effect of Lithium Ions on Rheology and Interfacial Forces in Ethylammonium Nitrate and Ethanolammonium Nitrate. J. Phys. Chem. C 2016, 120, 26960–26967. [Google Scholar] [CrossRef]
- Prabhu, S.R.; Dutt, G.B. Does Addition of an Electrolyte Influence the Rotational Diffusion of Nondipolar Solutes in a Protic Ionic Liquid? J. Phys. Chem. B 2015, 119, 6311–6316. [Google Scholar] [CrossRef]
- Evans, D.F.; Yamauchi, A.; Wei, G.J.; Bloomfleid, V.A. Micelle Size in Ethylammonium Nitrate as Determined by Classical and Quasi-Elastic Light Scattering. J. Phys. Chem. 1983, 87, 3537–3541. [Google Scholar] [CrossRef]
- Niga, P.; Wakeham, D.; Nelson, A.; Warr, G.G.; Rutland, M.; Atkin, R. Structure of the Ethylammonium Nitrate Surface: An X-ray Reflectivity and Vibrational Sum Frequency Spectroscopy Study. Langmuir 2010, 26, 8282–8288. [Google Scholar] [CrossRef]
- Wakeham, D.; Nelson, A.; Warr, G.G.; Atkin, R. Probing the protic ionic liquid surface using X-ray reflectivity. Phys. Chem. Chem. Phys. 2011, 13, 20828–20835. [Google Scholar] [CrossRef]
- Ridings, C.; Warr, G.G.; Andersson, G.G. Composition of the outermost layer and concentration depth profiles of ammonium nitrate ionic liquid surfaces. Phys. Chem. Chem. Phys. 2012, 14, 16088–16095. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Greaves, T.L.; Best, A.S. A comparative study of the electrodeposition of polyaniline from a protic opnic liquid, an aprotic ionic liquid and neutral aqueous solution using anilinium nitrate. J. Mater. Chem. 2011, 21, 7622–7629. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Evolving Structure−Property Relationships and Expanding Applications. Chem. Rev. 2015, 115, 11379–11448. [Google Scholar] [CrossRef] [PubMed]
- Benhlima, N.; Turmine, M.; Letellier, P.; Naejus, R.; Lemordant, D. Étude électrochimique du nitrate d’éthylammonium fondu à 298 K: Établissement d’une echelle de potentiel redox. J. Chim. Phys. 1998, 95, 25–44. [Google Scholar] [CrossRef]
- Zarrougui, R.; Dhahbi, M.; Lemordan, D. Electrochemical behaviour of iodine redox couples in aprotic and protic RTILs: 1-Butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide and ethylammonium nitrate. J. Electroanal. Chem. 2014, 717, 189–195. [Google Scholar] [CrossRef]
- Shotwell, J.B.; Flowers II, R.A. Electrochemical Investigation of the Solvolytic Properties of Ethylammonium Nitrate (EAN) and Propylammonium Nitrate (PAN). Electroanalysis 2000, 12, 223–226. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Gunawan, C.A.; Lu, X.; Zhao, C. Tuning the electrodeposition parameters of silver to yield micro/nano structures from room temperature protic ionic liquids. Electrochim. Acta 2012, 81, 98–105. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]



| Salt Cation | rion (Å) | Urion (e·nm−1) |
|---|---|---|
| Li+ | 0.59 | 1.35 |
| Ca2+ | 1.0 | 1.59 |
| Mg2+ | 0.72 | 2.21 |
| Al3+ | 0.54 | 4.42 |
| Sample | w (ppm) | [Men+] (mg·g−1) | [Men+] (m) | Aκ (mS·cm−1) | Bκ (K) | T0,κ (K) | s% |
|---|---|---|---|---|---|---|---|
| EAN + LiNO3 | 4730 ± 50 | 3.3 ± 0.1 | 0.49 | 664.4 | −432.4 | 175.8 | 0.4 |
| EAN + LiNO3 | 3430 ± 50 | 6.5 ± 0.3 | 1.00 | 649.2 | −430.5 | 179.9 | 0.7 |
| EAN + LiNO3 | 3270 ± 50 | 8.2 ± 0.4 | 1.29 | 680.8 | −445.5 | 178.9 | 0.5 |
| EAN + LiNO3 | 4300 ± 50 | 9.9 ± 0.6 | 1.59 | 782.0 | −474.7 | 177.6 | 0.7 |
| EAN + Ca(NO3)2 | 6600 ± 120 | 49 ± 1 | 1.54 | 700.4 | −512.1 | 190.9 | 0.8 |
| EAN + Mg(NO3)2 | 19,320 ± 60 | 32 ± 1 | 1.68 | 783.6 | −529.5 | 187.3 | 1.0 |
| EAN + Al(NO3)3 | 38,870 ± 70 | 28 ± 1 | 1.40 | 914.6 | −541.0 | 173.0 | 0.5 |
| PAN + LiNO3 | 3890 ± 50 | 8.8 ± 0.1 | 1.40 | 614.7 | −510.0 | 185.4 | 0.8 |
| PAN + Ca(NO3)2 | 4870 ± 50 | 62 ± 1 | 2.09 | - | - | - | - |
| PAN + Mg(NO3)2 | 11,350 ± 70 | 77 ± 1 | 6.11 | 2393 | −911.5 | 218.4 | 3 |
| PAN + Al(NO3)3 | 1960 ± 50 | 31 ± 2 | 1.53 | 607.5 | −581.2 | 176.6 | 1.0 |
| Sample | w (ppm) | [Men+] (mg·g−1) | [Men+] (mol·kgIL−1) | Aƞ (mPa·s) | Bƞ (K) | T0, ƞ (K) | s% | ρ0 (g·cm−3) | C × 104 (g·cm−3·°C−1) | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| EAN + LiNO3 | 4730 ± 50 | 3.3 ± 0.1 | 0.49 | 0.2116 | 793.4 | 150.1 | 0.2 | 1.243 | −6.029 | 0.9994 |
| EAN + LiNO3 | 3430 ± 50 | 6.5 ± 0.3 | 1.00 | 0.2094 | 803.5 | 152.4 | 0.2 | 1.260 | −6.124 | 0.9997 |
| EAN + LiNO3 | 3270 ± 50 | 8.2 ± 0.4 | 1.29 | 0.2645 | 743.9 | 158.9 | 0.2 | |||
| EAN + LiNO3 | 4300 ± 50 | 9.9 ± 0.6 | 1.59 | 0.2311 | 785.0 | 156.3 | 0.2 | 1.274 | −6.173 | 0.9997 |
| EAN + Mg(NO3)2 | 19,320 ± 60 | 32 ± 1 | 1.68 | 0.3094 | 775.1 | 174.6 | 0.3 | 1.356 | −6.945 | 0.9998 |
| EAN + Al(NO3)3 | 40,050 ± 70 | 28 ± 1 | 1.40 | 0.1548 | 898.3 | 157.5 | 0.4 | 1.349 | −7.113 | 0.9991 |
| PAN + LiNO3 | 3890 ± 50 | 8.8 ± 0.1 | 1.40 | 0.1740 | 893.3 | 158.8 | 0.3 | 1.217 | −6.407 | 0.9998 |
| PAN + Al(NO3)3 | 1960 ± 50 | 31 ± 2 | 1.53 | - | - | - | - | - | - | - |
| Sample | w (ppm) | [Men+] (mg·g−1) | [Men+] (mol·kgIL−1) | WE | Cathodic Limit (V) | Anodic Limit (V) | EPW (V) |
|---|---|---|---|---|---|---|---|
| EAN | 2380 ± 50 | - | - | DPt | −0.998 | 1.732 | 2.730 |
| EAN + LiNO3 | 2800 ± 50 | 12.2 ± 0.4 | 2.01 | DPt | −1.019 | 1.761 | 2.780 |
| EAN + Ca(NO3)2 | 6600 ± 120 | 49 ± 1 | 1.54 | DPt | −1.074 | 1.783 | 2.857 |
| EAN + Mg(NO3)2 | 19,320 ± 60 | 32 ± 1 | 1.68 | DPt | −1.024 | 1.821 | 2.845 |
| EAN + Al(NO3)3 | 38,870 ± 70 | 28 ± 1 | 1.40 | DPt | 0.429 | 1.774 | 1.345 |
| EAN | 5420 ± 50 | - | - | DGC | −1.357 | 1.670 | 3.027 |
| EAN + LiNO3 | 5590 ± 50 | 12.2 ± 0.4 | 2.01 | DGC | −1.645 | 1.694 | 3.339 |
| EAN + Ca(NO3)2 | 12,970 ± 60 | 49 ± 1 | 1.54 | DGC | −1.746 | 1.734 | 3.480 |
| EAN + Mg(NO3)2 | 25,820 ± 70 | 32 ± 1 | 1.68 | DGC | −1.560 | 1.764 | 3.324 |
| EAN + Al(NO3)3 | 41,350 ± 110 | 28 ± 1 | 1.40 | DGC | 0.493 | 1.732 | 1.239 |
| EAN | - | - | - | SPE | −2.060 | 1.900 | 3.960 |
| EAN + LiNO3 | - | 10.8 ± 0.2 | 1.75 | SPE | −1.972 | 1.826 | 3.798 |
| EAN + Ca(NO3)2 | - | 57.9 ± 1.0 | 1.91 | SPE | <−2.075 | >1.929 | >4.004 |
| EAN + Mg(NO3)2 | - | 33.1 ± 0.2 | 1.72 | SPE | −2.065 | 1.880 | 3.945 |
| EAN + Al(NO3)3 | - | 21.5 ± 0.8 | 0.98 | SPE | −1.806 | 1.758 | 3.564 |
| PAN | 1300 ± 50 | - | - | DPt | −1.031 | 1.694 | 2.725 |
| PAN + LiNO3 | 3890 ± 50 | 8.8 ± 0.1 | 1.40 | DPt | −1.066 | 1.714 | 2.780 |
| PAN + Ca(NO3)2 | 4870 ± 50 | 62 ± 1 | 2.09 | DPt | −1.424 | 2.095 | 3.519 |
| PAN + Mg(NO3)2 | 11,350 ± 70 | 77 ± 1 | 6.11 | DPt | <−8.750 | >6.071 | >14.821 |
| PAN + Al(NO3)3 | 8160 ± 60 | 31 ± 2 | 1.53 | DPt | −0.559 | 1.707 | 2.266 |
| PAN | 2530 ± 50 | - | - | DGC | −1.554 | 1.628 | 3.182 |
| PAN + LiNO3 | 7350 ± 60 | 8.8 ± 0.1 | 1.40 | DGC | −1.544 | 1.644 | 3.188 |
| PAN + Ca(NO3)2 | 21,620 ± 80 | 62 ± 1 | 2.09 | DGC | −1.761 | 1.749 | 3.510 |
| PAN + Mg(NO3)2 | 10,830 ± 70 | 77 ± 1 | 6.11 | DGC | <−5.748 | >6.255 | >12.003 |
| PAN + Al(NO3)3 | 8560 ± 60 | 31 ± 2 | 1.53 | DGC | −0.251 | 1.648 | 1.899 |
| PAN | - | - | - | SPE | −1.647 | 1.429 | 3.076 |
| PAN + LiNO3 | - | 7.7 ± 0.5 | 1.20 | SPE | −1.784 | 1.497 | 3.281 |
| PAN + Ca(NO3)2 | - | 105 ± 1 | 4.60 | SPE | −1.857 | 1.605 | 3.457 |
| PAN + Mg(NO3)2 | - | 66 ± 1 | 4.5 | SPE | <−2.038 | >1.966 | >4.004 |
| PAN + Al(NO3)3 | - | 21.7 ± 0.4 | 0.97 | SPE | −1.550 | 1.463 | 3.013 |
| PIL | Physical Property | Li+ | Ca2+ | Mg2+ | Al3+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 °C | 25 °C | 95 °C | 5 °C | 25 °C | 95 °C | 5 °C | 25 °C | 95 °C | 5 °C | 25 °C | 95 °C | ||
| EAN | κ | −25.76 | −21.15 | −9.91 | −60.82 | −53.20 | −34.60 | −67.57 | −58.98 | −36.61 | −86.05 | −74.26 | −42.95 |
| EAN | ρ | 2.55 | 2.52 | 2.57 | - | - | - | 6.48 | 6.41 | 6.34 | 7.55 | 7.44 | 7.39 |
| EAN | ƞ | 47.50 | 36.57 | 20.05 | - | - | - | 337.97 | 212.10 | 92.12 | 197.29 | 143.96 | 68.51 |
| EAN | nD | - | 0.21 | - | - | - | - | - | 0.42 | - | - | 0.62 | - |
| EAN | σ | - | 1.13 | - | - | - | - | - | 1.88 | - | - | −4.02 | - |
| PAN | κ | −27.76 | −19.58 | −10.62 | - | −49.93 * | - | −21.04 | −20.96 | −16.37 | −32.10 | −27.19 | −20.38 |
| PAN | ρ | 3.14 | 3.10 | - | - | - | - | - | - | - | - | 5.12 | - |
| PAN | ƞ | 45.62 | 34.68 | - | - | - | - | - | - | - | - | 120.66 | - |
| PAN | nD | - | 0.20 | - | - | - | - | - | - | - | - | 0.33 | - |
| PAN | σ | - | 0.46 | - | - | - | - | - | - | - | - | −1.69 | - |
| Material | Name | Supplier | Purity (%w/w) |
|---|---|---|---|
| EAN | Ethylammonium nitrate | IoLiTec (Heilbronn, Germany) | >97% |
| PAN | Propylammonium nitrate | IoLiTec (Heilbronn, Germany) | >97% |
| LiNO3 | Lithium nitrate | Alfa Aesar (Ward Hill, Massachusetts, USA) | 99.8% |
| Ca(NO3)2·4H2O | Calcium nitrate | PanReac AppliChem (Barcelona, Spain) | 99.7% |
| Mg(NO3)2·6H2O | Magnesium nitrate | Fluka (Sigma-Aldrich) (St. Louise, Missouri, USA) | ≥98.0% |
| Al(NO3)3·9H2O | Aluminum nitrate | Sigma-Aldrich (St. Louise, Missouri, USA) | 99.7% |
| Fc | Ferrocene | Sigma-Aldrich (St. Louise, Missouri, USA) | 99.9% |
| AgNO3 | Silver nitrate | PanReac AppliChem (Barcelona, Spain) | 99.8% |
| IL | WE | E’0 (Fc-Fc+) (V) | AWE (cm2) |
|---|---|---|---|
| EAN | SPE | 0.075 | 0.419 |
| EAN | DPt | −0.282 | 0.0432 |
| EAN | DGC | −0.280 | 0.0347 |
| PAN | SPE | 0.038 | 0.233 |
| PAN | DPt | −0.255 | 0.0351 |
| PAN | DGC | −0.255 | 0.0301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ausín, D.; Trenzado, J.L.; Turmine, M.; Varela, L.M.; Cabeza, O.; González Romero, E.; Segade, L. Influence of Metal Salts Addition on Physical and Electrochemical Properties of Ethyl and Propylammonium Nitrate. Int. J. Mol. Sci. 2022, 23, 16040. https://doi.org/10.3390/ijms232416040
Ausín D, Trenzado JL, Turmine M, Varela LM, Cabeza O, González Romero E, Segade L. Influence of Metal Salts Addition on Physical and Electrochemical Properties of Ethyl and Propylammonium Nitrate. International Journal of Molecular Sciences. 2022; 23(24):16040. https://doi.org/10.3390/ijms232416040
Chicago/Turabian StyleAusín, David, José L. Trenzado, Mireille Turmine, Luis M. Varela, Oscar Cabeza, Elisa González Romero, and Luisa Segade. 2022. "Influence of Metal Salts Addition on Physical and Electrochemical Properties of Ethyl and Propylammonium Nitrate" International Journal of Molecular Sciences 23, no. 24: 16040. https://doi.org/10.3390/ijms232416040
APA StyleAusín, D., Trenzado, J. L., Turmine, M., Varela, L. M., Cabeza, O., González Romero, E., & Segade, L. (2022). Influence of Metal Salts Addition on Physical and Electrochemical Properties of Ethyl and Propylammonium Nitrate. International Journal of Molecular Sciences, 23(24), 16040. https://doi.org/10.3390/ijms232416040







