Genome-Wide Identification of the SUN Gene Family in Melon (Cucumis melo) and Functional Characterization of Two CmSUN Genes in Regulating Fruit Shape Variation
Abstract
:1. Introduction
2. Results
2.1. CmSUN Family Gene Identification and Sequence Analyses
2.2. Phylogenetic Tree Analysis of SUN Family Proteins
2.3. Expression Profiling of CmSUN Genes
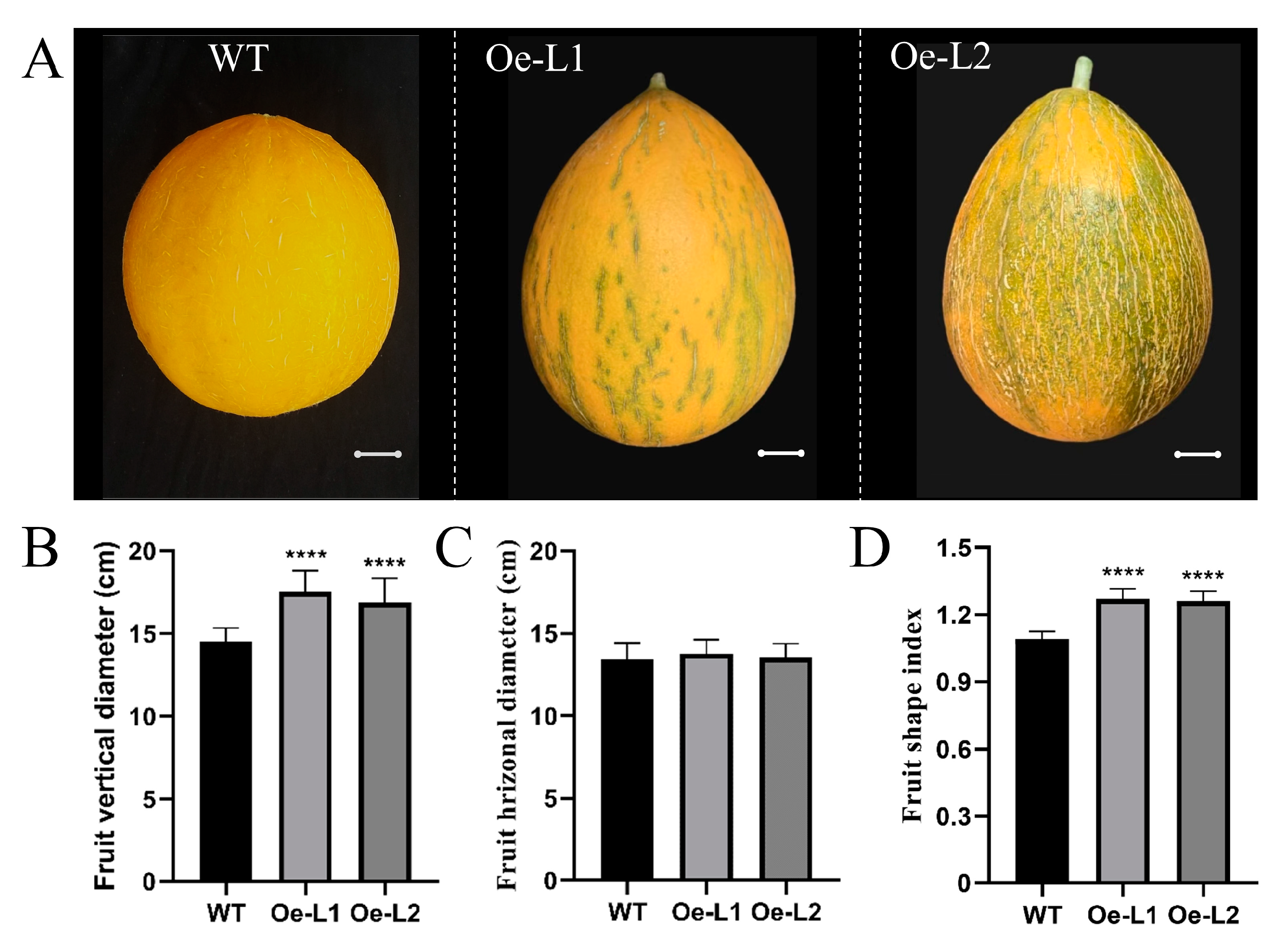
2.4. Overexpression of CmSUN23-24 and CmSUN25-26-27c Resulted in Melon Fruit Shape Variation
2.5. Yeast Two-Hybrid and Subcellular Localization
3. Discussion
4. Materials and Methods
4.1. Identification of SUN Gene Family Members
4.2. Sequence Analysis and Phylogenetic Tree Construction
4.3. Plant Materials and Growth Conditions
4.4. Expression Analysis and Quantitative Real-Time PCR
4.5. Gene Cloning and Plant Transformation
4.6. Subcellular Localization
4.7. Yeast Two-Hybrid Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolim, P.M.; Fidelis, G.P.; Padilha, C.E.A.; Santos, E.S.; Rocha, H.A.O.; Macedo, G.R. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Braz. J. Med. Biol. Res. 2018, 51, e6069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleshman, M.K.; Lester, G.E.; Riedl, K.M.; Kopec, R.E.; Narayanasamy, S.; Curley, R.W.; Schwartz, S.J.; Harrison, E.H. Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: Determinations of β-carotene bioaccessibility and bioavailability. J. Agric. Food Chem. 2011, 59, 4448. [Google Scholar] [CrossRef] [Green Version]
- Amanullah, S.; Liu, S.; Gao, P.; Zhu, Z.; Zhu, Q.; Fan, C.; Luan, F. QTL mapping for melon (Cucumis melo L.) fruit traits by assembling and utilization of novel SNPs based CAPS markers. Sci. Hortic. 2018, 236, 18–29. [Google Scholar] [CrossRef]
- Nunez-Palenius, H.G.; Gomez-Lim, M.; Ochoa-Alejo, N.; Grumet, R.; Lester, G.; Cantliffe, D.J. Melon fruits: Genetic diversity, physiology, and biotechnology features. Crit. Rev. Biotechnol. 2008, 28, 13–55. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, F.; Chen, Y.; Lian, Y. Research Progress on Inheritance of Fruit Shape in Horticultural Crops. Acta Hortic. Sin. 2011, 38, 1385–1396. [Google Scholar]
- Zhang, T.; Hong, Y.; Zhang, X.; Yuan, X.; Chen, S. Relationship between Key Environmental Factors and the Architecture of Fruit Shape and Size in Near-Isogenic Lines of Cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2022, 23, 14033. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Grumet, R. Transcriptional Profiling of Rapidly Growing Cucumber Fruit by 454-Pyrosequencing Analysis. J. Am. Soc. Hortic. Sci. 2010, 135, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Jiang, N.; Schaffner, E.; Stockinger, E.J.; van der Knaap, E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 2008, 319, 1527–1530. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, H.; Cabrera, A.; Meulia, T.; van der Knaap, E. SUN regulates vegetative and reproductive organ shape by changing cell division patterns. Plant Physiol. 2011, 157, 1175–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevenger, J.P.; Van Houten, J.; Blackwood, M.; Rodríguez, G.R.; Jikumaru, Y.; Kamiya, Y.; Kusano, M.; Saito, K.; Visa, S.; Esther, V.D.K. Network Analyses Reveal Shifts in Transcript Profiles and Metabolites That Accompany the Expression of SUN and an Elongated Tomato Fruit. Plant Physiol. 2015, 168, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Clevenger, J.P.; Illa-Berenguer, E.; Meulia, T.; van der Knaap, E.; Sun, L. A Comparison of sun, ovate, fs8.1 and Auxin Application on Tomato Fruit Shape and Gene Expression. Plant Cell Physiol. 2019, 60, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Savchenko, T.; Levy, M. Genome-wide comparative analysis of the IQD gene families in Arabidopsis thaliana and Oryza sativa. BMC Evol. Biol. 2005, 5, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.; Wang, Q.; Kaspi, R.; Parrella, M.P.; Abel, S. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J. 2005, 43, 79–96. [Google Scholar] [CrossRef]
- Snedden, W.A.; Fromm, H. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2010, 151, 35–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- McCormack, E.; Tsai, Y.C.; Braam, J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005, 10, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Abrams, C.; Wang, L.; Gizzi, A.; He, L.; Lin, R.; Yuan, C.; Loll, P.; Pascal, J.; Zhang, J.F. Structural basis for calmodulin as a dynamic calcium sensor. Structure 2012, 20, 911–923. [Google Scholar]
- Ranty, B.; Aldon, D.; Galaud, J.P. Plant calmodulins and calmodulin-related proteins: Multifaceted relays to decode calcium signals. Plant Signal Behav. 2006, 1, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, A.R.; Friedberg, F. Sequence motifs for calmodulin recognition. FASEB J. 1997, 11, 331–340. [Google Scholar] [CrossRef]
- Bahler, M.; Rhoads, A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002, 513, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Burstenbinder, K.; Moller, B.; Plotner, R.; Stamm, G.; Hause, G.; Mitra, D.; Abel, S. The IQD Family of Calmodulin-Binding Proteins Links Calcium Signaling to Microtubules, Membrane Subdomains, and the Nucleus. Plant Physiol. 2017, 173, 1692–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Yi, Z.; Pablo, M.; Carolyn, R.; Xu, T.; Yang, Z. The microtubule-associated protein IQ67 DOMAIN5 modulates microtubule dynamics and pavement cell shape. Plant Physiol. 2018, 177, 1555–1568. [Google Scholar] [CrossRef] [Green Version]
- Wendrich, J.R.; Yang, B.J.; Mijnhout, P.; Xue, H.W.; Weijers, D. IQD proteins integrate auxin and calcium signaling to regulate microtubule dynamics during Arabidopsis development. Cold Spring Harb. Lab. 2018, 275560. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, Y.; Wen, Y.; Wang, D.; Liu, H.; Li, Y.; Zhao, J.; An, L.; Yu, F.; Liu, X. The domain of unknown function 4005 (DUF4005) in an Arabidopsis IQD protein functions in microtubule binding. J. Biol. Chem. 2021, 297, 100849. [Google Scholar] [CrossRef]
- Huang, Z.; Houten, J.V.; Gonzalez, G.; Xiao, H.; Knaap, E. Genome-wide identification, phylogeny and expression analysis of SUN, OFP and YABBY gene family in tomato. Mol. Genet. Genom. 2013, 288, 111–129. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Z.; Ma, H.; Chen, X.; Li, Y.; Wang, Y.; Xiang, Y. The IQD gene family in soybean: Structure, phylogeny, evolution and expression. PLoS ONE 2014, 9, e110896. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, C.; Zhao, Y.; Zhu, K.; Wang, Y.; Jiang, H.; Xiang, Y.; Cheng, B. Genome-wide analysis of the IQD gene family in maize. Mol. Genet. Genom. 2016, 291, 543–558. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Mcgregor, C.; Liu, S.; Weng, Y. Genetic architecture of fruit size and shape variation in cucurbits: A comparative perspective. Theor. Appl. Genet. 2020, 133, 1–21. [Google Scholar] [CrossRef]
- Pan, Y.P.; Liang, X.J.; Gao, M.L.; Liu, H.Q.; Meng, H.W. Round fruit shape in WI7239 cucumber is controlled by two interacting quantitative trait loci with one putatively encoding a tomato SUN homolog. Theor. Appl. Genet. 2017, 130, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Zhao, S.; Lu, X.; He, N.; Zhang, L.; Ali, A.; Liu, W. Genetic mapping reveals a candidate gene (ClFS1) for fruit shape in watermelon (Citrullus lanatus L.). Theor. Appl. Genet. Int. J. Breed. Res. Cell Genet. 2018, 131, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Legendre, R.; Kuzy, J.; Mcgregor, C. Markers for selection of three alleles of ClSUN25-26-27a (Cla011257) associated with fruit shape in watermelon. Mol. Breed. 2020, 40, 19. [Google Scholar] [CrossRef]
- Monforte, A.J.; Diaz, A.; Caño-Delgado, A.; Van Der Knaap, E. The genetic basis of fruit morphology in horticultural crops: Lessons from tomato and melon. J. Exp. Bot. 2014, 65, 4625–4637. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wendrich, J.; Rybel, B.D.; Weijers, D.; Xue, H. Rice microtubule-associated protein IQ67-DOMAIN14 regulates grain shape by modulating microtubule cytoskeleton dynamics. Plant Biotechnol. J. 2020, 18, 1141–1152. [Google Scholar] [CrossRef]
- Zentella, R.; Zhang, Z.L.; Park, M.; Thomas, S.G.; Endo, A.; Murase, K.; Fleet, C.M.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, P.; Zhu, Q.; Zhu, Z.; Liu, H.; Wang, X.; Weng, Y.; Gao, M.; Luan, F. Resequencing of 297 melon accessions reveals the genomic history of improvement and loci related to fruit traits in melon. Plant Biotechnol. J. 2020, 18, 2545–2558. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, S.; Yang, W.; Li, Y.; Xia, M.; Chen, Z.; Wang, Q.; Yan, L.; Song, X.; Liu, R.; et al. Transcriptomic analysis reveals the roles of microtubule-related genes and transcription factors in fruit length regulation in cucumber (Cucumis sativus L.). Sci. Rep. 2015, 5, 8031. [Google Scholar] [CrossRef] [Green Version]
- Bürstenbinder, K.; Savchenko, T.; Müller, J.; Adamson, A.W.; Stamm, G.; Kwong, R.; Zipp, B.J.; Dinesh, D.C.; Abel, S. Arabidopsis Calmodulin-binding Protein IQ67-Domain 1 Localizes to Microtubules and Interacts with Kinesin Light Chain-related Protein-1. J. Biol. Chem. 2013, 288, 1822–1871. [Google Scholar] [CrossRef] [Green Version]
- Batistič, O.; Kudla, J. Analysis of calcium signaling pathways in plants. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1283–1293. [Google Scholar] [CrossRef]
- Alistair, M.H.; Colin, B. The generation of Ca2+ signals in plants. Annu. Rev. Plant Biol. 2004, 55, 401–427. [Google Scholar]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2009, 425, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, S.; Bai, Y.; Sun, H.; Jiao, C.; Guo, S.; Zhao, K.; Blanca, J.; Zhang, Z.; Huang, S.; et al. Cucurbit Genomics Database (CuGenDB): A central portal for comparative and functional genomics of cucurbit crops. Nucleic Acids Res. 2019, 47, D1128–D1136. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Baulcombe, D.C. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef] [Green Version]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Johnson, L.S.; Eddy, S.R.; Portugaly, E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinform. 2010, 11, 431. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 571–607. [Google Scholar]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Tian, Z.; Han, J.; Che, G.; Hasi, A. Genome-wide characterization and expression analysis of SAUR gene family in Melon (Cucumis melo L.). Planta 2022, 255, 123. [Google Scholar] [CrossRef]
- Chen, C.; Rui, X.; Hao, C.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. Cold Spring Harb. Lab. 2018, 289660, 289660. [Google Scholar]
- Bai, S.; Tian, Y.; Tan, C.; Bai, S.; Hasi, A. Genome-wide identification of microRNAs involved in the regulation of fruit ripening and climacteric stages in melon (Cucumis melo). Hortic. Res. 2020, 7, 13. [Google Scholar] [CrossRef]









| Name | Gene ID | Chromosome Distribution | ORF (bp) | Amino Acid Length (aa) | MW (kD) | pI |
|---|---|---|---|---|---|---|
| CmSUN1-2a | MELO3C014258.2.1 | Chrom05 | 1473 | 490 | 53.7 | 9.87 |
| CmSUN1-2b | MELO3C009321.2.1 | Chrom04 | 1491 | 496 | 56.4 | 10.34 |
| CmSUN3 | MELO3C025505.2.1 | Chrom09 | 1449 | 482 | 53.4 | 10.42 |
| CmSUN4 | MELO3C012442.2.1 | Chrom10 | 1395 | 464 | 52.1 | 10.06 |
| CmSUN5 | MELO3C009991.2.1 | Chrom02 | 1302 | 433 | 48.5 | 10.22 |
| CmSUN6 | MELO3C016880.2.1 | Chrom07 | 1338 | 445 | 49.6 | 10.47 |
| CmSUN7-8 | MELO3C024381.2.1 | Chrom01 | 1221 | 406 | 45.8 | 10.26 |
| CmSUN9-10a | MELO3C016091.2.1 | Chrom07 | 786 | 261 | 29.9 | 10.04 |
| CmSUN9-10b | MELO3C007235.2.1 | Chrom08 | 1107 | 368 | 41.9 | 10.31 |
| CmSUN11 | MELO3C005137.2.1 | Chrom09 | 1440 | 479 | 54.1 | 10.02 |
| CmSUN13-14a | MELO3C022423.2.1 | Chrom11 | 1614 | 537 | 60.5 | 10.87 |
| CmSUN13-14b | MELO3C017768.2.1 | Chrom07 | 1650 | 549 | 61.4 | 10.47 |
| CmSUN17-18a | MELO3C022253.2.1 | Chrom11 | 1539 | 512 | 57.5 | 10.42 |
| CmSUN19a | MELO3C014290.2.1 | Chrom05 | 1452 | 483 | 53.8 | 9.63 |
| CmSUN19b | MELO3C006504.2.1 | Chrom06 | 1269 | 422 | 46.9 | 9.81 |
| CmSUN19c | MELO3C004368.2.1 | Chrom05 | 1128 | 375 | 43.4 | 9.98 |
| CmSUN21a | MELO3C008499.2.1 | Chrom06 | 1410 | 469 | 52.8 | 9.60 |
| CmSUN21b | MELO3C005888.2.1 | Chrom09 | 1125 | 374 | 41.8 | 9.96 |
| CmSUN23-24 | MELO3C006884.2.1 | Chrom06 | 1413 | 470 | 51.5 | 10.28 |
| CmSUN25-26-27a | MELO3C015418.2.1 | Chrom02 | 1233 | 410 | 45.4 | 10.25 |
| CmSUN25-26-27b | MELO3C024434.2.1 | Chrom01 | 1293 | 430 | 48.3 | 9.66 |
| CmSUN25-26-27c | MELO3C013004.2.1 | Chrom04 | 1161 | 386 | 42.8 | 10.29 |
| CmSUN30-31 | MELO3C002201.2.1 | Chrom12 | 1800 | 599 | 65.9 | 9.80 |
| CmSUN32b | MELO3C010997.2.1 | Chrom03 | 2538 | 845 | 93.4 | 5.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Liu, S.; Wang, Z.; Shao, R.; Ye, J.; Yan, W.; Lv, H.; Hasi, A.; Che, G. Genome-Wide Identification of the SUN Gene Family in Melon (Cucumis melo) and Functional Characterization of Two CmSUN Genes in Regulating Fruit Shape Variation. Int. J. Mol. Sci. 2022, 23, 16047. https://doi.org/10.3390/ijms232416047
Ma M, Liu S, Wang Z, Shao R, Ye J, Yan W, Lv H, Hasi A, Che G. Genome-Wide Identification of the SUN Gene Family in Melon (Cucumis melo) and Functional Characterization of Two CmSUN Genes in Regulating Fruit Shape Variation. International Journal of Molecular Sciences. 2022; 23(24):16047. https://doi.org/10.3390/ijms232416047
Chicago/Turabian StyleMa, Ming, Suya Liu, Zhiwei Wang, Ran Shao, Jianrong Ye, Wei Yan, Hailing Lv, Agula Hasi, and Gen Che. 2022. "Genome-Wide Identification of the SUN Gene Family in Melon (Cucumis melo) and Functional Characterization of Two CmSUN Genes in Regulating Fruit Shape Variation" International Journal of Molecular Sciences 23, no. 24: 16047. https://doi.org/10.3390/ijms232416047
APA StyleMa, M., Liu, S., Wang, Z., Shao, R., Ye, J., Yan, W., Lv, H., Hasi, A., & Che, G. (2022). Genome-Wide Identification of the SUN Gene Family in Melon (Cucumis melo) and Functional Characterization of Two CmSUN Genes in Regulating Fruit Shape Variation. International Journal of Molecular Sciences, 23(24), 16047. https://doi.org/10.3390/ijms232416047





