Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families
Abstract
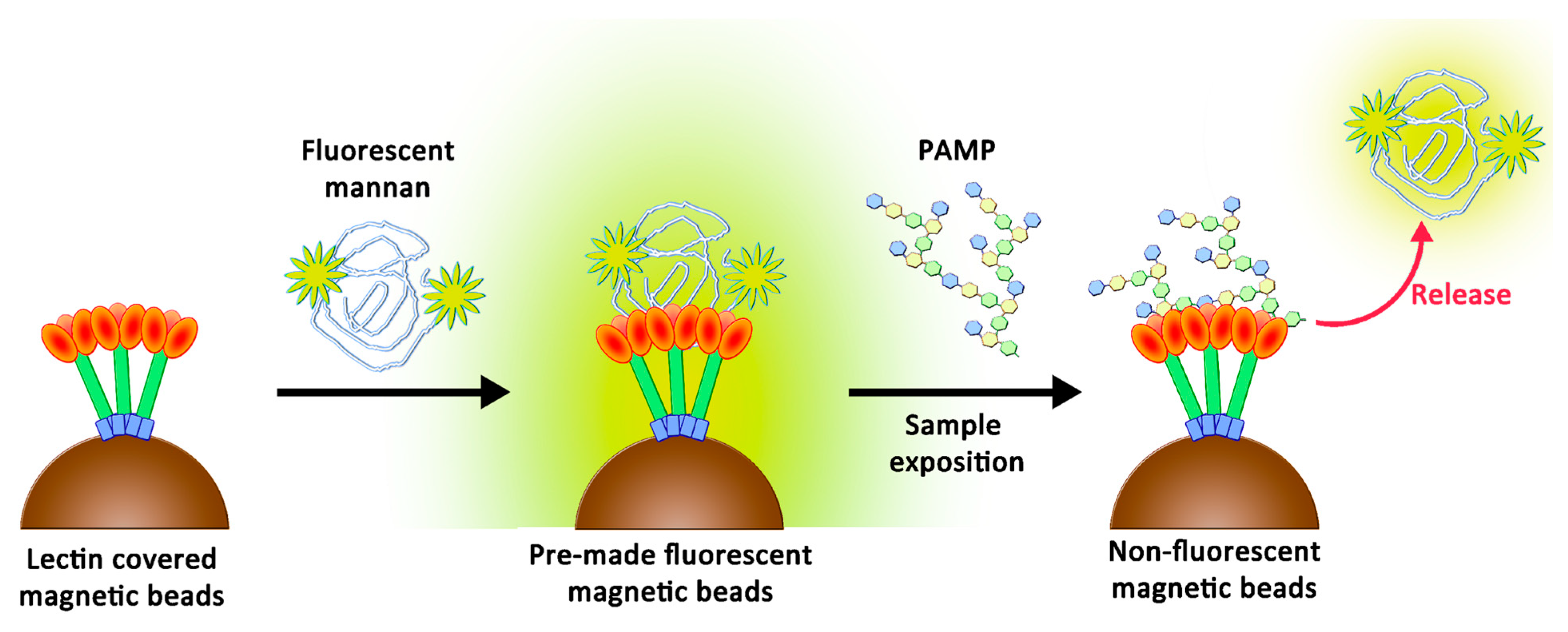
:1. Introduction
2. Results
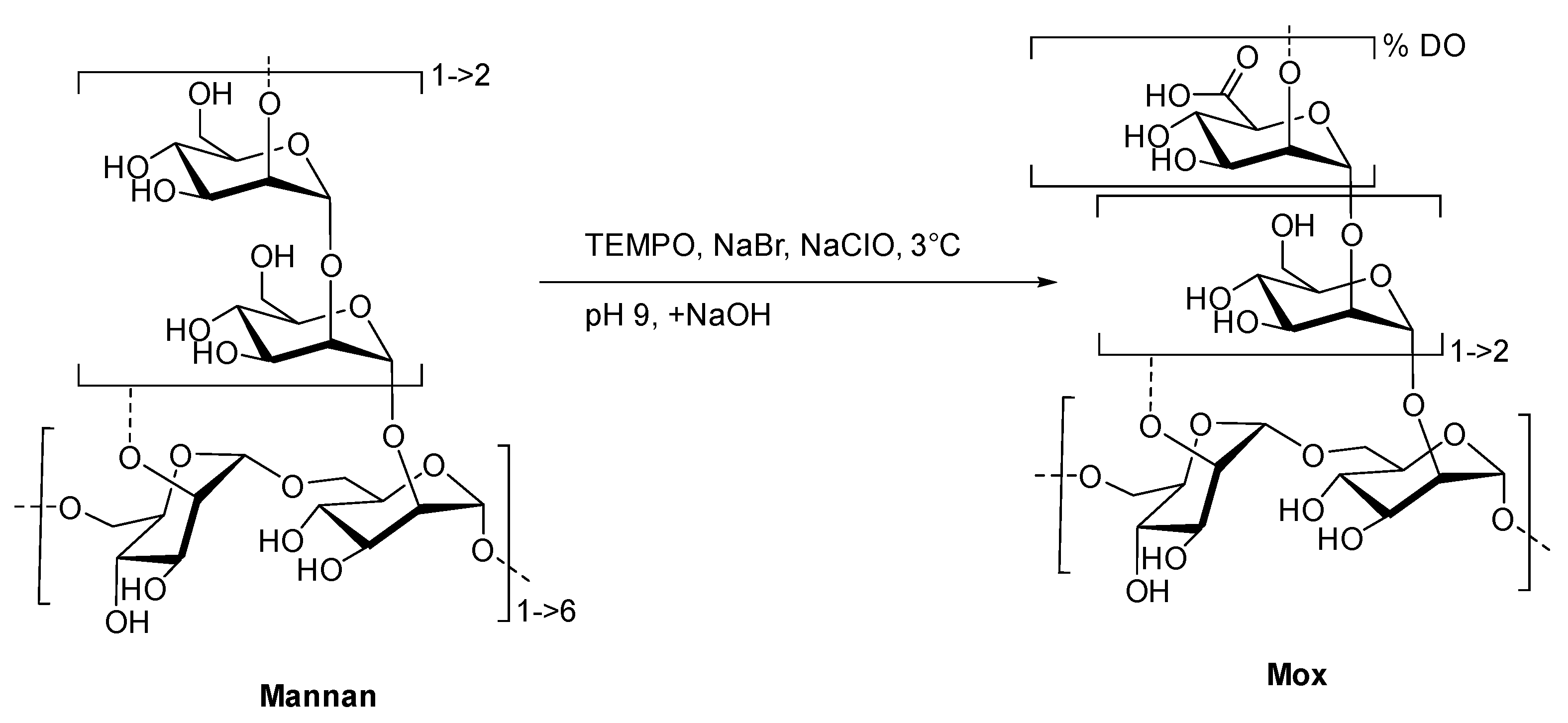
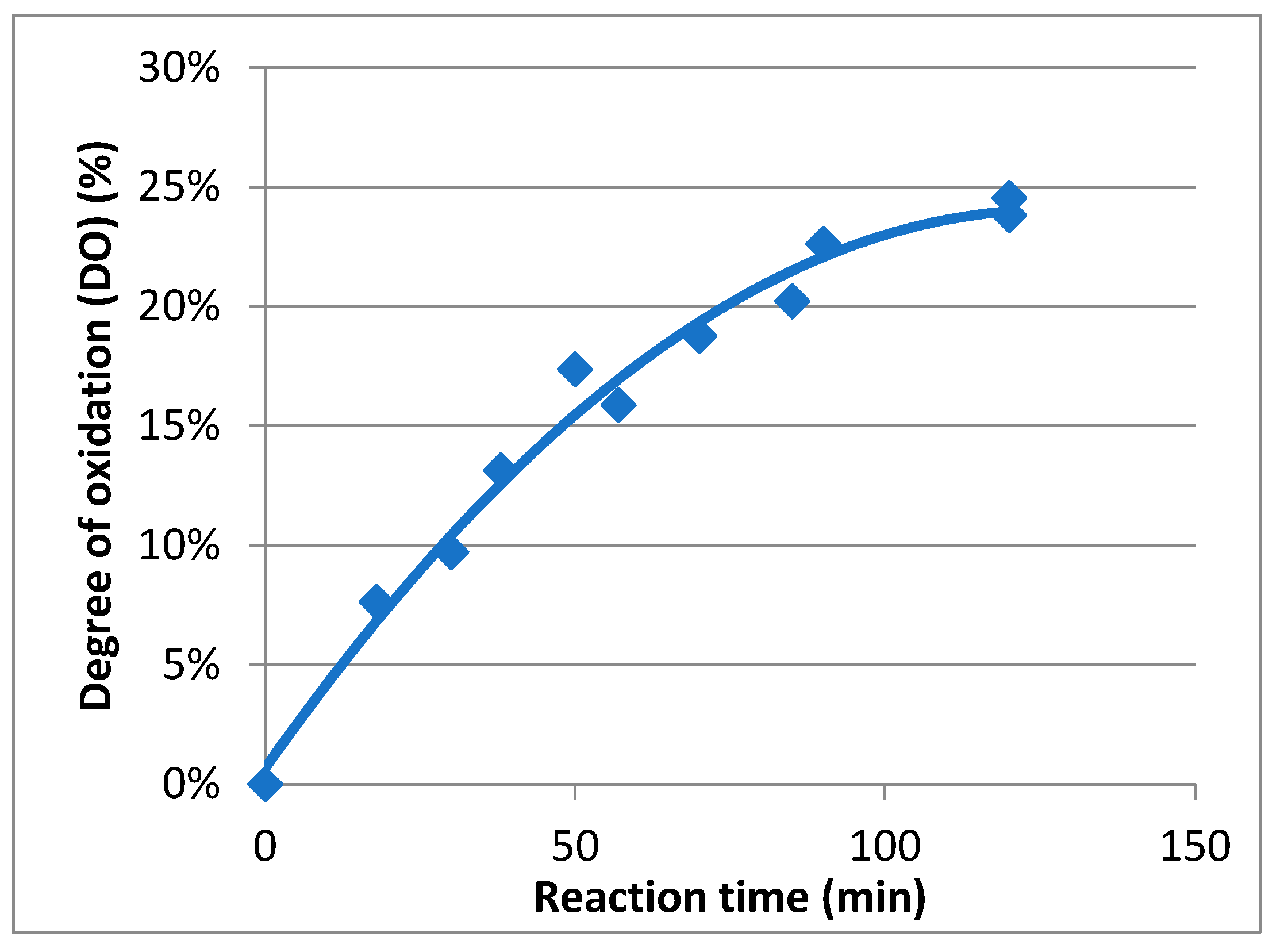
2.1. Preparation and Characterization of TEMPO-Oxidized Mannan
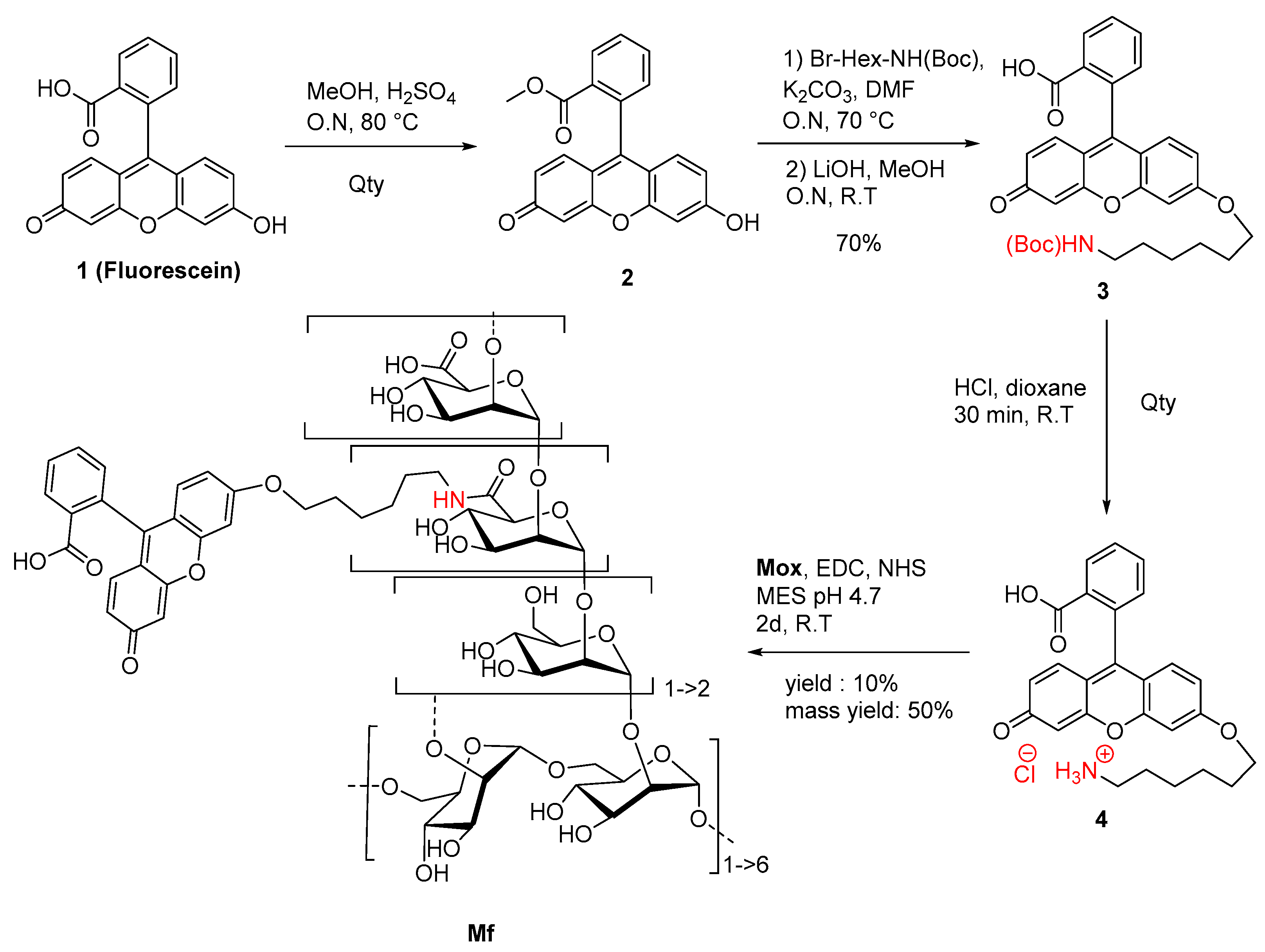
2.2. Preparation of Fluorescent Mannan
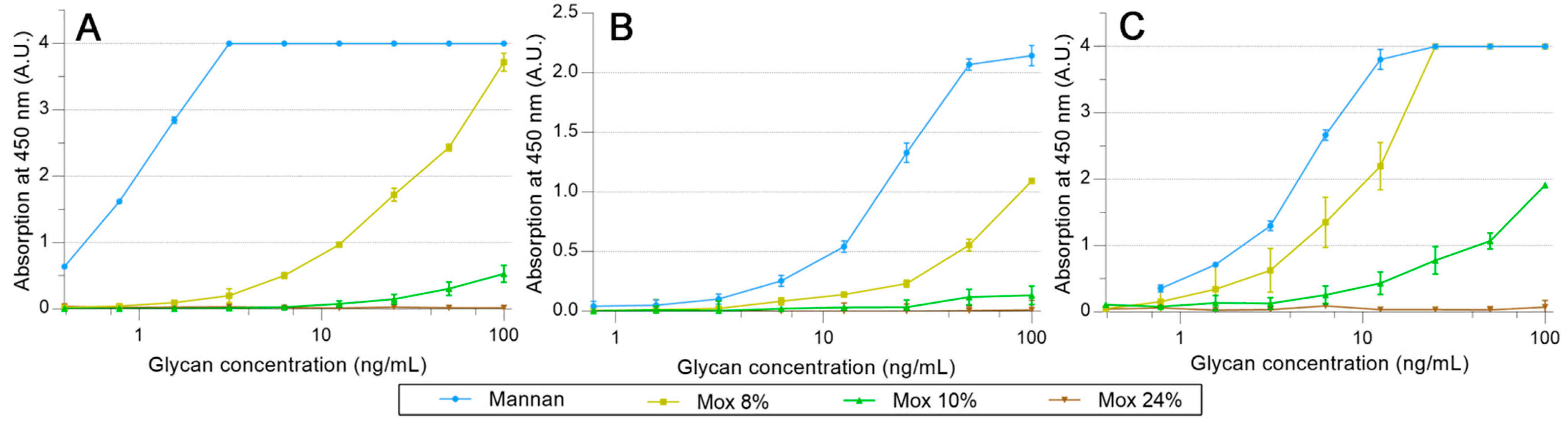
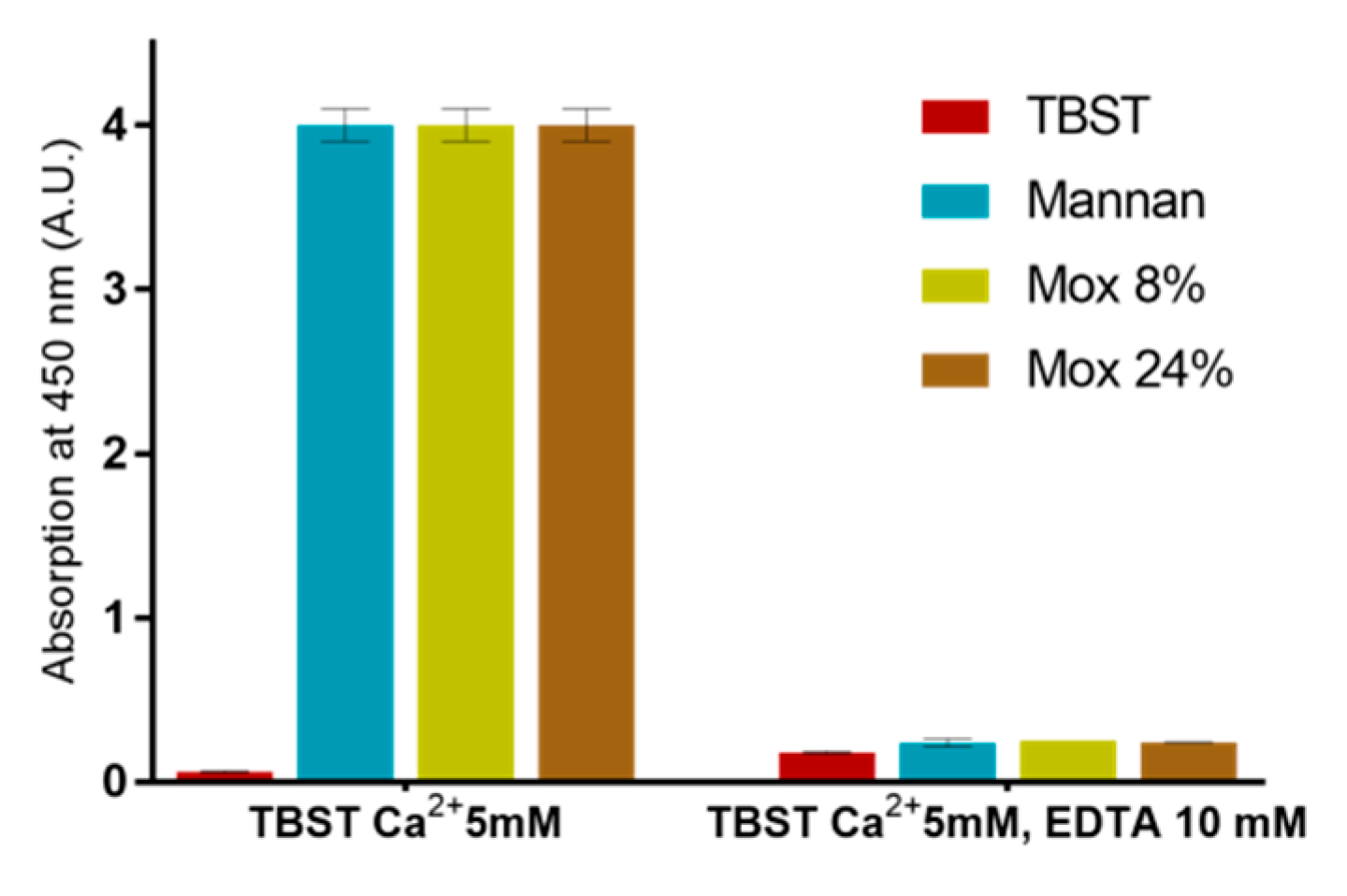
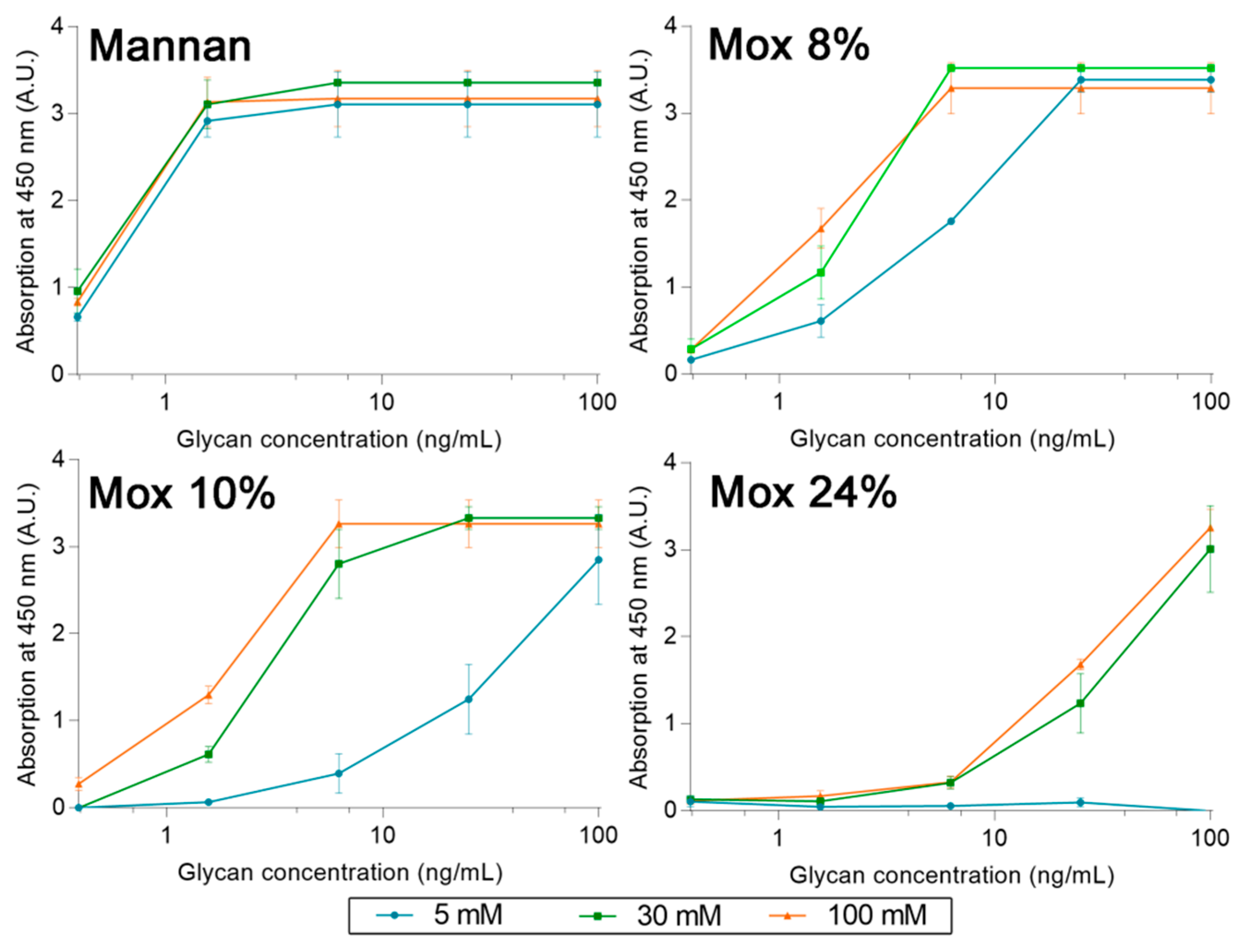
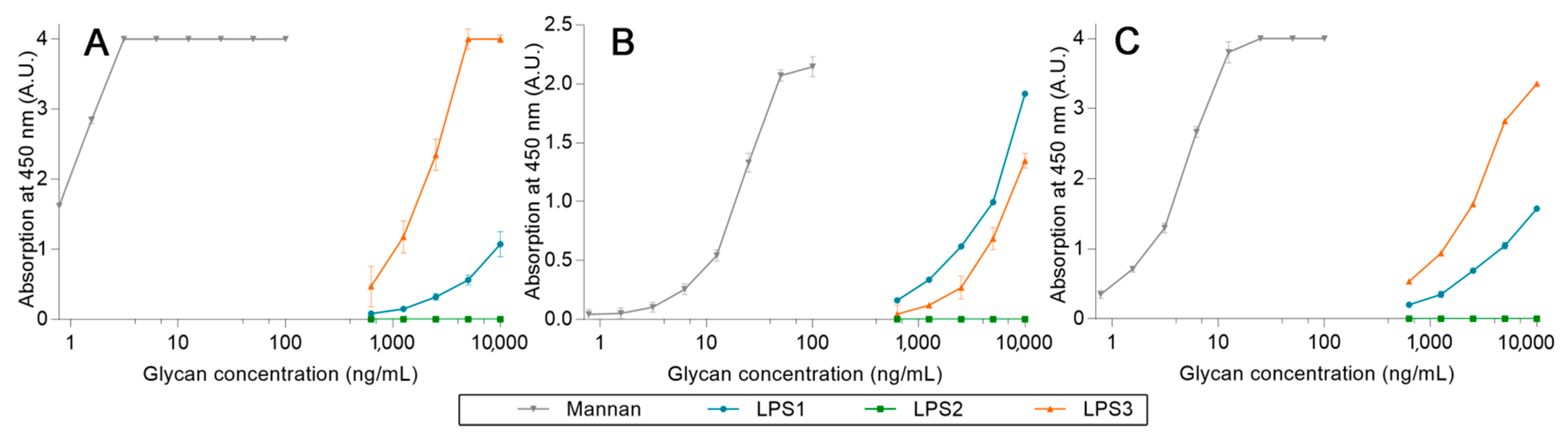
2.3. Avidities of Oxidized Mannan Derivatives
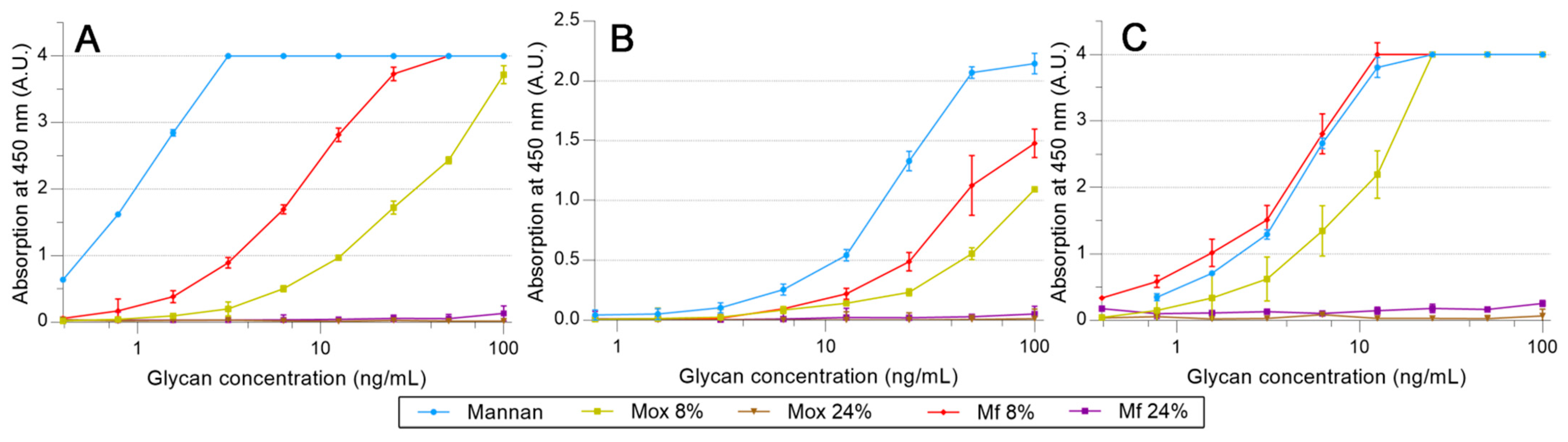
2.4. Avidities of Fluorescent Mannan Derivatives
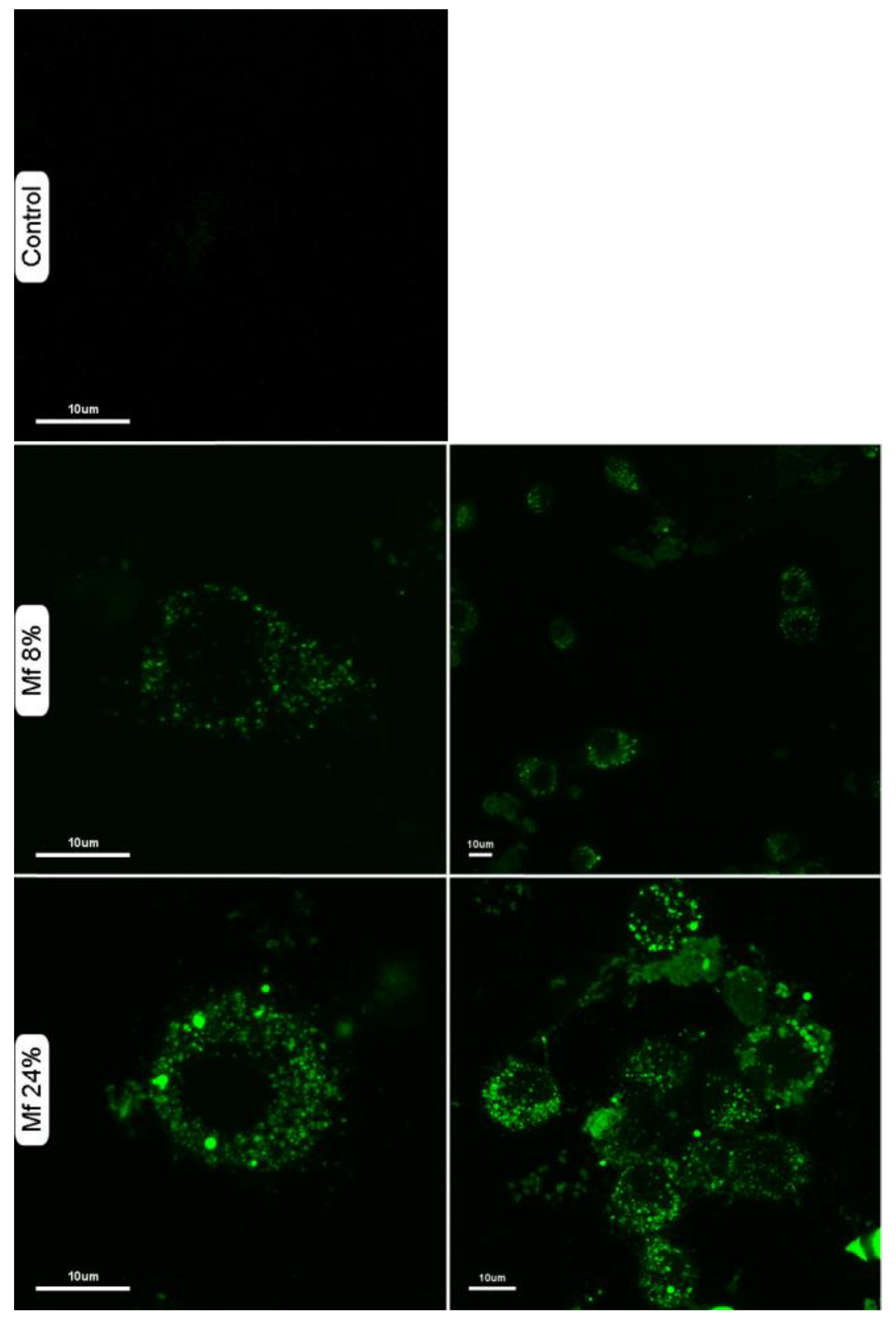
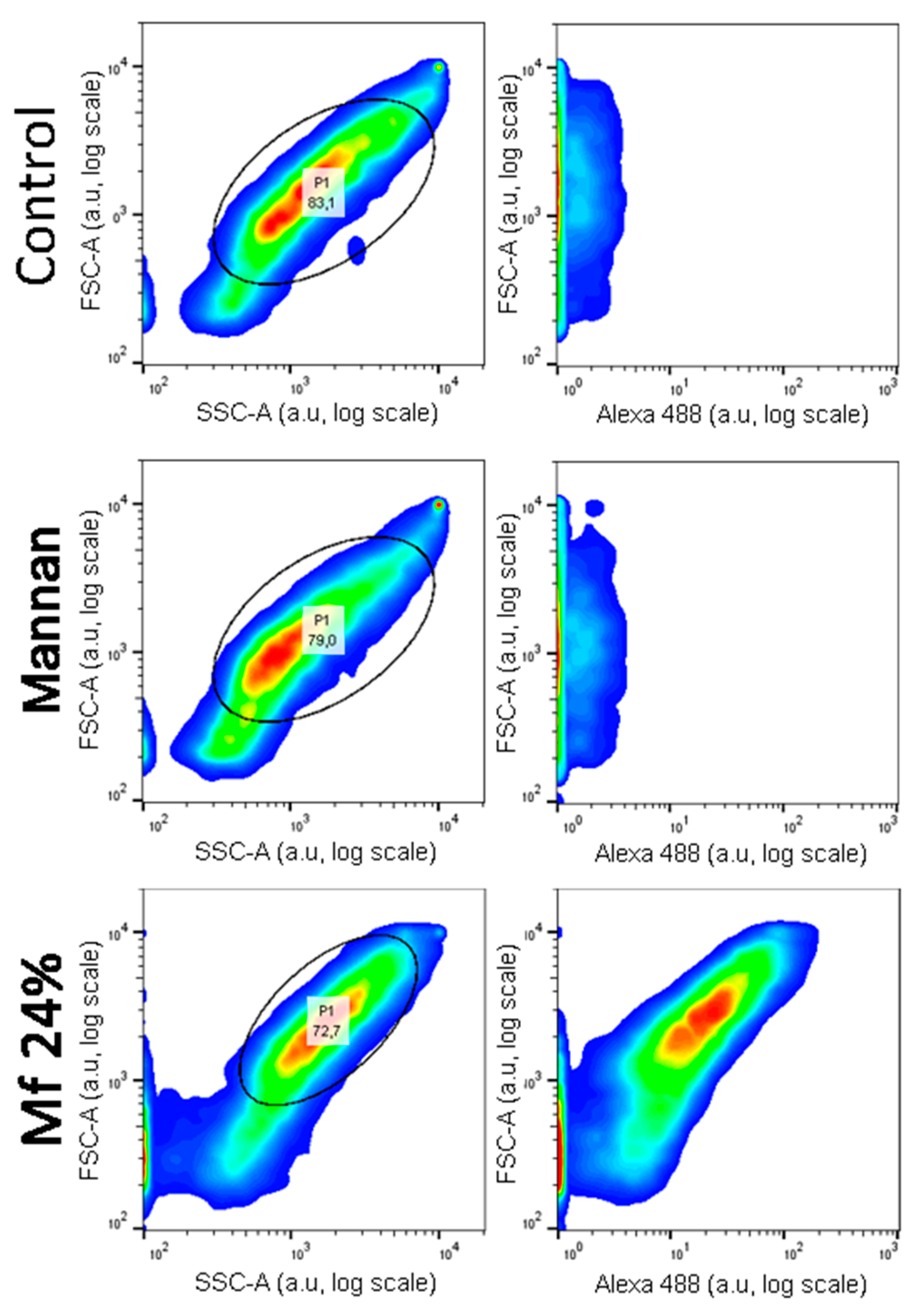
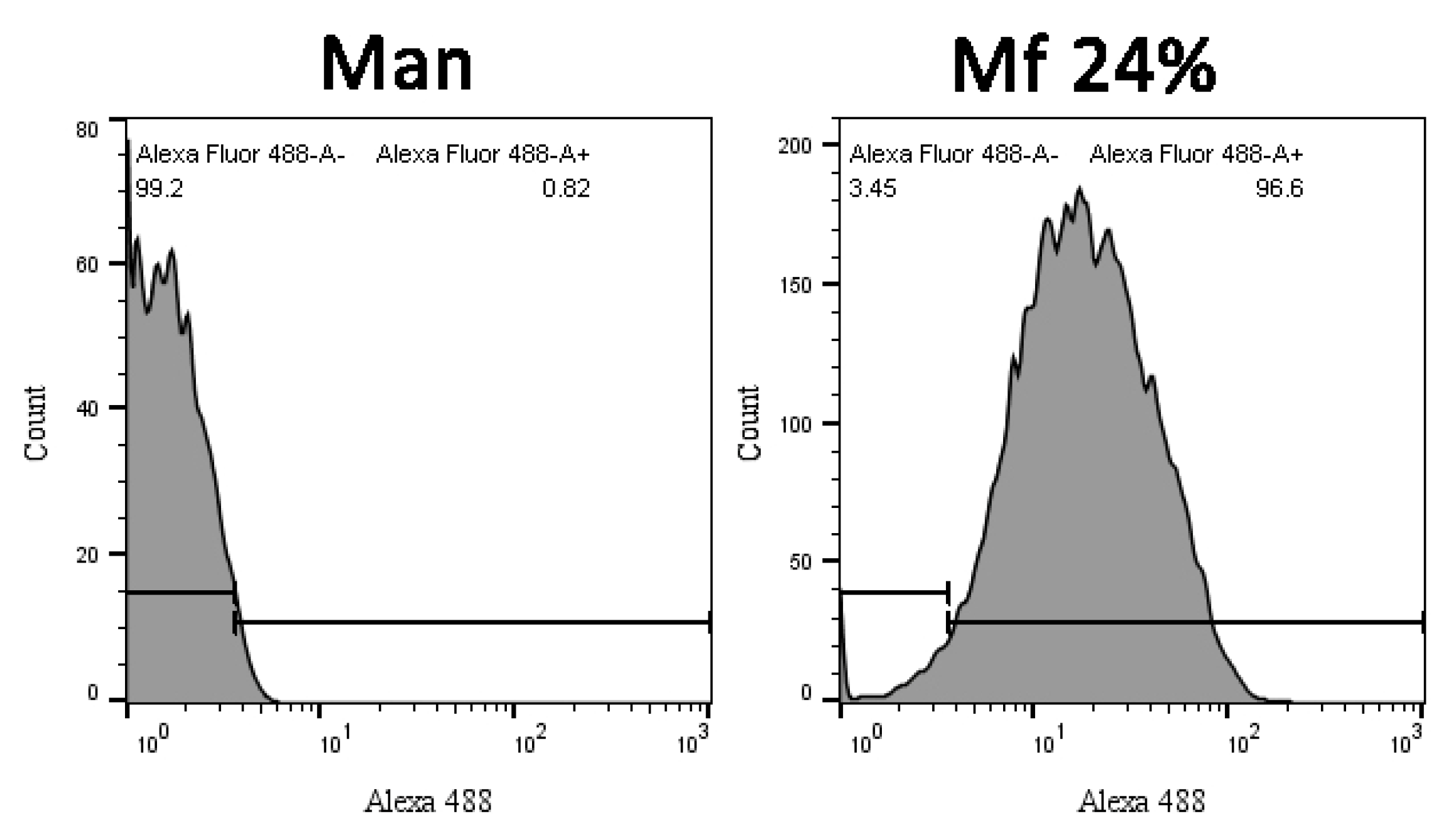
2.5. Additional Studies with the Novel Ligands
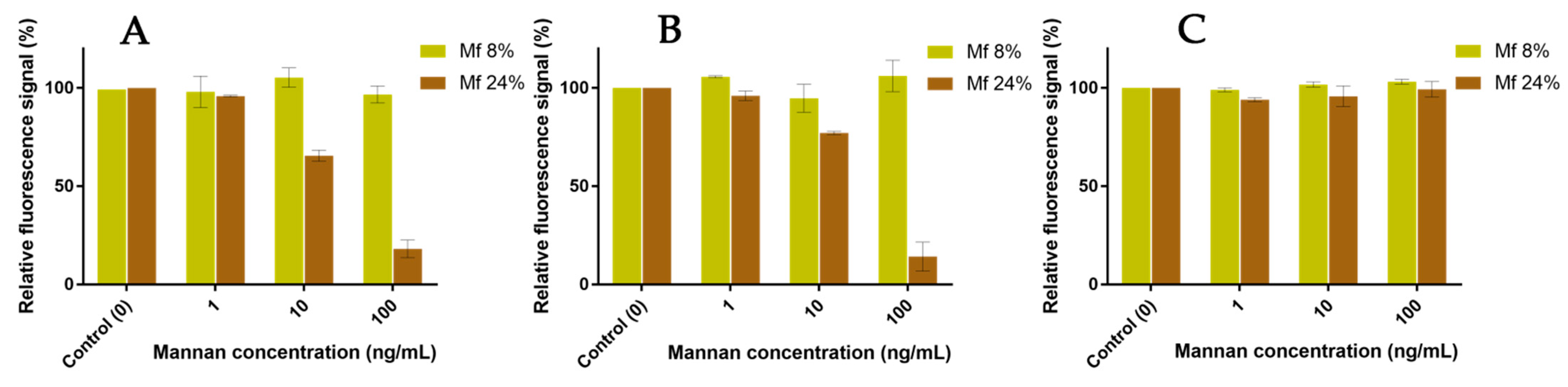
2.6. Competitions between Mannan Derivatives
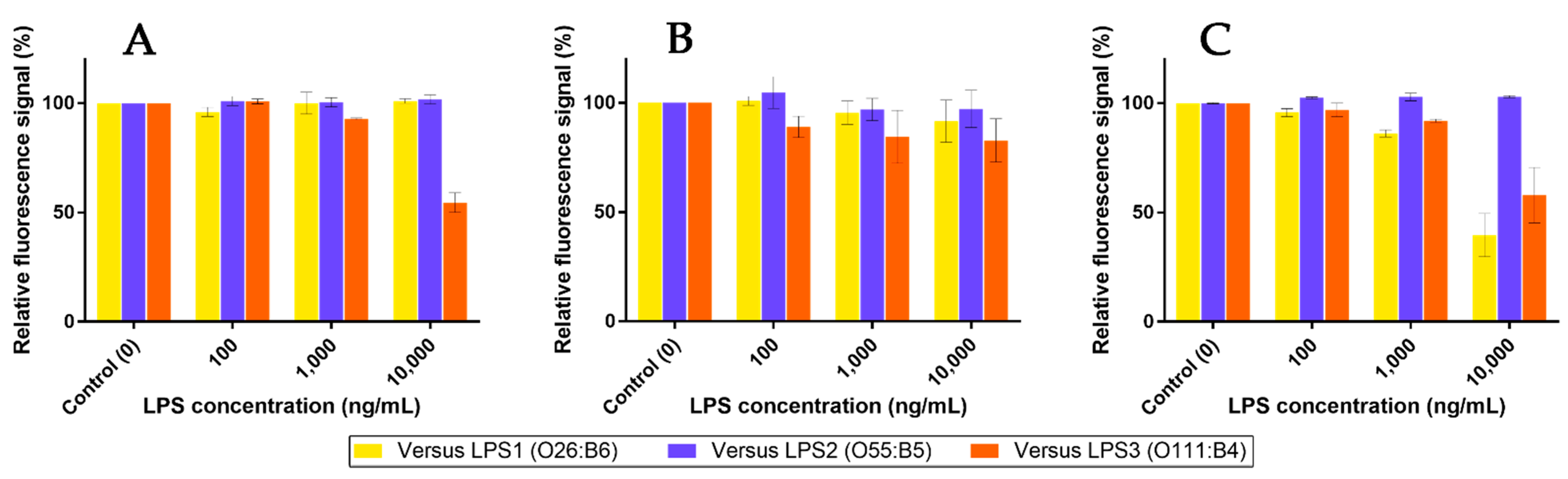
2.7. Competition Assays Using Endotoxins
3. Discussion
3.1. Preparation of the Fluorescent Ligands
3.2. Effect of the Oxidation on the Recognition of Mannan by Collectins
3.3. Recognition of the Fluorescent Ligands and Endotoxins
3.4. Two-Stage Competitions
4. Materials and Methods
4.1. Materials
4.2. Instrumentation
4.3. General Procedure for the TEMPO-Oxidation of Mannan (Mox)
4.4. Synthesis of Fluorescein Derivative and Fluorescent Mannan
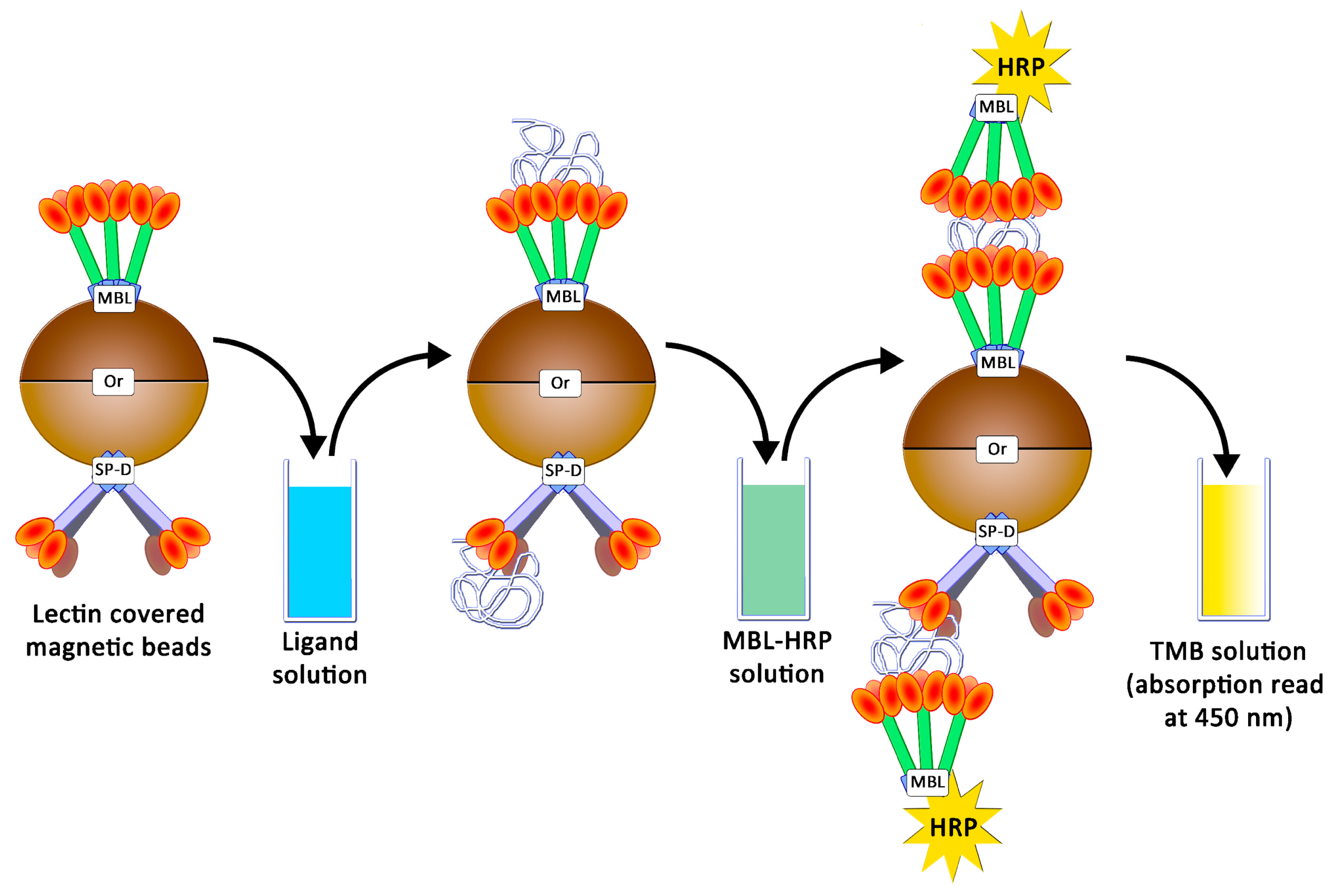
4.5. Preparation of Magnetic Beads and Avidity Assays
4.6. Competitions between Fluorescent Mannan Derivatives and Others Glycosyl Structures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.I.; Park, S. Sepsis: Early Recognition and Optimized Treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Wesener, D.A.; Dugan, A.; Kiessling, L.L. Recognition of Microbial Glycans by Soluble Human Lectins. Curr. Opin. Struct. Biol. 2017, 44, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Holmskov, U.; Thiel, S.; Jensenius, J.C. Collectins and Ficolins: Humoral Lectins of the Innate Immune Defense. Annu. Rev. Immunol. 2003, 21, 547–578. [Google Scholar] [CrossRef] [PubMed]
- Colin Hughes, R. Lectins as Cell Adhesion Molecules. Curr. Opin. Struct. Biol. 1992, 2, 687–692. [Google Scholar] [CrossRef]
- Słomińska-Wojewódzka, M.; Sandvig, K. The Role of Lectin-Carbohydrate Interactions in the Regulation of ER-Associated Protein Degradation. Molecules 2015, 20, 9816–9846. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Tizard, I. Lectin-Carbohydrate Interaction in the Immune System. Vet. Immunol. Immunopathol. 1996, 55, 205–223. [Google Scholar] [CrossRef]
- Mann, D.A.; Kanai, M.; Maly, D.J.; Kiessling, L.L. Probing Low Affinity and Multivalent Interactions with Surface Plasmon Resonance: Ligands for Concanavalin A. J. Am. Chem. Soc. 1998, 120, 10575–10582. [Google Scholar] [CrossRef]
- Neth, O.; Jack, D.L.; Dodds, A.W.; Holzel, H.; Klein, N.J.; Turner, M.W. Mannose-Binding Lectin Binds to a Range of Clinically Relevant Microorganisms and Promotes Complement Deposition. Infect. Immun. 2000, 68, 688. [Google Scholar] [CrossRef] [Green Version]
- Drickamer, K.; Taylor, M.E. Recent Insights into Structures and Functions of C-Type Lectins in the Immune System. Curr. Opin. Struct. Biol. 2015, 34, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Weis, W.I.; Drickamer, K.; Hendrickson, W.A. Structure of a C-Type Mannose-Binding Protein Complexed with an Oligosaccharide. Nature 1992, 360, 127–134. [Google Scholar] [CrossRef]
- Wang, H.; Head, J.; Kosma, P.; Brade, H.; Müller-Loennies, S.; Sheikh, S.; McDonald, B.; Smith, K.; Cafarella, T.; Seaton, B.; et al. Recognition of Heptoses and the Inner Core of Bacterial Lipopolysaccharides by Surfactant Protein D. Biochemistry 2008, 47, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Wehle, M.; Geissner, A.; Crouch, E.C.; Kang, Y.; Yang, Y.; Anish, C.; Santer, M.; Seeberger, P.H. Structure Binding Relationship of Human Surfactant Protein D and Various Lipopolysaccharide Inner Core Structures. J. Struct. Biol. 2016, 195, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.C.; Gordon, S. Dectin-1 is A Major β-Glucan Receptor on Macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkaš, P.; Čížová, A.; Bystrický, P.; Paulovičová, L.; Paulovičová, E.; Bystrický, S. One-Pot Preparation of Labelled Mannan–Peptide Conjugate, Model for Immune Cell Processing. Glycoconj. J. 2016, 33, 113–120. [Google Scholar] [CrossRef]
- Rabyk, M.; Galisova, A.; Jirotova, M.; Patsula, V.; Srbova, L.; Loukotova, L.; Parnica, J.; Jirak, D.; Stepanek, P.; Hruby, M. Mannan-Based Conjugates as a Multimodal Imaging Platform for Lymph Nodes. J. Mater. Chem. B 2018, 6, 2584–2596. [Google Scholar] [CrossRef]
- O’Connor, B.F.; Monaghan, D.; Cawley, J. Lectin Affinity Chromatography (LAC). In Protein Chromatography: Methods and Protocols; Methods in Molecular Biology; Walls, D., Loughran, S.T., Eds.; Springer: New York, NY, USA, 2017; pp. 411–420. ISBN 978-1-4939-6412-3. [Google Scholar]
- Kang, J.H.; Super, M.; Yung, C.W.; Cooper, R.M.; Domansky, K.; Graveline, A.R.; Mammoto, T.; Berthet, J.B.; Tobin, H.; Cartwright, M.J.; et al. An Extracorporeal Blood-Cleansing Device for Sepsis Therapy. Nat. Med. 2014, 20, 1211–1216. [Google Scholar] [CrossRef]
- Cartwright, M.; Rottman, M.; Shapiro, N.I.; Seiler, B.; Lombardo, P.; Gamini, N.; Tomolonis, J.; Watters, A.L.; Waterhouse, A.; Leslie, D.; et al. A Broad-Spectrum Infection Diagnostic That Detects Pathogen-Associated Molecular Patterns (PAMPs) in Whole Blood. EBioMedicine 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Beshr, G.; Sommer, R.; Hauck, D.; Siebert, D.C.B.; Hofmann, A.; Imberty, A.; Titz, A. Development of a Competitive Binding Assay for the Burkholderia cenocepacia Lectin BC2L-A and Structure Activity Relationship of Natural and Synthetic Inhibitors. Med. Chem. Commun. 2016, 7, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Sörme, P.; Kahl-Knutsson, B.; Huflejt, M.; Nilsson, U.J.; Leffler, H. Fluorescence Polarization as an Analytical Tool to Evaluate Galectin–Ligand Interactions. Anal. Biochem. 2004, 334, 36–47. [Google Scholar] [CrossRef]
- Marshall, J.C.; Walker, P.M.; Foster, D.M.; Harris, D.; Ribeiro, M.; Paice, J.; Romaschin, A.D.; Derzko, A.N. Measurement of Endotoxin Activity in Critically Ill Patients Using Whole Blood Neutrophil Dependent Chemiluminescence. Crit. Care 2002, 6, 342–348. [Google Scholar] [CrossRef]
- Gustot, T. Multiple Organ Failure in Sepsis: Prognosis and Role of Systemic Inflammatory Response. Curr. Opin. Crit. Care 2011, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Elshal, M.F.; McCoy, J.P. Multiplex Bead Array Assays: Performance Evaluation and Comparison of Sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Spier, V.C.; Sierakowski, M.R.; Reed, W.F.; de Freitas, R.A. Polysaccharide Depolymerization from TEMPO-Catalysis: Effect of TEMPO Concentration. Carbohydr. Polym. 2017, 170, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, F.; Ouk, T.-S.; Grenier, K.; Joly, N.; Lequart, V.; Sol, V. Enhancement of Photobactericidal Activity of Chlorin-E6-Cellulose Nanocrystals by Covalent Attachment of Polymyxin B. J. Mater. Chem. B 2017, 5, 6953–6962. [Google Scholar] [CrossRef]
- Isogai, A.; Bergström, L. Preparation of Cellulose Nanofibers Using Green and Sustainable Chemistry. Curr. Opin. Green Sustain. Chem. 2018, 12, 15–21. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic Oxidation of Cellulose with Nitroxyl Radicals under Aqueous Conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Cawley, T.N.; Ballou, C.E. Identification of Two Saccharomyces Cerevisiae Cell Wall Mannan Chemotypes. J. Bacteriol. 1972, 111, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, C.N.; Sierakowski, M.R.; Lucyszyn, N.; de Freitas, R.A. TEMPO-Mediated Oxidation on Galactomannan: Gal/Man Ratio and Chain Flexibility Dependence. Carbohydr. Polym. 2016, 153, 371–378. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular PH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Le Guern, F.; Mussard, V.; Gaucher, A.; Rottman, M.; Prim, D. Fluorescein Derivatives as Fluorescent Probes for PH Monitoring along Recent Biological Applications. Int. J. Mol. Sci. 2020, 21, 9217. [Google Scholar] [CrossRef] [PubMed]
- Hillaire, M.L.B.; van Eijk, M.; Vogelzang-van Trierum, S.E.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Haagsman, H.P.; Rimmelzwaan, G.F. Recombinant Porcine Surfactant Protein D Inhibits Influenza A Virus Replication Ex Vivo. Virus Res. 2014, 181, 22–26. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, M.; White, M.R.; Crouch, E.C.; Batenburg, J.J.; Vaandrager, A.B.; Van Golde, L.M.G.; Haagsman, H.P.; Hartshorn, K.L. Porcine Pulmonary Collectins Show Distinct Interactions with Influenza A Viruses: Role of the N-Linked Oligosaccharides in the Carbohydrate Recognition Domain. J. Immunol. 2003, 171, 1431–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eijk, M.; Rynkiewicz, M.J.; Khatri, K.; Leymarie, N.; Zaia, J.; White, M.R.; Hartshorn, K.L.; Cafarella, T.R.; van Die, I.; Hessing, M.; et al. Lectin-Mediated Binding and Sialoglycans of Porcine Surfactant Protein D Synergistically Neutralize Influenza A Virus. J. Biol. Chem. 2018, 293, 10646–10662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teillet, F.; Dublet, B.; Andrieu, J.-P.; Gaboriaud, C.; Arlaud, G.J.; Thielens, N.M. The Two Major Oligomeric Forms of Human Mannan-Binding Lectin: Chemical Characterization, Carbohydrate-Binding Properties, and Interaction with MBL-Associated Serine Proteases. J. Immunol. 2005, 174, 2870–2877. [Google Scholar] [CrossRef] [Green Version]
- Kjaer, T.R.; Jensen, L.; Hansen, A.; Dani, R.; Jensenius, J.C.; Dobó, J.; Gál, P.; Thiel, S. Oligomerization of Mannan-Binding Lectin Dictates Binding Properties and Complement Activation. Scand. J. Immunol. 2016, 84, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Super, M.; Cartwright, M.J.; Rottman, M.; Tomolonis, J. Methods and Compositions for Improving Detection and/or Capture of a Target Entity. WO 2014/144325 A1, 18 September 2014. [Google Scholar]
- van Eijk, M.; Bruinsma, L.; Hartshorn, K.L.; White, M.R.; Rynkiewicz, M.J.; Seaton, B.A.; Hemrika, W.; Romijn, R.A.; van Balkom, B.W.; Haagsman, H.P. Introduction of N-Linked Glycans in the Lectin Domain of Surfactant Protein D: Impact On Interactions With Influenza A Viruses. J. Biol. Chem. 2011, 286, 20137–20151. [Google Scholar] [CrossRef] [Green Version]
- van Eijk, M.; Hillaire, M.L.B.; Rimmelzwaan, G.F.; Rynkiewicz, M.J.; White, M.R.; Hartshorn, K.L.; Hessing, M.; Koolmees, P.A.; Tersteeg, M.H.; van Es, M.H.; et al. Enhanced Antiviral Activity of Human Surfactant Protein D by Site-Specific Engineering of the Carbohydrate Recognition Domain. Front. Immunol. 2019, 10, 2476. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Guern, F.; Gaucher, A.; Cosentino, G.; Lagune, M.; Haagsman, H.P.; Roux, A.-L.; Prim, D.; Rottman, M. Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families. Int. J. Mol. Sci. 2022, 23, 16067. https://doi.org/10.3390/ijms232416067
Le Guern F, Gaucher A, Cosentino G, Lagune M, Haagsman HP, Roux A-L, Prim D, Rottman M. Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families. International Journal of Molecular Sciences. 2022; 23(24):16067. https://doi.org/10.3390/ijms232416067
Chicago/Turabian StyleLe Guern, Florent, Anne Gaucher, Gina Cosentino, Marion Lagune, Henk P. Haagsman, Anne-Laure Roux, Damien Prim, and Martin Rottman. 2022. "Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families" International Journal of Molecular Sciences 23, no. 24: 16067. https://doi.org/10.3390/ijms232416067
APA StyleLe Guern, F., Gaucher, A., Cosentino, G., Lagune, M., Haagsman, H. P., Roux, A. -L., Prim, D., & Rottman, M. (2022). Labeled TEMPO-Oxidized Mannan Differentiates Binding Profiles within the Collectin Families. International Journal of Molecular Sciences, 23(24), 16067. https://doi.org/10.3390/ijms232416067





