New Insights into MdSPS4-Mediated Sucrose Accumulation under Different Nitrogen Levels Revealed by Physiological and Transcriptomic Analysis
Abstract
:1. Introduction
2. Results and Discussion
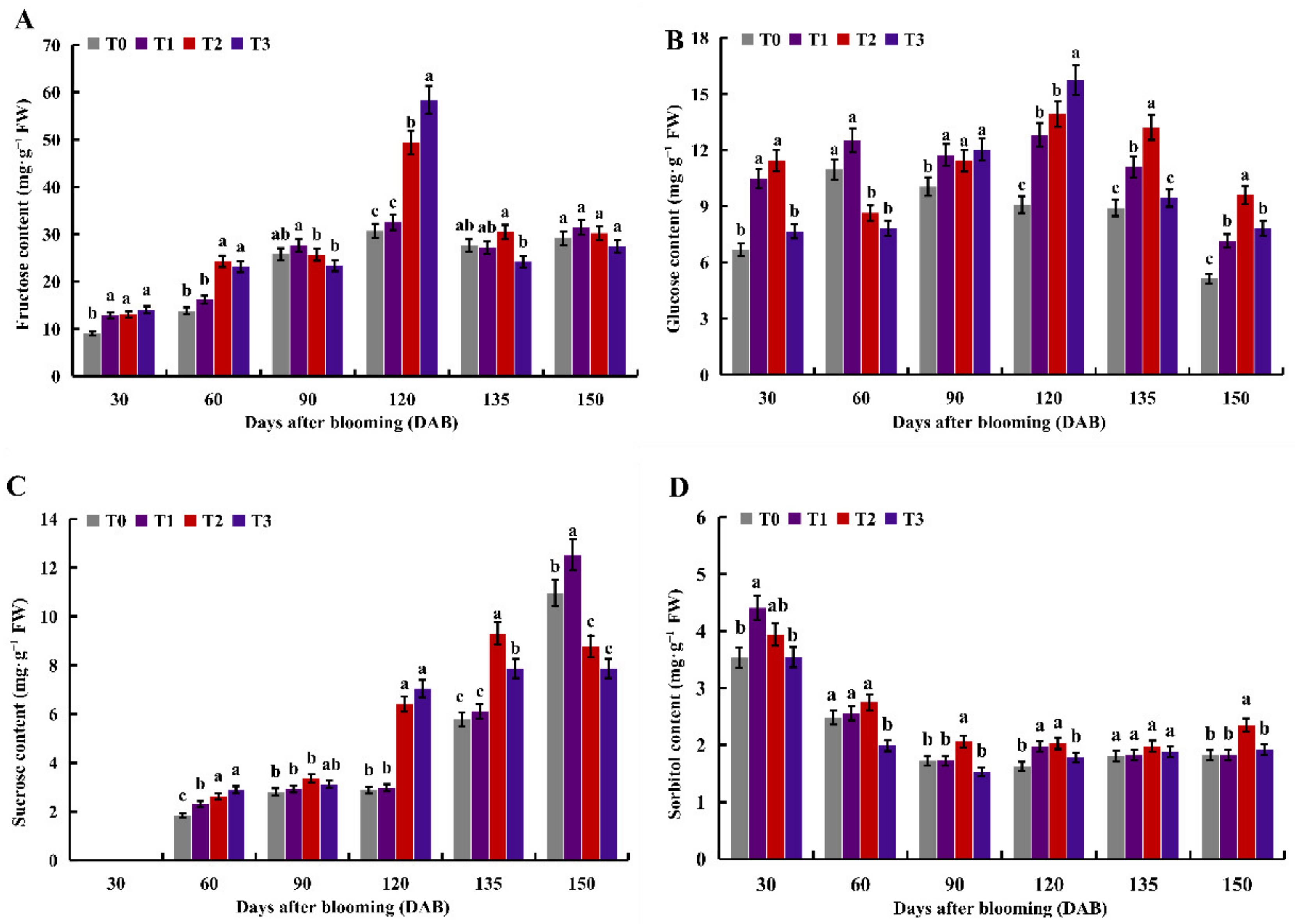
2.1. Effects of Different Nitrogen Levels on Soluble Sugar
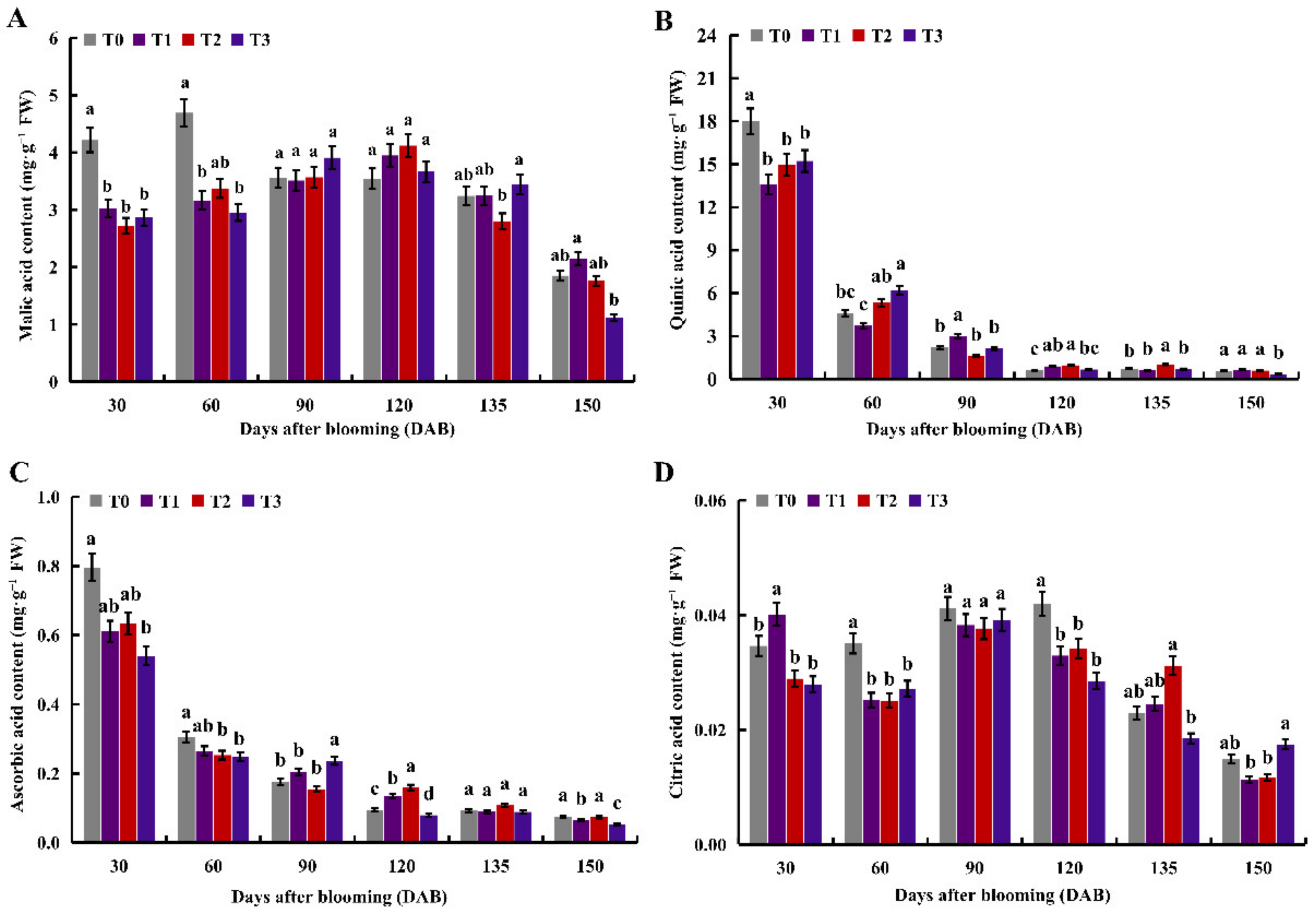
2.2. The Effects of under Different Nitrogen Levels on Organic Acids
2.3. Transcriptome Sequencing Analysis
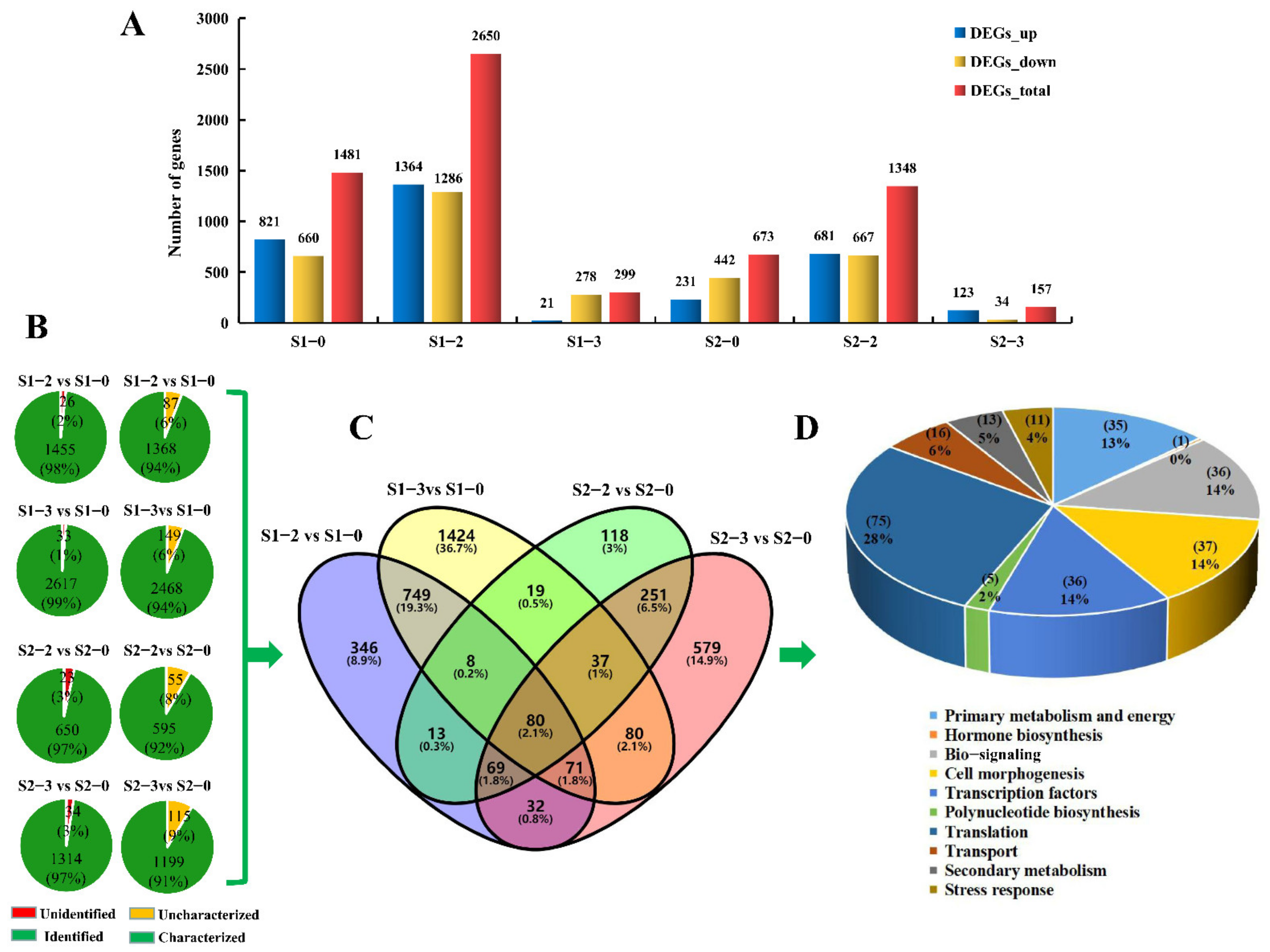
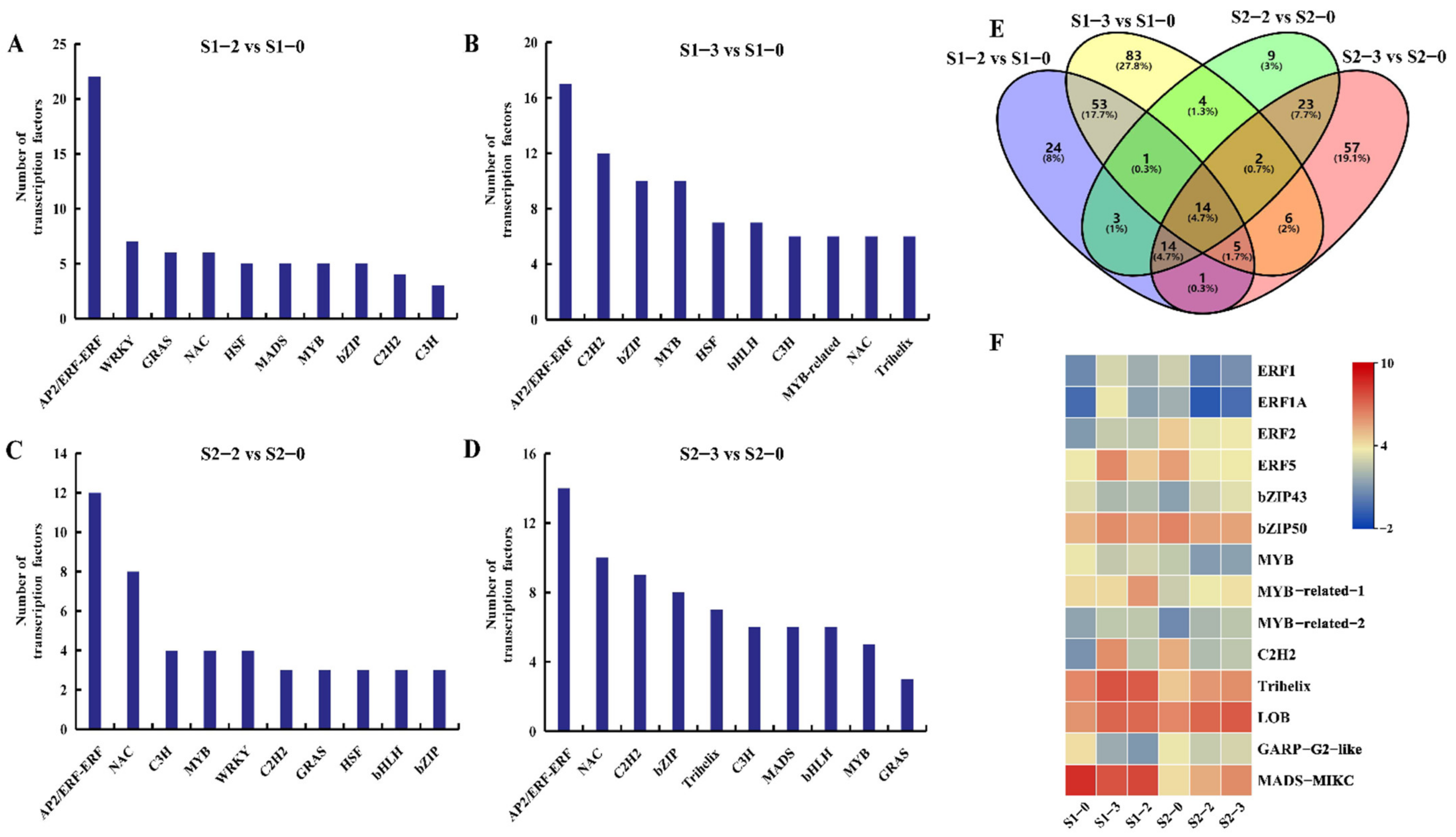
2.4. DEGs Analysis at Different Development Stages under Different Nitrogen Levels
2.5. GO Classification Analysis
2.6. KEGG Annotation Analysis
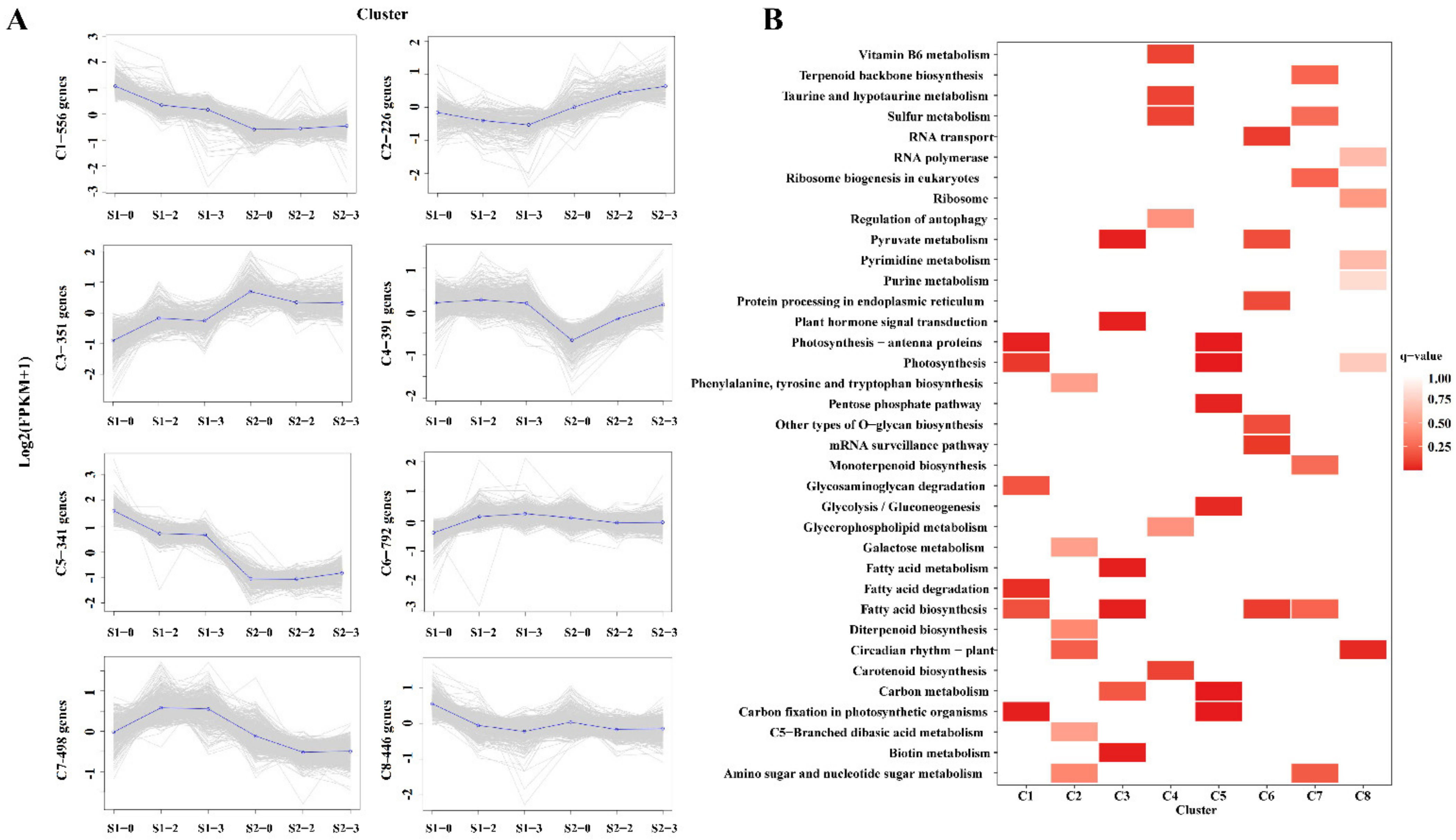
2.7. Expression Pattern and Functional Analysis of the DEGs in Different Nitrogen Levels
2.8. Differentially Expression Transcription Factors (TFs) under Different Nitrogen Levels
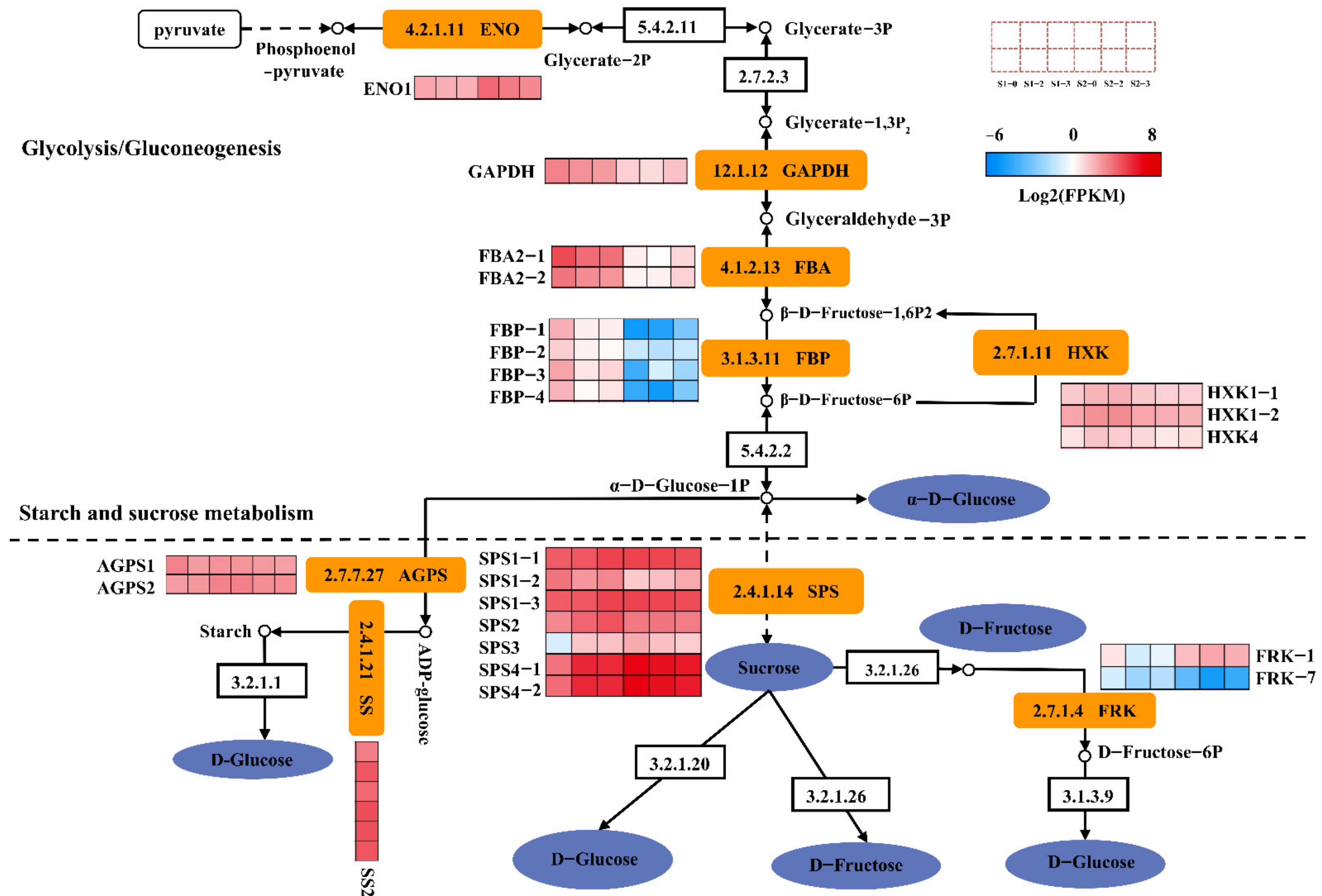
2.9. DEGs Involved in Sugar Metabolism
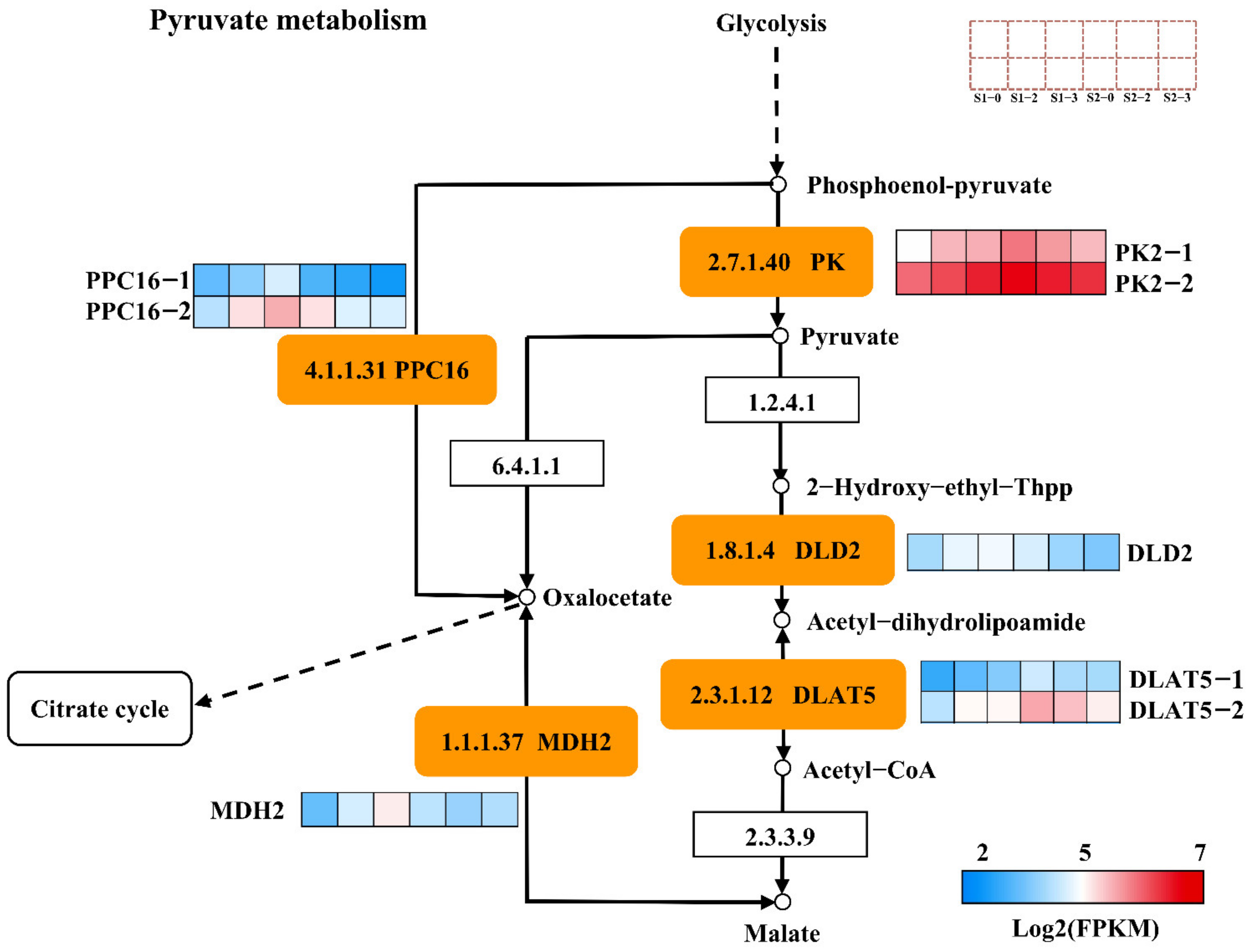
2.10. DEGs Associated with Pyruvate Metabolism
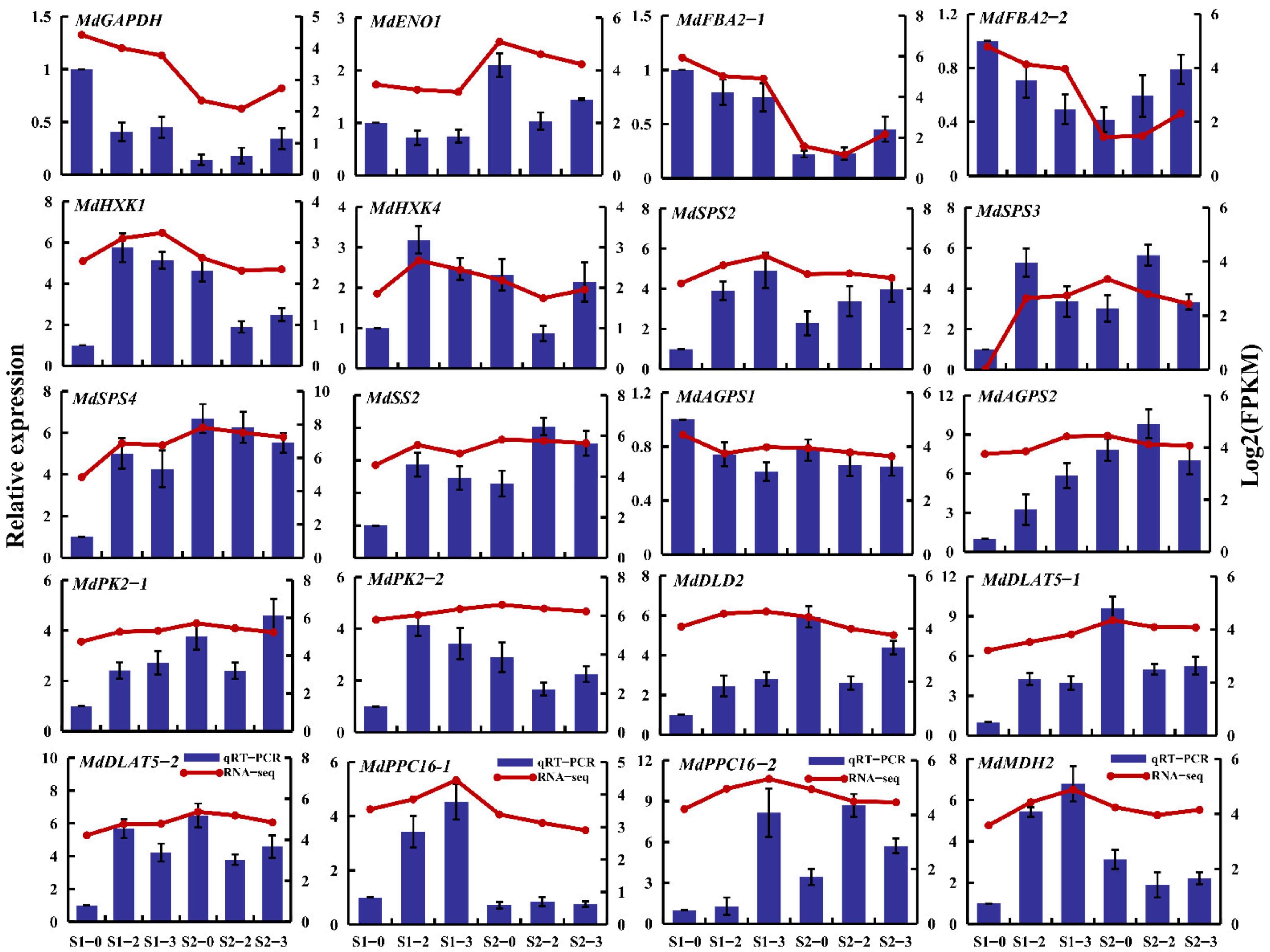
2.11. RNA-Seq Expression Validation by qRT-PCR
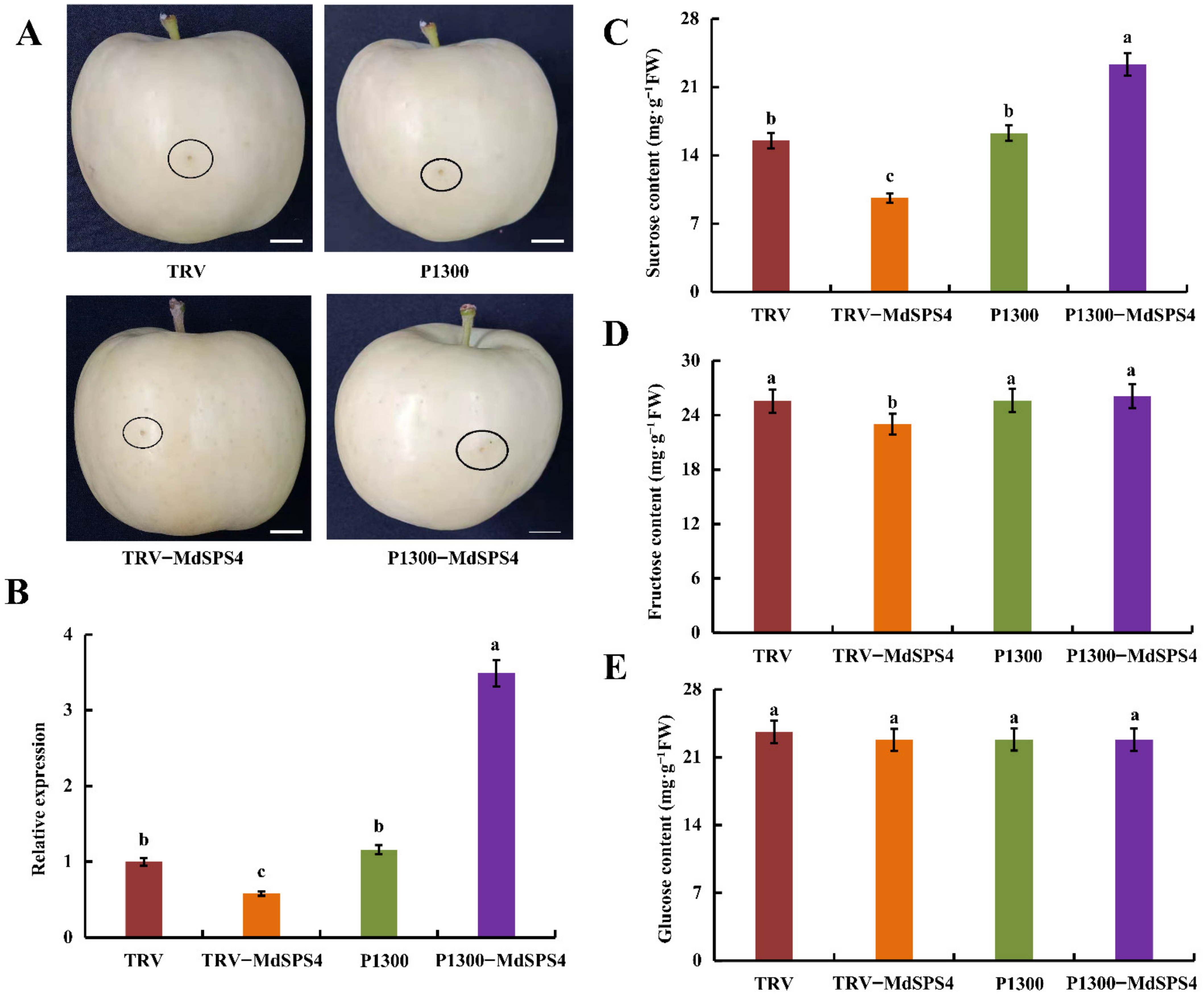
2.12. MdSPS4 Promotes Sucrose Accumulation in Apple Fruit
2.13. MdSPS4 Enhanced the Sucrose Content of Apple Callus in Response to Nitrogen
3. Materials and Methods
3.1. Plant Materials and Treatments
3.2. Determination of Soluble Sugars and Organic Acids
3.3. RNA Sequencing Analysis
3.4. Differentially Expression Analysis
3.5. Gene Functional Annotation
3.6. Validation of DEGs by qRT-PCR
3.7. Construction of the Plasmid and Genetic Transformation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sete, P.B.; Comin, J.J.; Ciotta, N.; Salume, J.A.; Brunetto, G. Nitrogen fertilization affects yield and fruit quality in pear. Sci. Hortic. 2019, 258, 108782. [Google Scholar] [CrossRef]
- Ruffel, S.; Gojon, A.; Lejay, L. Signal interactions in the regulation of root nitrate uptake. J. Exp. Bot. 2014, 65, 5509–5517. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sasaki, Y.; Li, X.; Mori, I.C.; Matsuura, T.; Hirayama, T.; Sato, T.; Yamaguchi, J. ABI1 regulates carbon/nitrogen-nutrient signal transduction independent of ABA biosynthesis and canonical ABA signalling pathways in Arabidopsis. J. Exp. Bot. 2015, 9, 2763–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, E.A.; Alvarez, J.M.; Viviana, A.; Eleodoro, R.; Brooks, M.D.; Gabriel, K.; Sandri, R.; Laurence, L. Nitrate in 2020: Thirty years from transport to signaling networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.L.; Zhao, X.R.; Yang, S.H.; Mulder, J.; Dörsch, P.; Zhang, G.L. Nitrate leaching and N accumulation in a typical subtropical red soil with N fertilization. Geoderma 2022, 407, 115559. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, B.; Chu, C. Towards understanding the hierarchical nitrogen signalling network in plants. Curr. Opin. Plant Biol. 2020, 55, 60–65. [Google Scholar] [CrossRef]
- Anur, R.M.; Mufithah, N.; Sawitri, W.D.; Sakakibara, H.; Sugiharto, B. Overexpression of sucrose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants 2020, 9, 200. [Google Scholar] [CrossRef] [Green Version]
- Sievenpiper, J.L.; Souza, D.; Russell, J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 291–304. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Chi, Y.H.; Wang, J.Z.; Zhou, J.X.; Cheng, Y.S.; Zhang, B.L.; Ma, A.; Vanitha, J.; Ramachandran, S. Sucrose metabolism gene families and their biological functions. Sci. Rep. 2015, 5, 17583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dun, B.Q.; Zhao, X.N.; Yue, M.Q.; Lu, M.; Li, G.Y. Correlation analysis between the key enzymes activies and sugar content in sweet sorghum (’Sorghum bicolor’ L. Moench) stems at physiological maturity state. Aust. J. Crop Sci. 2013, 7, 84–92. [Google Scholar]
- Bilska-Kos, A.; Mytych, J.; Suski, S.; Magoń, J.; Zebrowski, J. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of Miscanthus × giganteus and Zea mays at low temperature. Planta 2020, 23, 252. [Google Scholar] [CrossRef] [PubMed]
- Solís-Guzmán, M.; Argüello-Astorga, G.; López-Bucio, J.; Ruiz-Herrera, L.; López-Meza, J.; Sánchez-Calderón, L.; Carreón-Abud, Y.; Martínez-Trujillo, M. Arabidopsis thaliana sucrose phosphate synthase (sps) genes are expressed differentially in organs and tissues, and their transcription is regulated by osmotic stress. Gene Expr. Patterns 2017, 25, 92–101. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, H.; Zhang, Z.; Shi, J.; Timko, M.P.; Du, Y.; Liu, X.; Liu, Y. Organ- and growing stage-specific expression of solanesol biosynthesis genes in Nicotiana tabacum reveals their association with solanesol content. Molecules 2016, 21, 1536. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zeng, D.; Liu, Y.; Zhu, W. SlSPS, a sucrose phosphate synthase gene, mediates plant growth and thermotolerance in tomato. Horticulturae 2022, 8, 491. [Google Scholar] [CrossRef]
- Falter, C.; Voigt, C.A. Improving biomass production and saccharification in Brachypodium distachyon through overexpression of a sucrose-phosphate synthase from sugarcane. J. Plant Biochem. Biotechnol. 2016, 25, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Maloney, V.J.; Park, J.Y.; Unda, F.; Mansfield, S.D. Sucrose phosphate synthase and sucrose phosphate phosphatase interact in planta and promote plant growth and biomass accumulation. J. Exp. Bot. 2015, 66, 4383–4394. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Luo, Y.; Kong, X.; Huang, C.; Wang, Z. Interactions between exogenous cytokinin and nitrogen application regulate tiller bud growth via sucrose and nitrogen allocation in winter wheat. J. Plant Growth Regul. 2020, 40, 329–341. [Google Scholar] [CrossRef]
- Seger, M.; Gebril, S.; Tabilona, J.; Peel, A.; Sengupta-Gopalan, C. Impact of concurrent overexpression of cytosolic glutamine synthetase (GS1) and sucrose phosphate synthase (SPS) on growth and development in transgenic tobacco. Planta 2015, 241, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Baroja-Fernández, E.; Ricarte-Bermejo, A.; Sánchez-López, Á.M.; Muñoz, F.J.; Romero, J.M.; Ruiz, M.T.; Baslam, M.; Almagro, G.; Sesma, M.T.; et al. Characterization of multiple SPS knockout mutants reveals redundant functions of the four Arabidopsis sucrose phosphate synthase isoforms in plant viability, and strongly indicates that enhanced respiration and accelerated starch turnover can alleviate the blockage of sucrose biosynthesis. Plant Science 2015, 238, 135–147. [Google Scholar] [PubMed] [Green Version]
- Li, M.J.; Feng, F.J.; Cheng, L.L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tworkoski, T.; Fazio, G. Effects of Size-Controlling Apple Rootstocks on Growth, Abscisic Acid, and Hydraulic Conductivity of Scion of Different Vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Han, M.; Sun, Q.; Zhou, J.; Qiu, H.; Guo, J.; Lu, L.; Mu, W.; Sun, J. Insertion of a solo LTR retrotransposon associates with spur mutations in ‘Red Delicious’ apple (Malus × domestica). Plant Cell Rep. 2017, 36, 1375–1385. [Google Scholar] [CrossRef]
- Yang, M.; Wang, S.; Zhao, X.; Gao, X.; Liu, S. Soil properties of apple orchards on china’s loess plateau. Sci. Total Environ. 2020, 723, 138041. [Google Scholar] [CrossRef]
- Cavalcante, T.H.; Cunha, M.; Rocha, L.; Santos, E.M.; Silva, G. Physiological indexes of custard apple as a function of nitrogen fertilization and humic substances. Rev. Ciênc. Agrár. 2014, 57, 85–89. [Google Scholar] [CrossRef]
- Liao, L.; Dong, T.T.; Qiu, X.; Rong, Y.; Wang, Z.H.; Zhu, J. Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit. PLoS ONE 2019, 14, e0223356. [Google Scholar] [CrossRef]
- Fallahi, E.; Eichert, T. Principles and practices of foliar nutrients with an emphasis on nitrogen and calcium sprays in apple. HortTechnology 2013, 23, 542–547. [Google Scholar] [CrossRef]
- Vinod, K.J.; Vikas, K. Influence of different sugar sources, nitrogen sources and inocula on the quality characteristics of apple tea wine. J. Inst. Brew. 2017, 2, 268–276. [Google Scholar]
- Wang, F.; Ge, S.; Xu, X.; Xing, Y.; Jiang, Y. Multiomics analysis reveals new insights into the apple fruit quality decline under high nitrogen conditions. J. Agric. Food Chem. 2021, 69, 19. [Google Scholar] [CrossRef] [PubMed]
- Sokri, S.M.; Babalar, M.; Barker, A.V.; Lesani, H. Fruit quality and nitrogen, potassium, and calcium content of apple as influenced by nitrate: Ammonium ratios in tree nutrition. J. Plant Nutr. 2014, 38, 1619–1627. [Google Scholar] [CrossRef]
- Gao, Q.H.; Jia, S.S.; Miao, Y.M.; Lu, X.M.; Li, H.M. Effects of exogenous melatonin on nitrogen metabolism and osmotic adjustment substances of melon seedlings under sub-low temperature. J. Appl. Ecol. 2016, 27, 519–524. [Google Scholar]
- Minikaev, D.; Zurgel, U.; Tripler, E.; Gelfand, I. Effect of increasing nitrogen fertilization on soil nitrous oxide emissions and nitrate leaching in a young date palm (Phoenix dactylifera L.; cv. Medjool) orchard. Agric. Ecosyst. Environ. 2021, 319, 107569. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié, A.; Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [Green Version]
- Famiani, F.; Bonghi, C.; Chen, Z.H.; Drincovich, M.F.; Walker, R.P. Stone fruits: Growth and nitrogen and organic acid metabolism in the fruits and seeds—A review. Front. Plant Sci. 2020, 11, 572601. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Gu, K.D.; Cheng, L.; Wang, J.H.; Yu, J.Q.; Wang, X.F. BTB-TAZ Domain Protein MdBT2 Modulates Malate Accumulation and Vacuolar Acidification in Response to Nitrate. Plant Physiol. 2020, 183, 750–764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, H.; Yi, H.; Zhai, W.; Wang, G.; Fu, Q. Transcriptome profiling of Cucumis melo fruit development and ripening. Hortic. Res. 2016, 3, 16014. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Jia, W. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef]
- Leida, C.; Moser, C.; Esteras, C.; Sulpice, R.; Lunn, J.E.; Langen, F.; Monforte, A.J.; Picó, B. Variability of candidate genes, genetic structure and association with sugar accumulation and climacteric behavior in a broad germplasm collection of melon (Cucumis melo L.). BMC Genet. 2015, 16, 28. [Google Scholar] [CrossRef] [Green Version]
- Chayut, N.; Yuan, H.; Ohali, S.; Meir, A.; Yeselson, Y.; Portnoy, V.; Zheng, Y.; Fei, Z.J.; Lewinsohn, E.; Katzir, N.; et al. A bulk segregant transcriptome analysis reveals metabolic and cellular processes associated with Orange allelic variation and fruit β-carotene accumulation in melon fruit. BMC Plant Biol. 2015, 15, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Zhao, H.; Zheng, L.; Zhang, L.; Peng, Y.; Ma, W.; Tian, R.; Yua, Y.; Ma, F.; Li, M. Overexpression of the apple Ma12, a mitochondrial pyrophosphatase pump gene, leads to malic acid accumulation and the upregulation of malate dehydrogenase in tomato and apple calli. Hortic. Res. 2022, 9, uhab053. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Jeena, G.S.; Tripathi, V.; Shukla, R.K. MaRAP2-4, a waterlogging-responsive ERF, from Mentha, regulates bidirectional sugar transporter AtSWEET10 to modulate stress response in Arabidopsis. Plant Biotechnol. J. 2017, 16, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.J.; Wan, Q.Q.; Wang, Y.; Cheng, J.B.; Zhao, Y.; Zhu, W.S. A maize NAC transcription factor, ZmNAC34, negatively regulates starch synthesis in rice. Plant Cell Rep. 2019, 38, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Wang, Y.P.; Zhang, J.; Ren, Y.; Li, M.Y.; Tian, S.W.; Yu, Y.T.; Zuo, Y.; Gong, G.Y.; Zhang, H.Y.; et al. The NAC transcription factor ClNAC68 positively regulates sugar content and seed development in watermelon by repressing ClINV and ClGH3.6. Hortic. Res. 2021, 8, 1. [Google Scholar]
- Hu, D.G.; Sun, C.H.; Ma, J.; You, C.X.; Cheng, L.; Hao, Y.J. MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol. 2016, 170, 1315–1330. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.G.; Li, Y.Y.; Zhang, Q.Y.; Li, M.; Sun, C.H.; Yu, J.Q.; Hao, Y.J. The R2R3-MYB transcription factor Md MYB 73 is involved in malate accumulation and vacuolar acidification in apple. Plant J. 2017, 91, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Wiberley-Bradford, A.E.; Busse, J.S.; Jiang, J. Sugar metabolism, chip color, invertase activity, and gene expression during long-term cold storage of potato (Solanum tuberosum) tubers from wild-type and vacuolar invertase silencing lines of Katahdin. BMC Res. Notes 2014, 7, 801. [Google Scholar] [CrossRef] [Green Version]
- Liao, G.; Li, Y.; Wang, H.; Liu, Q.; Zhong, M.; Jia, D.; Huang, C.; Xu, X. Genome-wide identification and expression profiling analysis of sucrose synthase (sus) and sucrose phosphate synthase (sps) genes family in Actinidia chinensis and A. eriantha. BMC Plant Biol. 2022, 22, 215. [Google Scholar] [CrossRef]
- Fan, Y.; Qiu, L.; Chen, R.; Zhou, H.; Huang, X.; Lu, X. Effects of nitrogen application level on yield and sucrose content of different sugarcane cultivars. Sugar Crops China 2019, 41, 36–40. [Google Scholar]
- Ennab, H. Effect of Organic Manures, Biofertilizers and NPK on Vegetative Growth, Yield, Fruit Quality and Soil Fertility of Eureka Lemon Trees (Citrus limon (L.) Burm). J. Soil Sci. Agric. Eng. 2016, 7, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Zhu, Y.; Cao, S.; Wang, H.; Song, Y. Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food Bioprocess Technol. 2012, 6, 3490–3498. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, P.; Kang, T.L.; Xing, H.; Yang, D.L.; Zhang, J.L.; Par’e, P.W. Mapping podophyllotoxin biosynthesis and growth-related transcripts with high elevation in Sinopodophyllum hexandrum. Ind. Crop Prod. 2018, 124, 510–518. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, N.; Yuan, S.; Hai, Y.; Du, P.; Zhang, L. Identification of Differentially Expressed Genes for Disc Degeneration Using Gene Expression Profiling and Bioinformatics Analysis. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Deeplanaik, N.; Kumaran, R.C.; Venkatarangaiah, K.; Shivashankar, S.; Doddamani, D.; Telkar, S. Expression of drought responsive genes in pigeonpea and in silico comparison with soybean cdna library. J. Crop Sci. Biotechnol. 2013, 16, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Liu, J.; Huang, S.; Guo, T.; Deng, L.; Hua, W. Selection and evaluation of novel reference genes for quantitative reverse transcription pcr (qrt-pcr) based on genome and transcriptome data in Brassica napus L. Gene 2014, 538, 113–122. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Ma, F.F.; You, C.X.; Hu, D.G.; Hao, Y.J. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol. J. 2020, 19, 285–299. [Google Scholar] [CrossRef]

| DAB (Days) | N Fertilizer (kg·hm−2) | Clean Reads | Clean Bases | GC Content | %≥Q30 | Mapped Reads | Unique Match |
|---|---|---|---|---|---|---|---|
| 120 | 0 | 21,305,563 | 6,381,991,851 | 47.79% | 93.32% | 91.23 | 88.34 |
| 300 | 20,527,705 | 6,147,942,123 | 47.80% | 93.53% | 91.62 | 88.39 | |
| 600 | 21,972,666 | 6,581,184,203 | 47.67% | 93.37% | 91.51 | 88.17 | |
| 150 | 0 | 21,737,783 | 6,509,277,813 | 47.87% | 93.58% | 89.16 | 85.98 |
| 300 | 21,471,510 | 6,428,687,591 | 47.99% | 93.23% | 88.36 | 85.04 | |
| 600 | 20,316,939 | 6,085,634,954 | 48.04% | 93.37% | 90.92 | 87.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Li, W.; Wang, P.; Ma, Z.; Mao, J.; Chen, B. New Insights into MdSPS4-Mediated Sucrose Accumulation under Different Nitrogen Levels Revealed by Physiological and Transcriptomic Analysis. Int. J. Mol. Sci. 2022, 23, 16073. https://doi.org/10.3390/ijms232416073
Cao X, Li W, Wang P, Ma Z, Mao J, Chen B. New Insights into MdSPS4-Mediated Sucrose Accumulation under Different Nitrogen Levels Revealed by Physiological and Transcriptomic Analysis. International Journal of Molecular Sciences. 2022; 23(24):16073. https://doi.org/10.3390/ijms232416073
Chicago/Turabian StyleCao, Xuejing, Wenfang Li, Ping Wang, Zonghuan Ma, Juan Mao, and Baihong Chen. 2022. "New Insights into MdSPS4-Mediated Sucrose Accumulation under Different Nitrogen Levels Revealed by Physiological and Transcriptomic Analysis" International Journal of Molecular Sciences 23, no. 24: 16073. https://doi.org/10.3390/ijms232416073





