Contingent Synergistic Interactions between Non-Coding RNAs and DNA-Modifying Enzymes in Myelodysplastic Syndromes
Abstract
1. Introduction
2. The Course of Aberrant DNA Methylation Patterns in MDS
3. Long Non-Coding RNAs and MicroRNA Species’ Involvement in MDS
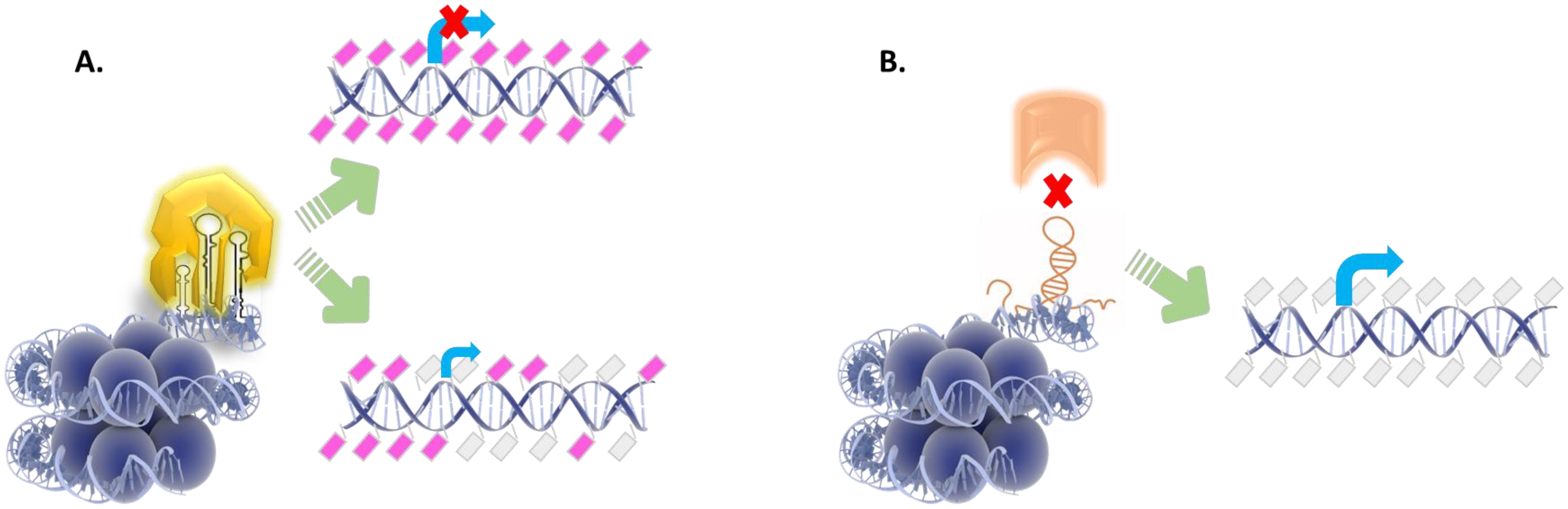
4. Non-Coding RNA Species Cooperate with DNA Modifying Enzymes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 5hmC | 5-hydroxy-methylcytocine |
| 5mC | 5-methylcytocine |
| AGO | Argonaute |
| AML | Acute Myeloid Leukemia |
| AZA | Azacytidine |
| BC | Breast cancer |
| BCL-2 | B-cell lymphoma 2 (protein) |
| BENC1 | Beclin-1 protein encoding gene |
| BMMC | bone marrow mononuclear cells |
| CASP8 | Caspase-8 |
| CDC2 | Cyclin-dependent kinase 2 |
| CDK6 | Cyclin Dependent Kinase 6 |
| CDKN1B | Cyclin-Dependent Kinase Inhibitor 1B |
| CDKN2A | Cyclin-Dependent Kinase 2A |
| CEBPA | CCAAT/Enhancer-Binding Protein Alpha |
| CpG | cytosine nucleotide followed by a guanine nucleotide |
| CRC | Colorectal cancer |
| DAC | Decitabine |
| DACOR1 | DNMT1-associated Colon Cancer Repressed 1 |
| DANCR-lncRNA | Differentiation antagonizing nonprotein coding RNA |
| DCs | dendritic cells |
| DICER | endoribonuclease Dicer or helicase with RNase motif |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| DROSHA | Drosha Ribonuclease III |
| DsDNA | double stranded Deoxyribonucleic Acid |
| DSB | DNA double strand breaks |
| E2F1 | E2F Transcription Factor 1 |
| EZH2 | Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit |
| FoxO | Forkhead box O |
| GB | Glioblastoma |
| H19 | H19 Imprinted Maternally Expressed Transcript |
| H3K9me | methylated histone H3 at lysine 9 |
| HCC | hepatocellular carcinoma |
| HDAC | Histone deacetylase |
| HIF-1 | Hypoxia-inducible factor 1 |
| HMAs | Hypomethylating agents |
| HOAX1 | Homeobox A1- protein coding gene |
| HOAX5 | Homeobox A5 |
| HOTAIR | Homeobox (HOX) transcript antisense RNA |
| HOTAIRM1 | HOXA Transcript Antisense RNA, Myeloid-Specific 1 |
| HOTTIP | HOXA Distal Transcript Antisense RNA |
| HOXB-AS3 | HOXB Cluster Antisense RNA 3 |
| HR | homologous recombination |
| HR-MDS | high-risk Myelodysplastic Syndromes |
| HSC | hematopoietic stem cell |
| IDH | Isocitrate dehydrogenase |
| IL-6 | Interleukin-6 |
| IPSS | International prognostic scoring system |
| IPSS-R | Revised international prognostic scoring system |
| jak-STAT | The Janus kinase—signal transducer and activator of transcription pathway |
| LEF1-AS1 | Lymphoid enhancer-binding factor 1 (LEF1) antisense RNA 1 |
| LncRNAs | long non-coding RNAs |
| LRIG1 | Leucine Rich Repeats And Immunoglobulin Like Domains 1 |
| LSCC | laryngeal squamous cell carcinoma |
| MDM2 | E3 Ubiquitin Protein Ligase |
| MAGI2-AS3 | MAGI2 Antisense RNA 3 |
| MALAT1 | Metastasis-Related Lung Adenocarcinoma Transcript 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| MBD-domain | Methyl-CpG-binding domain |
| MDS | Myelodysplastic syndromes |
| MEG3 | Maternally Expressed 3 |
| miRNAs | microRNAs |
| MM | multiple myeloma |
| Nanog | Nanog Homeobox |
| NcRNAs | non-coding RNAs |
| NEAT1 | Nuclear Paraspeckle Assembly Transcript 1 |
| Nt | Nucleotide |
| NPM1 | Nucleiphosmin-1 |
| Oct3 | Octamer binding transcription factor 3 |
| ORF | open reading frame |
| PC | prostate cancer |
| PKCd | protein kinase C delta |
| PI3K-Akt | Phosphatidyl-Inositol-3-Kinase- |
| PiRNAs | Piwi-interacting RNAs |
| Pol II | RNA polymerase II |
| PRKDC | Protein Kinase DNA-Activated Catalytic Subunit |
| PTEN | Phosphatase and tensin homolog |
| PWWP domain | ‘Pro-Trp-Trp-Pro’ core amino acid sequence |
| RNA | Ribonucleic Acid |
| RITS | RNA-induced transcriptional silencing |
| ROS | reactive oxygen species |
| SAM | S-adenosyl methionine |
| SCLC | Small cell lung carcinoma |
| SiRNAs | small interfering RNAs |
| SLE | Systemic Lupus Erythematosus |
| SnoRNP | Small nucleolar ribonucleoprotein |
| TET | Ten-Eleven Translocation or methyl-cytosine dioxygenase |
| TGFβ | Transforming Growth Factor-β |
| Th17 | T helper 17 cells (Th17)—a subset of pro-inflammatory T helper cells defined by their production of interleukin 17 (IL-17) |
| UCA1 | Urothelial Cancer Associated 1 |
| XIST | X-inactive specific transcript |
References
- Malcovati, L.; Hellstrom-Lindberg, E.; Bowen, D.; Ades, L.; Cermak, J.; Del Canizo, C.; Della Porta, M.G.; Fenaux, P.; Gattermann, N.; Germing, U.; et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood 2013, 122, 2943–2964. [Google Scholar] [CrossRef]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Skrabanek, L.; Li, Y.; Jiemjit, A.; Fandy, T.E.; Paietta, E.; Fernandez, H.; Tallman, M.S.; Greally, J.M.; Carraway, H.; et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood 2009, 114, 3448–3458. [Google Scholar] [CrossRef]
- Maegawa, S.; Gough, S.M.; Watanabe-Okochi, N.; Lu, Y.; Zhang, N.; Castoro, R.J.; Estecio, M.R.; Jelinek, J.; Liang, S.; Kitamura, T.; et al. Age-related epigenetic drift in the pathogenesis of MDS and AML. Genome Res. 2014, 24, 580–591. [Google Scholar] [CrossRef]
- Jiang, Y.; Dunbar, A.; Gondek, L.P.; Mohan, S.; Rataul, M.; O’Keefe, C.; Sekeres, M.; Saunthararajah, Y.; Maciejewski, J.P. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood 2009, 113, 1315–1325. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Li, S.; Liu, M.; Ying, S.; Jia, X.; Wang, X. CpG island methylator phenotype of myelodysplastic syndrome identified through genome-wide profiling of DNA methylation and gene expression. Br. J. Haematol. 2014, 165, 649–658. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef]
- Heuser, M.; Yun, H.; Thol, F. Epigenetics in myelodysplastic syndromes. Semin. Cancer Biol. 2018, 51, 170–179. [Google Scholar] [CrossRef]
- Madzo, J.; Vasanthakumar, A.; Godley, L.A. Perturbations of 5-hydroxymethylcytosine patterning in hematologic malignancies. Semin. Hematol. 2013, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Jin, G.; Karoly, E.D.; Spasojevic, I.; Yang, J.; Kinzler, K.W.; He, Y.; Bigner, D.D.; Vogelstein, B.; Yan, H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc. Natl. Acad. Sci. USA 2011, 108, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lee, H.J.; Enjeti, A.K. Unravelling the Epigenome of Myelodysplastic Syndrome: Diagnosis, Prognosis, and Response to Therapy. Cancers 2020, 12, 3128. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.A.; Tomuleasa, C.; Sahnoune, I.; Calin, G.A.; Berindan-Neagoe, I. Long Non-coding RNAs in Myeloid Malignancies. Front. Oncol. 2019, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Chen, F.Y.; Chou, W.C.; Hou, H.A.; Ko, B.S.; Lin, C.T.; Tang, J.L.; Li, C.C.; Yao, M.; Tsay, W.; et al. Long non-coding RNA HOXB-AS3 promotes myeloid cell proliferation and its higher expression is an adverse prognostic marker in patients with acute myeloid leukemia and myelodysplastic syndrome. BMC Cancer 2019, 19, 617. [Google Scholar] [CrossRef]
- Kuang, X.; Chi, J.; Wang, L. Deregulated microRNA expression and its pathogenetic implications for myelodysplastic syndromes. Hematology 2016, 21, 593–602. [Google Scholar] [CrossRef][Green Version]
- Kirimura, S.; Kurata, M.; Nakagawa, Y.; Onishi, I.; Abe-Suzuki, S.; Abe, S.; Yamamoto, K.; Kitagawa, M. Role of microRNA-29b in myelodysplastic syndromes during transformation to overt leukaemia. Pathology 2016, 48, 233–241. [Google Scholar] [CrossRef]
- Lyu, C.; Liu, K.; Jiang, Y.; Wang, T.; Wang, Y.; Xu, R. Integrated analysis on mRNA microarray and microRNA microarray to screen immune-related biomarkers and pathways in myelodysplastic syndrome. Hematology 2021, 26, 417–431. [Google Scholar] [CrossRef]
- Ghoshal, K.; Datta, J.; Majumder, S.; Bai, S.; Kutay, H.; Motiwala, T.; Jacob, S.T. 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol. Cell. Biol. 2005, 25, 4727–4741. [Google Scholar] [CrossRef]
- Hollenbach, P.W.; Nguyen, A.N.; Brady, H.; Williams, M.; Ning, Y.; Richard, N.; Krushel, L.; Aukerman, S.L.; Heise, C.; MacBeth, K.J. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS ONE 2010, 5, e9001. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Stahl, M.; DeVeaux, M.; Giri, S.; Huntington, S.; Podoltsev, N.; Wang, R.; Ma, X.; Davidoff, A.J.; Gore, S.D. Counseling patients with higher-risk MDS regarding survival with azacitidine therapy: Are we using realistic estimates? Blood Cancer J. 2018, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, T. DNA Methylation Reprogramming during Mammalian Development. Genes 2019, 10, 257. [Google Scholar] [CrossRef]
- Dodge, J.E.; Okano, M.; Dick, F.; Tsujimoto, N.; Chen, T.; Wang, S.; Ueda, Y.; Dyson, N.; Li, E. Inactivation of Dnmt3b in mouse embryonic fibroblasts results in DNA hypomethylation, chromosomal instability, and spontaneous immortalization. J. Biol. Chem. 2005, 280, 17986–17991. [Google Scholar] [CrossRef]
- Gowher, H.; Jeltsch, A. Mammalian DNA methyltransferases: New discoveries and open questions. Biochem. Soc. Trans. 2018, 46, 1191–1202. [Google Scholar] [CrossRef]
- Jurkowska, R.Z.; Jurkowski, T.P.; Jeltsch, A. Structure and function of mammalian DNA methyltransferases. ChemBioChem Eur. J. Chem. Biol. 2011, 12, 206–222. [Google Scholar] [CrossRef]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef]
- Margot, J.B.; Cardoso, M.C.; Leonhardt, H. Mammalian DNA methyltransferases show different subnuclear distributions. J. Cell. Biochem. 2001, 83, 373–379. [Google Scholar] [CrossRef]
- Dukatz, M.; Adam, S.; Biswal, M.; Song, J.; Bashtrykov, P.; Jeltsch, A. Complex DNA sequence readout mechanisms of the DNMT3B DNA methyltransferase. Nucleic Acids Res. 2020, 48, 11495–11509. [Google Scholar] [CrossRef]
- Lin, I.G.; Han, L.; Taghva, A.; O’Brien, L.E.; Hsieh, C.L. Murine de novo methyltransferase Dnmt3a demonstrates strand asymmetry and site preference in the methylation of DNA in vitro. Mol. Cell. Biol. 2002, 22, 704–723. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kamei, Y.; Ehara, T.; Yuan, X.; Suganami, T.; Takai-Igarashi, T.; Hatada, I.; Ogawa, Y. Analysis of DNA methylation change induced by Dnmt3b in mouse hepatocytes. Biochem. Biophys. Res. Commun. 2013, 434, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Mortusewicz, O.; Schermelleh, L.; Walter, J.; Cardoso, M.C.; Leonhardt, H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc. Natl. Acad. Sci. USA 2005, 102, 8905–8909. [Google Scholar] [CrossRef]
- Hervouet, E.; Vallette, F.M.; Cartron, P.F. Impact of the DNA methyltransferases expression on the methylation status of apoptosis-associated genes in glioblastoma multiforme. Cell Death Dis. 2010, 1, e8. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, E.; Peixoto, P.; Delage-Mourroux, R.; Boyer-Guittaut, M.; Cartron, P.F. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenetics 2018, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Vale, C.; Bhagat, T.; Verma, A. Role of DNA methylation in the pathogenesis and treatment of myelodysplastic syndromes. Semin. Hematol. 2013, 50, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.; Fernandez, A.F.; Fraga, M.F. The role of 5-hydroxymethylcytosine in development, aging and age-related diseases. Ageing Res. Rev. 2017, 37, 28–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C. Formation and biological consequences of 5-Formylcytosine in genomic DNA. DNA Repair 2019, 81, 102649. [Google Scholar] [CrossRef]
- Szulik, M.W.; Pallan, P.S.; Nocek, B.; Voehler, M.; Banerjee, S.; Brooks, S.; Joachimiak, A.; Egli, M.; Eichman, B.F.; Stone, M.P. Differential stabilities and sequence-dependent base pair opening dynamics of Watson-Crick base pairs with 5-hydroxymethylcytosine, 5-formylcytosine, or 5-carboxylcytosine. Biochemistry 2015, 54, 1294–1305. [Google Scholar] [CrossRef]
- Nimer, S.D. Myelodysplastic syndromes. Blood 2008, 111, 4841–4851. [Google Scholar] [CrossRef]
- Van Rompay, A.R.; Norda, A.; Linden, K.; Johansson, M.; Karlsson, A. Phosphorylation of uridine and cytidine nucleoside analogs by two human uridine-cytidine kinases. Mol. Pharmacol. 2001, 59, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Creusot, F.; Acs, G.; Christman, J.K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J. Biol. Chem. 1982, 257, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Datta, J.; Majumder, S.; Bai, S.; Dong, X.; Parthun, M.; Jacob, S.T. Inhibitors of histone deacetylase and DNA methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol. Cell. Biol. 2002, 22, 8302–8319. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Ghoshal, K.; Motiwala, T.; Jacob, S.T. Novel Insights into the Molecular Mechanism of Action of DNA Hypomethylating Agents: Role of Protein Kinase C delta in Decitabine-Induced Degradation of DNA Methyltransferase 1. Genes Cancer 2012, 3, 71–81. [Google Scholar] [CrossRef]
- Easwaran, H.P.; Schermelleh, L.; Leonhardt, H.; Cardoso, M.C. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 2004, 5, 1181–1186. [Google Scholar] [CrossRef]
- Diesch, J.; Zwick, A.; Garz, A.K.; Palau, A.; Buschbeck, M.; Gotze, K.S. A clinical-molecular update on azanucleoside-based therapy for the treatment of hematologic cancers. Clin. Epigenetics 2016, 8, 71. [Google Scholar] [CrossRef]
- Stomper, J.; Rotondo, J.C.; Greve, G.; Lubbert, M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: Mechanisms of resistance and novel HMA-based therapies. Leukemia 2021, 35, 1873–1889. [Google Scholar] [CrossRef]
- Enderle, D.; Spiel, A.; Coticchia, C.M.; Berghoff, E.; Mueller, R.; Schlumpberger, M.; Sprenger-Haussels, M.; Shaffer, J.M.; Lader, E.; Skog, J.; et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS ONE 2015, 10, e0136133. [Google Scholar] [CrossRef]
- Dong, L.; Lin, W.; Qi, P.; Xu, M.D.; Wu, X.; Ni, S.; Huang, D.; Weng, W.W.; Tan, C.; Sheng, W.; et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2016, 25, 1158–1166. [Google Scholar] [CrossRef]
- Beylerli, O.; Gareev, I.; Sufianov, A.; Ilyasova, T.; Guang, Y. Long noncoding RNAs as promising biomarkers in cancer. Non-Coding RNA Res. 2022, 7, 66–70. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Chen, L.L. Life without A tail: New formats of long noncoding RNAs. Int. J. Biochem. Cell Biol. 2014, 54, 338–349. [Google Scholar] [CrossRef]
- Yin, Q.F.; Yang, L.; Zhang, Y.; Xiang, J.F.; Wu, Y.W.; Carmichael, G.G.; Chen, L.L. Long noncoding RNAs with snoRNA ends. Mol. Cell 2012, 48, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hao, Q.; Prasanth, K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. TIG 2018, 34, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, J.E.; Freier, S.M.; Spector, D.L. 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 2008, 135, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Papaioannou, D.; Karunasiri, M.; Kohlschmidt, J.; Pepe, F.; Walker, C.J.; Walker, A.E.; Brannan, Z.; Pathmanathan, A.; Zhang, X.; et al. Expression and functional relevance of long non-coding RNAs in acute myeloid leukemia stem cells. Leukemia 2019, 33, 2169–2182. [Google Scholar] [CrossRef]
- Qi, X.; Jiao, Y.; Cheng, C.; Qian, F.; Chen, Z.; Wu, Q. H22954, a novel long non-coding RNA down-regulated in AML, inhibits cancer growth in a BCL-2-dependent mechanism. Cancer Lett. 2019, 454, 26–36. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Ishizu, H.; Siomi, H.; Siomi, M.C. Biology of PIWI-interacting RNAs: New insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012, 26, 2361–2373. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Buhler, M.; Verdel, A.; Moazed, D. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell 2006, 125, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Wen, J.; Liang, X.; Xie, Q.; Wu, W.; Wu, M.; Liu, Z. Identification of miR-320 family members as potential diagnostic and prognostic biomarkers in myelodysplastic syndromes. Sci. Rep. 2021, 11, 183. [Google Scholar] [CrossRef]
- Wen, J.; Huang, Y.; Li, H.; Zhang, X.; Cheng, P.; Deng, D.; Peng, Z.; Luo, J.; Zhao, W.; Lai, Y.; et al. Over-expression of miR-196b-5p is significantly associated with the progression of myelodysplastic syndrome. Int. J. Hematol. 2017, 105, 777–783. [Google Scholar] [CrossRef]
- Veryaskina, Y.A.; Titov, S.E.; Kovynev, I.B.; Fedorova, S.S.; Pospelova, T.I.; Zhimulev, I.F. MicroRNAs in the Myelodysplastic Syndrome. Acta Nat. 2021, 13, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, T.T.; Jin, S.; Liu, H.; Zhang, X.; Ruan, C.G.; Wu, D.P.; Han, Y.; Wang, X.Q. Pyrosequencing quantified methylation level of miR-124 predicts shorter survival for patients with myelodysplastic syndrome. Clin. Epigenetics 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.H.; Yao, D.M.; Yang, L.; Ma, J.C.; Wen, X.M.; Yang, J.; Guo, H.; Li, X.X.; Qian, W.; Lin, J.; et al. Hypomethylation of let-7a-3 is associated with poor prognosis in myelodysplastic syndrome. Leuk. Lymphoma 2017, 58, 96–103. [Google Scholar] [CrossRef]
- Luo, S.; Lu, J.Y.; Liu, L.; Yin, Y.; Chen, C.; Han, X.; Wu, B.; Xu, R.; Liu, W.; Yan, P.; et al. Divergent lncRNAs Regulate Gene Expression and Lineage Differentiation in Pluripotent Cells. Cell Stem Cell 2016, 18, 637–652. [Google Scholar] [CrossRef]
- Plath, K.; Mlynarczyk-Evans, S.; Nusinow, D.A.; Panning, B. Xist RNA and the mechanism of X chromosome inactivation. Annu. Rev. Genet. 2002, 36, 233–278. [Google Scholar] [CrossRef]
- Pabst, T.; Mueller, B.U.; Zhang, P.; Radomska, H.S.; Narravula, S.; Schnittger, S.; Behre, G.; Hiddemann, W.; Tenen, D.G. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat. Genet. 2001, 27, 263–270. [Google Scholar] [CrossRef]
- Di Ruscio, A.; Ebralidze, A.K.; Benoukraf, T.; Amabile, G.; Goff, L.A.; Terragni, J.; Figueroa, M.E.; De Figueiredo Pontes, L.L.; Alberich-Jorda, M.; Zhang, P.; et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 2013, 503, 371–376. [Google Scholar] [CrossRef]
- Esposito, C.; Autiero, I.; Basal, M.; Sandomenico, A.; Ummarino, S.; Borchiellini, M.; Ruvo, M.; Catuogno, S.; Ebralidze, A.; de Franciscis, V.; et al. Targeted systematic evolution of an RNA platform neutralizing DNMT1 function and controlling DNA methylation. bioRxiv 2020. bioRxiv:2020.07.29.226803. [Google Scholar]
- Yao, C.Y.; Chen, C.H.; Huang, H.H.; Hou, H.A.; Lin, C.C.; Tseng, M.H.; Kao, C.J.; Lu, T.P.; Chou, W.C.; Tien, H.F. A 4-lncRNA scoring system for prognostication of adult myelodysplastic syndromes. Blood Adv. 2017, 1, 1505–1516. [Google Scholar] [CrossRef]
- Merry, C.R.; Forrest, M.E.; Sabers, J.N.; Beard, L.; Gao, X.H.; Hatzoglou, M.; Jackson, M.W.; Wang, Z.; Markowitz, S.D.; Khalil, A.M. DNMT1-associated long non-coding RNAs regulate global gene expression and DNA methylation in colon cancer. Hum. Mol. Genet. 2015, 24, 6240–6253. [Google Scholar] [CrossRef]
- Fang, S.; Shen, Y.; Chen, B.; Wu, Y.; Jia, L.; Li, Y.; Zhu, Y.; Yan, Y.; Li, M.; Chen, R.; et al. H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann. Transl. Med. 2018, 6, 440. [Google Scholar] [CrossRef]
- Fang, S.; Gao, H.; Tong, Y.; Yang, J.; Tang, R.; Niu, Y.; Li, M.; Guo, L. Long noncoding RNA-HOTAIR affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells. Lab. Investig. J. Tech. Methods Pathol. 2016, 96, 60–68. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, S.; Chen, X.; Wang, C. HOTAIR suppresses PTEN via DNMT3b and confers drug resistance in acute myeloid leukemia. Hematology 2021, 26, 170–178. [Google Scholar] [CrossRef]
- Wang, S.L.; Huang, Y.; Su, R.; Yu, Y.Y. Silencing long non-coding RNA HOTAIR exerts anti-oncogenic effect on human acute myeloid leukemia via demethylation of HOXA5 by inhibiting Dnmt3b. Cancer Cell Int. 2019, 19, 114. [Google Scholar] [CrossRef]
- Lee, P.; Yim, R.; Miu, K.K.; Fung, S.H.; Liao, J.J.; Wang, Z.; Li, J.; Yung, Y.; Chu, H.T.; Yip, P.K.; et al. Epigenetic Silencing of PTEN and Epi-Transcriptional Silencing of MDM2 Underlied Progression to Secondary Acute Myeloid Leukemia in Myelodysplastic Syndrome Treated with Hypomethylating Agents. Int. J. Mol. Sci. 2022, 23, 5670. [Google Scholar] [CrossRef]
- Diaz-Beya, M.; Brunet, S.; Nomdedeu, J.; Pratcorona, M.; Cordeiro, A.; Gallardo, D.; Escoda, L.; Tormo, M.; Heras, I.; Ribera, J.M.; et al. The lincRNA HOTAIRM1, located in the HOXA genomic region, is expressed in acute myeloid leukemia, impacts prognosis in patients in the intermediate-risk cytogenetic category, and is associated with a distinctive microRNA signature. Oncotarget 2015, 6, 31613–31627. [Google Scholar] [CrossRef]
- Jing, Y.; Jiang, X.; Lei, L.; Peng, M.; Ren, J.; Xiao, Q.; Tao, Y.; Tao, Y.; Huang, J.; Wang, L.; et al. Mutant NPM1-regulated lncRNA HOTAIRM1 promotes leukemia cell autophagy and proliferation by targeting EGR1 and ULK3. J. Exp. Clin. Cancer Res. CR 2021, 40, 312. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, C.; Cui, J.; Wang, Y.; Hong, X. Over-expressed lncRNA HOTAIRM1 promotes tumor growth and invasion through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away from the HOXA1 gene in glioblastoma multiforme. J. Exp. Clin. Cancer Res. CR 2018, 37, 265. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.F.; Chen, L.T.; Wang, H.D.; Liu, Y.H.; Shiah, S.G. Transcriptional suppression of Dicer by HOXB-AS3/EZH2 complex dictates sorafenib resistance and cancer stemness. Cancer Sci. 2022, 113, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, S.; Li, L.; Zhang, S.; Liu, J.; Xu, X.; Zhang, M.; Du, T.; Du, Y.; Peng, X.; et al. LncRNA NR-104098 Inhibits AML Proliferation and Induces Differentiation Through Repressing EZH2 Transcription by Interacting with E2F1. Front. Cell Dev. Biol. 2020, 8, 142. [Google Scholar] [CrossRef]
- Saberwal, G.; Lucas, S.; Janssen, I.; Deobhakta, A.; Hu, W.Y.; Galili, N.; Raza, A.; Mundle, S.D. Increased levels and activity of E2F1 transcription factor in myelodysplastic bone marrow. Int. J. Hematol. 2004, 80, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Szikszai, K.; Krejcik, Z.; Klema, J.; Loudova, N.; Hrustincova, A.; Belickova, M.; Hruba, M.; Vesela, J.; Stranecky, V.; Kundrat, D.; et al. LncRNA Profiling Reveals That the Deregulation of H19, WT1-AS, TCL6, and LEF1-AS1 Is Associated with Higher-Risk Myelodysplastic Syndrome. Cancers 2020, 12, 2726. [Google Scholar] [CrossRef]
- Wang, J.; Xie, S.; Yang, J.; Xiong, H.; Jia, Y.; Zhou, Y.; Chen, Y.; Ying, X.; Chen, C.; Ye, C.; et al. The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J. Hematol. Oncol. 2019, 12, 81. [Google Scholar] [CrossRef]
- Hu, B.; Yue, Q.F.; Chen, Y.; Bu, F.D.; Sun, C.Y.; Liu, X.Y. Expression of autophagy related gene BECLIN-1 and number of autophagic vacuoles in bone marrow mononuclear cells from 40 myelodysplastic syndromes patients and their significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015, 23, 146–149. [Google Scholar]
- Chen, L.; Fan, X.; Zhu, J.; Chen, X.; Liu, Y.; Zhou, H. LncRNA MAGI2-AS3 inhibits the self-renewal of leukaemic stem cells by promoting TET2-dependent DNA demethylation of the LRIG1 promoter in acute myeloid leukaemia. RNA Biol. 2020, 17, 784–793. [Google Scholar] [CrossRef]
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.I.; Rendtlew Danielsen, J.M.; Yang, Y.G.; Qi, Y. A role for small RNAs in DNA double-strand break repair. Cell 2012, 149, 101–112. [Google Scholar] [CrossRef]
- Gao, M.; Wei, W.; Li, M.M.; Wu, Y.S.; Ba, Z.; Jin, K.X.; Li, M.M.; Liao, Y.Q.; Adhikari, S.; Chong, Z.; et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res. 2014, 24, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhu, S.; Yuan, M.; Cui, H.; Wang, L.; Luo, X.; Li, J.; Zhou, H.; Tang, Y.; Shen, N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 2010, 184, 6773–6781. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cheong, J.W.; Kim, Y.K.; Eom, J.I.; Jeung, H.K.; Kim, S.J.; Hwang, D.; Kim, J.S.; Kim, H.J.; Min, Y.H. Serum microRNA-21 as a potential biomarker for response to hypomethylating agents in myelodysplastic syndromes. PLoS ONE 2014, 9, e86933. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhang, W.; Zhang, B.; Zhao, Y.; Zhao, Y.; Li, S.; Liu, Y. miR-21 modulates the effect of EZH2 on the biological behavior of human lung cancer stem cells in vitro. Oncotarget 2017, 8, 85442–85451. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, H.; Li, Y. Preliminary study on the role of miR148a and DNMT1 in the pathogenesis of acute myeloid leukemia. Mol. Med. Rep. 2019, 19, 2943–2952. [Google Scholar]
- Garzon, R.; Liu, S.; Fabbri, M.; Liu, Z.; Heaphy, C.E.; Callegari, E.; Schwind, S.; Pang, J.; Yu, J.; Muthusamy, N.; et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 2009, 113, 6411–6418. [Google Scholar] [CrossRef]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C.; et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Y.; Jing, Z.; Wang, X.; Zha, X.; Zeng, C.; Chen, S.; Yang, L.; Luo, G.; Li, B.; et al. Altered expression pattern of miR-29a, miR-29b and the target genes in myeloid leukemia. Exp. Hematol. Oncol. 2014, 3, 17. [Google Scholar] [CrossRef]
- Qadir, X.V.; Han, C.; Lu, D.; Zhang, J.; Wu, T. miR-185 inhibits hepatocellular carcinoma growth by targeting the DNMT1/PTEN/Akt pathway. Am. J. Pathol. 2014, 184, 2355–2364. [Google Scholar] [CrossRef]
- Gurbuz, V.; Sozen, S.; Bilen, C.Y.; Konac, E. miR-148a, miR-152 and miR-200b promote prostate cancer metastasis by targeting DNMT1 and PTEN expression. Oncol. Lett. 2021, 22, 805. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Meng, X.; Ying, X.; Zuo, Y.; Liu, R.; Pan, Z.; Kang, T.; Huang, W. MicroRNA-342 inhibits colorectal cancer cell proliferation and invasion by directly targeting DNA methyltransferase 1. Carcinogenesis 2011, 32, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, H.; Yang, C. miR-342 suppresses the proliferation and invasion of acute myeloid leukemia by targeting Naa10p. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3671–3676. [Google Scholar] [CrossRef] [PubMed]
- Roscigno, G.; Quintavalle, C.; Donnarumma, E.; Puoti, I.; Diaz-Lagares, A.; Iaboni, M.; Fiore, D.; Russo, V.; Todaro, M.; Romano, G.; et al. MiR-221 promotes stemness of breast cancer cells by targeting DNMT3b. Oncotarget 2016, 7, 580–592. [Google Scholar] [CrossRef]
- Hussein, K.; Theophile, K.; Busche, G.; Schlegelberger, B.; Gohring, G.; Kreipe, H.; Bock, O. Significant inverse correlation of microRNA-150/MYB and microRNA-222/p27 in myelodysplastic syndrome. Leuk. Res. 2010, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Ji, Y.S.; Jang, G.H.; Lim, S.H.; Kim, S.H.; Kim, C.K.; Bae, S.B.; Won, J.H.; Park, S.K. TET2 Mutation and High miR-22 Expression as Biomarkers to Predict Clinical Outcome in Myelodysplastic Syndrome Patients Treated with Hypomethylating Therapy. Curr. Issues Mol. Biol. 2021, 43, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Ito, K.; Ala, U.; Kats, L.; Webster, K.; Sun, S.M.; Jongen-Lavrencic, M.; Manova-Todorova, K.; Teruya-Feldstein, J.; Avigan, D.E.; et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell 2013, 13, 87–101. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, W.; Wang, S.E.; Baltimore, D. Dual mechanisms of posttranscriptional regulation of Tet2 by Let-7 microRNA in macrophages. Proc. Natl. Acad. Sci. USA 2019, 116, 12416–12421. [Google Scholar] [CrossRef]
- Gonzales-Aloy, E.; Connerty, P.; Salik, B.; Liu, B.; Woo, A.J.; Haber, M.; Norris, M.D.; Wang, J.; Wang, J.Y. miR-101 suppresses the development of MLL-rearranged acute myeloid leukemia. Haematologica 2019, 104, e296–e299. [Google Scholar] [CrossRef]
- Castoro, R.J.; Dekmezian, M.; Saraf, A.J.; Watanabe, Y.; Chung, W.; Adhab, S.E.; Jelinek, J.; Issa, J.-P. Microrna 124 and Its Role in Response to Epigenetic Therapy in Patients with Acute Mylogenous Leukemia and Myelodysplastic Syndrome. Blood 2008, 112, 598. [Google Scholar] [CrossRef]
- Chen, L.; Jia, J.; Zang, Y.; Li, J.; Wan, B. MicroRNA-101 regulates autophagy, proliferation and apoptosis via targeting EZH2 in laryngeal squamous cell carcinoma. Neoplasma 2019, 66, 507–515. [Google Scholar] [CrossRef]
- Sabour Takanlu, J.; Aghaie Fard, A.; Mohammdi, S.; Hosseini Rad, S.M.A.; Abroun, S.; Nikbakht, M. Indirect Tumor Inhibitory Effects of MicroRNA-124 through Targeting EZH2 in The Multiple Myeloma Cell Line. Cell J. 2020, 22, 23–29. [Google Scholar] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Shahryari, V.; Arora, S.; Zaman, M.S.; Chang, I.; Yamamura, S.; Tanaka, Y.; Chiyomaru, T.; et al. miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Song, Y.; Zhang, Y.; Wang, H.; Xie, J. miR-34b Targets HSF1 to Suppress Cell Survival in Acute Myeloid Leukemia. Oncol. Res. 2016, 24, 109–116. [Google Scholar] [CrossRef] [PubMed]

| Upregulated miRNAs | Tissue | Impaired KEGG Pathways | Gene Targets within KEGG Pathway |
|---|---|---|---|
| miR-196b-5p | Bone marrow | PI3K-Akt/TGFβ signaling | BRAF, TGFBR1, E2F2, NRAS, AF1, CDKN1B, SMAD4, MYC, MAPK1, MDM2 |
| ECM-receptor interaction | ITGB1, ITGB8, THBS2, ITGA3, COL3A1, COL1A2, HMMR | ||
| cell cycle via MAPK signaling | ESPL1, CCNB1, E2F2, CDK2, CND2, SMC3, CDKN1B, STAG1, YWHAB, SMAD4, SKP2, MYC, PLK1, MDM2, MCM3 | ||
| miR-320c, miR-320d | Bone marrow | TGFβ signaling | PPP2CA, SMAD3, ACVR2B, SMAD7, MAPK1 |
| miR-422a | Bone marrow | GNG12, MAPK1, GNG5, YAP1 | |
| miR-617 | Bone marrow | RNA transport, translation initiation | XPO1, EIF5, EIF5B, EIF4G2 |
| miR-181a, further divided to miR-181a-2-3p, miR-181a-3p and miR-181a-5p | Bone marrow | Signaling pathways regulating pluripotency of stem cells | IGF1R, HESX1, WNT8A, POU5F1B, ACVR2B, JAK3 |
| miR-222, further dived to miR-222-3p and miR-222-5p | Bone marrow | PI3K-Akt/TGFβ signaling | SOS2, CDK4, E2F2, CRKL, RUNX1, CDKN1B, CDK6, E2F3, MYC, MAPK1, MDM2 |
| cell cycle via MAPK signaling | YWHAH, CDK4, E2F2, YWHAE, YWHAG, CDKN1B, WEE1, CDK1, CDK6, ATM, CDKN1C, E2F3, MYC, YWHAZ, CDC20, BUB1B, CDC27, PRKDC, MDM2, CDC25A | ||
| miR-210, further divided to miR-210-3p and miR-210-5p | Bone marrow | No related pathway | |
| let-7a, further divided to let-7a-2-3p, let-7a-3p and let-7a-5p | Bone marrow | cell cycle via MAPK signaling | 46 genes targeted/CREBBP, CDK6, WEE1, MAP3K1, ELK4, MAX and TAOK1 confirmed by more than 1 lists |
| FoxO, HIF-1 and PI3K-Akt signaling | Over than 100 genes targeted/IRS2, CREBBP, BCL2L11, SLC2A1, TFRC, EFNA1, PRKAA1, COL4A1 and CDK6 confirmed by more than 1 lists | ||
| miRNA-34a (further dived to miRNA-34a-3p and -5p) in del(5q) MDS | PI3K-Akt/TGFβ signaling | 35 genes targeted | |
| cell cycle via MAPK signaling | Over than 100 genes targeted/PRKDC, DUSP16 and MCM7 confirmed by more than 1 lists | ||
| Cell cycle and apoptosis via P53 signaling | 34 genes targeted/THBS1, confirmed by more than 1 lists | ||
| miRNA-125b-1-3p in translocation (p21;q23) MDS | Plasma/bone marrow | No related pathway | |
| miRNA-383 (further divided to miR-383-3p and -5p) in trisomy 8 MDS | Bone marrow | No related pathway | |
| downregulated miRNAs | |||
| miR-29b further divided to miR-29b-1-5, -2-5p and -3p | Bone marrow | PI3K-Akt/TGFβ signaling | 63 genes targeted/PKN2, SGK1, NRAS, CCND1, YWHAB, CDK6, MCL1 and MDM2 confirmed by more than 1 lists |
| cell cycle via MAPK signaling | 33 genes targeted/MDM2, SMC1A, YWHAB, CCND1, CDK6, HDAC1 and WEE1 confirmed by more than 1 lists | ||
| Cell cycle and apoptosis via P53 signaling | 22 genes targeted/CCND1, CDK6 and MDM2 confirmed by more than 1 lists | ||
| FoxO signaling | 27 genes targeted/GF1R, NRAS, STK4, SGK1, MDM2, CCND1 and GABARAP confirmed by more than 1 lists | ||
| miR-130b-5p | Plasma | Cell cycle and apoptosis via P53 signaling | ZMAT3, CDK6, CHEK1, ATM, CCND1, MDM4, RRM2, SESN3, SERPINE1, PPM1D, MDM2 |
| miR-30e further divided to miR-30e-3p and -5p | Bone marrow | Cell cycle and apoptosis via TGFβ signaling | FST, TGFBR1,SMAD2, THBS1, CUL1, INHBA, DCN,MYC, PPP2R1A, SMURF1, ZFYVE9, BMP2, SP1, EP300, SMAD7, E2F4, MAPK1, PPP2R1B, TGFBR2, BMPR2, RPS6KB1, the underlined confirmed by more than 1 lists |
| cell cycle via MAPK signaling | 39 genes targeted/MDM2, SMAD2, YWHAZ, PRKDC, STAG2, MYC, CDK6, CCNB1 and E2F3 confirmed by more than 1 lists | ||
| Cell cycle and apoptosis via P53 signaling | 24 genes targeted/MDM2, CDK6, CCNB1, CCNG1, CASP3 and SIAH1 confirmed by more than 1 lists | ||
| RNA transport, translation initiation | 37 genes targeted/XPO1, NCBP1 and RANBP2 confirmed by more than 1 lists | ||
| miR-221 further divided to miR-221-3p and -5p | Plasma | mRNA surveillance pathway | 21 genes targeted/ACIN1 and PABPC1 confirmed by more than 1 lists |
| cell cycle via MAPK signaling | 26 genes targeted/CCND1 confirmed by more than 1 lists | ||
| Cell cycle and apoptosis via P53 signaling | 50 genes targeted/EFNA1, CDKN1B, CCND1 and ATF4 confirmed by more than 1 lists | ||
| miRNA-194-5p | Bone marrow | Ubiquitin mediated proteolysis | WWP1, TRIP12, CBLB, BIRC6, SMURF1, DDB1, CDC23, RBX1, UBE2W, UBE2B, PIAS1, CUL4B |
| miRNA-146a (further dived to miRNA-146a-3p and -5p) in del(5q) MDS | Peripheral blood mononuclear cells | cell cycle via MAPK signaling | GSK3B, CCNB1, CDC25B, YWHAG, CDKN1B, SMAD4, RBL1, CDC23, CDKN1A, PRKDC, MDM2, ABL1, CDC25A |
| miRNA-125b-1 | No related pathway |
| Non-Coding RNA | Epigenetic Enzyme Targeted | Disease/Tissue of Investigation | Gene/Function Implicated | Possible Relation in MDS/AML Pathogenesis |
|---|---|---|---|---|
| MAGI2-AS3-lncRNA | ↑TET2 | AML | ↑LRIG1 [89] | - |
| HOTAIR-lncRNA | ↑DNMT3B ↑EZH2, DNMT1/3B | AML SCLC | ↓PTEN [77], ↓HOXA5 [78] ↓HOAX1 [76] | - |
| HOXB-AS3-lncRNA | ↕EZH2 | Liver cancer | ↑DICER1 [83] | Implicated in myeloid cell proliferation with adverse prognosis in AML and MDS [15] |
| H19-lncRNA | ↨DNMT3B | BC | ↑BECN1/ autophagy [87] | Associated with adverse prognosis in MDS patients [86] BECN1 is also implicated in MDS autophagy [88] |
| HOTAIRM1-lncRNA | ↨EZH2/DNMTs | GB | ↑HOAX1 [82] | Associated with leukemia cell autophagy and proliferation and adverse prognosis in NPM1-mutated AML [80,81] |
| NR-104098-lncRNA | ↓EZH2 (via E2F1) | AML | Inhibits AML proliferation & Induces differentiation [84] | - |
| CEBPA-lncRNA | ↨DNMT1 | AML | ↑CEBPA [71,72] | - |
| DACOR1-lncRNA | ↨DNMT1 | CRC | Whole genome demethylation [74] | MDS have abnormal methylation patterns across many genomic regions [4,5,6,7] |
| miR-22 | ↓TET2 | HSC | provoke MDS in mice [106] | Significant prognostic value in MDS treated with HMAs [105] |
| Let-7 family | ↓TET2 | murine macrophages | ↑ IL-6 [107] | Altered methylation and expression profiles in MDS [10,11] |
| miR-101 | ↓EZH2 | LSCC | ↓autophagy, proliferation & ↑apoptosis [110] | Reduces incidence and delays the onset and progression of AML in mice [108] |
| miR-124 | ↓EZH2 | MM | ↓CDKN2A ↓proliferation &viability of myeloma cell line [111] | Potential marker of response to DAC in MDS/AML [109] |
| miR-21 | ↓EZH2 ↓DNMT1 | Lung cancer SLE | ↓Cdc2, cyclinB1, BLC-2 [94] aberrant DNA hypomethylation [92] | Potential biomarker of epigenetic therapy in MDS [93] |
| miR-29b | ↓DNTM3A/B, ↓DNMT1 | AML | global DNA hypomethylation [96] | - |
| miR-29a | ↓DNTM3A/B | Lung cancer | Re-expression of methylation-silenced tumor suppressor genes [97] | Downregulated in AML [98] |
| miR-221 | ↓DNTM3B | BC | ↓ methylation levels of Nanog & Oct 3 [103] | Decreased levels in AML evolving from MDS [104] |
| miR-185 | ↓DNMT1 | HCC | ↑PTEN/Akt pathway | PTEN is associated with HMA resistance in MDS and progression to AML [79] |
| miR-152 | ↓DNMT1 | PC | ↑PTEN | PTEN is associated with HMA resistance in MDS and progression to AML [79] |
| miR-148a | ↓DNMT1 | SLE AML | aberrant DNA hypomethylation [92] ↑miR-148a, mutual negative feedback loop/↓cell proliferation & ↑ apoptosis [95] | - |
| miR-34b | ↓DNMT1, HDAC1, HDAC2 & HDAC4 | PC | ↓proliferation through demethylation/active chromatin modifications/Akt pathway [112] | Cell viability inhibition/enhance cell apoptosis in AML cell lines [113] |
| miR-342 | ↓DNMT1 | CRC | ↓ cancer cell proliferation & invasion | Downregulation in AML [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symeonidis, A.; Chatzilygeroudi, T.; Chondrou, V.; Sgourou, A. Contingent Synergistic Interactions between Non-Coding RNAs and DNA-Modifying Enzymes in Myelodysplastic Syndromes. Int. J. Mol. Sci. 2022, 23, 16069. https://doi.org/10.3390/ijms232416069
Symeonidis A, Chatzilygeroudi T, Chondrou V, Sgourou A. Contingent Synergistic Interactions between Non-Coding RNAs and DNA-Modifying Enzymes in Myelodysplastic Syndromes. International Journal of Molecular Sciences. 2022; 23(24):16069. https://doi.org/10.3390/ijms232416069
Chicago/Turabian StyleSymeonidis, Argiris, Theodora Chatzilygeroudi, Vasiliki Chondrou, and Argyro Sgourou. 2022. "Contingent Synergistic Interactions between Non-Coding RNAs and DNA-Modifying Enzymes in Myelodysplastic Syndromes" International Journal of Molecular Sciences 23, no. 24: 16069. https://doi.org/10.3390/ijms232416069
APA StyleSymeonidis, A., Chatzilygeroudi, T., Chondrou, V., & Sgourou, A. (2022). Contingent Synergistic Interactions between Non-Coding RNAs and DNA-Modifying Enzymes in Myelodysplastic Syndromes. International Journal of Molecular Sciences, 23(24), 16069. https://doi.org/10.3390/ijms232416069






