Spinal Canal and Spinal Cord in Rat Continue to Grow Even after Sexual Maturation: Anatomical Study and Molecular Proposition
Abstract
:1. Introduction
2. Results
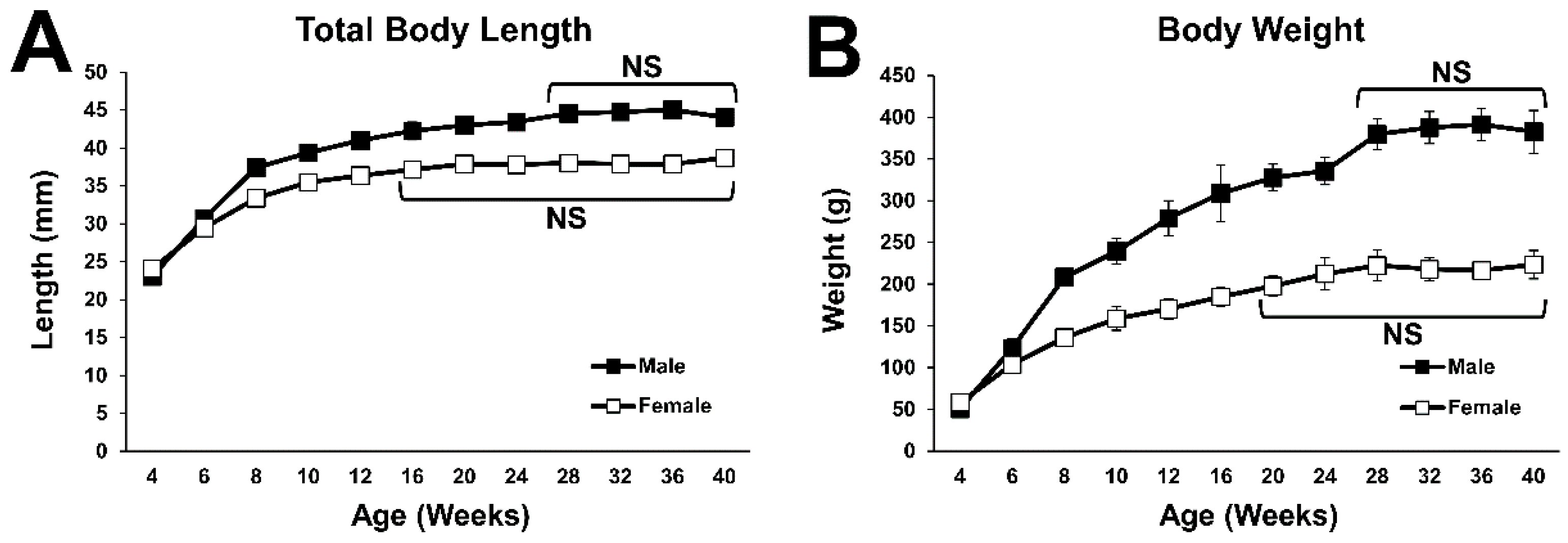
2.1. General Body Growth
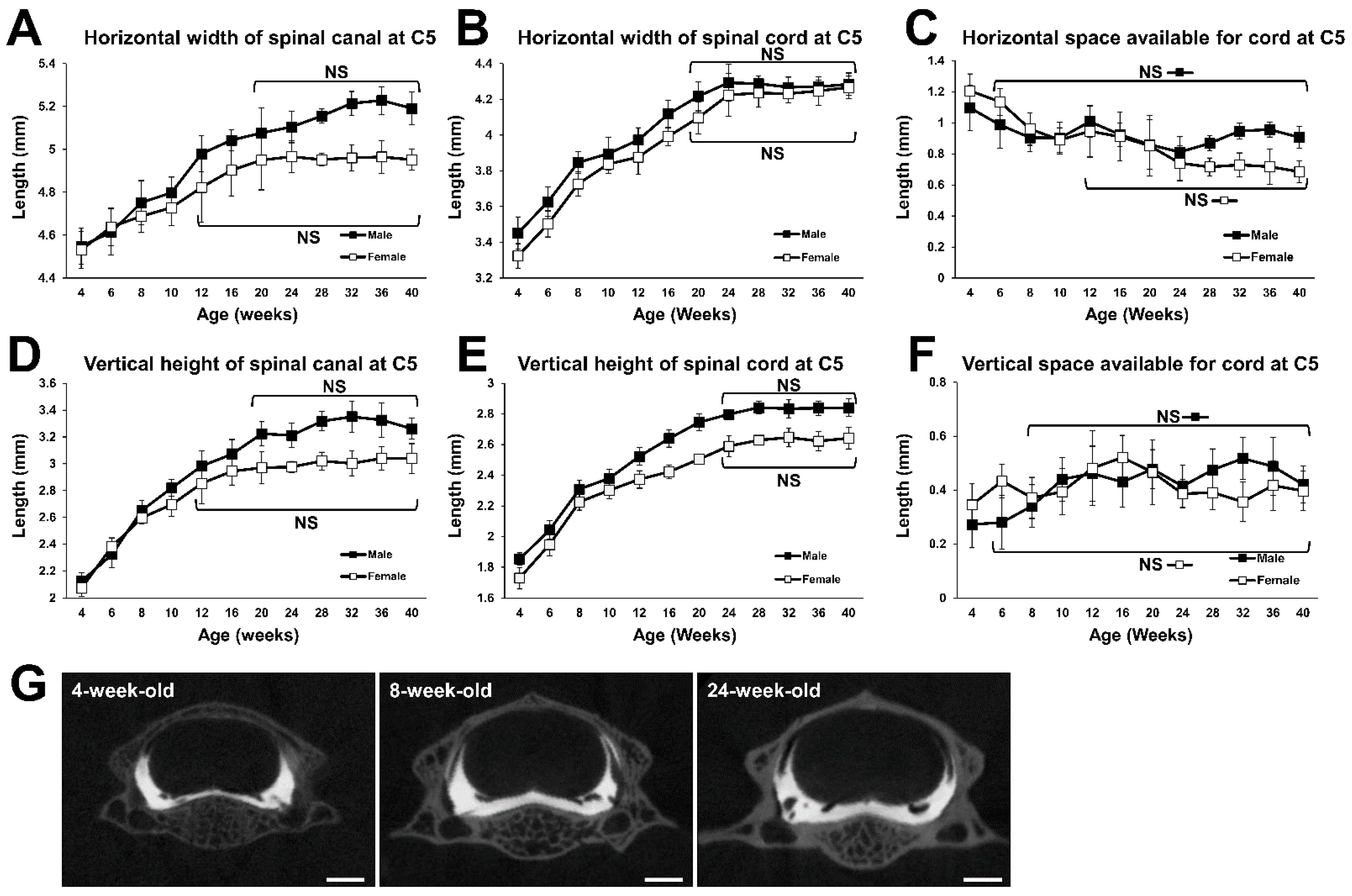
2.2. Growth Curve of the Fifth Cervical (C5) Spine
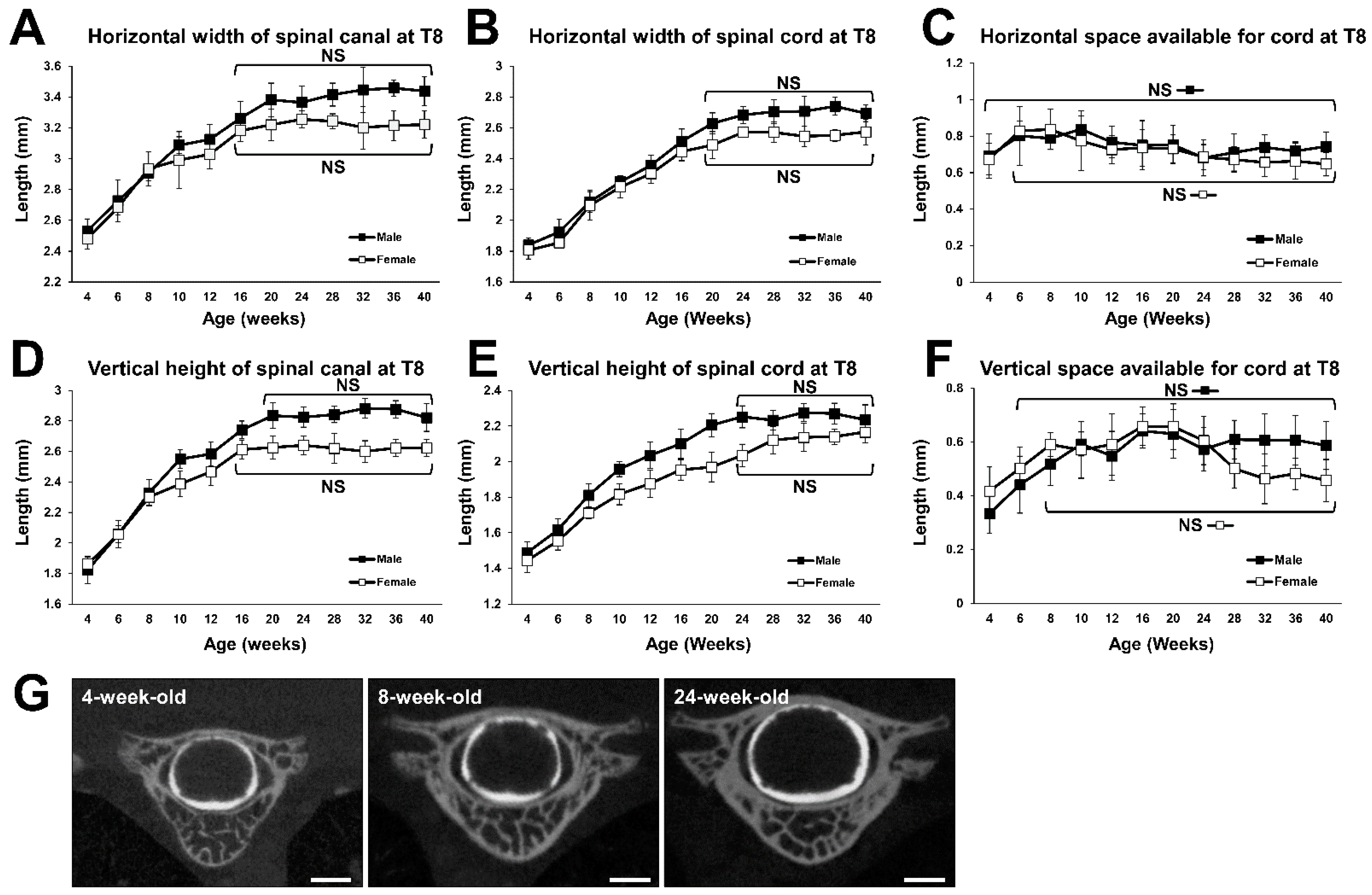
2.3. Growth Curve of the 8th Thoracic (T8) Spine
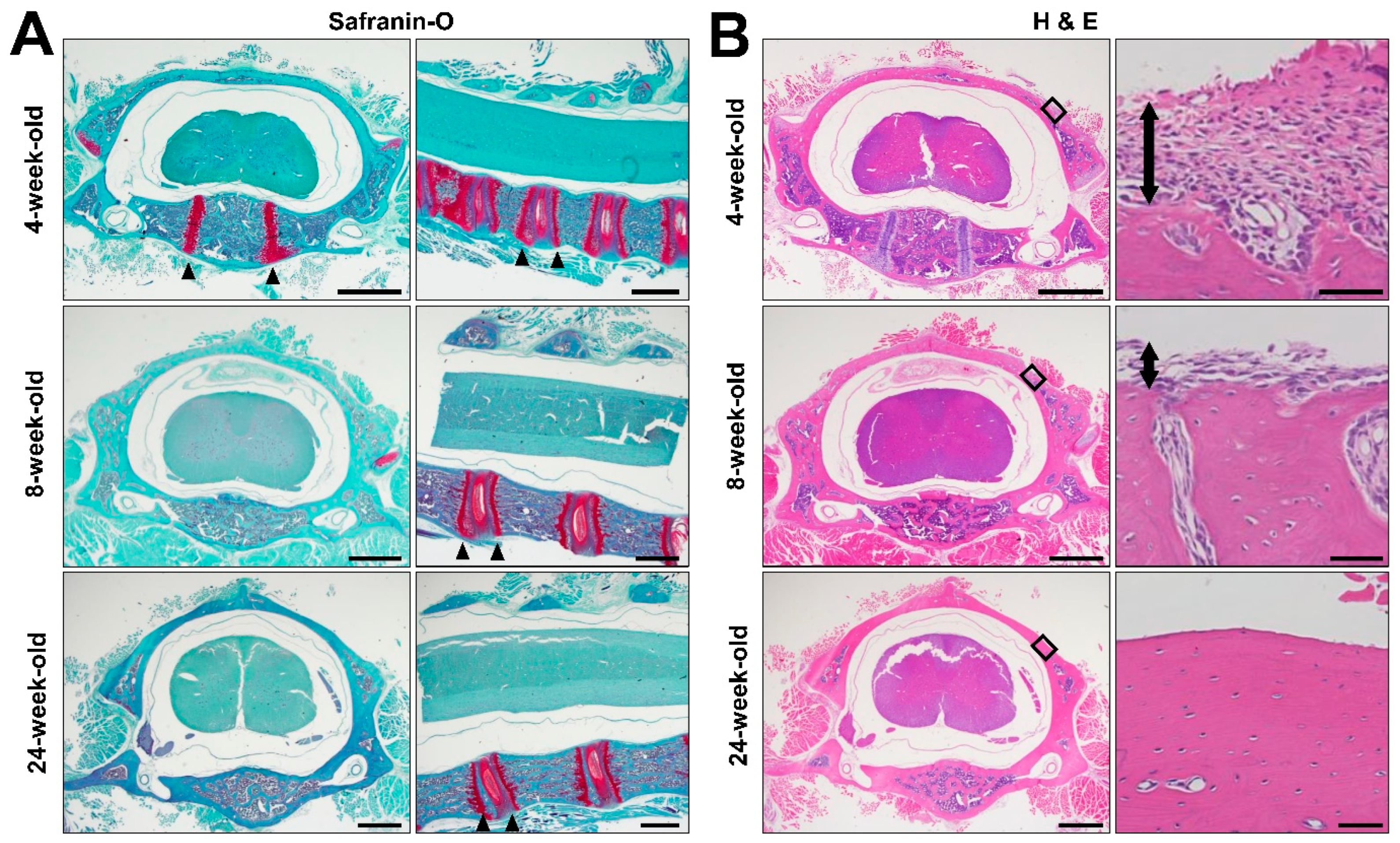
2.4. Histology
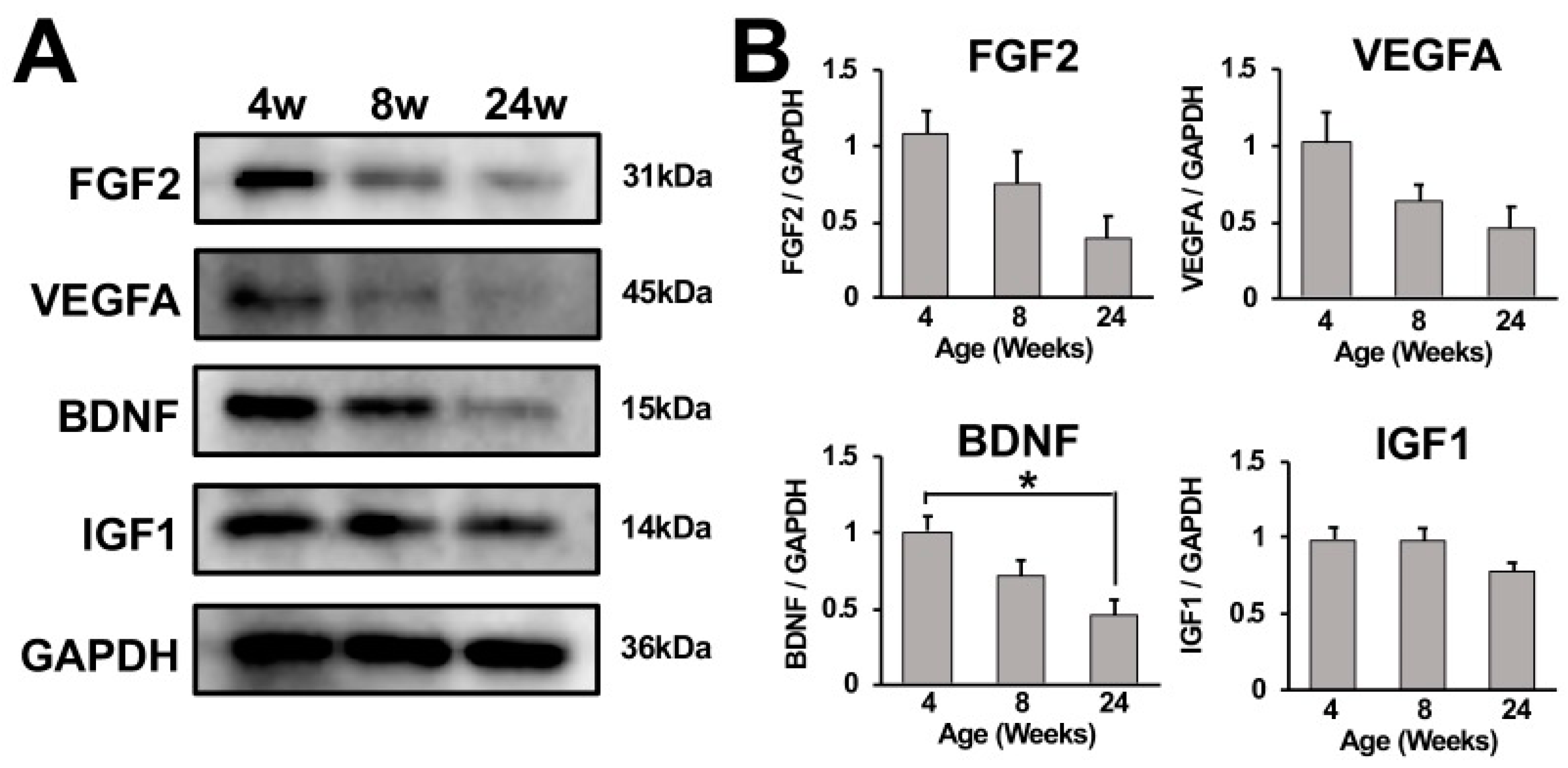
2.5. Growth Factor Expression in Cervical Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Animals
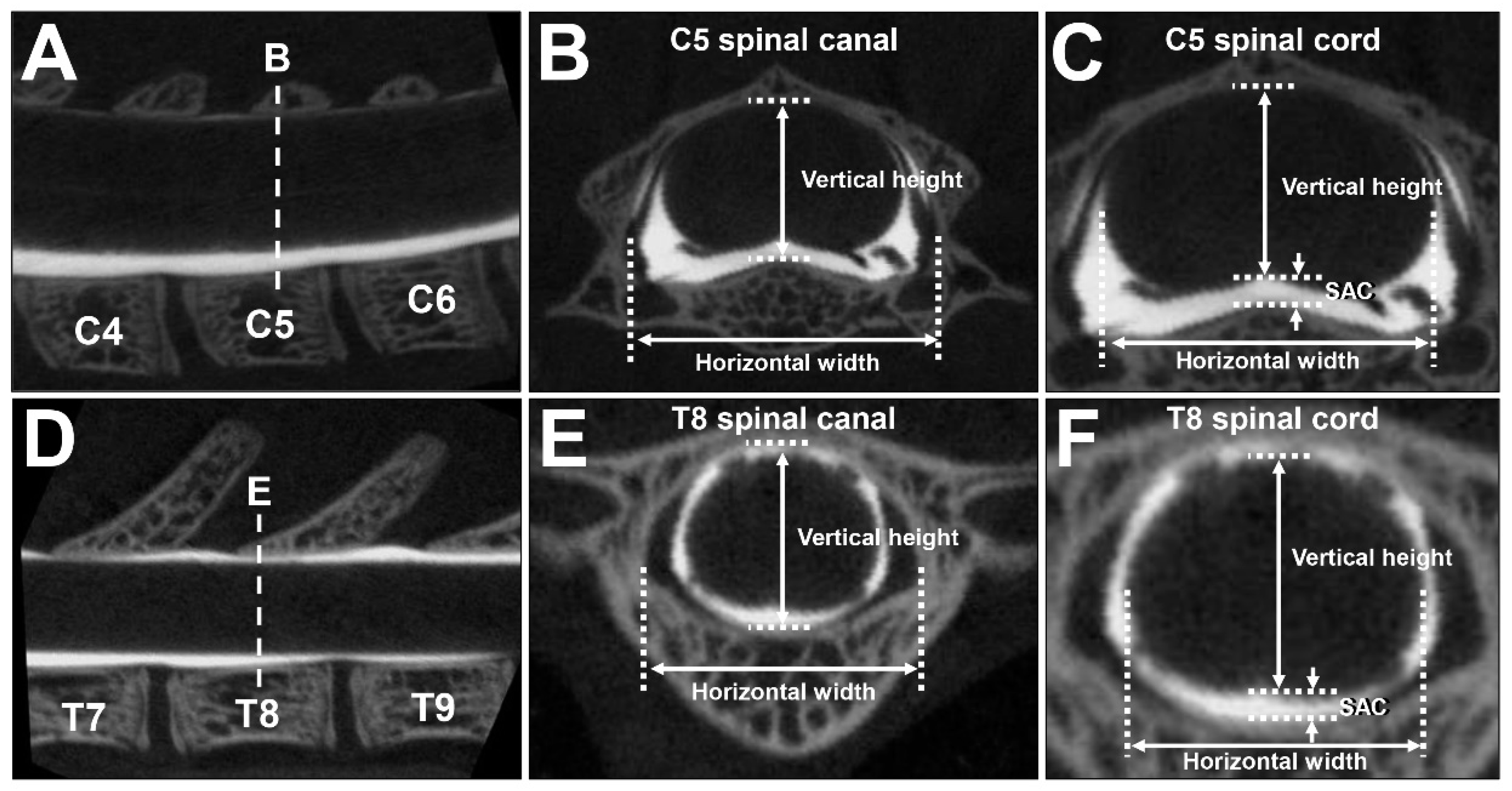
4.2. CT Myelography
4.3. Measurements
4.4. Histology
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iyer, A.; Azad, T.D.; Tharin, S. Cervical Spondylotic Myelopathy. Clin Spine Surg. 2016, 29, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Nakajima, H.; Watanabe, S.; Yayama, T.; Guerrero, A.R.; Inukai, T.; Hirai, T.; Sugita, D.; Johnson, W.E.; Baba, H. Apoptosis of neurons and oligodendrocytes in the spinal cord of spinal hyperostotic mouse (twy/twy): Possible pathomechanism of human cervical compressive myelopathy. Eur. Spine J. 2012, 21, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Hosoda, Y.; Kojimahara, K.; Yamazaki, T.; Yoshimura, Y. Arthritis and ankylosis in twy mice with hereditary multiple osteochondral lesions: With special reference to calcium deposition. Pathol. Int. 1994, 44, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Klironomos, G.; Karadimas, S.; Mavrakis, A.; Mirilas, P.; Savvas, I.; Papadaki, E.; Papachristou, D.J.; Gatzounis, G. New experimental rabbit animal model for cervical spondylotic myelopathy. Spinal Cord. 2011, 49, 1097–1102. [Google Scholar] [CrossRef] [Green Version]
- Karadimas, S.K.; Moon, E.S.; Yu, W.-R.; Satkunendrarajah, K.; Kallitsis, J.K.; Gatzounis, G.; Fehlings, M.G. A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol. Dis. 2013, 54, 43–58. [Google Scholar] [CrossRef]
- Long, H.; Li, G.; Lin, E.; Xie, W.; Chen, W.; Luk, K.D.; Hu, Y. Is the speed of chronic compression an important factor for chronic spinal cord injury rat model? Neurosci. Lett. 2013, 545, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Haisa, T.; Kawamoto, T.; Kirino, T.; Wakai, S. Delayed myelopathy induced by chronic compression in the rat spinal cord. Ann. Neurol. 2004, 55, 503–511. [Google Scholar] [CrossRef]
- Kurokawa, R.; Murata, H.; Ogino, M.; Ueki, K.; Kim, P. Altered blood flow distribution in the rat spinal cord under chronic compression. Spine 2011, 36, 1006–1009. [Google Scholar] [CrossRef]
- Ijima, Y.; Furuya, T.; Koda, M.; Matsuura, Y.; Saito, J.; Kitamura, M.; Miyamoto, T.; Orita, S.; Inage, K.; Suzuki, T.; et al. Experimental rat model for cervical compressive myelopathy. Neuroreport 2017, 28, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Chen, Y.; Zhou, Z.; Yang, S.; Zhong, C.; Li, Z. A progressive compression model of thoracic spinal cord injury in mice: Function assessment and pathological changes in spinal cord. Neural. Regen. Res. 2017, 12, 1365–1374. [Google Scholar]
- Laliberte, A.M.; Karadimas, S.K.; Vidal, P.M.; Satkunendrarajah, K.; Fehlings, M.G. Mir21 modulates inflammation and sensorimotor deficits in cervical myelopathy: Data from humans and animal models. Brain Commun. 2021, 3, fcaa234. [Google Scholar] [CrossRef]
- Karadimas, S.K.; Klironomos, G.; Papachristou, D.J.; Papanikolaou, S.; Papadaki, E.; Gatzounis, G. Immunohistochemical profile of NF-κB/p50, NF-κB/p65, MMP-9, MMP-2, and u-PA in experimental cervical spondylotic myelopathy. Spine 2013, 38, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Chandar, K.; Freeman, B.K. Spinal Cord Anatomy. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 254–263. [Google Scholar]
- Markuske, H. Sagittal diameter measurements of the bony cervical spinal canal in children. Pediatr. Radiol. 1977, 6, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, H. Adolescent Development. In Encyclopedia of Educational Theory and Philosophy; Phillips, D.C., Ed.; SAGE Reference: Los Angeles, CA, USA, 2014; pp. 18–19. [Google Scholar]
- Ogden, C.L.; Kuczmarski, R.J.; Flegal, K.M.; Mei, Z.; Guo, S.; Wei, R.; Grummer-Strawn, L.M.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002, 109, 45–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawcett, J.W. The Struggle to Make CNS Axons Regenerate: Why Has It Been so Difficult? Neurochem Res. 2020, 45, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Yun, M. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Åberg, M.A.I.; Åberg, N.D.; Hedbäcker, H.; Oscarsson, J.; Eriksson, P.S. Peripheral Infusion of IGF-I Selectively Induces Neurogenesis in the Adult Rat Hippocampus. J. Neurosci. 2000, 20, 2896–2903. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jin, K.; Childs, J.T.; Xie, L.; Mao, X.O.; Greenberg, D.A. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: Evidence from knockout mice and growth factor administration. Dev. Biol. 2006, 289, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Hebert, J.M. FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice. J. Neurosci. 2015, 35, 10217–10223. [Google Scholar] [CrossRef]
- Taliaz, D.; Stall, N.; Dar, D.E.; Zangen, A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 2010, 15, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, R.; Rahardjo, P. Bone Development and Growth. In Osteogenesis and Bone Regeneration; Yang, H., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Andreollo, N.A.; dos Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef] [Green Version]
- Gwak, Y.S.; Hains, B.C.; Johnson, K.M.; Hulsebosch, C.E. Effect of age at time of spinal cord injury on behavioral outcomes in rat. J. Neurotrauma 2004, 21, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, M.J.; Galvan, M.D.; Partida, E.; Anderson, A.J. Characterization of recovery, repair, and inflammatory processes following contusion spinal cord injury in old female rats: Is age a limitation? Immun. Ageing 2014, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.M.; Weinberg, B.D.; Hoch, M.J. CT myelography: Clinical Indications and Imaging Findings. Radiographics 2020, 40, 470–484. [Google Scholar] [CrossRef]
- Wilen, R.; Naftolin, F. Pubertal food intake and body length, weight, and composition in the feed-restricted female rat: Comparison with well fed animals. Pediatr. Res. 1978, 12, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Brower, M.; Grace, M.; Kotz, C.M.; Koya, V. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Lab. Anim. Res. 2015, 31, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Kuzmicz-Kowalska, K.; Kicheva, A. Regulation of size and scale in vertebrate spinal cord development. WIREs Dev. Biol. 2021, 10, e383. [Google Scholar] [CrossRef]
- Shechter, R.; Ziv, Y.; Schwartz, M. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem Cells. 2007, 25, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of new glial cells in intact and injured adult spinal cord. Cell. Stem Cell. 2010, 7, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef] [PubMed]
- Rusanescu, G.; Mao, J. Immature spinal cord neurons are dynamic regulators of adult nociceptive sensitivity. J. Cell. Mol. Med. 2015, 19, 2352–2364. [Google Scholar] [CrossRef]
- Horner, P.J.; Power, A.E.; Kempermann, G.; Kuhn, H.G.; Palmer, T.D.; Winkler, J.; Thal, L.J.; Gage, F.H. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 2000, 20, 2218–2228. [Google Scholar] [CrossRef] [Green Version]
- Portiansky, E.L.; Nishida, F.; Barbeito, C.G.; Gimeno, E.J.; Goya, R.G. Increased number of neurons in the cervical spinal cord of aged female rats. PLoS ONE 2011, 6, e22537. [Google Scholar] [CrossRef] [Green Version]
- Shetty, A.K.; Hattiangady, B.; Shetty, G.A. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia 2005, 51, 173–186. [Google Scholar] [CrossRef]
- Lepack, A.E.; Fuchikami, M.; Dwyer, J.M.; Banasr, M.; Duman, R.S. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 2015, 18, pyu033. [Google Scholar] [CrossRef] [Green Version]
- Mashima, T.; Wakatsuki, T.; Kawata, N.; Jang, M.-K.; Nagamori, A.; Yoshida, H.; Nakamura, K.; Migita, T.; Seimiya, H.; Yamaguchi, K. Neutralization of the induced VEGF-A potentiates the therapeutic effect of an anti-VEGFR2 antibody on gastric cancer in vivo. Sci. Rep. 2021, 11, 15125. [Google Scholar] [CrossRef]
- Sasaki, T.; Jyo, Y.; Tanda, N.; Kawakami, Y.; Nohno, T.; Tamai, H.; Osawa, G. Changes in glomerular epithelial cells induced by FGF2 and FGF2 neutralizing antibody in puromycin aminonucleoside nephropathy. Kidney Int. 1997, 51, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.-P.; Gjata, B.; Lafont, H.; Sebille, A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta1. J. Neuroimmunol. 1996, 70, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, G.; Penniman, C.M.; Jena, J.; Suarez Beltran, P.A.; Foster, C.; Poro, K.; Junck, T.L.; Hinton, A.O.; Souvenir, R.; Fuqua, J.D.; et al. Insulin and IGF-1 receptors regulate complex I–dependent mitochondrial bioenergetics and supercomplexes via FoxOs in muscle. J. Clin. Investig. 2021, 131, e146415. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, J.; Liu, C.; Papudesu, C.; Ward, P.J.; Wilhelm, J.C.; English, A.W. Neuronal BDNF signaling is necessary for the effects of treadmill exercise on synaptic stripping of axotomized motoneurons. Neural. Plast. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, T.; Hosaka, K.; Fischer, C.; Lim, S.; Andersson, P.; Abe, M.; Iwamoto, H.; Gao, Y.; Wang, X.; Fong, G.-H.; et al. Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning. J. Exp. Med. 2018, 215, 611–626. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotome, A.; Kadoya, K.; Suzuki, Y.; Iwasaki, N. Spinal Canal and Spinal Cord in Rat Continue to Grow Even after Sexual Maturation: Anatomical Study and Molecular Proposition. Int. J. Mol. Sci. 2022, 23, 16076. https://doi.org/10.3390/ijms232416076
Sotome A, Kadoya K, Suzuki Y, Iwasaki N. Spinal Canal and Spinal Cord in Rat Continue to Grow Even after Sexual Maturation: Anatomical Study and Molecular Proposition. International Journal of Molecular Sciences. 2022; 23(24):16076. https://doi.org/10.3390/ijms232416076
Chicago/Turabian StyleSotome, Akihito, Ken Kadoya, Yuki Suzuki, and Norimasa Iwasaki. 2022. "Spinal Canal and Spinal Cord in Rat Continue to Grow Even after Sexual Maturation: Anatomical Study and Molecular Proposition" International Journal of Molecular Sciences 23, no. 24: 16076. https://doi.org/10.3390/ijms232416076
APA StyleSotome, A., Kadoya, K., Suzuki, Y., & Iwasaki, N. (2022). Spinal Canal and Spinal Cord in Rat Continue to Grow Even after Sexual Maturation: Anatomical Study and Molecular Proposition. International Journal of Molecular Sciences, 23(24), 16076. https://doi.org/10.3390/ijms232416076






