Structural and Functional Characterization of β−lytic Protease from Lysobacter capsici VKM B−2533T
Abstract
1. Introduction
2. Results
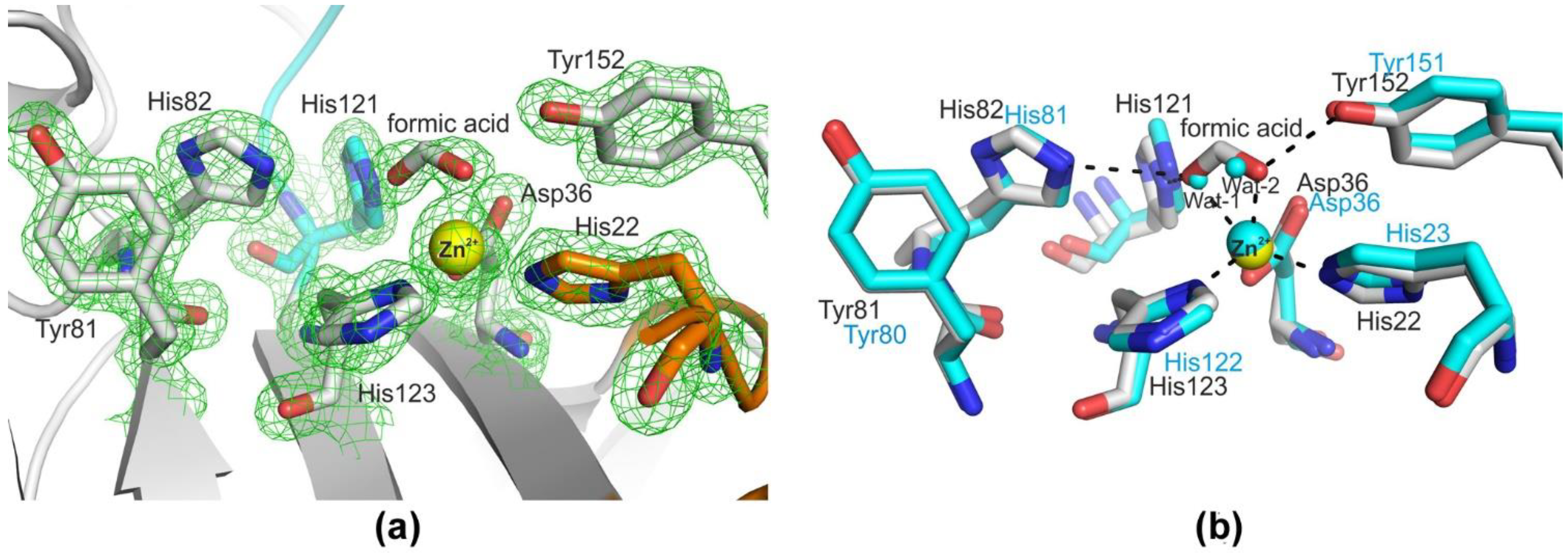
2.1. Structural Characterization of Blp from L. capsici VKM B−2533T
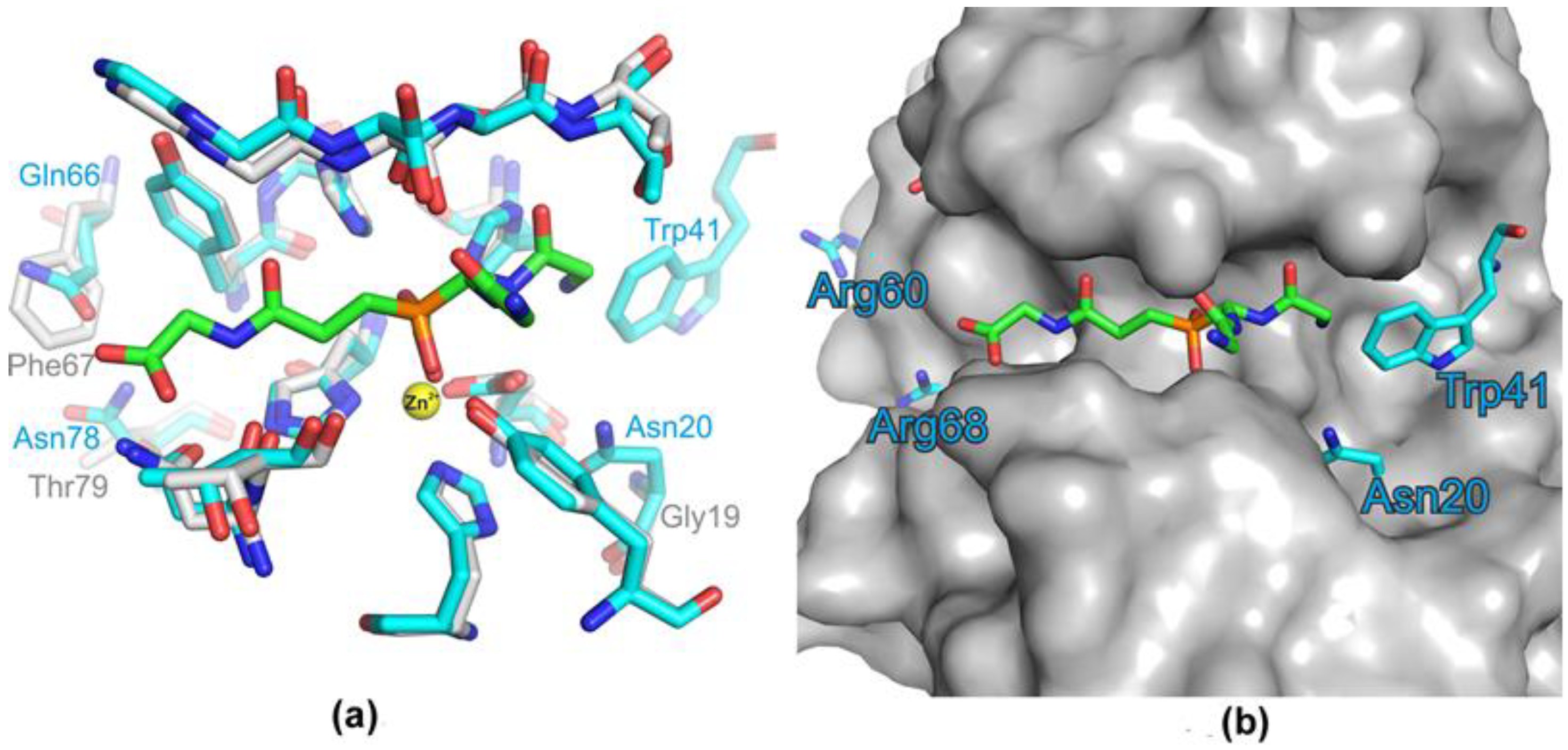
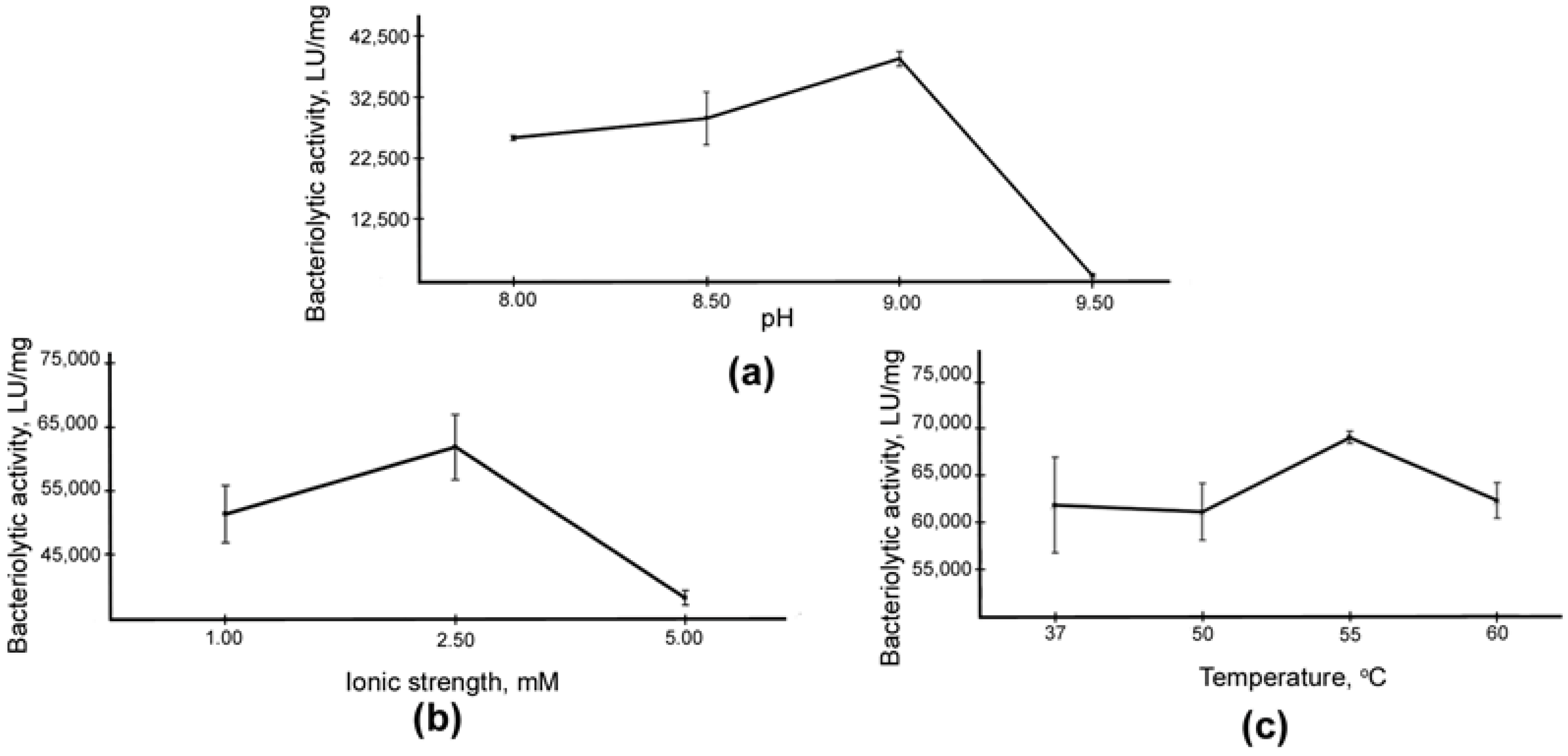
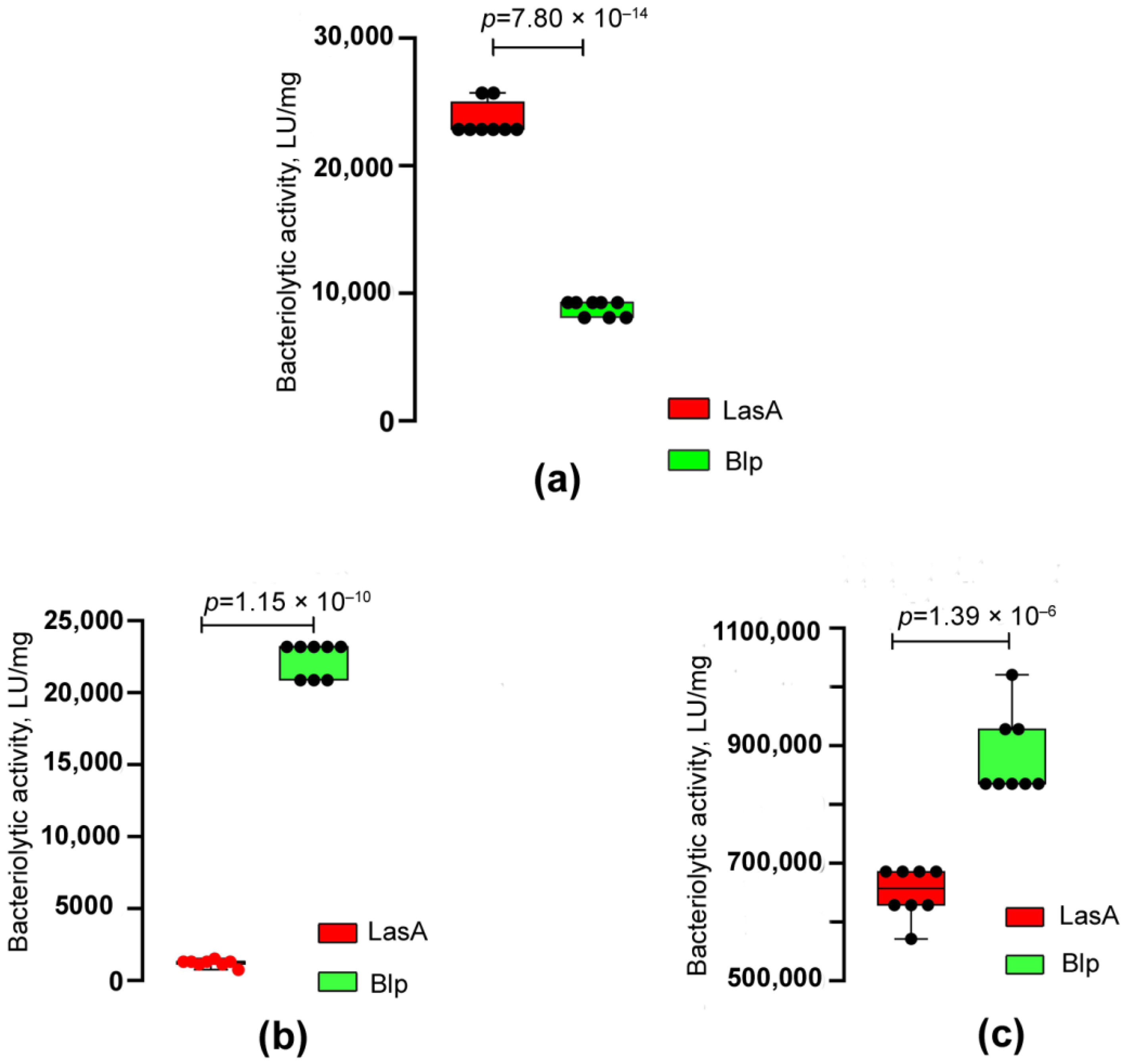
2.2. Comparative Characterization of LasA and Blp Physicochemical and Bacteriolytic Properties
3. Discussion
4. Materials and Methods
4.1. Strains and Cultivation Conditions
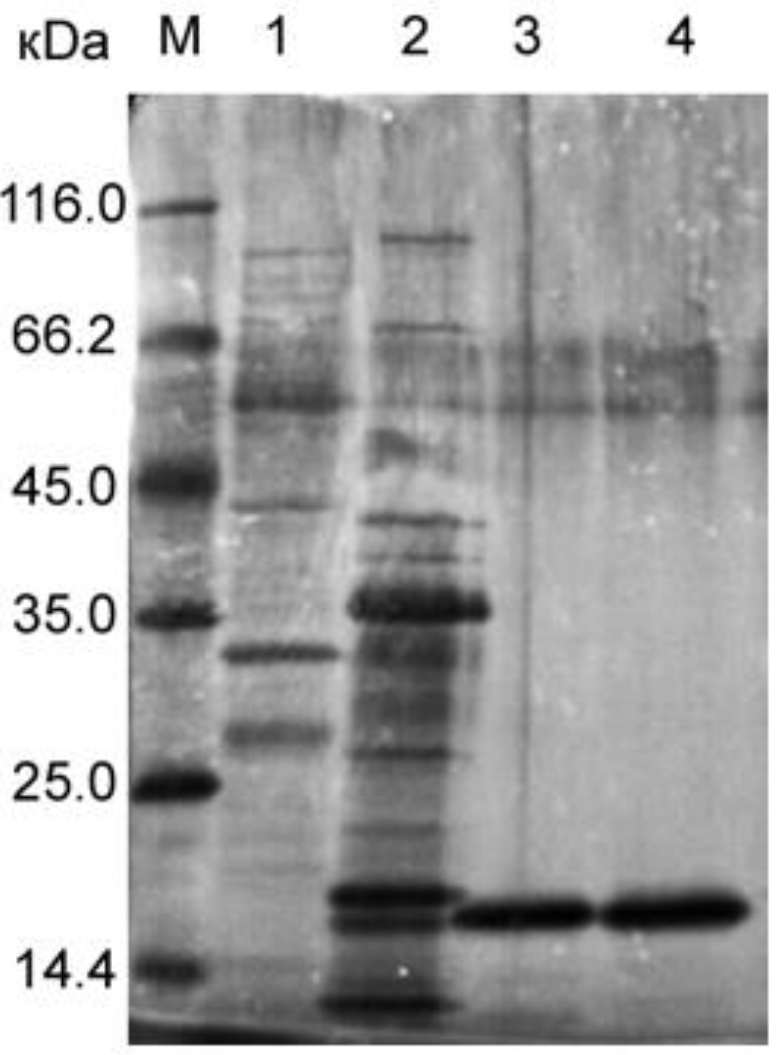
4.2. Purification of Bacteriolytic Proteins L. capsici VKM B−2533T Blp and P. aeruginosa LasA
4.3. Protein Measurement by Bradford Method
4.4. Determination of Optimal Conditions for LasA Bacteriolytic Activity
4.5. Comparison of Bacteriolytic Activity of L. capsici VKM B−2533T Blp and P. aeruginosa LasA Proteins with Respect to Target Cells
4.6. SDS−PAGE
4.7. MALDI−TOF Mass Spectrometry
4.8. Crystallization and Crystallography
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitaker, D.R. Lytic enzymes of Sorangium sp. Isolation and enzymatic properties of the alpha− and beta−lytic proteases. Can. J. Biochem. 1965, 43, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. 1922, 93, 306–317. [Google Scholar]
- Ahmed, K.; Chohnan, S.; Ohashi, H.; Hirata, T.; Masaki, T.; Sakiyama, F. Purification, bacteriolytic activity, and specificity of beta−lytic protease from Lysobacter sp. IB−9374. J. Biosci. Bioeng. 2003, 95, 27–34. [Google Scholar] [CrossRef]
- Li, S.; Norioka, S.; Sakiyama, F. Bacteriolytic activity and specificity of Achromobacter beta−lytic protease. J. Biochem. 1998, 124, 332–339. [Google Scholar] [CrossRef]
- Afoshin, A.S.; Kudryakova, I.V.; Borovikova, A.O.; Suzina, N.E.; Toropygin, I.Y.; Shishkova, N.A.; Vasilyeva, N.V. Lytic potential of Lysobacter capsici VKM B−2533T: Bacteriolytic enzymes and outer membrane vesicles. Sci. Rep. 2020, 10, 9944. [Google Scholar] [CrossRef]
- Afoshin, A.S.; Konstantinov, M.A.; Toropygin, I.Y.; Kudryakova, I.V.; Vasilyeva, N.V. β−Lytic protease of Lysobacter capsici VKM B−2533T. Antibiotics 2020, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Loewy, A.G.; Santer, U.V.; Wieczorek, M.; Blodgett, J.K.; Jones, S.W.; Cheronis, J.C. Purification and characterization of a novel zinc−proteinase from cultures of Aeromonas hydrophila. J. Biol. Chem. 1993, 268, 9071–9078. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.; Falcón, M.A.; Carnicero, A.; Gutiérrez−Navarro, A.M.; Mansito, T.B. Purification and peptidase activity of a bacteriolytic extracellular enzyme from Pseudomonas aeruginosa. Res. Microbiol. 1989, 140, 125–137. [Google Scholar] [CrossRef]
- Zhao, H.L.; Chen, X.L.; Xie, B.B.; Zhou, M.Y.; Gao, X.; Zhang, X.Y.; Zhou, B.C.; Weiss, A.S.; Zhang, Y.Z. Elastolytic mechanism of a novel M23 metalloprotease pseudoalterin from deep−sea Pseudoalteromonas sp. CF6−2: Cleaving not only glycyl bonds in the hydrophobic regions but also peptide bonds in the hydrophilic regions involved in cross−linking. J. Biol. Chem. 2012, 287, 39710–39720. [Google Scholar] [CrossRef]
- Kessler, E. Chapter 348—β−Lytic Metalloendopeptidase. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press, Elsevier: London, UK, 2013; pp. 1550–1553. [Google Scholar]
- Kessler, E.; Ohman, D.E. Chapter 349—Staphylolysin. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press, Elsevier: London, UK, 2013; pp. 1553–1558. [Google Scholar]
- Wysocka, A.; Jagielska, E.; Łężniak, Ł.; Sabała, I. Two new M23 peptidoglycan hydrolases with distinct net charge. Front. Microbiol. 2021, 12, 719689. [Google Scholar] [CrossRef]
- Xiang, Y.; Morais, M.C.; Cohen, D.N.; Bowman, V.D.; Anderson, D.L.; Rossmann, M.G. Crystal and cryoEM structural studies of a cell wall degrading enzyme in the bacteriophage phi29 tail. Proc. Natl. Acad. Sci. USA 2008, 105, 9552–9557. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Flint, S.H.; Yu, P.L. Determination of the mode of action of enterolysin A, produced by Enterococcus faecalis B9510. J. Appl. Microbiol. 2013, 115, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Simmonds, R.S.; Young, J.K.; Timkovich, R. Solution structure of the recombinant target recognition domain of zoocin A. Proteins 2013, 81, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Park, P.W.; Mecham, R.P. Chapter 350—Lysostaphin. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press, Elsevier: London, UK, 2013; pp. 1558–1560. [Google Scholar]
- Sugai, M.; Fujiwara, T.; Akiyama, T.; Ohara, M.; Komatsuzawa, H.; Inoue, S.; Suginaka, H. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitis EPK1. J. Bacteriol. 1997, 179, 1193–1202. [Google Scholar] [CrossRef]
- Ramadurai, L.; Jayaswal, R.K. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J. Bacteriol. 1997, 179, 3625–3631. [Google Scholar] [CrossRef]
- Raulinaitis, V.; Tossavainen, H.; Aitio, O.; Juuti, J.T.; Hiramatsu, K.; Kontinen, V.; Permi, P. Identification and structural characterization of LytU, a unique peptidoglycan endopeptidase from the lysostaphin family. Sci. Rep. 2017, 7, 6020. [Google Scholar] [CrossRef]
- Wu, J.W.; Chen, X.L. Extracellular metalloproteases from bacteria. Appl. Microbiol. Biotechnol. 2011, 92, 253–262. [Google Scholar] [CrossRef]
- Spencer, J.; Murphy, L.M.; Conners, R.; Sessions, R.B.; Gamblin, S.J. Crystal structure of the LasA virulence factor from Pseudomonas aeruginosa: Substrate specificity and mechanism of M23 metallopeptidases. J. Mol. Biol. 2010, 396, 908–923. [Google Scholar] [CrossRef]
- Xing, M.; Simmonds, R.S.; Timkovich, R. Solution structure of the Cys74 to Ala74 mutant of the recombinant catalytic domain of Zoocin A. Proteins 2017, 85, 177–181. [Google Scholar] [CrossRef]
- Simmonds, R.S.; Pearson, L.; Kennedy, R.C.; Tagg, J.R. Mode of action of a lysostaphin−like bacteriolytic agent produced by Streptococcus zooepidemicus 4881. Appl. Environ. Microbiol. 1996, 62, 4536–4541. [Google Scholar] [CrossRef]
- Grabowska, M.; Jagielska, E.; Czapinska, H.; Bochtler, M.; Sabala, I. High resolution structure of an M23 peptidase with a substrate analogue. Sci. Rep. 2015, 5, 14833. [Google Scholar] [CrossRef] [PubMed]
- Sabala, I.; Jagielska, E.; Bardelang, P.T.; Czapinska, H.; Dahms, S.O.; Sharpe, J.A.; James, R.; Than, M.E.; Thomas, N.R.; Bochtler, M. Crystal structure of the antimicrobial peptidase lysostaphin from Staphylococcus simulans. FEBS J. 2014, 281, 4112–4122. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez−Delgado, L.S.; Walters−Morgan, H.; Salamaga, B.; Robertson, A.J.; Hounslow, A.M.; Jagielska, E.; Sabała, I.; Williamson, M.P.; Lovering, A.L.; Mesnage, S. Two−site recognition of Staphylococcus aureus peptidoglycan by lysostaphin SH3b. Nat. Chem. Biol. 2020, 16, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L.; Yang, J.; Chen, X.L.; Wang, P.; Zhao, H.L.; Su, H.N.; Li, C.Y.; Yu, Y.; Zhong, S.; Wang, L.; et al. A predator-prey interaction between a marine Pseudoalteromonas sp. and Gram-positive bacteria. Nat. Commun. 2020, 11, 285. [Google Scholar] [CrossRef]
- Bochtler, M.; Odintsov, S.G.; Marcyjaniak, M.; Sabala, I. Similar active sites in lysostaphins and D−Ala−D−Ala metallopeptidases. Protein Sci. 2004, 13, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Cruse, W.B.; Whitaker, D.R. Preliminary crystallographic data for beta−lytic protease. J. Mol. Biol. 1976, 102, 173–175. [Google Scholar] [CrossRef]
- Kudryakova, I.; Afoshin, A.; Leontyevskaya, E.; Leontyevskaya (Vasilyeva), N. The first homologous expression system for the β−lytic protease of Lysobacter capsici VKM B−2533T, a promising antimicrobial agent. Int. J. Mol. Sci. 2022, 23, 5722. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fernandez−Patron, C.; Castellanos−Serra, L.; Rodriguez, P. Reverse staining of sodium dodecyl sulfate polyacrylamide gels by imidazole−zinc salts: Sensitive detection of unmodified proteins. Biotechniques 1992, 12, 564–573. [Google Scholar] [PubMed]
- Kraft, P.; Bergamaschi, A.; Broennimann, C.; Dinapoli, R.; Eikenberry, E.F.; Henrich, B.; Johnson, I.; Mozzanica, A.; Schlepütz, C.M.; Willmott, P.R.; et al. Performance of single−photon−counting PILATUS detector modules. J. Synchrotron Radiat. 2009, 16, 368–375. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse−Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse−Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef]

| Data Collection | |
|---|---|
| Space group | P21212 |
| a, b, c, Å | 70.58, 44.13, 52.70 |
| α = β = γ, ° | 90.0 |
| Resolution limits, Å | 50.0–1.57 (1.61–1.57) |
| Rsigma, % | 18.5 (111.8) |
| Mean I/σ (I) | 11.16 (2.10) |
| Completeness, % | 100.0 (100.0) |
| Redundancy | 12.53 (12.52) |
| CC1/2, % | 99.7 (69.9) |
| Unique reflections | 23 631 (1 701) |
| Refinement statistics | |
| Resolution, Å | 42.23–1.57 (1.64–1.57) |
| Total number of reflections | 23 623 (2 761) |
| Rwork/Rfree, % | 14.8/20.4 (21.8/28.5) |
| Average B−factor, Å2 | 14.0 |
| Ramachandran plot | |
| Most favourable regions, % | 98.9 |
| Allowed regions, % | 1.1 |
| R.m.s. deviations | |
| Bond length, Å | 0.006 |
| Bond angle, ° | 0.902 |
| Blp | LasA | Location |
|---|---|---|
| Gly19 | Asn20 | Loop1 |
| – | Trp41 | |
| Lys61 | Arg60 | β3 |
| His63 | Leu62 | β3 |
| Phe67 | Glu66 | β4 |
| Glu69 | Arg68 | β4 |
| Ser77 | Ala76 | β5 |
| Thr79 | Asn78 | β5 |
| Asn113 | Glu112 | Loop3 |
| Lys127 | Leu126 | β7 |
| Target Cells | Blp, LU/mg | LasA, LU/mg |
|---|---|---|
| S. aureus 209P living cells S. aureus 209P autoclaved cells | 52,214 ± 3441 8846 ± 600 | 55,626 ± 3002 ns 23,571 ± 1323 *** |
| M. luteus Ac−2230T autoclaved cells | 22,332 ± 1201 | 1238 ± 28 *** |
| M. luteus Ac−2230T living cells K. rosea Ac−2200T living cells | 888,300 ± 73,020 1817 ± 77 | 644,898 ± 43,196 *** 0 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afoshin, A.; Tishchenko, S.; Gabdulkhakov, A.; Kudryakova, I.; Galemina, I.; Zelenov, D.; Leontyevskaya, E.; Saharova, S.; Leontyevskaya, N. Structural and Functional Characterization of β−lytic Protease from Lysobacter capsici VKM B−2533T. Int. J. Mol. Sci. 2022, 23, 16100. https://doi.org/10.3390/ijms232416100
Afoshin A, Tishchenko S, Gabdulkhakov A, Kudryakova I, Galemina I, Zelenov D, Leontyevskaya E, Saharova S, Leontyevskaya N. Structural and Functional Characterization of β−lytic Protease from Lysobacter capsici VKM B−2533T. International Journal of Molecular Sciences. 2022; 23(24):16100. https://doi.org/10.3390/ijms232416100
Chicago/Turabian StyleAfoshin, Alexey, Svetlana Tishchenko, Azat Gabdulkhakov, Irina Kudryakova, Inna Galemina, Dmitry Zelenov, Elena Leontyevskaya, Sofia Saharova, and Natalya Leontyevskaya (Vasilyeva). 2022. "Structural and Functional Characterization of β−lytic Protease from Lysobacter capsici VKM B−2533T" International Journal of Molecular Sciences 23, no. 24: 16100. https://doi.org/10.3390/ijms232416100
APA StyleAfoshin, A., Tishchenko, S., Gabdulkhakov, A., Kudryakova, I., Galemina, I., Zelenov, D., Leontyevskaya, E., Saharova, S., & Leontyevskaya, N. (2022). Structural and Functional Characterization of β−lytic Protease from Lysobacter capsici VKM B−2533T. International Journal of Molecular Sciences, 23(24), 16100. https://doi.org/10.3390/ijms232416100









