Varicocele, Functional Foods and Nutraceuticals: From Mechanisms of Action in Animal Models to Therapeutic Application
Abstract
:1. Introduction
2. Materials and Methods
3. Varicocele Experimental Models
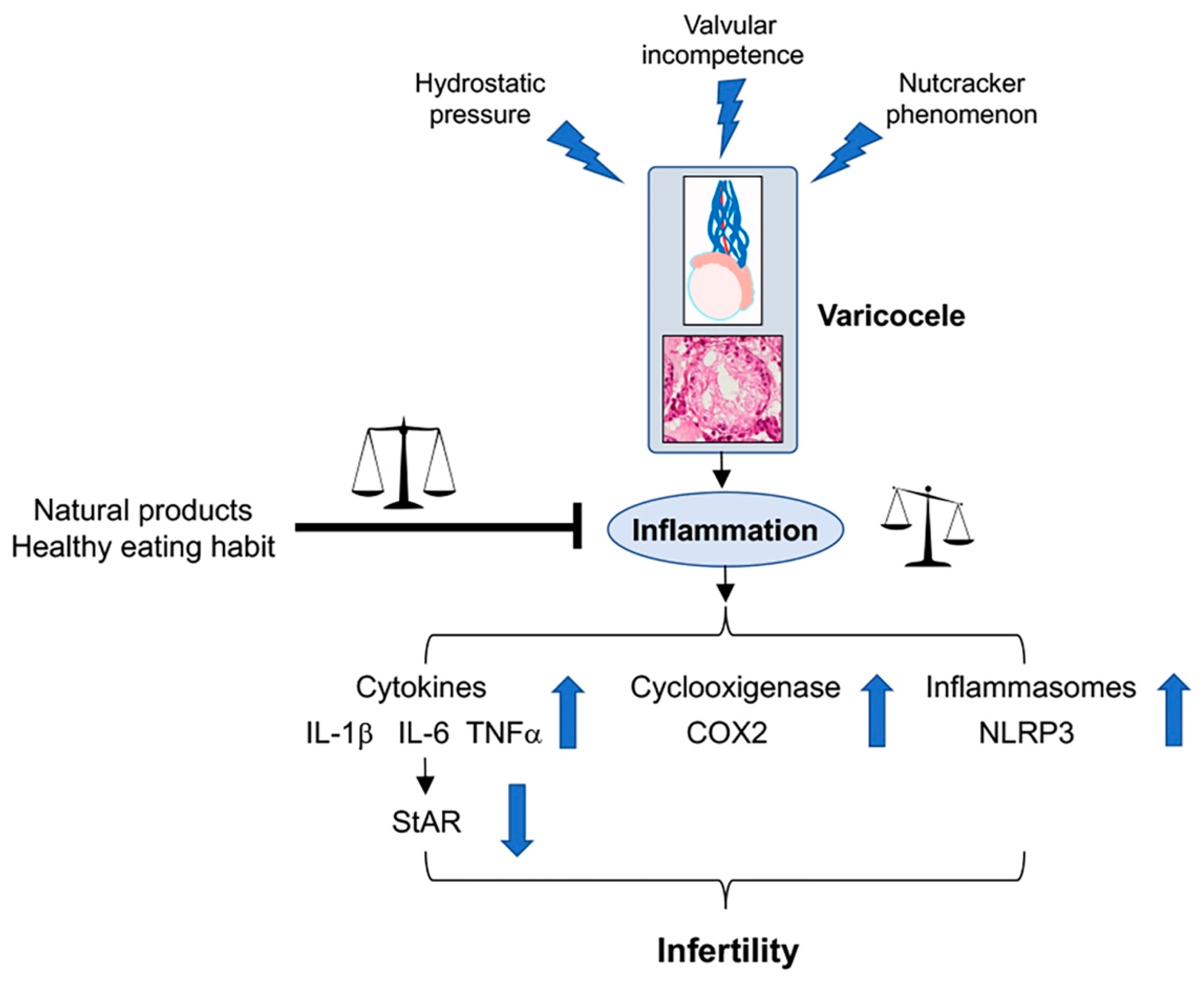
4. Varicocele and Inflammation
5. Dietary Habits and Their Effects in Varicocele
6. Anti-Inflammatory Effects of Nutraceuticals in Experimental Varicocele
| Animal | Treatment | Results | Reference |
|---|---|---|---|
| Rat | Aescin (20–40 mg/kg intragastric) for 7 weeks after 4 weeks from surgery | Lower interstitial edema Reduced polymorphonuclear leucocytes density | [54] |
| Rat | Morinda officinalis Polysaccharide (200–300–400 mg/kg gavage) for 4 weeks after 8 weeks from surgery | Reduction of TGF-β3 and TNF-α Upregulation of tight junction protein expression | [55] |
| Rat | Resveratrol (20–50 mg/kg gavage) for one month after 3 months from surgery | Reduction of NLRP3 inflammasome | [28] |
| Rat | Silymarin (50 mg/kg orally) for 60 days after surgery | Reduction of COX-2 | [27] |
| Rat | MOTILIPERM (monotropein, astragalin, spiraeoside) (200 mg/kg gavage) for 28 days after 4 weeks from surgery | Reduction of IL-6 and TNF-α | [2] |
| Rat | Berberine (50–100 mg/kg i.p.) for 60 days after surgery | Reduction of IL-6 and TNF-α Diminished immune cells infiltration | [18] |
| Rat | Gui-A-Gra (1.63–6.5 g/kg orally) for 42 days after 4 weeks from surgery | Reduction of IL-6 and TNF-α | [29] |
| Rat | Erbal combination (200–400 mg/kg orally) for 4 weeks after surgery | Reduction of IL-6, IL-1β and TNF-α | [60] |
| Rat | PDRN (8 mg/kg i.p.)-Se (0.4 mg/kg i.p.) for 30 days after 28 days from surgery | Reduction of NLRP3 inflammasome and IL-1β | [13] |
| Mouse | N-Palmitoylethanolamide (PEA) (10 mg/kg i.p.) for 21 days after 28 days from surgery | Reduction of NLRP3 inflammasome, pERK 1/2, TGF-β3 Improvement of tubular and extratubular organization | [56] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albano Nogueira, G.A.K.; Maciel Junior, V.L.; Minas, A.; Antoniassi, M.P. Characterization of varicocele-induced animal models: Potential role of inflammasome complex in the varicocele pathophysiology. J. Reprod. Immunol. 2022, 149, 103442. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.K.; Choi, B.R.; You, J.H.; Shin, Y.S.; Cui, W.S.; Lee, S.W.; Kim, J.H.; Kim, C.Y.; Kim, H.K.; Park, J.K. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement. Altern. Med. 2019, 19, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef]
- Abo El Gheit, R.E.; Soliman, N.A.; Nagla, S.A.; El-Sayed, R.M.; Badawi, G.A.; Emam, M.N.; Abdel Ghafar, M.T.; Ibrahim, M.A.A.; Elswaidy, N.R.M.; Radwan, D.A.; et al. Melatonin epigenetic potential on testicular functions and fertility profile in varicocele rat model is mediated by silent information regulator 1. Br. J. Pharmacol. 2022, 179, 3363–3381. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Sabanegh, E., Jr. Anatomic Theories of Varicocele Origin. In Varicocele and Male Infertility; Esteves, S.C., Cho, C.L., Majzoub, A., Agarwal, A., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 17–26. [Google Scholar]
- Goren, M.; Gat, Y. Varicocele is the root cause of BPH: Destruction of the valves in the spermatic veins produces elevated pressure which diverts undiluted testosterone directly from the testes to the prostate. Andrologia 2018, 50, e12992. [Google Scholar] [CrossRef]
- Dunphy, L.; Penna, M.; Tam, E.; El-Kafsi, J. Left renal vein entrapment syndrome: Nutcracker syndrome! BMJ Case Rep. 2019, 12, e230877. [Google Scholar] [CrossRef] [PubMed]
- Englund, K.M.; Rayment, M. Nutcracker syndrome: A proposed ultrasound protocol. Australas J. Ultrasound Med. 2018, 21, 75–78. [Google Scholar] [CrossRef]
- Macey, M.R.; Owen, R.C.; Ross, S.S.; Coward, R.M. Best practice in the diagnosis and treatment of varicocele in children and adolescents. Ther. Adv. Urol. 2018, 10, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Paick, S.; Choi, W.S. Varicocele and Testicular Pain: A Review. World J. Mens Health. 2019, 37, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.K.; Zhang, L.T.; Choi, B.R.; Karna, K.K.; You, J.H.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Zhao, C.; Chae, H.J.; et al. Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm. Biol. 2018, 56, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lorian, K.; Kadkhodaee, M.; Kianian, F.; Abdi, A.; Sadeghipour, H.; Seifi, B. Oxidative stress, nitric oxide and inflammation in the pathophysiology of varicocele and the effect of hydrogen sulfide as a potential treatment. Physiol. Pharmacol. 2019, 23, 249–260. [Google Scholar]
- Antonuccio, P.; Micali, A.G.; Romeo, C.; Freni, J.; Vermiglio, G.; Puzzolo, D.; Squadrito, F.; Irrera, N.; Marini, H.R.; Rana, R.A.; et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. Int. J. Mol. Sci. 2021, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Saypol, D.C.; Howards, S.S.; Turner, T.T.; Miller, E.D. Influence of surgically induced varicocele on testicular blood flow, temperature, and histology in adult rats and dogs. J. Clin. Investig. 1981, 68, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najari, B.B.; Li, P.S.; Ramasamy, R.; Katz, M.; Sheth, S.; Robinson, B.; Chen, H.; Zirkin, B.; Schlegel, P.N.; Goldstein, M. Microsurgical rat varicocele model. J. Urol. 2014, 191, 548–553. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Hadian Amree, A.; Khorramdelazad, H.; Karami, H.; Abedinzadeh, M. Inflammatory and anti-inflammatory cytokines in the seminal plasma of infertile men suffering from varicocele. Andrologia 2017, 49, e12685. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Bafrani, H.; Najaran, H.; Razi, M.; Rashtbari, H. Berberine ameliorates experimental varicocele-induced damages at testis and sperm levels; evidences for oxidative stress and inflammation. Andrologia 2019, 51, e13179. [Google Scholar] [CrossRef]
- Potashnik, H.; Elhija, M.A.; Lunenfeld, E.; Potashnik, G.; Schlatt, S.; Nieschlag, E.; Huleihel, M. Interleukin-6 expression during normal maturation of the mouse testis. Eur. Cytokine Netw. 2005, 16, 161–165. [Google Scholar]
- Huang, G.; Yuan, M.; Zhang, J.; Li, J.; Gong, D.; Li, Y.; Zhang, J.; Lin, P.; Huang, L. IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway. Sci. Rep. 2016, 6, 28012. [Google Scholar] [CrossRef] [Green Version]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Villiger, P.M.; Caliezi, G.; Cottin, V.; Förger, F.; Senn, A.; Østensen, M. Effects of TNF antagonists on sperm characteristics in patients with spondyloarthritis. Ann. Rheum. Dis. 2010, 69, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, Z.; Xu, G.; Niu, L.; Yu, L.; Luo, Z.; Yan, J. Expression of claudin-11 in a rat model of varicocele and its effects on the blood-testis barrier. Mol. Med. Rep. 2018, 18, 5647–5651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y. Action and Interaction between Retinoic Acid Signaling and Blood-Testis Barrier Function in the Spermatogenesis Cycle. Cells 2022, 11, 352. [Google Scholar] [CrossRef]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2A Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2017, 7, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Su, Y.; Xu, J.; Hu, Z.; Zhao, K.; Liu, C.; Zhang, H. Varicocele-Mediated Male Infertility: From the Perspective of Testicular Immunity and Inflammation. Front. Immunol. 2021, 12, 729539. [Google Scholar] [CrossRef]
- Mazhari, S.; Razi, M.; Sadrkhanlou, R. Silymarin and celecoxib ameliorate experimental varicocele-induced pathogenesis: Evidences for oxidative stress and inflammation inhibition. Int. Urol. Nephrol. 2018, 50, 1039–1052. [Google Scholar] [CrossRef]
- Hajipour, E.; Jalali Mashayekhi, F.; Mosayebi, G.; Baazm, M.; Zendedel, A. Resveratrol decreases apoptosis and NLRP3 complex expressions in experimental varicocele rat model. Iran. J. Basic Med. Sci. 2018, 21, 225–229. [Google Scholar]
- Karna, K.K.; Choi, N.Y.; Kim, C.Y.; Kim, H.K.; Shin, Y.S.; Park, J.K. Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway. Int. J. Mol. Sci. 2020, 21, 9231. [Google Scholar] [CrossRef]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower seed oil improves steroidogenesis and spermatogenesis in rats with type II diabetes mellitus by modulating the genes expression involved in steroidogenesis, inflammation and oxidative stress. J. Ethnopharmacol. 2021, 275, 114139. [Google Scholar] [CrossRef]
- Arab, D.; Doustmohammadi, H.; Ardestani Zadeh, A. Dietary supplements in the management of varicocele-induced infertility: A review of potential mechanisms. Andrologia 2021, 53, e13879. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef] [PubMed]
- Varani, J. Healthful eating, the western style diet and chronic disease. Approaches Poult. Dairy Vet. Sci. 2017, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Radwan, P.; Bochenek, M.; Hanke, W. Dietary patterns and their relationship with semen quality. Am. J. Men Health 2018, 12, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, E.; Bravi, F.; Noli, S.; Ferrari, S.; De Cosmi, V.; La Vecchia, I.; Cavadini, M.; La Vecchia, C.; Parazzini, F. Mediterranean diet and the risk of poor semen quality: Cross-sectional analysis of men referring to an Italian fertility clinic. Andrology 2019, 7, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Caputo, M.; Cirillo, P.; Scappaticcio, L.; Longo, M.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Effects of Mediterranean diet on semen parameters in healthy young adults: A randomized controlled trial. Minerva Endocrinol. 2020, 45, 280–287. [Google Scholar] [CrossRef]
- Orzylowska, E.M.; Jacobson, J.D.; Bareh, G.M.; Ko, E.Y.; Corselli, J.U.; Chan, P.J. Food intake diet and sperm characteristics in a blue zone: A Loma Linda study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 112–115. [Google Scholar] [CrossRef]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and nutritional factors in male (in)fertility—Underestimated factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef]
- Bachir, B.G.; Jarvi, K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol. Clin. N. Am. 2014, 41, 67–81. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Ferramosca, A.; Conte, A.; Moscatelli, N.; Zara, V. A high-fat diet negatively affects rat sperm mitochondrial respiration. Andrology 2016, 4, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) production alters sperm quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Llopis, S.; Bañuls, C.; de Marañon, A.M.; Diaz-Morales, N.; Jover, A.; Garzon, S.; Rocha, M.; Victor, V.M.; Hernandez-Mijares, A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 2017, 108, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lunetti, P.; Capobianco, L.; Zara, V.; Ferramosca, A. Physical activity and male reproductive function: A new role for gamete mitochondria. Exerc. Sport Sci. Rev. 2021, 49, 99–106. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Temple, N.; Woodside, J.V. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef]
- Di Giacomo, M.; Zara, V.; Bergamo, P.; Ferramosca, A. Crosstalk between mitochondrial metabolism and oxidoreductive homeostasis: A new perspective for understanding the effects of bioactive dietary compounds. Nutr. Res. Rev. 2020, 33, 90–101. [Google Scholar] [CrossRef]
- Ferramosca, A.; Moscatelli, N.; Di Giacomo, M.; Zara, V. Dietary fatty acids influence sperm quality and function. Andrology 2017, 5, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Rozati, R.; Reddy, P.P.; Reddanna, P.; Mujtaba, R. Role of environmental estrogens in the deterioration of male factor fertility. Fertil. Steril. 2002, 78, 1187–1194. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [Green Version]
- Turner, T.T. The study of varicocele through the use of animal models. Hum. Reprod. Update 2001, 7, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofikitis, N.; Miyagawa, I. Effects of surgical repair of experimental left varicocele on testicular temperature, spermatogenesis, sperm maturation, endocrine function, and fertility in rabbits. Arch. Androl. 1992, 29, 163–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, R.H.; Ma, M.; Zhu, Y.; Yang, S.; Wang, Z.Q.; Zhang, Z.S.; Wan, C.F.; Li, P.; Liu, Y.F.; Wang, J.L.; et al. Effects of aescin on testicular repairment in rats with experimentally induced varicocele. Andrologia 2014, 46, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.; Wang, F.; Lin, Q.; Wang, W. Effects of Morinda officinalis Polysaccharide on Experimental Varicocele Rats. Evid. Based Complement. Alternat. Med. 2016, 2016, 5365291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonuccio, P.; Marini, H.R.; Micali, A.; Romeo, C.; Granese, R.; Retto, A.; Martino, A.; Benvenga, S.; Cuzzocrea, S.; Impellizzeri, D.; et al. The Nutraceutical N-Palmitoylethanolamide (PEA) Reveals Widespread Molecular Effects Unmasking New Therapeutic Targets in Murine Varicocele. Nutrients 2021, 13, 734. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xin, H.L.; Xu, Y.M.; Shen, Y.; He, Y.Q.; Hsien-Yeh; Lin, B.; Song, H.T.; Juan-Liu; Yang, H.Y.; et al. Morinda officinalis How—A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 213, 230–255. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.K.; Zhang, L.T.; You, J.H.; Lee, S.W.; Kim, C.Y.; Cui, W.S.; Chae, H.J.; Kim, H.K.; Park, J.K. The effects of MOTILIPERM on cisplatin induced testicular toxicity in Sprague-Dawley rats. Cancer Cell. Int. 2015, 15, 121. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.Y.; Han, J.W.; Hwang, J.S.; Yun, E.Y.; Lee, B.M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Health A 2014, 77, 1332–1345. [Google Scholar] [CrossRef]
- Kim, S.J.; Jeon, S.H.; Kwon, E.B.; Jeong, H.C.; Choi, S.W.; Bae, W.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; et al. Early and Synergistic Recovery Effect of Herbal Combination on Surgically Corrected Varicocele. Altern. Ther. Health Med. 2020, 26, 24–31. [Google Scholar]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Saalu, L.; Oguntola, J.; Babalola, O.; Oyewopo, A. Reversal of experimental varicocele-induced testicular toxicity by L-ascorbate in rats. Afr. J. Biotechnol. 2009, 8, 965–970. [Google Scholar]
- Cyrus, A.; Kabir, A.; Goodarzi, D.; Moghimi, M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: A double blind placebo controlled clinical trial. Int. Braz. J. Urol. 2015, 41, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ağtürk, G.; Demir, E.A.; Tutuk, O.; Doğan, H.; Bayraktar, S.; Duran, G.G.; Temiz, M.; Tümer, C. Investigation of the effect of vitamin D in experimental varicocele model in rats. J. Cell. Neurosci. Oxidative Stress 2018, 10, 759–760. [Google Scholar]
- Khosravanian, H.; Razi, M.; Farokhi, F.; Khosravanian, N. Simultaneous Administration of Dexamethasone and Vitamin E Reversed Experimental Varicocele-induced Impact in testicular tissue in Rats; Correlation with Hsp70-2 Chaperone Expression. Int. Braz. J. Urol. 2015, 41, 773–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardestani Zadeh, A.; Arab, D.; Kia, N.S.; Heshmati, S.; Amirkhalili, S.N. The role of Vitamin E—Selenium—Folic Acid Supplementation in Improving Sperm Parameters After Varicocelectomy: A Randomized Clinical Trial. Urol. J. 2019, 16, 495–500. [Google Scholar]
- Festa, R.; Giacchi, E.; Raimondo, S.; Tiano, L.; Zuccarelli, P.; Silvestrini, A.; Meucci, E.; Littarru, G.P.; Mancini, A. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia 2014, 46, 805–807. [Google Scholar] [CrossRef]
- Al-Rubiey, F.K. Effect of L-carnitine and meloxicam treatment on testicular Leydig cell numbers of varicocelized rats. Middle East Fertil. Soc. J. 2012, 17, 47–53. [Google Scholar] [CrossRef]
- Sofimajidpour, H.; Ghaderi, E.; Ganji, O. Comparison of the Effects of Varicocelectomy and Oral L-carnitine on Sperm Parameters in Infertile Men with Varicocele. J. Clin. Diagn. Res. 2016, 10, PC07–PC10. [Google Scholar] [CrossRef]
- Nematollahi-Mahani, S.N.; Azizollahi, G.H.; Baneshi, M.R.; Safari, Z.; Azizollahi, S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia 2014, 46, 240–245. [Google Scholar] [CrossRef]
- Asghari, A.; Akbari, G.; Galustanian, G. Magnesium sulfate protects testis against unilateral varicocele in rat. Anim. Reprod. 2018, 14, 442–451. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, H.R.; Micali, A.; Puzzolo, D.; Minutoli, L.; Antonuccio, P. Varicocele, Functional Foods and Nutraceuticals: From Mechanisms of Action in Animal Models to Therapeutic Application. Int. J. Mol. Sci. 2022, 23, 16118. https://doi.org/10.3390/ijms232416118
Marini HR, Micali A, Puzzolo D, Minutoli L, Antonuccio P. Varicocele, Functional Foods and Nutraceuticals: From Mechanisms of Action in Animal Models to Therapeutic Application. International Journal of Molecular Sciences. 2022; 23(24):16118. https://doi.org/10.3390/ijms232416118
Chicago/Turabian StyleMarini, Herbert Ryan, Antonio Micali, Domenico Puzzolo, Letteria Minutoli, and Pietro Antonuccio. 2022. "Varicocele, Functional Foods and Nutraceuticals: From Mechanisms of Action in Animal Models to Therapeutic Application" International Journal of Molecular Sciences 23, no. 24: 16118. https://doi.org/10.3390/ijms232416118
APA StyleMarini, H. R., Micali, A., Puzzolo, D., Minutoli, L., & Antonuccio, P. (2022). Varicocele, Functional Foods and Nutraceuticals: From Mechanisms of Action in Animal Models to Therapeutic Application. International Journal of Molecular Sciences, 23(24), 16118. https://doi.org/10.3390/ijms232416118







