PHF21A Related Disorder: Description of a New Case
Abstract
:1. Introduction
2. Case Presentation
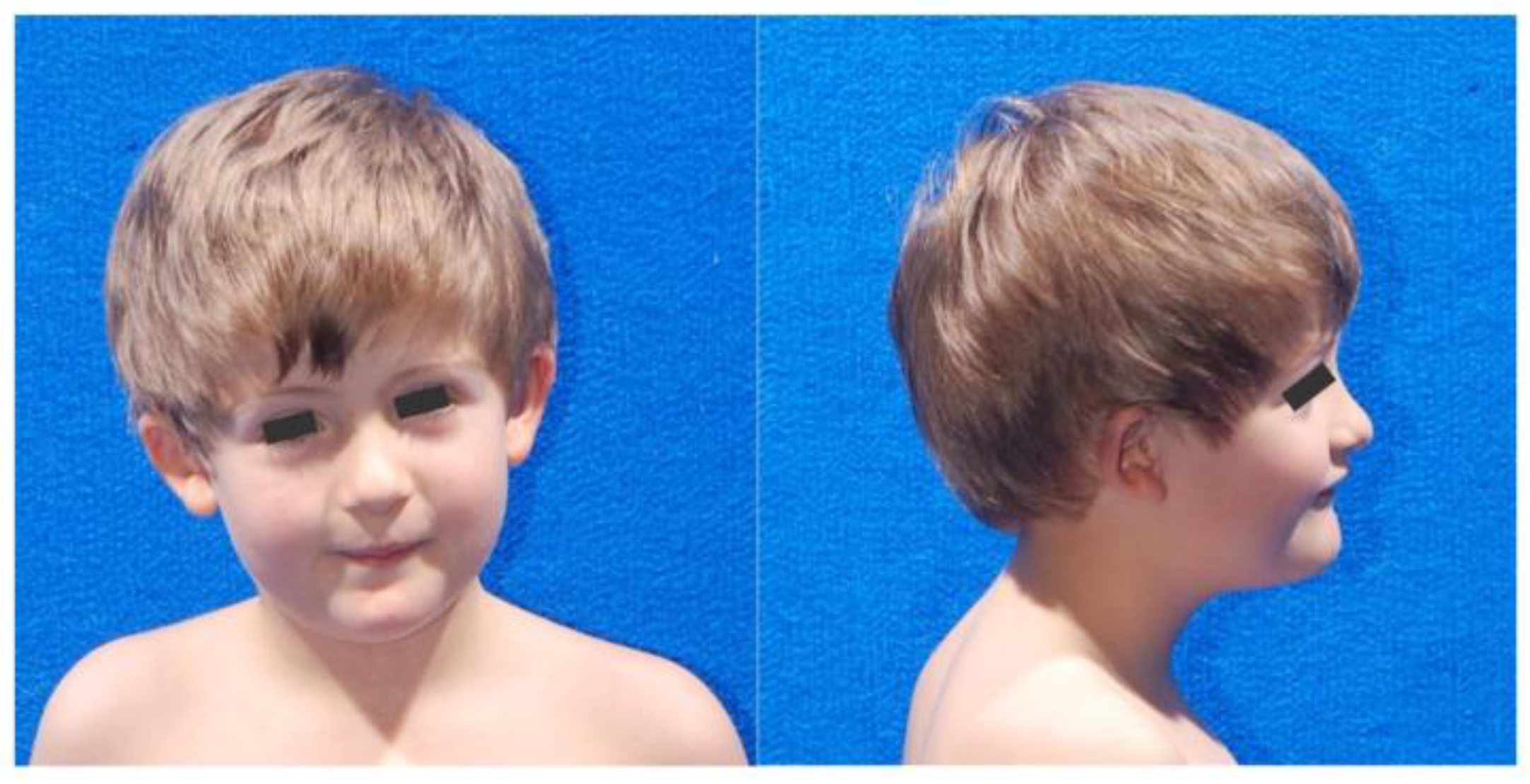
2.1. Patient
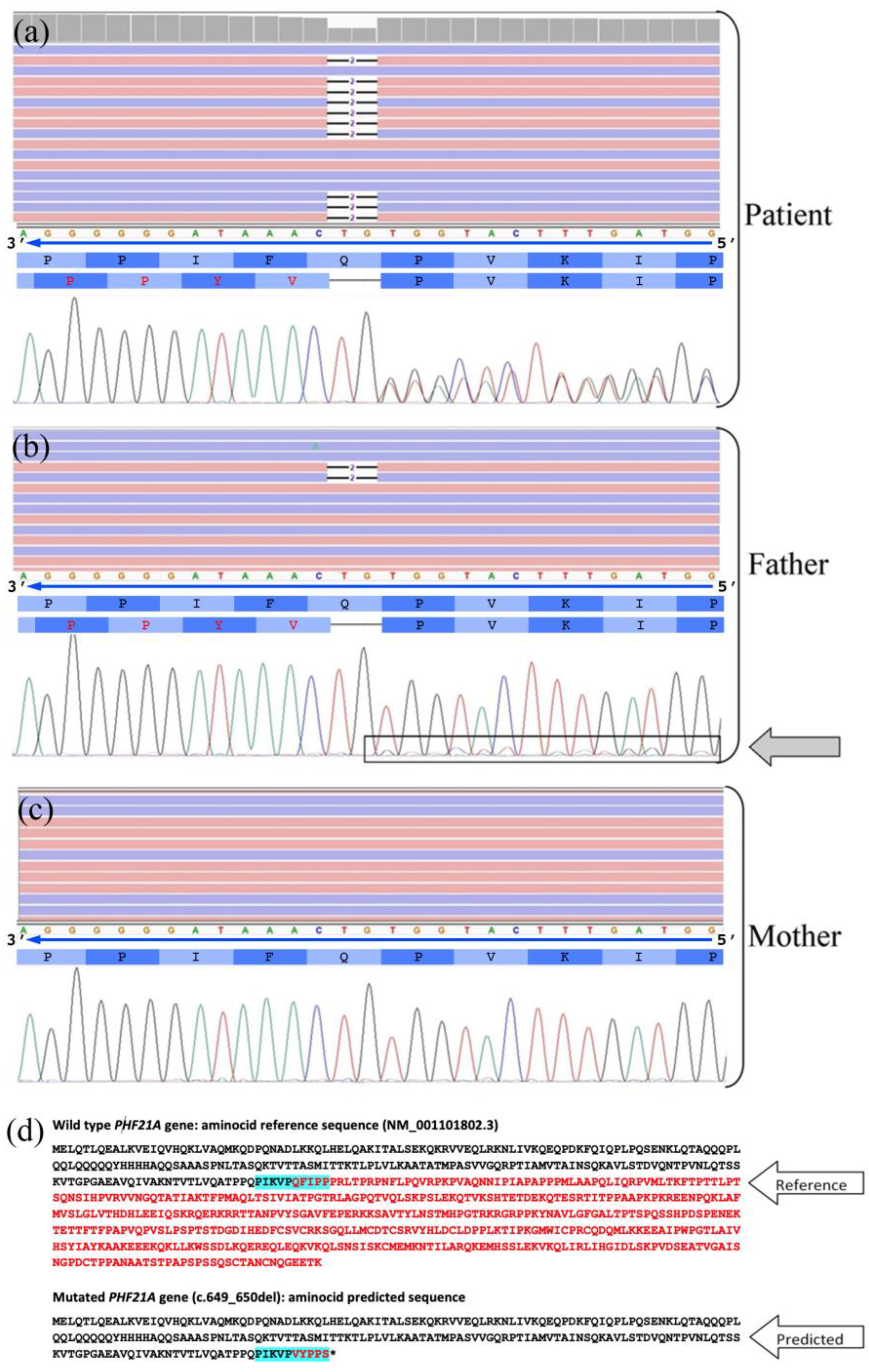
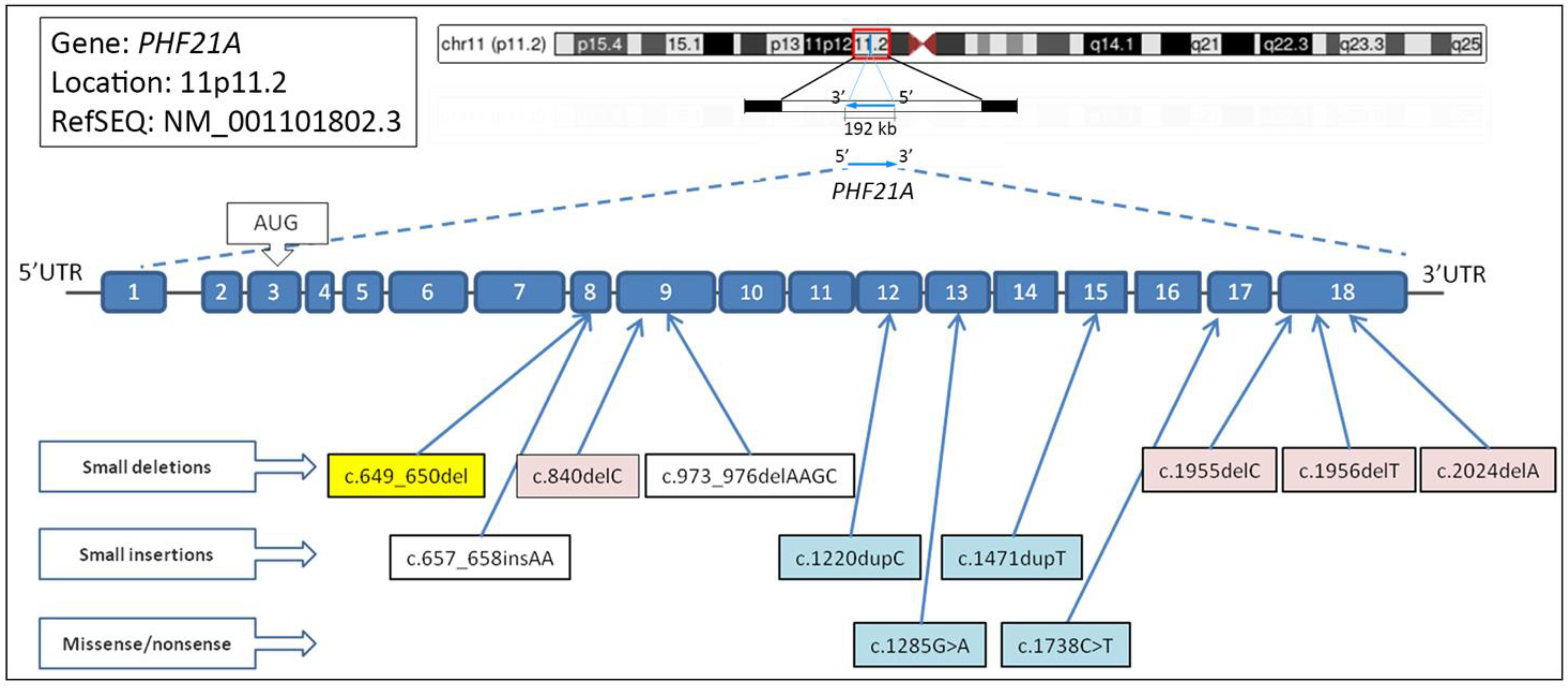
2.2. Genetic Data
2.3. Materials and Methods
2.3.1. DNA Preparation
2.3.2. Array-CGH and NGS Sequencing
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hakimi, M.-A.; Bochar, D.A.; Chenoweth, J.; Lane, W.S.; Mandel, G.; Shiekhattar, R. A core–BRAF35 complex containing histone deacetylase mediates repression of neuronal-specific genes. Proc. Natl. Acad. Sci. USA 2002, 99, 7420–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.-J.; Matson, C.; Lan, F.; Iwase, S.; Baba, T.; Shi, Y. Regulation of LSD1 Histone Demethylase Activity by Its Associated Factors. Mol. Cell 2005, 19, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lakowski, B.; Roelens, I.; Jacob, S. CoREST-Like Complexes Regulate Chromatin Modification and Neuronal Gene Expression. J. Mol. Neurosci. 2006, 29, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.S.; Murata-Nakamura, Y.; Nagasu, H.; Kim, H.-G.; Iwase, S. Transcriptome Analysis Revealed Impaired cAMP Responsiveness in PHF21A-Deficient Human Cells. Neuroscience 2017, 370, 170–180. [Google Scholar] [CrossRef]
- Kim, H.-G.; Rosenfeld, J.A.; Scott, D.A.; Bénédicte, G.; LaBonne, J.D.J.; Brown, J.; McGuire, M.; Mahida, S.; Naidu, S.R.; Gutierrez, J.; et al. Disruption of PHF21A causes syndromic intellectual disability with craniofacial anomalies, epilepsy, hypotonia, and neurobehavioral problems including autism. Mol. Autism. 2019, 10, 35. [Google Scholar] [CrossRef]
- Swarr, D.; Bloom, D.; Lewis, R.A.; Elenberg, E.; Friedman, E.M.; Glotzbach, C.; Wissman, S.D.; Shaffer, L.G.; Potocki, L. Potocki-Shaffer syndrome: Comprehensive clinical assessment, review of the literature, and proposals for medical management. Am. J. Med. Genet. Part A 2010, 152A, 565–572. [Google Scholar] [CrossRef]
- Garay, P.M.; Wallner, M.A.; Iwase, S. Yin–yang actions of histone methylation regulatory complexes in the brain. Epigenomics 2016, 8, 1689–1708. [Google Scholar] [CrossRef] [Green Version]
- Trajkova, S.; Di Gregorio, E.; Ferrero, G.; Carli, D.; Pavinato, L.; Delplancq, G.; Kuentz, P.; Brusco, A. New Insights into Potocki-Shaffer Syndrome: Report of Two Novel Cases and Literature Review. Brain Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Shi, W.; Wang, F.; Xu, F.; Zhang, Y.; Gan, J.; Tian, X.; Chen, B.; Dai, M. 11p11.12p12 duplication in a family with intellectual disability and craniofacial anomalies. BMC Med. Genom. 2021, 14, 99. [Google Scholar] [CrossRef]
- Hamanaka, K.; Sugawara, Y.; Shimoji, T.; Nordtveit, T.I.; Kato, M.; Nakashima, M.; Saitsu, H.; Suzuki, T.; Yamakawa, K.; Aukrust, I.; et al. De novo truncating variants in PHF21A cause intellectual disability and craniofacial anomalies. Eur. J. Hum. Genet. 2018, 27, 378–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-Q.; Badano, J.L.; McCaskill, C.; Vogel, H.; Potocki, L.; Shaffer, L.G. Haploinsufficiency of ALX4 as a Potential Cause of Parietal Foramina in the 11p11.2 Contiguous Gene–Deletion Syndrome. Am. J. Hum. Genet. 2000, 67, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Labonne, J.D.J.; Vogt, J.; Reali, L.; Kong, I.-K.; Layman, L.C.; Kim, H.-G. A microdeletion encompassing PHF21A in an individual with global developmental delay and craniofacial anomalies. Am. J. Med. Genet. Part A 2015, 167, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Potocki, L.; Shaffer, L.G. Interstitial deletion of 11 (P11.2p12): A newly described contiguous gene deletion syndrome involving the gene for hereditary multiple exostoses (EXT2). Am. J. Med. Genet. 1996, 62, 319–325. [Google Scholar] [CrossRef]
- Wakui, K.; Gregato, G.; Ballif, B.C.; Glotzbach, C.D.; A Bailey, K.; Kuo, P.-L.; Sue, W.-C.; Sheffield, L.J.; Irons, M.; Gomez, E.G.; et al. Construction of a natural panel of 11p11.2 deletions and further delineation of the critical region involved in Potocki–Shaffer syndrome. Eur. J. Hum. Genet. 2005, 13, 528–540. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, N.D.; Turcott, C.M.; Tepperberg, J.H.; McDonald, M.T.; Aylsworth, A.S. A 137-kb deletion within the Potocki-Shaffer syndrome interval on chromosome 11p11.2 associated with developmental delay and hypotonia. Am. J. Med. Genet. Part A 2012, 161, 198–202. [Google Scholar] [CrossRef]
- McCool, C.; Spinks-Franklin, A.; Noroski, L.M.; Potocki, L. Potocki-Shaffer syndrome in a child without intellectual disability-The role of PHF21A in cognitive function. Am. J. Med. Genet. Part A 2017, 173, 716–720. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, H.-T.; Leach, N.T.; Lan, F.; Ullmann, R.; Silahtaroglu, A.; Kurth, I.; Nowka, A.; Seong, I.S.; Shen, Y.; et al. Translocations Disrupting PHF21A in the Potocki-Shaffer-Syndrome Region Are Associated with Intellectual Disability and Craniofacial Anomalies. Am. J. Hum. Genet. 2012, 91, 56–72. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, A.; Calì, F.; Scuderi, C.; Vinci, M.; Vitello, G.A.; Musumeci, S.A.; Chiavetta, V.; Federico, C.; Amore, G.; Saccone, S.; et al. Identification of a Novel Missense Mutation of POLR3A Gene in a Cohort of Sicilian Patients with Leukodystrophy. Biomedicines 2022, 10, 2276. [Google Scholar] [CrossRef]
- Lan, F.; Collins, R.E.; De Cegli, R.; Alpatov, R.; Horton, J.R.; Shi, X.; Gozani, O.; Cheng, X.; Shi, Y. Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature 2007, 448, 718–722. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Yoon, J.; Park, B.G.; Eun, B.-L.; Kwon, J.A. Novel Pathogenic Variant (c.1171A>T) in PHF21A in a Female with Intellectual Disability and Craniofacial Anomalies. Mol. Syndr. 2022, 13, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.-Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Bertoli-Avella, A.M.; Kandaswamy, K.K.; Khan, S.; Ordonez-Herrera, N.; Tripolszki, K.; Beetz, C.; Rocha, M.E.; Urzi, A.; Hotakainen, R.; Leubauer, A.; et al. Combining exome/genome sequencing with data repository analysis reveals novel gene–disease associations for a wide range of genetic disorders. Genet. Med. 2021, 23, 1551–1568. [Google Scholar] [CrossRef]
- Ung, C.; Sanchez, A.V.; Shen, L.; Davoudi, S.; Ahmadi, T.; Navarro-Gomez, D.; Chen, C.J.; Hancock, H.; Penman, A.; Hoadley, S.; et al. Whole exome sequencing identification of novel candidate genes in patients with proliferative diabetic retinopathy. Vis. Res. 2017, 139, 168–176. [Google Scholar] [CrossRef]
- McTague, A.; Howell, K.B.; Cross, J.H.; A Kurian, M.; E Scheffer, I. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016, 15, 304–316. [Google Scholar] [CrossRef]
- Spoto, G.; Saia, M.C.; Amore, G.; Gitto, E.; Loddo, G.; Mainieri, G.; Nicotera, A.G.; Di Rosa, G. Neonatal Seizures: An Overview of Genetic Causes and Treatment Options. Brain Sci. 2021, 11, 1295. [Google Scholar] [CrossRef]
- Amore, G.; Butera, A.; Spoto, G.; Valentini, G.; Saia, M.C.; Salpietro, V.; Calì, F.; Di Rosa, G.; Nicotera, A.G. KCNQ2-Related Neonatal Epilepsy Treated With Vitamin B6: A Report of Two Cases and Literature Review. Front. Neurol. 2022, 13, 826225. [Google Scholar] [CrossRef]
| Pt. 1 | Pt. 2 | Pt. 3 | Pt. 4 | Pt. 5 | Pt. 6 | Pt. 7 | Pt. 8 | Pt. 9 | Pt. 10 | Pt. 11 | Pt. 12 | Pt. 13 | Pt. 14 | Pt. 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 16 | 13 | 3 | 9 | 10 | 18 | 6 | 18 | N/A | N/A | N/A | N/A | 9 | 3 | 26 |
| Sex | Male | Female | Male | Female | Male | Male | Male | Female | Female | N/A | Female | N/A | Male | Male | Female |
| Nucleotide change | c.649_650 del | c.1955 delC | c.1285 G > A | c.1956 delT | c.1738 C > T | c.1471 dupT | c.1738 C > T | c.2024 delA | c.840 delC | c.976_979 delAAGC | c.1153 delA | c.1545 delA | c.657_658 insAA | c.1220 dupC | c.1171 A > T |
| Effect on protein | p.Q217V fsTer6 | p.P652L fsX104 | p.G429S | p.P652P fsX104 | p.R580Ter | p.C491L fsX81 | p.R580Ter | p.Q675R fsX81 | p.I281S fs*14 | p.K326fs | p.S385A fs*30 | p.E517K fsTer14 | p.P220Nfs*48 | p.E408R fs*3 | p.K391Ter |
| Inheritance (b) | P.I. (5%) | D.N. | D.N. | D.N. | D.N. | N/A (c) | D.N. | D.N. | N/A | D.N. | D.N. | D.N. | D.N. | D.N. | D.N. |
| Developmental delay | + | + | + | + | + | + | + | + | N/A | + | N/A | N/A | + | + | + |
| Intellectual disability | + | + | + | + | + | + | + | + | N/A | + | N/A | N/A | + | + | + |
| Facial dysmorphism | + | + | + | + | + | − | + | + | N/A | + | N/A | N/A | + | + | + |
| Cranial anomalies (d) | Mic | Mac | − | − | Plc | − | − | Mac | N/A | + (e) | N/A | N/A | Mac | Mac | Plc |
| Autism | − | + | N/A | − | − | + | − | + | + | N/A | + | N/A | − | N/A | − |
| Epilepsy/seizures/spasms | + | + | + | + | + | − | − | − | − | − | − | − | − | + | + |
| Language delay | + | + | + | + | + | + | + | + | N/A | + | N/A | N/A | + | + | + |
| Tapering fingers | − | + | + | + | − | − | − | − | N/A | N/A | N/A | N/A | − | + | − |
| Clinodactily | − | + | + | + | − | + | − | − | N/A | N/A | N/A | N/A | N/A | N/A | − |
| Syndactily | − | − | + | − | + | − | − | − | N/A | N/A | N/A | N/A | N/A | N/A | − |
| Impaired motor skills | − | + | + | + | + | + | + | + | N/A | + | N/A | N/A | + | + | + |
| Hypotonia | + | + | + | + | N/A | − | − | − | N/A | N/A | N/A | N/A | + | + | + |
| ADHD | − | + | N/A | + | + | + | − | N/A | N/A | N/A | N/A | N/A | + | N/A | + |
| Anxiety disorder | + | + | N/A | + | N/A | − | + | + | N/A | N/A | N/A | N/A | N/A | N/A | − |
| Neurobehavioral problems | − | + | + | + | + | + | − | + | N/A | + | N/A | N/A | N/A | N/A | + |
| Obesity | − | + | − | + | − | + | − | + | N/A | N/A | N/A | N/A | N/A | N/A | + |
| Reference | (a) | [6] | [6] | [6] | [6] | [6] | [6] | [6] | [23] | [24] | [21] | [25] | [11] | [11] | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butera, A.; Nicotera, A.G.; Di Rosa, G.; Musumeci, S.A.; Vitello, G.A.; Musumeci, A.; Vinci, M.; Gloria, A.; Federico, C.; Saccone, S.; et al. PHF21A Related Disorder: Description of a New Case. Int. J. Mol. Sci. 2022, 23, 16130. https://doi.org/10.3390/ijms232416130
Butera A, Nicotera AG, Di Rosa G, Musumeci SA, Vitello GA, Musumeci A, Vinci M, Gloria A, Federico C, Saccone S, et al. PHF21A Related Disorder: Description of a New Case. International Journal of Molecular Sciences. 2022; 23(24):16130. https://doi.org/10.3390/ijms232416130
Chicago/Turabian StyleButera, Ambra, Antonio Gennaro Nicotera, Gabriella Di Rosa, Sebastiano Antonino Musumeci, Girolamo Aurelio Vitello, Antonino Musumeci, Mirella Vinci, Angelo Gloria, Concetta Federico, Salvatore Saccone, and et al. 2022. "PHF21A Related Disorder: Description of a New Case" International Journal of Molecular Sciences 23, no. 24: 16130. https://doi.org/10.3390/ijms232416130
APA StyleButera, A., Nicotera, A. G., Di Rosa, G., Musumeci, S. A., Vitello, G. A., Musumeci, A., Vinci, M., Gloria, A., Federico, C., Saccone, S., & Calì, F. (2022). PHF21A Related Disorder: Description of a New Case. International Journal of Molecular Sciences, 23(24), 16130. https://doi.org/10.3390/ijms232416130











