The BCO2 Genotype and the Expression of BCO1, BCO2, LRAT, and TTPA Genes in the Adipose Tissue and Brain of Rabbits Fed a Diet with Marigold Flower Extract
Abstract
:1. Introduction
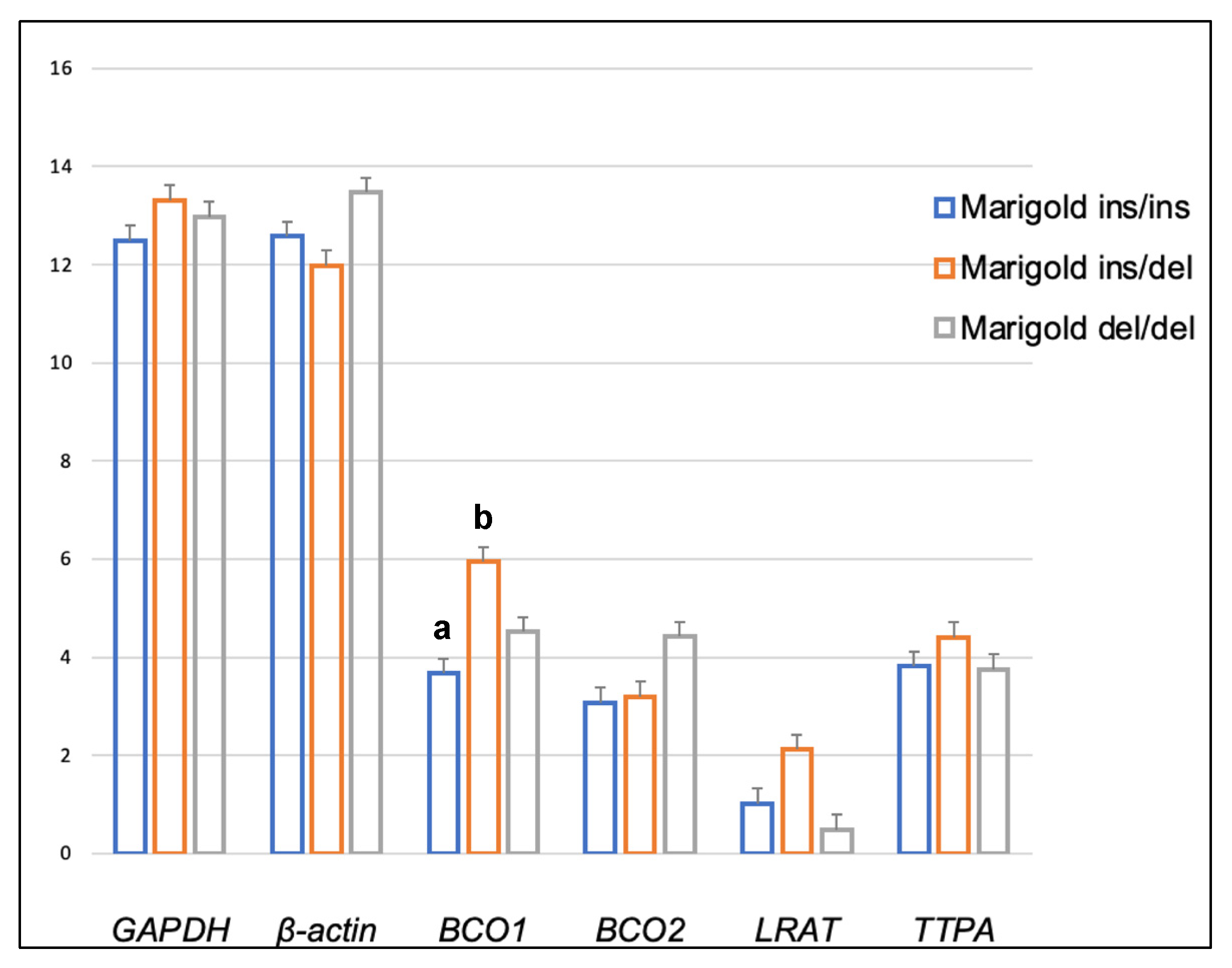
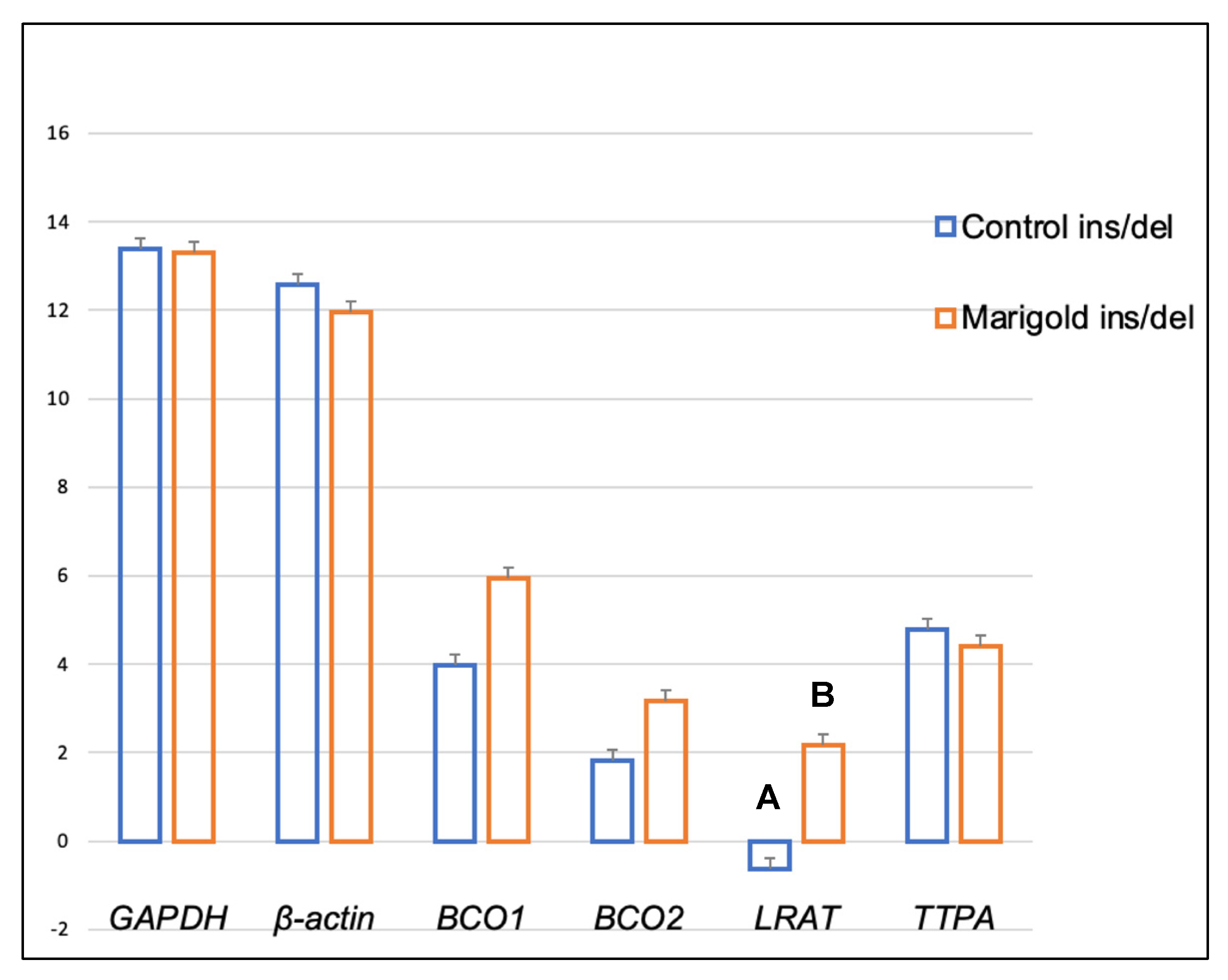
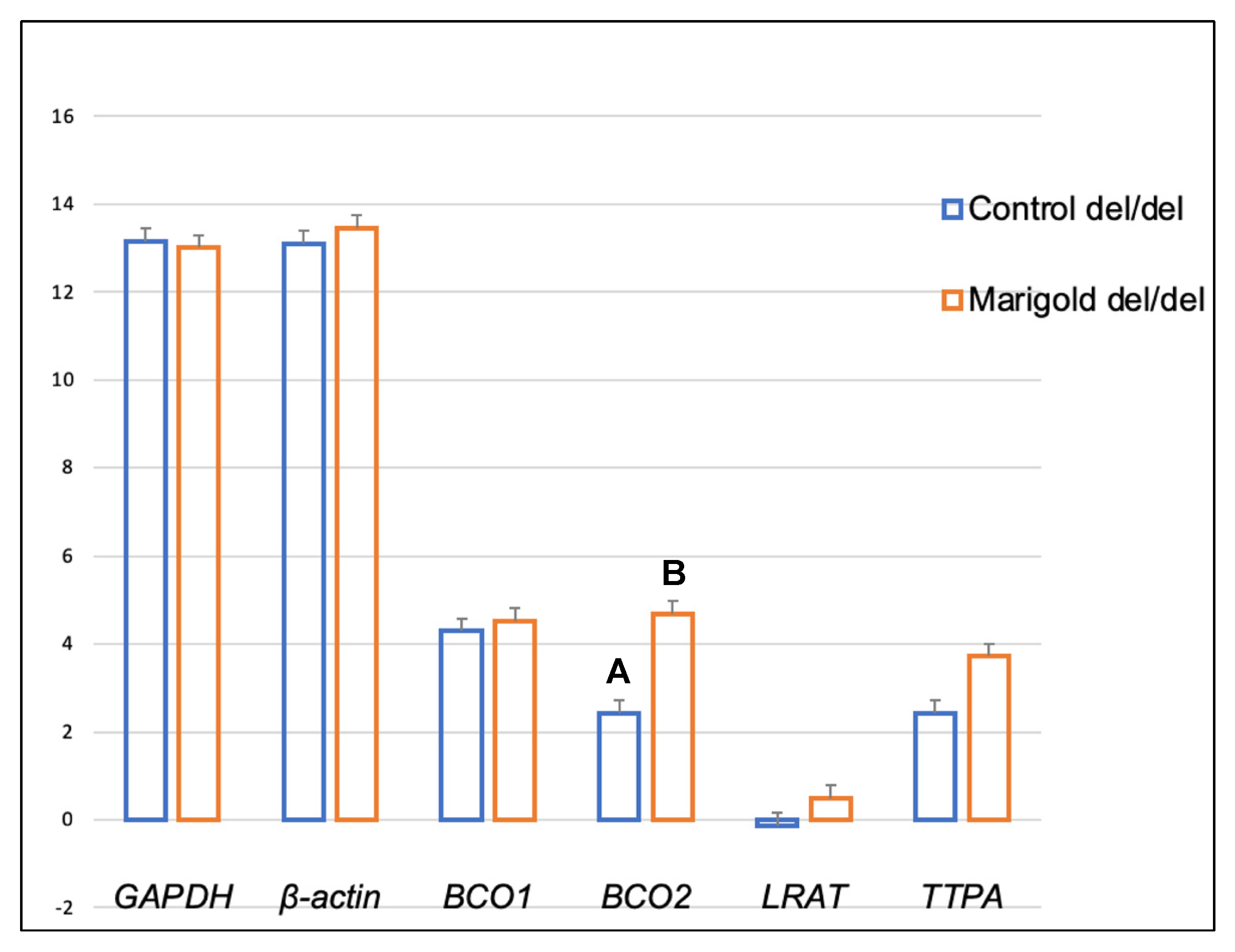
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotake-Nara, E.; Nagao, A. Absorption and metabolism of xanthophylls. Mar. Drugs 2011, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Sola, M.A.; Rodríguez-Concepción, M. Carotenoid biosynthesis in arabidopsis: A colorful pathway. Arab. Book 2012, 10, e0158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Quirós, A.R.-B.; Costa, H.S. Analysis of carotenoids in vegetable and plasma samples: A review. J. Food Compos. Anal. 2006, 19, 97–111. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.A. Benefits and liabilities of vitamin A and carotenoids. Rev. J. Nutr. 1996, 126, 1208S–1212S. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthinbinding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of StARD3 as a luteinbinding protein in the macula of the primate retina. Biochemistry 2011, 50, 2541–2549. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Jin, M.; Li, Y.; Li, P.; Zhou, J.; Wang, X.; Chen, Y. Biallelic β-carotene oxygenase 2 knockout results in yellow fat in sheep via CRISPR/Cas9. Anim. Gen. 2017, 48, 242–244. [Google Scholar] [CrossRef]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Molec. Nutr. Food Res. 2012, 56, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Lietz, G.; Oxley, A.; Boesch-Saadatmandi, C.; Kobayashi, D. Importanceof β,β-carotene 15,15′-monooxygenase 1 (BCMO1) and β,β-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. Molec. Nutr. Food Res. 2012, 56, 241–250. [Google Scholar] [CrossRef]
- Lietz, G.; Lange, J.; Rimbach, G. Molecular and dietary regulation of beta, beta-carotene 15,15-monooxygenase 1 (BCMO1). Arch. Biochem. Biophys. 2010, 502, 8–16. [Google Scholar] [CrossRef]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; von Lintig, J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef] [Green Version]
- Våge, D.I.; Boman, I.A. A nonsense mutation in the betacarotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z.; Chwastowska-Siwiecka, I. Polymorphism of the BCO2 gene and the content of carotenoids, retinol and α-tocopherol in the liver and fat of rabbits. Braz. J. Anim. Sci. 2019, 48, e20180243. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.; Cullen, N.G.; Morris, C.A.; Fisher, P.J.; Pitchford, W.S.; Bottema, C.D.K. Major effect of retinal short-chain dehydrogenase reductase (RDHE2) on bovine fat colour. Mamm. Genome 2012, 23, 378–386. [Google Scholar] [CrossRef]
- Eriksson, J.; Larson, G.; Gunnarsson, U.; Bed’hom, B.; Tixier-Boichard, M.; Strömstedt, L.; Wright, D.; Jungerius, A.; Vereijken, A.; Randi, E.; et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008, 4, e1000010. [Google Scholar] [CrossRef] [Green Version]
- Strychalski, J.; Brym, P.; Czarnik, U.; Gugołek, A. A novel AAT-deletion mutation in the coding sequence of the BCO2 gene in yellow-fat rabbits. J. Appl. Gen. 2015, 56, 535–537. [Google Scholar] [CrossRef]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z. Effect of the β-carotene oxygenase 2 genotype on the content of carotenoids, retinol and α-tocopherol in the liver, fat and milk of rabbit does, reproduction parameters and kitten growth. J. Anim. Phys. Anim. Nutr. 2019, 103, 1585–1593. [Google Scholar] [CrossRef]
- Abdul-Wasea, A.A.; Khalid, M. Elhindi. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Piccaglia, R.; Marotti, M.; Grandi, S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998, 8, 45–51. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, Y.; Ding, M.; Xi, Q.; Liu, G.; Li, Y.; Liu, D.; Chen, X. Effects of Moringa oleifera leaves as a substitute for alfalfa meal on nutrient digestibility, growth performance, carcass trait, meat quality, antioxidant capacity and biochemical parameters of rabbits. J. Anim. Physiol. Anim. Nutr. 2018, 102, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Prache, S.; Priolo, A.; Grolier, P. Persistence of carotenoid pigments in the blood of concentrate-finished grazing sheep: Its significance for the traceability of grass-feeding. J. Anim. Sci. 2003, 81, 360–367. [Google Scholar] [CrossRef]
- Strychalski, J.; Gugołek, A.; Antoszkiewicz, Z.; Fopp-Bayat, D.; Kaczorek-Łukowska, E.; Snarska, A.; Zwierzchowski, G.; Król-Grzymała, A.; Matusevičius, P. The Effect of the BCO2 Genotype on the Expression of Genes Related to Carotenoid, Retinol, and α-Tocopherol Metabolism in Rabbits Fed a Diet with Aztec Marigold Flower Extract. Int. J. Mol. Sci. 2022, 23, 10552. [Google Scholar] [CrossRef]
- Ruiz, A.; Winston, A.; Lim, Y.H.; Gilbert, B.A.; Rando, R.R.; Bok, D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 1999, 274, 3834–3841. [Google Scholar] [CrossRef] [Green Version]
- Arita, M.; Sato, Y.; Miyata, A.; Tanabe, T.; Takahashi, E.; Kayden, H.J.; Arai, H.; Inoue, K. Human alpha-tocopherol transfer protein: cDNA cloning, expression and chromosomal localization. Biochem. J. 1995, 306, 437–443. [Google Scholar] [CrossRef]
- Koh, M.; Takitani, K.; Miyazaki, H.; Yamaoka, S.; Tamai, H. Liver X receptor up-regulates α-tocopherol transfer protein expression and α-tocopherol status. J. Nutr. Biochem. 2013, 24, 2158–2167. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Wang, D.; Wen, J.; Chen, J. Vitamin A deficiency indicating as low expression of LRAT may be a novel biomarker of primary hypertension. Clin. Exp. Hypertens 2021, 43, 151–163. [Google Scholar] [CrossRef]
- Buechter, M.; Gerken, G. Liver function—How to screen and to diagnose: Insights from personal experiences, controlled clinical studies and future perspectives. J. Pers. Med. 2022, 12, 1657. [Google Scholar] [CrossRef]
- Terenzio, M.; Schiavo, G.; Fainzilber, M. Compartmentalized signaling in neurons: From cell biology to neuroscience. Neuron 2017, 96, 667–679. [Google Scholar] [CrossRef]
- Pereira, S.; Alvarez-Leite, J. Adipokines: Biological functions and metabolically healthy obese profile. J. Recept. Ligand Channel Res. 2014, 7, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Baradaran, A.; Rafieian, M. Oxidative stress and the paradoxical effects of antioxidants. J. Res. Med. Sci. 2013, 18, 628. [Google Scholar]
- Strychalski, J.; Gugołek, A.; Antoszkiewicz, Z.; Kowalska, D.; Konstantynowicz, M. Biologically active compounds in selected tissues of white-fat and yellow-fat rabbits and their production performance parameters. Livest. Sci. 2016, 183, 92–97. [Google Scholar] [CrossRef]
- Zwolińska, D.; Grzeszczak, W.; Szczepańska, M.; Kiliś-Pstrusińska, K.; Szprynger, K. Vitamins A, E and C as non-enzymatic antioxidants and their relation to lipid peroxidation in children with chronic renal failure. Nephron Clin. Pract. 2006, 103, 12–18. [Google Scholar] [CrossRef]
- Napoli, J.L.; McCormick, A.M.; O’Meara, B.; Dratz, E.A. Vitamin A metabolism: α-tocopherol modulates tissue retinol levels in vivo, and retinyl palmitate hydrolysis in vitro. Arch. Biochem. Biophys. 1984, 230, 194–202. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 18th ed.; Association of Analytical Communities: Arlington, VA, USA, 2006. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Högberg, A.; Pickova, J.; Babol, J.; Andersson, K.; Dutta, P.C. Muscle lipids, vitamins E and A, and lipid oxidation as affected by diet and RN genotype in female and castrated male Hampshire crossbreed pigs. Meat Sci. 2002, 60, 411–420. [Google Scholar] [CrossRef]
- Xu, Z. Comparison of extraction methods for quantifying vitamin E from animal tissues. Bioresour. Technol. 2008, 99, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 10 December 2021).
- Matz, M.V.; Wright, R.M.; Scott, J.G. No control genes required: Bayesian analysis of qRT-PCR data. PLoS One 2013, 8, e71448. [Google Scholar] [CrossRef] [PubMed]
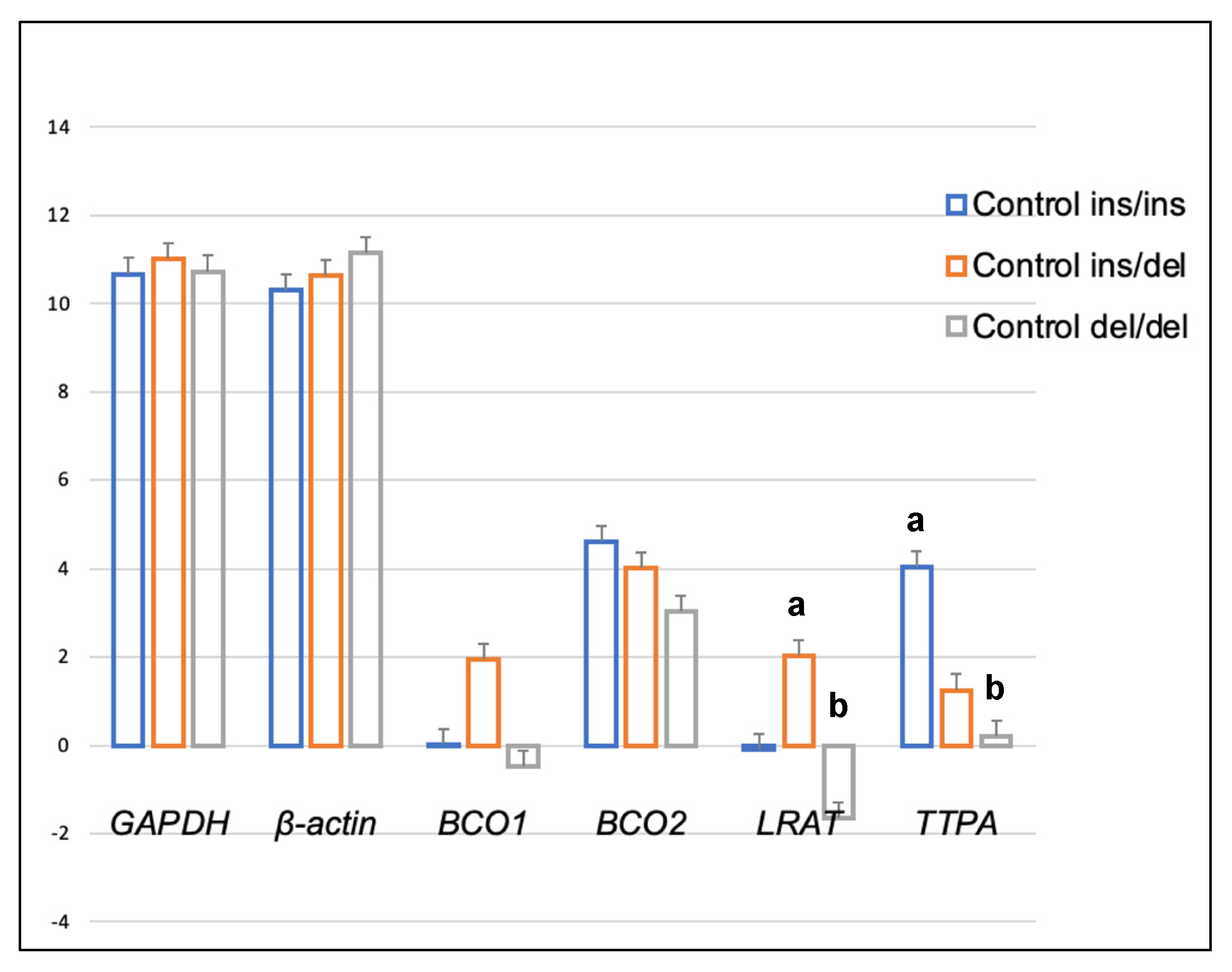
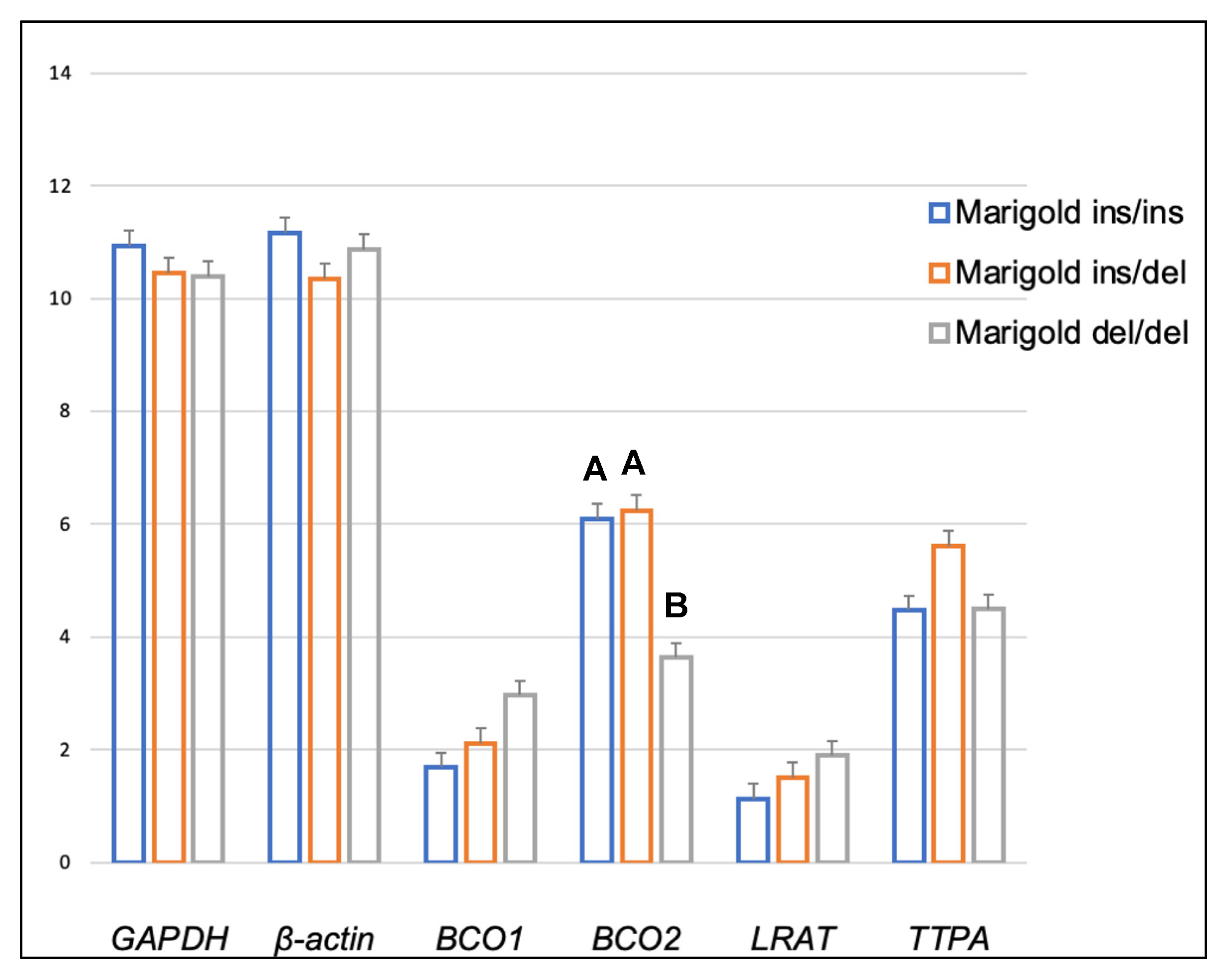
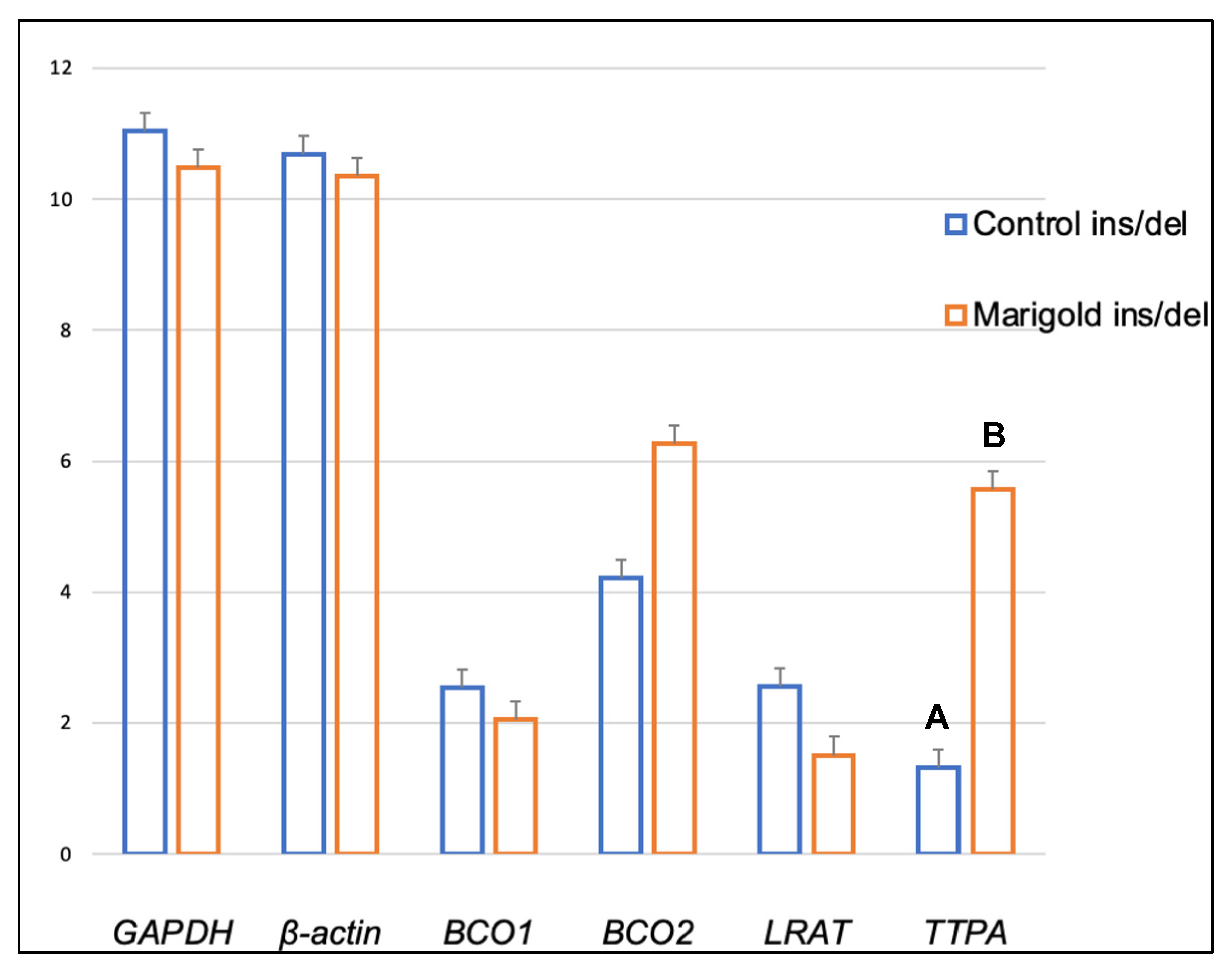
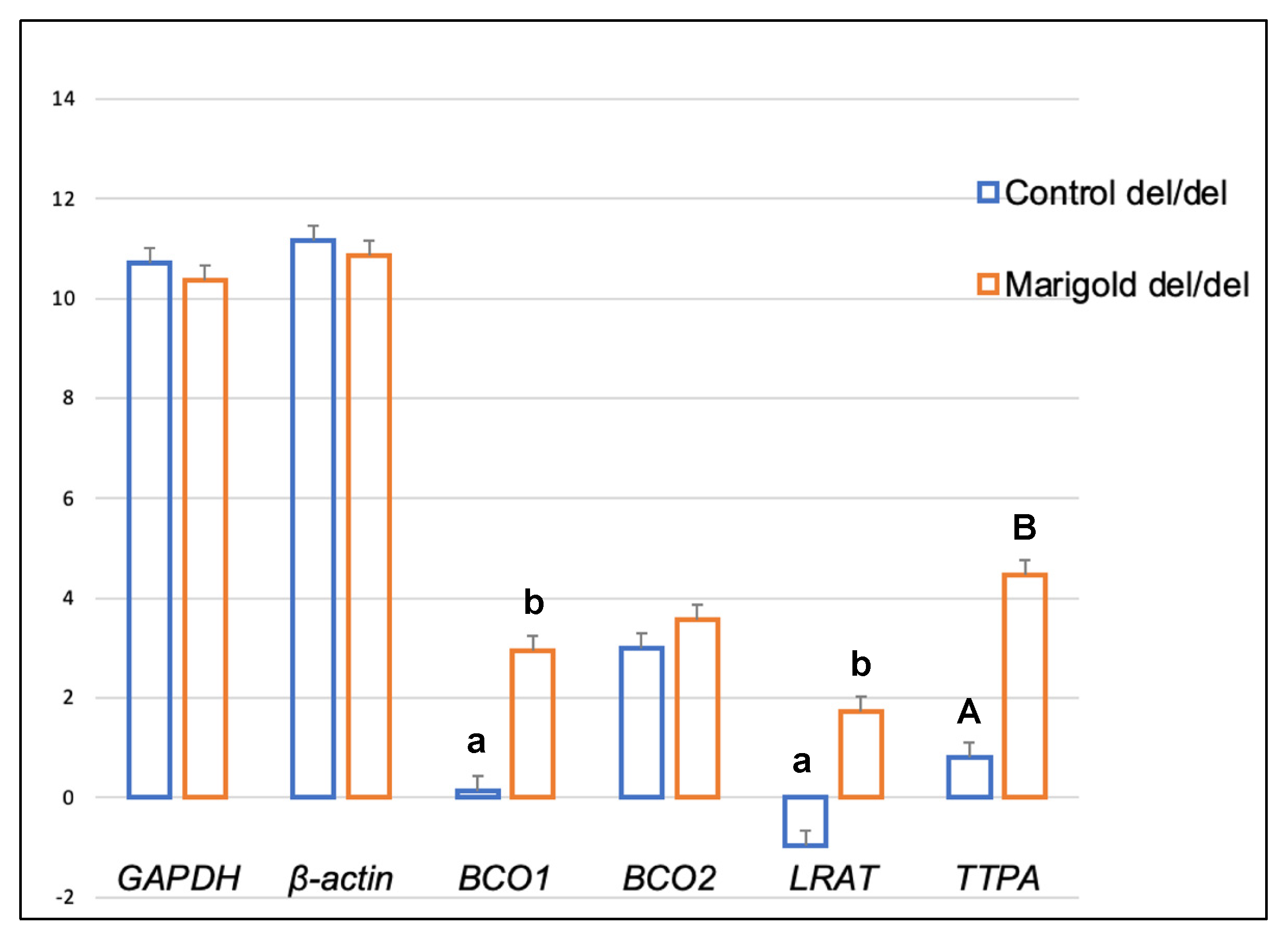

| Compound | Diet | BCO2 Genotypes | p-Value | ||
|---|---|---|---|---|---|
| ins/ins | ins/del | del/del | |||
| Lutein | Control | 0.06±0.05 A | 0.07±0.07 A | 0.58±0.24 B | <0.001 |
| Marigold | 0.04±0.02 A | 0.05±0.05 A | 5.37±3.16 B | <0.001 | |
| p-value | 0.304 | 0.655 | <0.001 | ||
| Zeaxanthin | Control | 0.01±0.01 A | 0.01±0.01 A | 0.05±0.04 B | <0.001 |
| Marigold | 0.01±0.01 A | 0.01±0.01 A | 0.57±0.40 B | <0.001 | |
| p-value | 0.093 | 0.031 | <0.001 | ||
| β-carotene | Control | 0.08±0.04 A | 0.13±0.07 a | 0.25±0.14 Bb | <0.001 |
| Marigold | 0.08±0.04 A | 0.08±0.03 A | 0.56±0.39 B | <0.001 | |
| p-value | 0.854 | 0.219 | 0.034 | ||
| Retinol | Control | 6.48±3.89 | 6.01±5.41 | 7.03±6.15 | 0.772 |
| Marigold | 6.19±4.82 | 6.39±6.55 | 6.80±4.78 | 0.965 | |
| p-value | 0.886 | 0.864 | 0.851 | ||
| α-tocopherol | Control | 4.02±2.33 A | 6.90±3.31 | 9.63±4.99 B | 0.009 |
| Marigold | 6.65±3.81 a | 7.60±4.15 a | 13.32±6.34 b | 0.011 | |
| p-value | 0.079 | 0.616 | 0.165 | ||
| Compound | Diet | BCO2 Genotypes | p-Value | ||
|---|---|---|---|---|---|
| ins/ins | ins/del | del/del | |||
| Lutein | Control | 0.15±0.05A | 0.15±0.07A | 0.66±0.47B | <0.001 |
| Marigold | 0.18±0.18A | 0.26±0.16A | 1.89±1.39B | <0.001 | |
| p-value | 0.662 | 0.054 | 0.016 | ||
| Zeaxanthin | Control | 0.02±0.01a | 0.01±0.01A | 0.04±0.02Bb | <0.001 |
| Marigold | 0.01±0.01A | 0.01±0.01A | 0.21±0.20B | <0.001 | |
| p-value | 0.153 | 0.542 | <0.001 | ||
| β-carotene | Control | 0.06±0.04a | 0.09±0.05 | 0.13±0.06b | 0.019 |
| Marigold | 0.07±0.03A | 0.07±0.04A | 0.21±0.11B | <0.001 | |
| p-value | 0.520 | 0.320 | 0.074 | ||
| Retinol | Control | 0.33±0.24 | 0.28±0.22 | 0.39±0.37 | 0.691 |
| Marigold | 0.53±0.28 | 0.68±0.46 | 0.73±0.46 | 0.455 | |
| p-value | 0.100 | 0.025 | 0.052 | ||
| α-tocopherol | Control | 0.00±0.00A | 0.00±0.00A | 0.04±0.02B | <0.001 |
| Marigold | 0.00±0.00A | 0.00±0.00A | 0.02±0.01B | <0.001 | |
| p-value | 0.938 | 0.964 | 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strychalski, J.; Gugołek, A.; Kaczorek-Łukowska, E.; Antoszkiewicz, Z.; Matusevičius, P. The BCO2 Genotype and the Expression of BCO1, BCO2, LRAT, and TTPA Genes in the Adipose Tissue and Brain of Rabbits Fed a Diet with Marigold Flower Extract. Int. J. Mol. Sci. 2023, 24, 2304. https://doi.org/10.3390/ijms24032304
Strychalski J, Gugołek A, Kaczorek-Łukowska E, Antoszkiewicz Z, Matusevičius P. The BCO2 Genotype and the Expression of BCO1, BCO2, LRAT, and TTPA Genes in the Adipose Tissue and Brain of Rabbits Fed a Diet with Marigold Flower Extract. International Journal of Molecular Sciences. 2023; 24(3):2304. https://doi.org/10.3390/ijms24032304
Chicago/Turabian StyleStrychalski, Janusz, Andrzej Gugołek, Edyta Kaczorek-Łukowska, Zofia Antoszkiewicz, and Paulius Matusevičius. 2023. "The BCO2 Genotype and the Expression of BCO1, BCO2, LRAT, and TTPA Genes in the Adipose Tissue and Brain of Rabbits Fed a Diet with Marigold Flower Extract" International Journal of Molecular Sciences 24, no. 3: 2304. https://doi.org/10.3390/ijms24032304
APA StyleStrychalski, J., Gugołek, A., Kaczorek-Łukowska, E., Antoszkiewicz, Z., & Matusevičius, P. (2023). The BCO2 Genotype and the Expression of BCO1, BCO2, LRAT, and TTPA Genes in the Adipose Tissue and Brain of Rabbits Fed a Diet with Marigold Flower Extract. International Journal of Molecular Sciences, 24(3), 2304. https://doi.org/10.3390/ijms24032304





