Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects
Abstract
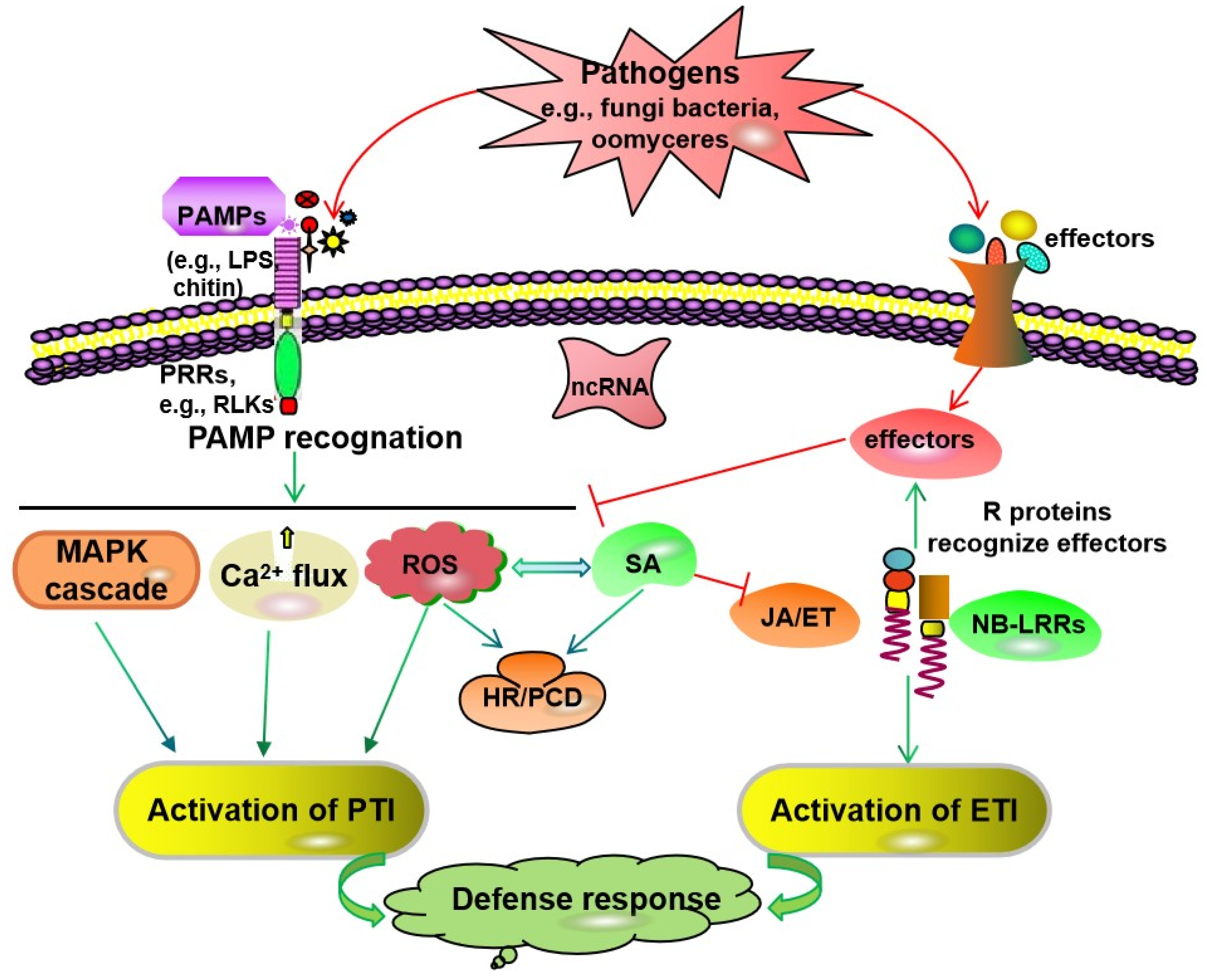
:1. Introduction
2. Plant Messenger Signal Molecule-Mediated Signaling Pathways
2.1. Calcium-Mediated Disease Resistance Pathway
2.2. ROS-Mediated Disease Resistance Signaling Pathway
2.3. NO-Mediated Disease Resistance Signaling Pathway
3. Plant Defense Hormone-Mediated Signaling Pathways
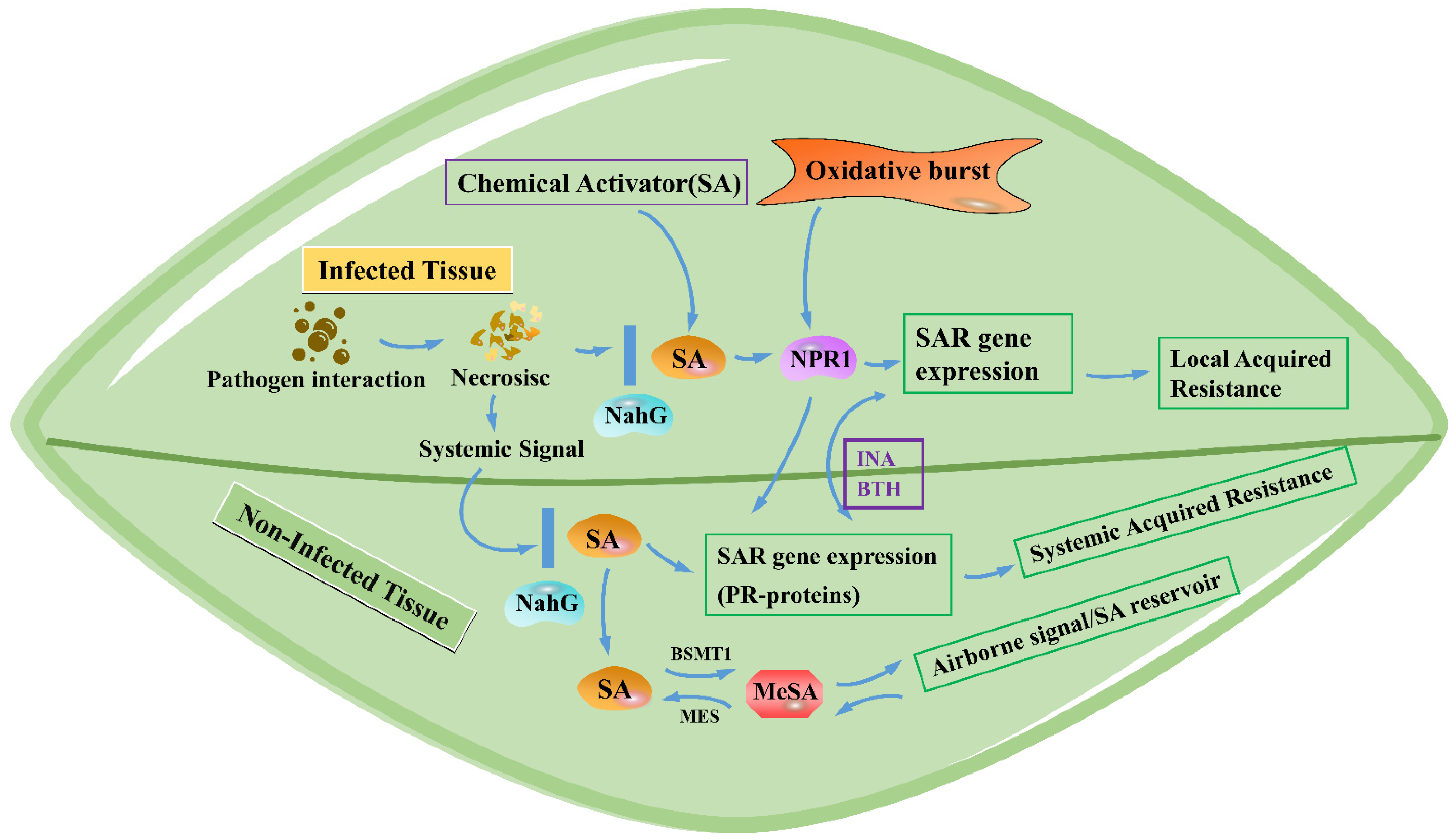
3.1. SA-Mediated Disease Resistance Signaling Pathway
3.2. JA-Mediated Disease Resistance Signaling Pathway
3.3. ET-Mediated Disease Resistance Signaling Pathway
4. Heterotrimeric G Protein-Mediated Disease Resistance Signaling Pathway
5. MAPK Cascade-Mediated Disease Resistance Signaling Pathway
6. ncRNA-Mediated Disease Resistance Pathway
7. Interaction and Complexity of Disease Resistance Signaling Pathways
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zacchino, S.A.; Butassi, E.; Liberto, M.D.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; de Souza, C.M.; de Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The role of antimicrobial peptides in plant immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho-Coronel, X.; Molina-Torres, J.; Heil, M. Sequestration of exogenous volatiles by plant cuticular waxes as a mechanism of passive associational resistance: A Proof of Concept. Front. Plant Sci. 2020, 11, 121. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, T.; Guo, X.J.; Li, M.; Liu, X.Y.; Cao, J.; Tan, X.L. Sclerotinia Stem Rot Resistance in Rapeseed: Recent Progress and Future Prospects. J. Agric. Food Chem. 2021, 69, 2965–2978. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef] [Green Version]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Nicaise, V.; Candresse, T. Plum pox virus capsid protein suppresses plant pathogen-associated molecular pattern (PAMP)-triggered immunity. Mol. Plant Pathol. 2017, 18, 878–886. [Google Scholar] [CrossRef]
- Schreiber, K.J.; Chau-Ly, I.J.; Lewis, J.D. What the Wild Things Do: Mechanisms of Plant Host Manipulation by Bacterial Type III-Secreted Effector Proteins. Microorganisms 2021, 9, 1029. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.J.; Seo, P.J.; Kim, J.H.; Sim, H.J.; Kim, S.G.; Park, C.M. Systemic Immunity Requires SnRK2.8-Mediated Nuclear Import of NPR1 in Arabidopsis. Plant Cell 2015, 27, 3425–3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overmyer, K.; Vuorinen, K.; Brosché, M. Interaction points in plant stress signaling pathways. Physiol. Plant 2018, 162, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Bentham, A.R.; De la Concepcion, J.C.; Mukhi, N.; Zdrzałek, R.; Draeger, M.; Gorenkin, D.; Hughes, R.K.; Banfield, M.J. A molecular roadmap to the plant immune system. J. Biol. Chem. 2020, 295, 14916–14935. [Google Scholar] [CrossRef]
- Jacob, P.; Kim, N.H.; Wu, F.; El-Kasmi, F.; Chi, Y.; Walton, W.G.; Furzer, O.J.; Lietzan, A.D.; Sunil, S.; Kempthorn, K.; et al. Plant “helper” immune receptors are Ca2+-permeable nonselective cation channels. Science 2021, 373, 420–425. [Google Scholar] [CrossRef]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [Green Version]
- Hilleary, R.; Paez-Valencia, J.; Vens, C.; Toyota, M.; Palmgren, M.; Gilroy, S. Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2020, 117, 18849–18857. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, X.; Li, W.; Hussain, J.; Qi, G.; Liu, S. Calcium Signaling in Plant Programmed Cell Death. Cells 2021, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Yadav, D.; Khan, A.L.; Hashem, A.; Abd Allah, E.F.; Al-Harrasi, A. Molecular Players of EF-hand Containing Calcium Signaling Event in Plants. Int. J. Mol. Sci. 2019, 20, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Huang, W. Calcium-Dependent Protein Kinases in Phytohormone Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Smigel, A.; Tsai, Y.C.; Braam, J.; Berkowitz, G.A. Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008, 148, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.P.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; An, C.; Park, S.; Gilmour, S.J.; Wang, L.; Renna, L.; Brandizzi, F.; Grumet, R.; Thomashow, M.F. CAMTA-Mediated Regulation of Salicylic Acid Immunity Pathway Genes in Arabidopsis Exposed to Low Temperature and Pathogen Infection. Plant Cell 2017, 29, 2465–2477. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Robe, E.; Jomat, L.; Aldon, D.; Mazars, C.; Galaud, J.P. CML8, an Arabidopsis Calmodulin-Like Protein, Plays a Role in Pseudomonas syringae Plant Immunity. Plant Cell Physiol. 2017, 58, 307–319. [Google Scholar]
- Shen, L.; Yang, S.; Guan, D.; He, S. CaCML13 Acts Positively in Pepper Immunity Against Ralstonia solanacearum Infection Forming Feedback Loop with CabZIP63. Int. J. Mol. Sci. 2020, 21, 4186. [Google Scholar] [CrossRef]
- Zhu, X.; Mazard, J.; Robe, E.; Pignoly, S.; Aguilar, M.; San Clemente, H.; Lauber, E.; Berthomé, R.; Galaud, J.P. The Same against Many: AtCML8, a Ca2+ Sensor Acting as a Positive Regulator of Defense Responses against Several Plant Pathogens. Int. J. Mol. Sci. 2021, 22, 10469. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [Green Version]
- Eichstädt, B.; Lederer, S.; Trempel, F.; Jiang, X.; Guerra, T.; Waadt, R.; Lee, J.; Liese, A.; Romeis, T. Plant Immune Memory in Systemic Tissue Does Not Involve Changes in Rapid Calcium Signaling. Front. Plant Sci. 2021, 12, 798230. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Coca, M. Calcium-dependent protein kinase OsCPK10 mediates both drought tolerance and blast disease resistance in rice plants. J. Exp. Bot. 2017, 68, 2963–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Liu, Y.; He, Q.; Chai, M.; Huang, Y.; Chen, F.; Wang, X.; Liu, Y.; Cai, H.; Qin, Y. Genome-wide investigation of calcium-dependent protein kinase gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2020, 21, 72. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Zhou, J.; Zhang, X.; Feng, Z.; Wei, F.; Zhao, L.; Zhang, Y.; Feng, H.; Zhu, H. Calcium-Dependent Protein Kinase GhCDPK28 Was Dentified and Involved in Verticillium Wilt Resistance in Cotton. Front. Plant Sci. 2021, 12, 772649. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Bouteau, F.; Kawano, T. Discovery of oxidative burst in the field of plant immunity: Looking back at the early pioneering works and towards the future development. Plant Signal Behav. 2008, 3, 153–155. [Google Scholar] [CrossRef] [Green Version]
- Averyanov, A. Oxidative burst and plant disease resistance. Front. Biosci. (Elite Ed.) 2009, 1, 142–152. [Google Scholar] [PubMed]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Pitzschke, A.; Hirt, H. Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol. 2006, 141, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Kasa, S.; Sakamoto, M.; Aoki, N.; Kai, K.; Yuasa, T.; Hanada, A.; Yamaguchi, S.; Iwaya-Inoue, M. A Role for Reactive Oxygen Species Produced by NADPH Oxidases in the Embryo and Aleurone Cells in Barley Seed Germination. PLoS ONE 2015, 10, e0143173. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Lal, N.K.; Lin, Z.D.; Ma, S.; Liu, J.; Castro, B.; Toruño, T.; Dinesh-Kumar, S.P.; Coaker, G. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 2020, 11, 1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S.; Hunter, K.; Vaahtera, L.; Tran, H.C.; Citterico, M.; Vaattovaara, A.; Rokka, A.; Stolze, S.C.; Harzen, A.; Meißner, L.; et al. CRK2 and C-terminal Phosphorylation of NADPH Oxidase RBOHD Regulate Reactive Oxygen Species Production in Arabidopsis. Plant Cell 2020, 32, 1063–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolupaev Iu, E.; Karpets Iu, V. Reactive oxygen species and stress signaling in plants. Ukr Biochem. J. 2014, 86, 18–35. [Google Scholar] [CrossRef] [Green Version]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.C. Antimicrobial actions of reactive oxygen species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef] [Green Version]
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Jwa, N.S.; Hwang, B.K. Convergent Evolution of Pathogen Effectors toward Reactive Oxygen Species Signaling Networks in Plants. Front. Plant Sci. 2017, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fernando, W.G.D. Analysis of the Oxidative Burst and Its Relevant Signaling Pathways in Leptosphaeria maculans-Brassica napus Pathosystem. Int. J. Mol. Sci. 2021, 22, 4812. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH Oxidase RBOHD During Plant Immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Mittler, R. Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J. 2021, 107, 7–20. [Google Scholar] [CrossRef]
- Yu, M.; Lamattina, L.; Spoel, S.H.; Loake, G.J. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol. 2014, 202, 1142–1156. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kolbert, Z.; Durner, J.; Lindermayr, C.; Corpas, F.J.; Brouquisse, R.; Barroso, J.B.; Umbreen, S.; Palma, J.M.; Hancock, J.T.; et al. Regulating the regulator: Nitric oxide control of post-translational modifications. New Phytol. 2020, 227, 1319–1325. [Google Scholar] [CrossRef]
- Skelly, M.J.; Malik, S.I.; Le Bihan, T.; Bo, Y.; Jiang, J.; Spoel, S.H.; Loake, G.J. A role for S-nitrosylation of the SUMO-conjugating enzyme SCE1 in plant immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 17090–17095. [Google Scholar] [CrossRef] [Green Version]
- Lubega, J.; Umbreen, S.; Loake, G.J. Recent advances in the regulation of plant immunity by S-nitrosylation. J. Exp. Bot. 2021, 72, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Liu, Z.; Shao, Y.; Su, J.; Li, X.; Sun, F.; Zhang, Y.; Li, S.; Zhang, Y.; Cui, J.; et al. Nitric Oxide Enhances Rice Resistance to Rice Black-Streaked Dwarf Virus Infection. Rice 2020, 13, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falak, N.; Imran, Q.M.; Hussain, A.; Yun, B.W. Transcription Factors as the “Blitzkrieg” of Plant Defense: A Pragmatic View of Nitric Oxide’s Role in Gene Regulation. Int. J. Mol. Sci. 2021, 22, 522. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz-Tohid, V.; Taheri, P.; Taghavi, S.M.; Tarighi, S. The role of nitric oxide in basal and induced resistance in relation with hydrogen peroxide and antioxidant enzymes. J. Plant Physiol. 2016, 199, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Río, L.A.D.; Palma, J.M. Impact of Nitric Oxide (NO) on the ROS Metabolism of Peroxisomes. Plants 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Jedelská, T.; Luhová, L.; Petřivalský, M. Nitric oxide signalling in plant interactions with pathogenic fungi and oomycetes. J. Exp. Bot. 2021, 72, 848–863. [Google Scholar] [CrossRef]
- Asai, S.; Mase, K.; Yoshioka, H. Role of nitric oxide and reactive oxygen [corrected] species in disease resistance to necrotrophic pathogens. Plant Signal. Behav. 2010, 5, 872–874. [Google Scholar] [CrossRef] [Green Version]
- Bellin, D.; Asai, S.; Delledonne, M.; Yoshioka, H. Nitric oxide as a mediator for defense responses. Mol. Plant Microbe Interact. 2013, 26, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.A.; Laarhoven, L.J.; Harren, F.J.; Hall, M.A.; Smith, A.R. Nitric oxide interacts with salicylate to regulate biphasic ethylene production during the hypersensitive response. Plant Physiol. 2008, 148, 1537–1546. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, B.; Zheng, X.Y.; Li, J.; Yang, M.; Dong, X.; He, G.; An, C.; Deng, X.W. Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat. Commun. 2015, 6, 7309. [Google Scholar] [CrossRef] [Green Version]
- Filgueiras, C.C.; Martins, A.D.; Pereira, R.V.; Willett, D.S. The Ecology of Salicylic Acid Signaling: Primary, Secondary and Tertiary Effects with Applications in Agriculture. Int. J. Mol. Sci. 2019, 20, 5851. [Google Scholar] [CrossRef] [PubMed]
- Janda, T.; Szalai, G.; Pál, M. Salicylic Acid Signalling in Plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, J.; Zeier, J. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernsdorff, F.; Döring, A.C.; Gruner, K.; Schuck, S.; Bräutigam, A.; Zeier, J. Pipecolic Acid Orchestrates Plant Systemic Acquired Resistance and Defense Priming via Salicylic Acid-Dependent and -Independent Pathways. Plant Cell 2016, 28, 102–129. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, K.; Sato, M.; Glazebrook, J.; Cohen, J.D.; Katagiri, F. Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 2008, 53, 763–775. [Google Scholar] [CrossRef]
- Shine, M.B.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. New Phytol. 2016, 212, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Nawrath, C.; Métraux, J.P. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 1999, 11, 1393–1404. [Google Scholar]
- Zhou, N.; Tootle, T.L.; Tsui, F.; Klessig, D.F.; Glazebrook, J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 1998, 10, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Willits, M.G.; Glazebrook, J. Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of defense responses: Evidence for inhibition of jasmonic acid signaling by SA. Mol. Plant Microbe. Interact. 2000, 13, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.D.; Zhang, C. The role of NDR1 in avirulence gene-directed signaling and control of programmed cell death in Arabidopsis. Plant Physiol. 2001, 127, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Wiermer, M.; Feys, B.J.; Parker, J.E. Plant immunity: The EDS1 regulatory node. Curr. Opin. Plant Biol. 2005, 8, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jeong, R.D.; Venugopal, S.C.; Lapchyk, L.; Navarre, D.; Kachroo, A.; Kachroo, P. SAG101 forms a ternary complex with EDS1 and PAD4 and is required for resistance signaling against turnip crinkle virus. PLoS Pathog. 2011, 7, e1002318. [Google Scholar] [CrossRef] [Green Version]
- Makandar, R.; Nalam, V.J.; Chowdhury, Z.; Sarowar, S.; Klossner, G.; Lee, H.; Burdan, D.; Trick, H.N.; Gobbato, E.; Parker, J.E.; et al. The Combined Action of ENHANCED DISEASE SUSCEPTIBILITY1, PHYTOALEXIN DEFICIENT4, and SENESCENCE-ASSOCIATED101 Promotes Salicylic Acid-Mediated Defenses to Limit Fusarium graminearum Infection in Arabidopsis thaliana. Mol. Plant Microbe. Interact. 2015, 28, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, S.C.; Jeong, R.D.; Mandal, M.K.; Zhu, S.; Chandra-Shekara, A.C.; Xia, Y.; Hersh, M.; Stromberg, A.J.; Navarre, D.; Kachroo, A.; et al. Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet. 2009, 5, e1000545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Zhang, C.; Albrecht, U.; Shimizu, R.; Wang, G.; Bowman, K.D. Overexpression of a citrus NDR1 ortholog increases disease resistance in Arabidopsis. Front. Plant Sci. 2013, 4, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Clarke, J.D.; Zhang, Y.; Dong, X. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol. Plant Microbe Interact. 2001, 14, 1131–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, J.; Kachroo, P.; Klessig, D.F. The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell 1999, 11, 191–206. [Google Scholar] [CrossRef]
- Desveaux, D.; Subramaniam, R.; Després, C.; Mess, J.N.; Lévesque, C.; Fobert, P.R.; Dangl, J.L.; Brisson, N. A “Whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev. Cell 2004, 6, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Desveaux, D.; Maréchal, A.; Brisson, N. Whirly transcription factors: Defense gene regulation and beyond. Trends Plant Sci. 2005, 10, 95–102. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.e15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, T.; Sun, Y.; Zhang, Y.; Radojičić, A.; Ding, Y.; Tian, H.; Huang, X.; Lan, J.; Chen, S.; et al. Diverse Roles of the Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Plant Immunity. Plant Cell 2020, 32, 4002–4016. [Google Scholar] [CrossRef]
- Trang Nguyen, H.; Thi Mai To, H.; Lebrun, M.; Bellafiore, S.; Champion, A. Jasmonates-the Master Regulator of Rice Development, Adaptation and Defense. Plants 2019, 8, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Zhao, J.; Tzeng, D.T.W.; Liu, Y.; Deng, L.; Yang, T.; Zhai, Q.; Wu, F.; Huang, Z.; Zhou, M.; et al. MYC2 Orchestrates a Hierarchical Transcriptional Cascade That Regulates Jasmonate-Mediated Plant Immunity in Tomato. Plant Cell 2017, 29, 1883–1906. [Google Scholar] [CrossRef] [Green Version]
- Oblessuc, P.R.; Obulareddy, N.; DeMott, L.; Matiolli, C.C.; Thompson, B.K.; Melotto, M. JAZ4 is involved in plant defense, growth, and development in Arabidopsis. Plant J. 2020, 101, 371–383. [Google Scholar] [CrossRef]
- Iñigo, S.; Alvarez, M.J.; Strasser, B.; Califano, A.; Cerdán, P.D. PFT1, the MED25 subunit of the plant Mediator complex, promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis. Plant J. 2012, 69, 601–612. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Yang, J.; Yao, S.; Zhao, K.; Wang, D.; Qin, Q.; Bian, Z.; Li, Y.; Lan, Y.; et al. Jasmonate Signaling Enhances RNA Silencing and Antiviral Defense in Rice. Cell Host Microbe 2020, 28, 89–103.e8. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, H.; Sun, Z.; Li, J.; Hong, G.; Zhu, Q.; Zhou, X.; MacFarlane, S.; Yan, F.; Chen, J. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017, 214, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, J.; Xu, H.; Wang, Y.; Li, C.; Wen, P.; You, X.; Zhang, X.; Pan, G.; Li, Q.; et al. Rice stripe virus suppresses jasmonic acid-mediated resistance by hijacking brassinosteroid signaling pathway in rice. PLoS Pathog. 2020, 16, e1008801. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.N.; Liu, R.; Li, T.; Li, M.; Liu, X.Y.; Wang, W.J.; Yu, Y.K.; Cao, J.; Tan, X.L. Physiological and comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in a rapeseed anthocyanin-more mutant. Biotechnol. Biofuels Bioprod. 2022, 15, 55. [Google Scholar] [CrossRef]
- Pantelides, I.S.; Tjamos, S.E.; Pappa, S.; Kargakis, M.; Paplomatas, E.J. The ethylene receptor ETR1 is required for Fusarium oxysporum pathogenicity. Plant Pathol. 2013, 62, 1302–1309. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Y.F.; Randlett, M.D.; Zhao, X.C.; Findell, J.L.; Kieber, J.J.; Schaller, G.E. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J. Biol. Chem. 2003, 278, 34725–34732. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [Green Version]
- Berrocal-Lobo, M.; Molina, A. Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Mol. Plant Microbe Interact. 2004, 17, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping Ethylene Response: The Role of EIN3/EIL1 Transcription Factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef] [Green Version]
- Azhar, B.J.; Zulfiqar, A.; Shakeel, S.N.; Schaller, G.E. Amplification and Adaptation in the Ethylene Signaling Pathway. Small Methods 2019, 4, 1900452. [Google Scholar] [CrossRef]
- Ton, J.; Van Pelt, J.A.; Van Loon, L.C.; Pieterse, C.M. Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol Plant Microbe Interact 2002, 15, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-Dependent Signaling Pathway and Activates PAMP-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.H.; Huang, Z.Y.; Xie, P.; Gu, C.; Li, K.; Wang, D.C.; Yu, Y.Y.; Fan, Z.H.; Wang, C.J.; Wang, Y.P.; et al. Transcription factors WRKY70 and WRKY11 served as regulators in rhizobacterium Bacillus cereus AR156-induced systemic resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. J. Exp. Bot. 2016, 67, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.Y.; Catinot, J.; Zimmerli, L. Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 2016, 67, 1231–1241. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, F.; Wang, Z.; Peng, W.; Huang, R.; Huang, D.; Xie, D. An Arabidopsis mutant cex1 exhibits constant accumulation of jasmonate-regulated AtVSP, Thi2.1 and PDF1.2. FEBS Lett. 2001, 494, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Zarei, A.; Körbes, A.P.; Younessi, P.; Montiel, G.; Champion, A.; Memelink, J. Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol. Biol. 2011, 75, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Ausubel, F.M.; Dong, X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 2000, 12, 2175–2190. [Google Scholar] [CrossRef] [Green Version]
- Stateczny, D.; Oppenheimer, J.; Bommert, P. G protein signaling in plants: Minus times minus equals plus. Curr. Opin. Plant Biol. 2016, 34, 127–135. [Google Scholar] [CrossRef]
- Ofoe, R. Signal transduction by plant heterotrimeric G-protein. Plant Biol. 2021, 23, 3–10. [Google Scholar] [CrossRef]
- Katz, A.; Wu, D.; Simon, M.I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature 1992, 360, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Milde, M.; Werthmann, R.C.; von Hayn, K.; Bünemann, M. Dynamics of adenylate cyclase regulation via heterotrimeric G-proteins. Biochem. Soc. Trans. 2014, 42, 239–243. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruta, N.; Trusov, Y.; Jones, A.M.; Botella, J.R. Heterotrimeric G Proteins in Plants: Canonical and Atypical Gα Subunits. Int. J. Mol. Sci. 2021, 22, 11841. [Google Scholar] [CrossRef] [PubMed]
- Brenya, E.; Trusov, Y.; Dietzgen, R.G.; Botella, J.R. Heterotrimeric G-proteins facilitate resistance to plant pathogenic viruses in Arabidopsis thaliana (L.) Heynh. Plant Signal. Behav. 2016, 11, e1212798. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.L.; Zhang, C.; Liu, J.Z. Heterotrimeric G protein signaling in plant immunity. J. Exp. Bot. 2019, 70, 1109–1118. [Google Scholar] [CrossRef]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Ding, P.; Lian, K.; Wang, J.; Ma, M.; Li, L.; Li, L.; Li, M.; Zhang, X.; Chen, S.; et al. Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. Elife 2016, 5, e13568. [Google Scholar] [CrossRef]
- Trusov, Y.; Sewelam, N.; Rookes, J.E.; Kunkel, M.; Nowak, E.; Schenk, P.M.; Botella, J.R. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J. 2009, 58, 69–81. [Google Scholar] [CrossRef]
- Escudero, V.; Torres, M.; Delgado, M.; Sopeña-Torres, S.; Swami, S.; Morales, J.; Muñoz-Barrios, A.; Mélida, H.; Jones, A.M.; Jordá, L.; et al. Mitogen-Activated Protein Kinase Phosphatase 1 (MKP1) Negatively Regulates the Production of Reactive Oxygen Species During Arabidopsis Immune Responses. Mol. Plant Microbe Interact. 2019, 32, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Xu, J.; Zhang, S. RACK1, scaffolding a heterotrimeric G protein and a MAPK cascade. Trends Plant Sci. 2015, 20, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.J.; Cobb, M.H. Protein kinases. Curr. Opin. Struct. Biol. 1994, 4, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Qian, Y.; Zhang, W.; Qian, L.; Wang, Y.; Cailin, G.; Ding, H. Mitogen-activated protein kinase action in plant response to high-temperature stress: A mini review. Protoplasma 2021, 258, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant Mitogen-Activated Protein Kinase Cascades in Environmental Stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Singh, D.; Sinha, A.K. Plant cell cycle regulators: Mitogen-activated protein kinase, a new regulating switch? Plant Sci. 2020, 301, 110660. [Google Scholar] [CrossRef]
- Melvin, P.; Prabhu, S.A.; Anup, C.P.; Shailasree, S.; Shetty, H.S.; Kini, K.R. Involvement of mitogen-activated protein kinase signalling in pearl millet-downy mildew interaction. Plant Sci. 2014, 214, 29–37. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Xu, L.; Wang, Q.; Lou, Y. Expressing OsMPK4 Impairs Plant Growth but Enhances the Resistance of Rice to the Striped Stem Borer Chilo suppressalis. Int. J. Mol. Sci. 2018, 19, 1182. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.; Colcombet, J. Sustained Incompatibility between MAPK Signaling and Pathogen Effectors. Int. J. Mol. Sci. 2020, 21, 7954. [Google Scholar] [CrossRef]
- Ekengren, S.K.; Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P.; Martin, G.B. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003, 36, 905–917. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Y.; Xiong, Y.; DeFraia, C.; Li, J.; Dong, X.; Mou, Z. Overexpression of Arabidopsis MAP kinase kinase 7 leads to activation of plant basal and systemic acquired resistance. Plant J. 2007, 52, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Zhang, X.; Zhao, L.; Feng, Z.; Wei, F.; Zhang, Y.; Feng, H.; Zhou, Y.; Zhu, H. MPK homolog GhNTF6 was involved in cotton against Verticillium wilt by interacted with VdEPG1. Int. J. Biol. Macromol. 2022, 195, 456–465. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Yan, S.; Yu, T.; Yang, J.; Dong, J.; Zhang, S.; Zhao, J.; Yang, T.; Mao, X.; et al. OsWRKY67 positively regulates blast and bacteria blight resistance by direct activation of PR genes in rice. BMC Plant Biol. 2018, 18, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, W.; Qasim, M.; Noman, A.; Adnan, M.; Tayyab, M.; Farooq, T.H.; Wei, H.; Wang, L. Plant microRNAs: Front line players against invading pathogens. Microb Pathog. 2018, 118, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Fang, Y.; Chen, L.; Wang, J.; Chen, X. Role of non-coding RNAs in plant immunity. Plant Commun. 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, P.; Mei, H.; Wang, D.; Sun, J.; Yang, C.; Hao, L.; Cao, S.; Chu, C.; Hu, S.; et al. Fine-Tuning of MiR528 Accumulation Modulates Flowering Time in Rice. Mol Plant. 2019, 12, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Hao, M.; Wang, L.; Liu, J.; Zhang, Z.; Tang, Y.; Peng, Q.; Yang, Z.; Wu, J. The Cotton miR477-CBP60A Module Participates in Plant Defense Against Verticillium dahlia. Mol. Plant Microbe Interact. 2020, 33, 624–636. [Google Scholar] [CrossRef]
- Boccara, M.; Sarazin, A.; Thiébeauld, O.; Jay, F.; Voinnet, O.; Navarro, L.; Colot, V. The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog. 2014, 10, e1003883. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wang, J.; Tung, J.; Liu, D.; Zhou, Y.; He, S.; Du, Y.; Baker, B.; Li, F. A role for small RNA in regulating innate immunity during plant growth. PLoS Pathog. 2018, 14, e1006756. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, X.; Liu, D.; Xu, W.; Wise, R.; Shen, Q.H. The miR9863 family regulates distinct Mla alleles in barley to attenuate NLR receptor-triggered disease resistance and cell-death signaling. PLoS Genet. 2014, 10, e1004755. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wang, S.; Zhou, Y.; Bai, J.; Huang, G.; Liu, X.; Zhang, Y.; Tang, D.; Lu, D. Transcriptional Regulation of the Immune Receptor FLS2 Controls the Ontogeny of Plant Innate Immunity. Plant Cell 2018, 30, 2779–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tao, Z.; Hong, H.; Chen, Z.; Wu, C.; Li, X.; Xiao, J.; Wang, S. Transposon-derived small RNA is responsible for modified function of WRKY45 locus. Nat. Plants 2016, 2, 16016. [Google Scholar] [CrossRef]
- Yang, L.; Huang, H. Roles of small RNAs in plant disease resistance. J Integr. Plant Biol. 2014, 56, 962–970. [Google Scholar] [CrossRef]
- Chandran, V.; Wang, H.; Gao, F.; Cao, X.L.; Chen, Y.P.; Li, G.B.; Zhu, Y.; Yang, X.M.; Zhang, L.L.; Zhao, Z.X.; et al. miR396-OsGRFs Module Balances Growth and Rice Blast Disease-Resistance. Front. Plant Sci. 2019, 9, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.K.; Megha, S.; Basu, U.; Rahman, M.H.; Kav, N.N. Genome Wide Identification and Functional Prediction of Long Non-Coding RNAs Responsive to Sclerotinia sclerotiorum Infection in Brassica napus. PLoS ONE 2016, 11, e0158784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gai, Y.P.; Yuan, S.S.; Zhao, Y.N.; Zhao, H.N.; Zhang, H.L.; Ji, X.L. A Novel LncRNA, MuLnc1, Associated With Environmental Stress in Mulberry (Morus multicaulis). Front. Plant Sci. 2018, 9, 669. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Cui, J.; Liu, W.; Jiang, N.; Zhou, X.; Qi, H.; Meng, J.; Luan, Y. LncRNA39026 Enhances Tomato Resistance to Phytophthora infestans by Decoying miR168a and Inducing PR Gene Expression. Phytopathology 2020, 110, 873–880. [Google Scholar]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato—Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Cui, J.; Luan, Y.; Jiang, N.; Bao, H.; Meng, J. Comparative transcriptome analysis between resistant and susceptible tomato allows the identification of lncRNA16397 conferring resistance to Phytophthora infestans by co-expressing glutaredoxin. Plant J. 2017, 89, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Cui, J.; Hou, X.; Yang, G.; Xiao, Y.; Han, L.; Meng, J.; Luan, Y. Sl-lncRNA15492 interacts with Sl-miR482a and affects Solanum lycopersicum immunity against Phytophthora infestans. Plant J. 2020, 103, 1561–1574. [Google Scholar] [CrossRef]
- Hong, Y.H.; Meng, J.; Zhang, M.; Luan, Y.S. Identification of tomato circular RNAs responsive to Phytophthora infestans. Gene 2020, 746, 144652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.L.; Zhao, J.H.; Wang, S.; Jin, Y.; Chen, Z.Q.; Fang, Y.Y.; Hua, C.L.; Ding, S.W.; Guo, H.S. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Tisserant, C.; Tulinski, M.; Weiberg, A.; Feldbrugge, M. Inside-out: From endosomes to extracellular vesicles in fungal RNA transport. Fungal Biol. Rev. 2020, 34, 89–99. [Google Scholar] [CrossRef]
- Prins, M.; Laimer, M.; Noris, E.; Schubert, J.; Wassenegger, M.; Tepfer, M. Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 2008, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, X.; Zhang, F.; Xu, M.; Ye, Z.; Wang, K.; Liu, S.; Han, X.; Cheng, Y.; Zhong, K.; et al. A virus-derived siRNA activates plant immunity by interfering with ROS scavenging. Mol. Plant 2021, 14, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Zhang, W.; Corwin, J.A.; Copeland, D.; Feusier, J.; Eshbaugh, R.; Chen, F.; Atwell, S.; Kliebenstein, D.J. Plastic Transcriptomes Stabilize Immunity to Pathogen Diversity: The Jasmonic Acid and Salicylic Acid Networks within the Arabidopsis/Botrytis Pathosystem. Plant Cell 2017, 29, 2727–2752. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mi, X.; Chen, C.; Wang, H.; Guo, W. Identification on mitogen-activated protein kinase signaling cascades by integrating protein interaction with transcriptional profiling analysis in cotton. Sci. Rep. 2018, 8, 8178. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, S.; Yang, D.; Fan, Z. Revealing Shared and Distinct Genes Responding to JA and SA Signaling in Arabidopsis by Meta-Analysis. Front. Plant Sci. 2020, 11, 908. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D. Pathological hormone imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 2015, 56, 2158–2168. [Google Scholar] [PubMed]
- Chandrashekar, N.; Ali, S.; Grover, A. Exploring expression patterns of PR-1, PR-2, PR-3, and PR-12 like genes in Arabidopsis thaliana upon Alternaria brassicae inoculation. 3 Biotech 2018, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, L.; Wei, X.; Zhou, Y.; Dai, Y.; Zhu, Z. OsJAZ13 Negatively Regulates Jasmonate Signaling and Activates Hypersensitive Cell Death Response in Rice. Int. J. Mol. Sci. 2020, 21, 4379. [Google Scholar] [CrossRef]
- Song, S.; Huang, H.; Gao, H.; Wang, J.; Wu, D.; Liu, X.; Yang, S.; Zhai, Q.; Li, C.; Qi, T.; et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 2014, 26, 263–279. [Google Scholar] [CrossRef] [Green Version]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Choudhary, P.; Gupta-Bouder, P.; Chatterjee, S.; Liu, P.P.; Klessig, D.F.; Raina, R. Arabidopsis SMALL DEFENSE-ASSOCIATED PROTEIN 1 Modulates Pathogen Defense and Tolerance to Oxidative Stress. Front. Plant Sci. 2020, 11, 703. [Google Scholar] [CrossRef]
- Li, H.; Jiao, Z.; Zhang, P.; Ni, Y.; Wang, T.; Zhang, J.; Li, J.; Jiang, Y.; Yang, X.; Li, L.; et al. Enhanced SA and Ca2+ signaling results in PCD-mediated spontaneous leaf necrosis in wheat mutant wsl. Mol. Genet. Genomics 2021, 296, 1249–1262. [Google Scholar] [CrossRef]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.; Korzelius, J.P.; Van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Brown, R.; Kazan, K.; et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Dongus, J.A.; Parker, J.E. EDS1 signalling: At the nexus of intracellular and surface receptor immunity. Curr. Opin. Plant Biol. 2021, 62, 102039. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, P.; Petersen, M.; Bjørn Nielsen, H.; Zhu, S.; Newman, M.A.; Shokat, K.M.; Rietz, S.; Parker, J.; Mundy, J. Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J. 2006, 47, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Kariola, T.; Palva, E.T. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Guo, R.; Tu, M.; Wang, D.; Guo, C.; Wan, R.; Li, Z.; Wang, X. Ectopic Expression of the Wild Grape WRKY Transcription Factor VqWRKY52 in Arabidopsis thaliana Enhances Resistance to the Biotrophic Pathogen Powdery Mildew But Not to the Necrotrophic Pathogen Botrytis cinerea. Front. Plant Sci. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, P.; Duan, M.; Wei, C.; Li, Y. WRKY62 transcription factor acts downstream of cytosolic NPR1 and negatively regulates jasmonate-responsive gene expression. Plant Cell Physiol. 2007, 48, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Xiao, J.; Ding, X.; Xiong, M.; Cai, M.; Cao, Y.; Li, X.; Xu, C.; Wang, S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 2007, 20, 492–499. [Google Scholar]
- Ndamukong, I.; Abdallat, A.A.; Thurow, C.; Fode, B.; Zander, M.; Weigel, R.; Gatz, C. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 2007, 50, 128–139. [Google Scholar] [CrossRef]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Kachroo, A.; Lapchyk, L.; Fukushige, H.; Hildebrand, D.; Klessig, D.; Kachroo, P. Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell 2003, 15, 2952–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis WRKY33 Is a Key Transcriptional Regulator of Hormonal and Metabolic Responses toward Botrytis cinerea Infection. Plant Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mang, H.G.; Laluk, K.A.; Parsons, E.P.; Kosma, D.K.; Cooper, B.R.; Park, H.C.; AbuQamar, S.; Boccongelli, C.; Miyazaki, S.; Consiglio, F. The Arabidopsis RESURRECTION1 gene regulates a novel antagonistic interaction in plant defense to biotrophs and necrotrophs. Plant Physiol. 2009, 151, 290–305. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Species | Biological Function | Molecular Switch Function | Citations |
|---|---|---|---|---|
| NPR1 | A. thaliana | SA-dependent PR gene expression and SAR | SA signaling (+) JA/ET signaling (−) | [180] |
| EDS1 | A. thaliana | R-mediated resistance; regulation of HR/PCD and SA accumulation | SA signaling (+) JA/ET signaling (−) | [80,82,181] |
| PAD4 | A. thaliana | EDS1 interactor, basal and receptor-triggered immunity, and SA accumulation | SA signaling (+) JA/ET signaling (−) | [84,182,183] |
| WRKY70 | A. thaliana | Regulator of developmental senescence and plant defense | SA signaling (+) JA/ET signaling (−) | [184,185] |
| WRKY52 | Vitis quinquangularis | SA-dependent signaling pathway and in HR cell death | SA signaling (+) JA/ET signaling (−) | [186] |
| WRKY62 | A. thaliana | Downstream of cytosolic NPR1 | SA signaling (+) JA/ET signaling (−) | [167,187] |
| WRKY13 | O. sativa | Enhances rice resistance to bacterial blight and fungal blast | SA signaling (+) JA/ET signaling (−) | [188] |
| GRX480 | A. thaliana | TGA-interacting protein, controlling the redox state of regulatory proteins | SA signaling (+) JA/ET signaling (−) | [189] |
| MPK4 | A. thaliana | JA-responsive gene expression; represses SAR | SA signaling (−) JA/ET signaling (+) | [182,190] |
| SSI2 | A. thaliana | Regulates the overall level of desaturated FAs | SA signaling (−) JA/ET signaling (+) | [191] |
| WRKY33 | A. thaliana | Defense toward necrotrophic fungi | SA signaling (−) JA/ET signaling (+) | [192] |
| RST1 | A. thaliana | Lipid synthesis; cuticular wax with embryo development | SA signaling (+) JA/ET signaling (−) | [193] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.-N.; Li, Y.-T.; Wu, Y.-Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. https://doi.org/10.3390/ijms232416200
Ding L-N, Li Y-T, Wu Y-Z, Li T, Geng R, Cao J, Zhang W, Tan X-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. International Journal of Molecular Sciences. 2022; 23(24):16200. https://doi.org/10.3390/ijms232416200
Chicago/Turabian StyleDing, Li-Na, Yue-Tao Li, Yuan-Zhen Wu, Teng Li, Rui Geng, Jun Cao, Wei Zhang, and Xiao-Li Tan. 2022. "Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects" International Journal of Molecular Sciences 23, no. 24: 16200. https://doi.org/10.3390/ijms232416200
APA StyleDing, L.-N., Li, Y.-T., Wu, Y.-Z., Li, T., Geng, R., Cao, J., Zhang, W., & Tan, X.-L. (2022). Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. International Journal of Molecular Sciences, 23(24), 16200. https://doi.org/10.3390/ijms232416200






