iADRGSE: A Graph-Embedding and Self-Attention Encoding for Identifying Adverse Drug Reaction in the Earlier Phase of Drug Development
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation Metrics
2.2. Parameter Setting
2.3. Feature Evaluation
2.4. Comparison of Feature-Extraction Methods of iADRGSE and Several Classic Feature-Extraction Methods
2.5. Comparison with Existing Predictor
2.6. Case study
3. Materials and Methods
3.1. Dataset
3.2. Problem Formulation
3.3. Overview of iADRGSE
3.4. Drug Molecular Representation
3.5. Feature Learning
3.5.1. Graph Channel
3.5.2. Sequence Channel
3.5.3. Multi-Label Classification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004, 329, 15. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.; Gagnon, J.P. The cost of new drug discovery and development. Discov. Med. 2009, 4, 172–179. [Google Scholar]
- Whitebread, S.; Hamon, J.; Bojanic, D.; Urban, L. Keynote review: In vitro safety pharmacology profiling: An essential tool for successful drug development. Drug Discov. Today 2005, 10, 1421–1433. [Google Scholar] [CrossRef]
- Harpaz, R.; Vilar, S.; Dumouchel, W.; Salmasian, H.; Haerian, K.; Shah, N.H.; Chase, H.S.; Friedman, C. Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions. J. Am. Med. Inform. Assoc. 2013, 20, 413–419. [Google Scholar] [CrossRef]
- Tutubalina, E.; Alimova, I.; Miftahutdinov, Z.; Sakhovskiy, A.; Malykh, V.; Nikolenko, S. The Russian Drug Reaction Corpus and neural models for drug reactions and effectiveness detection in user reviews. Bioinformatics 2021, 37, 243–249. [Google Scholar] [CrossRef]
- Jagannatha, A.N.; Yu, H. Bidirectional RNN for medical event detection in electronic health records. In Proceedings of the Association for Computational Linguistics, Berlin, Germany, 7–12 August 2016; p. 473. [Google Scholar]
- Cocos, A.; Fiks, A.G.; Masino, A.J. Deep learning for pharmacovigilance: Recurrent neural network architectures for labeling adverse drug reactions in Twitter posts. J. Am. Med. Inform. Assoc. 2017, 24, 813–821. [Google Scholar] [CrossRef]
- Ding, P.; Zhou, X.; Zhang, X.; Wang, J.; Lei, Z. An attentive neural sequence labeling model for adverse drug reactions mentions extraction. IEEE Access 2018, 6, 73305–73315. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, H.; Ren, Y.; Yang, L.; Xu, B.; Yang, Z.; Wang, J.; Zhang, Y. Adverse drug reaction detection via a multihop self-attention mechanism. BMC Bioinform. 2019, 20, 479. [Google Scholar] [CrossRef]
- El-Allaly, E.-D.; Sarrouti, M.; En-Nahnahi, N.; El Alaoui, S.O. An adverse drug effect mentions extraction method based on weighted online recurrent extreme learning machine. Comput. Methods Programs Biomed. 2019, 176, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, T.; Kim, M.-H.; Yoon, Y. Prediction of Side Effects Using Comprehensive Similarity Measures. Biomed Res. Int. 2020, 2020, 1357630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yue, X.; Liu, F.; Chen, Y.; Tu, S.; Zhang, X. A unified frame of predicting side effects of drugs by using linear neighborhood similarity. BMC Syst. Biol. 2017, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Peng, H.; Ghosh, S.; Lan, C.; Li, J. Inverse similarity and reliable negative samples for drug side-effect prediction. BMC Bioinform. 2019, 19, 554. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, P.; Li, J.; Fu, Y.; Qu, L.; Chen, Y.; Chen, Z. Learning important features from multi-view data to predict drug side effects. J. Cheminformatics 2019, 11, 79. [Google Scholar] [CrossRef]
- Emir Muñoz, M.; Nováček, V.; Vandenbussche, P.-Y. Using Drug Similarities for Discovery of Possible Adverse Reactions. AMIA Annu Symp Proc 2017, 2016, 924–933. [Google Scholar]
- Bean, D.M.; Wu, H.; Iqbal, E.; Dzahini, O.; Ibrahim, Z.M.; Broadbent, M.; Stewart, R.; Dobson, R.J. Knowledge graph prediction of unknown adverse drug reactions and validation in electronic health records. Sci. Rep. 2017, 7, 16416. [Google Scholar] [CrossRef]
- Emir, M.; Nováček, V.; Vandenbussche, P.-Y. Facilitating prediction of adverse drug reactions by using knowledge graphs and multi-label learning models. Brief. Bioinform. 2019, 20, 190–202. [Google Scholar]
- Zhang, F.; Sun, B.; Diao, X.; Zhao, W.; Shu, T. Prediction of adverse drug reactions based on knowledge graph embedding. BMC Med. Inform. Decis. Mak. 2021, 21, 38. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, P. Towards early detection of adverse drug reactions: Combining pre-clinical drug structures and post-market safety reports. BMC Med. Inform. Decis. Mak. 2019, 19, 279. [Google Scholar] [CrossRef]
- Timilsina, M.; Tandan, M.; d’Aquin, M.; Yang, H. Discovering Links Between Side Effects and Drugs Using a Diffusion Based Method. Sci. Rep. 2019, 9, 10436. [Google Scholar] [CrossRef] [PubMed]
- Xuan, P.; Song, Y.; Zhang, T.; Jia, L. Prediction of Potential Drug–Disease Associations through Deep Integration of Diversity and Projections of Various Drug Features. Int. J. Mol. Sci. 2019, 20, 4102. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Xiong, Z.; Chen, K.; Jiang, H.; Zheng, M. Graph neural networks for automated de novo drug design. Drug Discov. Today 2021, 26, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Withnall, M.; Lindelöf, E.; Engkvist, O.; Chen, H. Building attention and edge message passing neural networks for bioactivity and physical–chemical property prediction. J. Cheminformatics 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; p. 30. [Google Scholar]
- Schwarz, K.; Allam, A.; Perez Gonzalez, N.A.; Krauthammer, M. AttentionDDI: Siamese attention-based deep learning method for drug–drug interaction predictions. BMC Bioinform. 2021, 22, 412. [Google Scholar] [CrossRef]
- Dey, S.; Luo, H.; Fokoue, A.; Hu, J.; Zhang, P. Predicting adverse drug reactions through interpretable deep learning framework. BMC Bioinform. 2018, 19, 476. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.; Chang, M.-W.; Lee, K.; Toutanova, K. Bert: Pre-training of deep bidirectional transformers for language understanding. arXiv 2018, arXiv:1810.04805. [Google Scholar]
- Xiong, Z.; Wang, D.; Liu, X.; Zhong, F.; Wan, X.; Li, X.; Li, Z.; Luo, X.; Chen, K.; Jiang, H.; et al. Pushing the Boundaries of Molecular Representation for Drug Discovery with the Graph Attention Mechanism. J. Med. Chem. 2020, 63, 8749–8760. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.; Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Modeling 2010, 50, 742–754. [Google Scholar] [CrossRef]
- DuMouchel, W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 1999, 53, 177–190. [Google Scholar]
- Xiao, C.; Li, Y.; Baytas, I.M.; Zhou, J.; Wang, F. An MCEM Framework for Drug Safety Signal Detection and Combination from Heterogeneous Real World Evidence. Sci. Rep. 2018, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Byun, J.M.; Yoon, S.-S.; Koh, Y.; Yoon, S.-W.; Shin, D.-Y.; Hong, J.; Kim, I. Cyclophosphamide addition to pomalidomide/dexamethasone is not necessarily associated with universal benefits in RRMM. PLoS ONE 2022, 17, e0260113. [Google Scholar] [CrossRef] [PubMed]
- Toropov, A.A.; Toropova, A.P.; Mukhamedzhanova, D.V.; Gutman, I. Simplified molecular input line entry system (SMILES) as an alternative for constructing quantitative structure-property relationships (QSPR). Indian J. Chemistry. Sect. A Inorg. Phys. Theor. Anal. 2005, 44, 1545–1552. [Google Scholar]
- Landrum, G. RDKit: Open-Source Cheminformatics and Machine Learning. Available online: https://www.rdkit.org (accessed on 12 September 2022).
- Chen, Y.; Ma, T.; Yang, X.; Wang, J.; Song, B.; Zeng, X. MUFFIN: Multi-scale feature fusion for drug–drug interaction prediction. Bioinformatics 2021, 37, 2651–2658. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef]
- Veličković, P.; Fedus, W.; Hamilton, W.L.; Liò, P.; Bengio, Y.; Hjelm, R.D. Deep Graph Infomax. arXiv 2018, arXiv:1809.10341. [Google Scholar]
- Hamilton, W.L.; Ying, R.; Leskovec, J. Representation learning on graphs: Methods and applications. arXiv 2017, arXiv:1709.05584. [Google Scholar]
- Hu, W.; Liu, B.; Gomes, J.; Zitnik, M.; Liang, P.; Pande, V.; Leskovec, J. Strategies for Pre-training Graph Neural Networks. arXiv 2020, arXiv:1905.12265. [Google Scholar]
- Ba, J.L.; Kiros, J.R.; Hinton, G.E. Layer normalization. arXiv 2016, arXiv:1607.06450. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Ioffe, S.; Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. In Proceedings of the International Conference on Machine Learning 2015, Lille, France, 6–11 July 2015; pp. 448–456. [Google Scholar]


 represents the drug node, while
represents the drug node, while  represents the adverse reaction node; the more drugs connected to the ADR, the larger the node of the ADR; the line connecting the drug node and the ADR node indicates that the drug has these adverse reactions, and the thickened line indicates the potential adverse reactions of the drug.
represents the adverse reaction node; the more drugs connected to the ADR, the larger the node of the ADR; the line connecting the drug node and the ADR node indicates that the drug has these adverse reactions, and the thickened line indicates the potential adverse reactions of the drug.
 represents the drug node, while
represents the drug node, while  represents the adverse reaction node; the more drugs connected to the ADR, the larger the node of the ADR; the line connecting the drug node and the ADR node indicates that the drug has these adverse reactions, and the thickened line indicates the potential adverse reactions of the drug.
represents the adverse reaction node; the more drugs connected to the ADR, the larger the node of the ADR; the line connecting the drug node and the ADR node indicates that the drug has these adverse reactions, and the thickened line indicates the potential adverse reactions of the drug.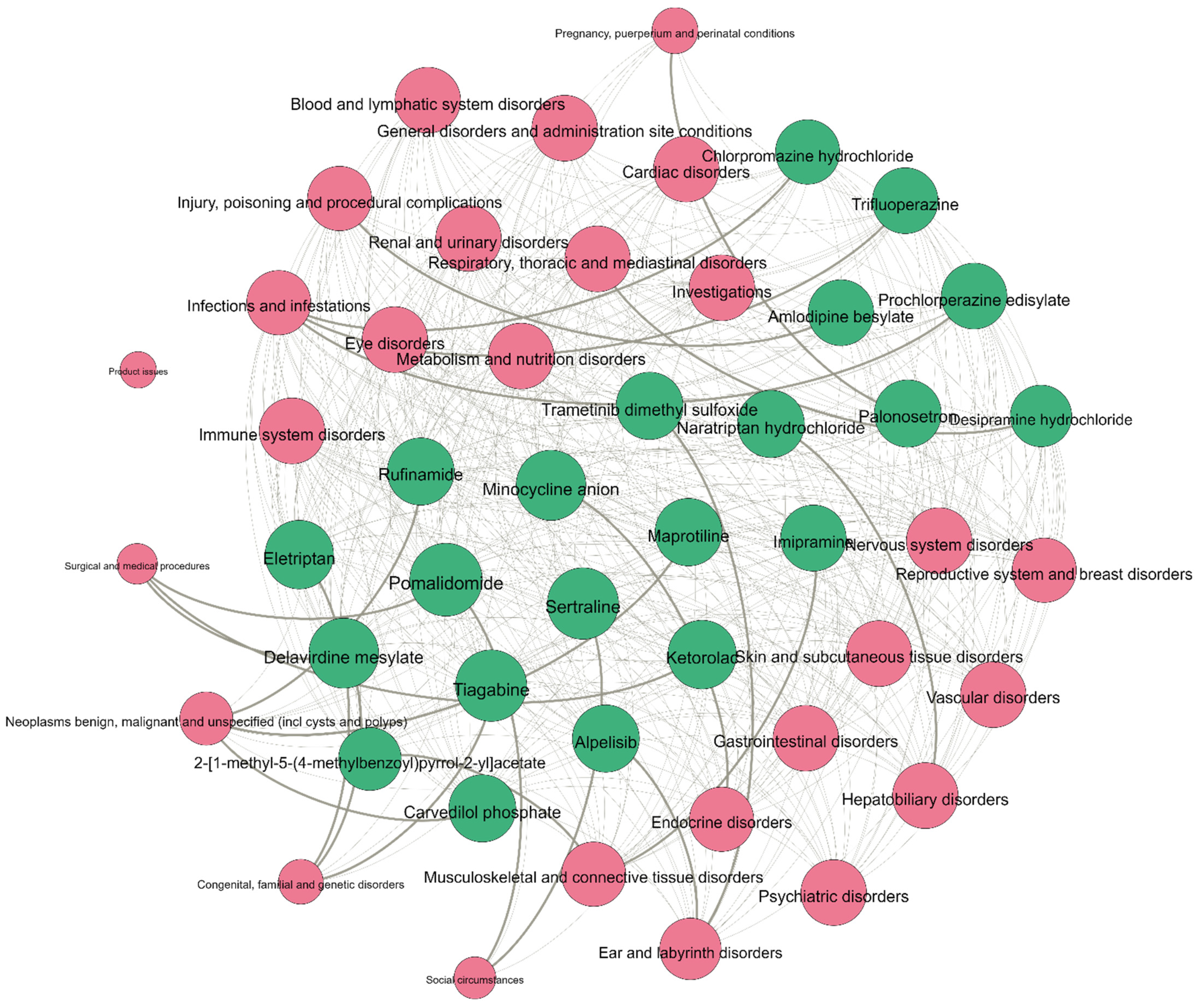

 represents positive samples, while
represents positive samples, while  is negative samples.
is negative samples.
 represents positive samples, while
represents positive samples, while  is negative samples.
is negative samples.


| Features Set | Accuracy | Precision (Macro) | Recall (Macro) | AUC (Macro) | AUPR (Macro) |
|---|---|---|---|---|---|
| CNN_FP2 | 0.7802 ± 0.0089 | 0.6474 ± 0.0213 | 0.7255 ± 0.0125 | 0.6726 ± 0.0145 | 0.7037 ± 0.0156 |
| BERT_smiles | 0.7754 ± 0.0084 | 0.6266 ± 0.0251 | 0.7246 ± 0.0091 | 0.6587 ± 0.0202 | 0.6987 ± 0.0150 |
| Attentive_FP | 0.7638 ± 0.0099 | 0.6748 ± 0.0130 | 0.7431 ± 0.0153 | 0.5669 ± 0.0234 | 0.6362 ± 0.0137 |
| E + S | 0.8074 ± 0.0083 | 0.7241 ± 0.0280 | 0.7350 ± 0.0145 | 0.7519 ± 0.0210 | 0.7590 ± 0.0156 |
| C + S | 0.8008 ± 0.0071 | 0.7251 ± 0.0120 | 0.7342 ± 0.0260 | 0.7545 ± 0.0163 | 0.7605 ± 0.0810 |
| I + S | 0.8044 ± 0.0081 | 0.7136 ± 0.0286 | 0.7282 ± 0.0172 | 0.7479 ± 0.0172 | 0.7533 ± 0.0172 |
| E + C + S | 0.8100 ± 0.0081 | 0.7369 ± 0.0256 | 0.7384 ± 0.0136 | 0.7628 ± 0.0148 | 0.7709 ± 0.0135 |
| E + I + S | 0.8078 ± 0.0081 | 0.7251 ± 0.0234 | 0.7342 ± 0.0138 | 0.7545 ± 0.0181 | 0.7605 ± 0.0138 |
| C + I + S | 0.8065 ± 0.0083 | 0.7245 ± 0.0305 | 0.7393 ± 0.0123 | 0.7580 ± 0.0125 | 0.7682 ± 0.0145 |
| iADRGSE_Gin | 0.7992 ± 0.0022 | 0.7450 ± 0.0103 | 0.7235 ± 0.0063 | 0.7358 ± 0.0113 | 0.7526 ± 0.0088 |
| iADRGSE_no_Gin | 0.7900 ± 0.0057 | 0.6888 ± 0.0323 | 0.7506 ± 0.0176 | 0.7098 ± 0.0179 | 0.7428 ± 0.0120 |
| iADRGSE_ no_attention | 0.8028 ± 0.011 | 0.7451 ± 0.0257 | 0.7302 ± 0.0117 | 0.7410 ± 0.0192 | 0.7619 ± 0.0139 |
| iADRGSE_mean | 0.7938 ± 0.0062 | 0.6793 ± 0.0352 | 0.7441 ± 0.0132 | 0.7206 ± 0.0161 | 0.7426 ± 0.0156 |
| iADRGSE (ours) | 0.8117 ± 0.0089 | 0.7434 ± 0.0266 | 0.7421 ± 0.0105 | 0.7674 ± 0.0147 | 0.7760 ± 0.0130 |
| Features Set | Accuracy | Precision (Macro) | Recall (Macro) | AUC (Macro) | AUPR (Macro) |
|---|---|---|---|---|---|
| CNN_FP2 | 0.8021 | 0.6960 | 0.7391 | 0.6990 | 0.7566 |
| BERT_smiles | 0.7949 | 0.6436 | 0.7523 | 0.6547 | 0.7196 |
| Attentive_FP | 0.7794 | 0.5791 | 0.7314 | 0.5398 | 0.6507 |
| iADRGSE | 0.8196 | 0.7632 | 0.7461 | 0.7735 | 0.7950 |
| Drug Name | ADR | Evidence |
|---|---|---|
| Pomalidomide | Surgical and medical procedures | clinicaltrials.gov (all accessed on 2 December 2022) |
| Pomalidomide | Social circumstances | PMID: 35085238 |
| Ketorolac | Surgical and medical procedures | cdek.liu.edu |
| Prochlorperazine edisylate | Infections and infestations | baxter.ca |
| Trametinib dimethyl sulfoxide | Ear and labyrinth disorders | clinicaltrials.gov |
| Trifluoperazine | Infections and infestations | healthline.com |
| Desipramine hydrochloride | Respiratory, thoracic and mediastinal disorders | rxlist.com |
| Chlorpromazine hydrochloride | Infections and infestations | Unconfirmed |
| Eletriptan | Congenital, familial and genetic disorders | Unconfirmed |
| 2-[1-methyl-5-(4-methylbenzoyl) 3-pyrrol-2-yl]acetate | Musculoskeletal and connective-tissue disorders | medthority.com |
| Alpelisib | Ear and labyrinth disorders | clinicaltrials.gov |
| Imipramine | Musculoskeletal and connective-tissue disorders | cchr.org.au |
| Delavirdine mesylate | Congenital, familial and genetic disorders | rochecanada.com |
| Delavirdine mesylate | Delavirdine mesylate | Unconfirmed |
| Tiagabine | Congenital, familial and genetic disorders | Unconfirmed |
| Minocycline anion | Endocrine disorders | medthority.com |
| Naratriptan hydrochloride | Hepatobiliary disorders | medthority.com |
| Sertraline | Social circumstances | medthority.com |
| Amlodipine besylate | Injury, poisoning and procedural complications | PMID: 25097362 |
| Palonosetron | Pregnancy, puerperium and perinatal conditions | Unconfirmed |
| Rufinamide | Neoplasms: benign, malignant and unspecified (incl cysts and polyps) | clinicaltrials.gov |
| Carvedilol phosphate | Neoplasms: benign, malignant and unspecified (incl cysts and polyps) | clinicaltrials.gov |
| Maprotiline | Neoplasms: benign, malignant and unspecified (incl cysts and polyps) | Unconfirmed |
| Datasets | Drug | ADRS Labels |
|---|---|---|
| ADRECS | 2248 | 27 |
| OMOP | 171 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Cheng, M.; Yu, L.; Xiao, X. iADRGSE: A Graph-Embedding and Self-Attention Encoding for Identifying Adverse Drug Reaction in the Earlier Phase of Drug Development. Int. J. Mol. Sci. 2022, 23, 16216. https://doi.org/10.3390/ijms232416216
Cheng X, Cheng M, Yu L, Xiao X. iADRGSE: A Graph-Embedding and Self-Attention Encoding for Identifying Adverse Drug Reaction in the Earlier Phase of Drug Development. International Journal of Molecular Sciences. 2022; 23(24):16216. https://doi.org/10.3390/ijms232416216
Chicago/Turabian StyleCheng, Xiang, Meiling Cheng, Liyi Yu, and Xuan Xiao. 2022. "iADRGSE: A Graph-Embedding and Self-Attention Encoding for Identifying Adverse Drug Reaction in the Earlier Phase of Drug Development" International Journal of Molecular Sciences 23, no. 24: 16216. https://doi.org/10.3390/ijms232416216
APA StyleCheng, X., Cheng, M., Yu, L., & Xiao, X. (2022). iADRGSE: A Graph-Embedding and Self-Attention Encoding for Identifying Adverse Drug Reaction in the Earlier Phase of Drug Development. International Journal of Molecular Sciences, 23(24), 16216. https://doi.org/10.3390/ijms232416216






