A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan
Abstract
1. Introduction
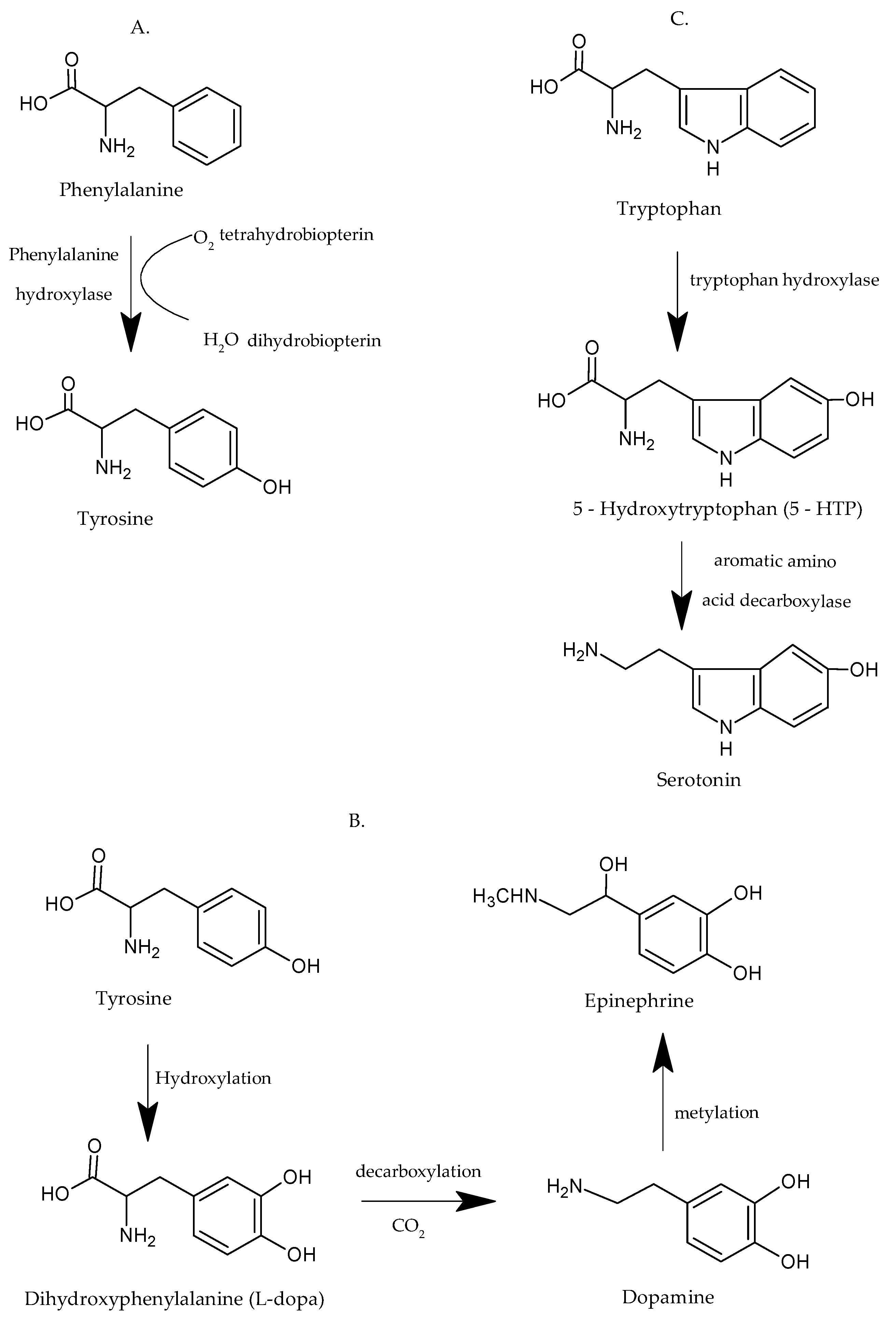
2. Phe-Tyr-Trypt. Properties and Importance for the Human Body
3. CPs and MIPs Used to Determine Phe, Tyr, and Trypt
4. Sensors and Biosensors Based on CPs and MIPs to Quantitatively Determine AAs (Phe, Tyr, and Trypt)
4.1. General Methods Used to Determine AAs
4.2. CPs and MIPs Involved in Developing Electrochemical Sensors to Detect AAs: Phe, Tyr, and Trypt
4.3. CPs and MIPs Involved in Developing Electrochemical Biosensors to Detect AAs: Phe, Tyr, Trypt
5. Conclusions and Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Neurauter, G.; Scholl-Bürgi, S.; Haara, A.; Geisler, S.; Mayersbach, P.; Schennach, H.; Fuchs, D. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin. Biochem. 2013, 46, 1848–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.-F.; Bao, G.-M.; Xia, Y.-F.; Peng, X.-X.; Peng, J.-F.; He, J.-X.; Lin, S.; Zeng, L.; Fan, Q.; Xiao, W.; et al. Recyclable europium functionalized metal-organic fluorescent probe for detection of tryptophan in biological fluids and food products. Anal. Chim. Acta 2021, 1180, 338897. [Google Scholar] [CrossRef] [PubMed]
- Bech-Andersen, S. Determination of Tryptophan with HPLC after Alkaline Hydrolysis in Autoclave using α-methyl-tryptophan as Internal Standard. Acta Agric. Scand. 1991, 41, 305–309. [Google Scholar] [CrossRef]
- Boulet, L.; Faure, P.; Flore, P.; Montérémal, J.; Ducros, V. Simultaneous determination of tryptophan and 8 metabolites in human plasma by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B 2017, 1054, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Whiley, L.; Nye, L.C.; Grant, I.; Andreas, N.J.; Chappell, K.E.; Sarafian, M.H.; Misra, R.; Plumb, R.S.; Lewis, M.R.; Nicholson, J.K.; et al. Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry with Electrospray Ionization Quantification of Tryptophan Metabolites and Markers of Gut Health in Serum and Plasma—Application to Clinical and Epidemiology Cohorts. Anal. Chem. 2019, 91, 5207–5216. [Google Scholar] [CrossRef]
- Chen, S.; Fu, Y.; Bian, X.; Zhao, M.; Zuo, Y.; Ge, Y.; Xiao, Y.; Xiao, J.; Li, N.; Wu, J.-L. Investigation and dynamic profiling of oligopeptides, free amino acids and derivatives during Pu-erh tea fermentation by ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2021, 371, 131176. [Google Scholar] [CrossRef]
- De Silva, V.; Oldham, C.D.; May, S.W. L-Phenylalanine concentration in blood of phenylketonuria patients: A modified enzyme colorimetric assay compared with amino acid analysis, tandem mass spectrometry, and HPLC methods. Clin. Chem. Lab. Med. 2010, 48, 1271–1279. [Google Scholar] [CrossRef]
- Kawana, S.; Nakagawa, K.; Hasegawa, Y.; Yamaguchi, S. Simple and rapid analytical method for detection of amino acids in blood using blood spot on filter paper, fast-GC/MS and isotope dilution technique. J. Chromatogr. B 2010, 878, 3113–3118. [Google Scholar] [CrossRef]
- Orhan, H.; Vermeulen, N.P.; Tump, C.; Zappey, H.; Meerman, J.H. Simultaneous determination of tyrosine, phenylalanine and deoxyguanosine oxidation products by liquid chromatography–tandem mass spectrometry as non-invasive biomarkers for oxidative damage. J. Chromatogr. B 2004, 799, 245–254. [Google Scholar] [CrossRef]
- Rigobello-Masini, M.; MasiniJ, C. Sequential Injection Chromatography for Fluorimetric Determination of Intracellular Amino Acids in Marine Microalgae. In Advanced Structural Safety Studies; Springer: Singapore, 2012; Volume 828, pp. 305–315. [Google Scholar]
- Shokrollah, A.; Refahi, M. Development of Cloud Point Extraction-Scanometry, for the Preconcentration and Determination of Colorless Species: Application for the Determination of Phenylalanine. Quim. Nova 2019, 42, 36–41. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemistry and voltammetric determination of L-tryptophan and L-tyrosine using a glassy carbon electrode modified with a Nafion/TiO2-graphene composite film. Mikrochim. Acta 2011, 173, 241–247. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Alam, A.-M.; Kim, K.M.; Lee, S.H.; Kim, Y.H.; Kim, G.-M.; Dang, T.D. Microfluidic chip based chemiluminescence detection of L-phenylalanine in pharmaceutical and soft drinks. Food Chem. 2012, 135, 57–62. [Google Scholar] [CrossRef]
- Li, S.; Xing, M.; Wang, H.; Zhang, L.; Zhong, Y.; Chen, L. Determination of tryptophan and tyrosine by chemiluminescence based on a luminol–N-bromosuccinimide–ZnS quantum dots system. RSC Adv. 2015, 5, 59286–59291. [Google Scholar] [CrossRef]
- Dinu, A.; Apetrei, C. A review on electrochemical sensors and biosensors used in Phenylalanine Electroanalysis. Sensors 2020, 20, 2496. [Google Scholar] [CrossRef]
- Da Silva, K.P.; Ptak, M.; Pizani, P.; Filho, J.M.; Melo, F.; Freire, P. Raman spectroscopy of l -phenylalanine nitric acid submitted to high pressure. Vib. Spectrosc. 2016, 85, 97–103. [Google Scholar] [CrossRef]
- Sereda, V.; Ralbovsky, N.M.; Vasudev, M.C.; Naik, R.R.; Lednev, I.K. Polarized raman spectroscopy for determining the orientation of di-d-phenylalanine molecules in a nanotube. J. Raman Spectrosc. 2016, 47, 1056–1062. [Google Scholar] [CrossRef]
- Li, Q.Q.; Duan, J.; Wu, L.J.; Huang, Y.; Tang, G.; Min, S.G. Sucrose as chiral selector for determining enantiomeric composition of phenylalanine by UV–vis spectroscopy and chemometrics. Chin. Chem. Lett. 2012, 23, 1055–1058. [Google Scholar] [CrossRef]
- Dailey, C.A.; Garnier, N.; Rubakhin, S.S.; Sweedler, J.V. Automated method for analysis of tryptophan and tyrosine metabolites using capillary electrophoresis with native fluorescence detection. Anal. Bioanal. Chem. 2013, 405, 2451–2459. [Google Scholar] [CrossRef]
- Hawkins, G.; Zipkin, I.; Marshall, L. Determination of Uric Acid, Tyrosine, Tryptophan, and Protein in whole Human Parotid Saliva by Ultraviolet Absorption Spectrophotometry. J. Dent. Res. 1963, 42, 1015–1022. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, M.-F.; Feng, H.; Cong, X.-X.; Wang, X.-L. Determination of Tryptophan, Glutathione, and Uric Acid in Human Whole Blood Extract by Capillary Electrophoresis with a One-Step Electrochemically Reduced Graphene Oxide Modified Microelectrode. Chromatographia 2016, 79, 911–918. [Google Scholar] [CrossRef]
- Moscetti, I.; Cannistraro, S.; Bizzarri, A.R. Probing direct interaction of oncomiR-21-3p with the tumor suppressor p53 by fluorescence, FRET and atomic force spectroscopy. Arch. Biochem. Biophys. 2019, 671, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hashkavayi, A.B.; Raoof, J.B.; Park, K.S. Sensitive Electrochemical Detection of Tryptophan Using a Hemin/G-Quadruplex Aptasensor. Chemosensors 2020, 8, 100. [Google Scholar] [CrossRef]
- D’Souza, E.S.; Manjunatha, J.G.; Chenthatti, R.; Tigari, G.; Ravishankar, D.K. Rapid Electrochemical Monitoring of Tyrosine by Poly (Riboflavin) Modified Carbon Nanotube Paste Electrode as a Sensitive Sensor and its Applications in Pharmaceutical Samples. Biointerface Res. Appl. Chem. 2021, 11, 14661–14672. [Google Scholar] [CrossRef]
- Zhang, J.-L. Electrochemical Determination of Tyrosine and Nitrite Using CS/CMWNTs/GCE-modified Electrode. Int. J. Electrochem. Sci. 2018, 13, 3527–3534. [Google Scholar] [CrossRef]
- Dinu, A.; Apetrei, C. Voltammetric Determination of Phenylalanine Using Chemically Modified Screen-Printed Based Sensors. Chemosensors 2020, 8, 113. [Google Scholar] [CrossRef]
- Dinu, A.; Apetrei, C. Development of Polypyrrole Modified Screen-Printed Carbon Electrode Based Sensors for Determination of L-Tyrosine in Pharmaceutical Products. Int. J. Mol. Sci. 2021, 22, 7528. [Google Scholar] [CrossRef] [PubMed]
- Dinu, A.; Apetrei, C. Development of a Novel Sensor Based on Polypyrrole Doped with Potassium Hexacyanoferrate (II) for Detection of L-Tryptophan in Pharmaceutics. Inventions 2021, 6, 56. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, C.; Qu, H.; Chen, W.; Ma, A.; Zheng, L. Highly sensitive real-time detection of tyrosine based on organic electrochemical transistors with poly-(diallyldimethylammonium chloride), gold nanoparticles and multi-walled carbon nanotubes. J. Electroanal. Chem. 2017, 799, 321–326. [Google Scholar] [CrossRef]
- Tığ, G.A. Development of electrochemical sensor for detection of ascorbic acid, dopamine, uric acid and l-tryptophan based on Ag nanoparticles and poly(l-arginine)-graphene oxide composite. J. Electroanal. Chem. 2017, 807, 19–28. [Google Scholar] [CrossRef]
- Yıldız, C.; Bayraktepe, D.E.; Yazan, Z. Electrochemical low-level detection of l-tryptophan in human urine samples: Use of pencil graphite leads as electrodes for a fast and cost-effective voltammetric method. Monatsh. Chem. 2020, 151, 871–879. [Google Scholar] [CrossRef]
- Kavitha, C.; Bramhaiah, K.; John, N.S. Low-cost electrochemical detection of l-tyrosine using an rGO–Cu modified pencil graphite electrode and its surface orientation on a Ag electrode using an ex situ spectroelectrochemical method. RSC Adv. 2020, 10, 22871–22880. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical Sensor for Rapid and Sensitive Detection of Tryptophan by a Cu2O Nanoparticles-Coated Reduced Graphene Oxide Nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Hajian, R. Determination of tryptophan and histidine by adsorptive cathodic stripping voltammetry using H-point standard addition method. Anal. Chim. Acta 2006, 580, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Kraatz, H.-B. Electrochemical detection of protein tyrosine kinase-catalysed phosphorylation using gold nanoparticles. Biosens. Bioelectron. 2009, 24, 1484–1489. [Google Scholar] [CrossRef]
- Feng, J.; Deng, P.; Xiao, J.; Li, J.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. New voltammetric method for determination of tyrosine in foodstuffs using an oxygen-functionalized multi-walled carbon nanotubes modified acetylene black paste electrode. J. Food Compos. Anal. 2020, 96, 103708. [Google Scholar] [CrossRef]
- Liu, M.; Lao, J.; Wang, H.; Xu, Z.; Li, J.; Wen, L.; Yin, Z.; Luo, C.; Peng, H. Electrochemical Determination of Tyrosine Using Graphene and Gold Nanoparticle Composite Modified Glassy Carbon Electrode. Russ. J. Electrochem. 2021, 57, 41–50. [Google Scholar] [CrossRef]
- Liguori, C.; Pierantozzi, M.; Spanetta, M.; Sarmati, L.; Cesta, N.; Iannetta, M.; Ora, J.; Mina, G.G.; Puxeddu, E.; Balbi, O.; et al. Depressive and anxiety symptoms in patients with SARS-CoV2 infection. J. Affect. Disord. 2020, 278, 339–340. [Google Scholar] [CrossRef]
- Varmira, K.; Mohammadi, G.; Mahmoudi, M.; Khodarahmi, R.; Rashidi, K.; Hedayati, M.; Goicoechea, H.C.; Jalalvand, A.R. Fabrication of a novel enzymatic electrochemical biosensor for determination of tyrosine in some food samples. Talanta 2018, 183, 1–10. [Google Scholar] [CrossRef]
- Kane-Maguire, L.A.P.; Wallace, G.G. Chiral conducting polymers. Chem. Soc. Rev. 2010, 39, 2545–2576. [Google Scholar] [CrossRef]
- Namazi, H. Polymers in our daily life. BioImpacts 2017, 7, 73–74. [Google Scholar] [CrossRef]
- Wang, J.; Liang, R.; Qin, W. Molecularly imprinted polymer-based potentiometric sensors. TrAC Trends Anal. Chem. 2020, 130, 115980. [Google Scholar] [CrossRef]
- Ermiş, N.; Uzun, L.; Denizli, A. Preparation of molecularly imprinted electrochemical sensor for l-phenylalanine detection and its application. J. Electroanal. Chem. 2017, 807, 244–252. [Google Scholar] [CrossRef]
- Roy, S.; Nagabooshanam, S.; Wadhwa, S.; Chauhan, N.; Mathur, A.; Khan, S.A.; Davis, J. Ultra-sensitive detection of l-tyrosine using molecularly imprinted electrochemical sensor towards diabetic foot ulcer detection. Electrochem. Commun. 2020, 117, 106782. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, Y.M.; Galal, A. Layered-designed composite sensor based on crown ether/Nafion®/polymer/carbon nanotubes for determination of norepinephrine, paracetamol, tyrosine and ascorbic acid in biological fluids. J. Electroanal. Chem. 2018, 828, 11–23. [Google Scholar] [CrossRef]
- Duan, S.; Wang, W.; Yu, C.; Liu, M.; Yu, L. Development of Electrochemical Sensor for Detection of L-Tryptophan Based on Exfoliated Graphene/PEDOT:PSS. Nano 2019, 14, 1950058. [Google Scholar] [CrossRef]
- Chena, B.; Zhanga, Y.; Lina, L.; Chenb, H.; Zhaoa, M. Au nanoparticles @metal organic framework/polythionine loaded with molecularly imprinted polymer sensor: Preparation, characterization, and electrochemical detection of tyrosine. J. Electroanal. Chem. 2020, 863, 114052. [Google Scholar] [CrossRef]
- Rahman, M.; Lopa, N.S.; Kim, K.; Lee, J.-J. Selective detection of l-tyrosine in the presence of ascorbic acid, dopamine, and uric acid at poly(thionine)-modified glassy carbon electrode. J. Electroanal. Chem. 2015, 754, 87–93. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, F.; Zeng, B. A molecularly imprinted copolymer based electrochemical sensor for the highly sensitive detection of L-Tryptophan. Talanta 2019, 206, 120245. [Google Scholar] [CrossRef]
- Nan, A.; Bunge, A.; Turcu, R. Hybride magnetic nanostructure based on amino acids functionalized polypyrrole. AIP Conf. Proc. 2015, 1700, 060007. [Google Scholar]
- Kisa, P.T.; Erkmen, S.E.; Bahceci, H.; Gulten, Z.A.; Aydogan, A.; Pekuz, O.K.K.; Inel, T.Y.; Ozturk, T.; Uysal, S.; Arslan, N. Efficacy of Phenylalanine- and Tyrosine-Restricted Diet in Alkaptonuria Patients on Nitisinone Treatment: Case Series and Review of Literature. Ann. Nutr. Metab. 2021, 78, 48–60. [Google Scholar] [CrossRef]
- Doma, K.; Singh, U.; Boullosa, D.; Connor, J.D. The effect of branched-chain amino acid on muscle damage markers and performance following strenuous exercise: A systematic review and meta-analysis. Appl. Physiol. Nutr. Metab. 2021, 46, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Dong, Q.; Hong, D.; Lu, R. Amino Acid Encoding Methods for Protein Sequences: A Comprehensive Review and Assessment. IEEE/ACM Trans. Comput. Biol. Bioinform. 2019, 17, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Marks, A.D. Marks’ Basic Medical Biochemistry: A Clinical Approach; Lippincott Williams & Wilkins; IUBMB: Philadelphia, PA, USA, 2009; ISBN 0-7817-7022-X. [Google Scholar] [CrossRef]
- Litwack, G. Human Biochemistry; Academic Press: Los Angeles, CA, USA, 2018. [Google Scholar] [CrossRef]
- Sadok, I.; Tyszczuk-Rotko, K.; Mroczka, R.; Staniszewska, M. Simultaneous voltammetric analysis of tryptophan and kynurenine in culture medium from human cancer cells. Talanta 2019, 209, 120574. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Turner, J.; Del Mar, C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst. Rev. 2002, 1, CD003198. [Google Scholar] [CrossRef]
- Hudson, C.; Hudson, S.; MacKenzie, J. Protein-source tryptophan as an efficacious treatment for social anxiety disorder: A pilot studyThis article is one of a selection of papers published in this special issue (part 1 of 2) on the Safety and Efficacy of Natural Health Products. Can. J. Physiol. Pharmacol. 2007, 85, 928–932. [Google Scholar] [CrossRef]
- Schneider-Helmert, D.; Spinweber, C. Evaluation of l-tryptophan for treatment of insomnia: A review. Psychopharmacology 1986, 89, 1–7. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, J.; Wang, C.E.; Hu, W.; Krischek, B. There may be no significant increase of cerebrospinal fluid tyrosine levels in patients with Parkinson’s disease. Eur. J. Neurol. 2020, 28, e15–e16. [Google Scholar] [CrossRef]
- Mette, C.; Zimmermann, M.; Grabemann, M.; Abdel-Hamid, M.; Uekermann, J.; Biskup, C.S.; Wiltfang, J.; Zepf, F.D.; Kis, B. The impact of acute tryptophan depletion on attentional performance in adult patients with ADHD. Acta Psychiatr. Scand. 2013, 128, 124–132. [Google Scholar] [CrossRef]
- Prabhu, P.R.; Hudson, A.O. Identification and Partial Characterization of an L-Tyrosine Aminotransferase (TAT) fromArabidopsis thaliana. Biochem. Res. Int. 2010, 2010, 549572. [Google Scholar] [CrossRef]
- Bross, R.; Ball, R.O.; Clarke, J.T.R.; Pencharz, P.B. Tyrosine requirements in children with classical PKU determined by indicator amino acid oxidation. Am. J. Physiol. Metab. 2000, 278, E195–E201. [Google Scholar] [CrossRef]
- Harding, C.O.; Winn, S.R.; Gibson, K.M.; Arning, E.; Bottiglieri, T.; Grompe, M. Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU). J. Inherit. Metab. Dis. 2014, 37, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Idili, A.; Parolo, C.; Ortega, G.; Plaxco, K.W. Calibration-Free Measurement of Phenylalanine Levels in the Blood Using an Electrochemical Aptamer-Based Sensor Suitable for Point-of-Care Applications. ACS Sensors 2019, 4, 3227–3233. [Google Scholar] [CrossRef] [PubMed]
- Bangaleh, Z.; Sadeghi, H.B.; Ebrahimi, S.A.; Najafizadeh, P. A New Potentiometric Sensor for Determination and Screening Phenylalanine in Blood Serum Based on Molecularly Imprinted Polymer. Iran. J. Pharm. Res. IJPR 2019, 18, 61–71. [Google Scholar] [PubMed]
- Shen, Y.-P.; Niu, F.-X.; Yan, Z.-B.; Fong, L.S.; Huang, Y.-B.; Liu, J.-Z. Recent Advances in Metabolically Engineered Microorganisms for the Production of Aromatic Chemicals Derived From Aromatic Amino Acids. Front. Bioeng. Biotechnol. 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.; Sharon, I.; Béjà, O.; Kuhn, J.C. The tryptophan pathway genes of the Sargasso Sea metagenome: New operon structures and the prevalence of non-operon organization. Genome Biol. 2008, 9, R20. [Google Scholar] [CrossRef]
- Storga, D.; Vrecko, K.; Birkmayer, J.; Reibnegger, G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett. 1996, 203, 29–32. [Google Scholar] [CrossRef]
- Shi, D.; Li, Z.; Li, Y.; Jiang, Q. Variables associated with self-reported anxiety and depression symptoms in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitor therapy. Leuk. Lymphoma 2020, 62, 640–648. [Google Scholar] [CrossRef]
- Eroğlu, I.; Eroğlu, B.; Güven, G.S. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 2021, 90, 111308. [Google Scholar] [CrossRef]
- Schopman, S.M.E.; Bosman, R.C.; Muntingh, A.D.T.; van Balkom, A.J.L.M.; Batelaan, N.M. Effects of tryptophan depletion on anxiety, a systematic review. Transl. Psychiatry 2021, 11, 118. [Google Scholar] [CrossRef]
- Negut, C.C.; Staden, R.I.S.-V. Review—Recent Trends in Supramolecular Recognition of Dopamine, Tyrosine, and Tryptophan, Using Electrochemical Sensors. J. Electrochem. Soc. 2021, 168, 067517. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Mood effects of the amino acids tryptophan and tyrosine. Acta Psychiatr. Scand. 2011, 124, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; ElNesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Q. 2020, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- King, J.M.; Muthian, G.; Mackey, V.; Smith, M.; Charlton, C. L-Dihydroxyphenylalanine modulates the steady-state expression of mouse striatal tyrosine hydroxylase, aromatic L-amino acid decarboxylase, dopamine and its metabolites in an MPTP mouse model of Parkinson’s disease. Life Sci. 2011, 89, 638–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moja, E.; Lucini, V.; Benedetti, F.; Lucca, A. Decrease in plasma phenylalanine and tyrosine after phenylalanine-tyrosine free amino acid solutions in man. Life Sci. 1996, 58, 2389–2395. [Google Scholar] [CrossRef]
- Reiner, C.; Nicholson, G.J.; Nagel, U.; Schurig, V. Evaluation of enantioselective gas chromatography for the determination of minute deviations from racemic composition of α-amino acids with emphasis on tyrosine: Accuracy and precision of the method. Chirality 2007, 19, 401–414. [Google Scholar] [CrossRef]
- Biskup, C.S.; Helmbold, K.; Baurmann, D.; Klasen, M.; Gaber, T.J.; Bubenzer-Busch, S.; Königschulte, W.; Fink, G.R.; Zepf, F.D. Resting state default mode network connectivity in children and adolescents with ADHD after acute tryptophan depletion. Acta Psychiatr. Scand. 2016, 134, 161–171. [Google Scholar] [CrossRef]
- Boros, F.A.; Vécsei, L. Tryptophan 2,3-dioxygenase, a novel therapeutic target for Parkinson’s disease. Expert Opin. Ther. Targets 2021, 25, 877–888. [Google Scholar] [CrossRef]
- Zhai, G.; Sun, X.; Randell, E.W.; Liu, M.; Wang, N.; Tolstykh, I.; Rahman, P.; Torner, J.; Lewis, C.E.; Nevitt, M.C.; et al. Phenylalanine Is a Novel Marker for Radiographic Knee Osteoarthritis Progression: The MOST Study. J. Rheumatol. 2020, 48, 123–128. [Google Scholar] [CrossRef]
- Pešić, N.; Dapčević, A.; Ivković, B.; Kachrimanis, K.; Mitrić, M.; Ibrić, S.; Medarević, D. Potential application of low molecular weight excipients for amorphization and dissolution enhancement of carvedilol. Int. J. Pharm. 2021, 608, 121033. [Google Scholar] [CrossRef]
- Moreno, L.S.S.; Junior, H.V.N.; da Silva, A.R.; Nascimento, F.B.S.A.D.; da Silva, C.R.; Neto, J.B.D.A.; Cavalcanti, B.C.; de Moraes, M.O.; Pinazo, A.; Pérez, L. Arginine-phenylalanine and arginine-tryptophan-based surfactants as new biocompatible antifungal agents and their synergistic effect with Amphotericin B against fluconazole-resistant Candida strains. Colloids Surf. B Biointerfaces 2021, 207, 112017. [Google Scholar] [CrossRef]
- Nosrati, H.; Hamzehei, H.; Afroogh, S.; Ashabi, S.F.; Attari, E.; Manjili, H.K. Phenyl alanine & Tyrosine Amino acids Coated Magnetic Nanoparticles: Preparation and Toxicity study. Drug Res. 2018, 69, 277–283. [Google Scholar] [CrossRef]
- Demeester, J.; Bracke, M.; Vochten, R.; Lauwers, A. Differential Spectrophotometric Determination of Tyrosine and Tryptophan in Pharmaceutical Amino Acid Solutions. J. Pharm. Sci. 1978, 67, 729–730. [Google Scholar] [CrossRef]
- Unger, N.; Ferraro, A.; Holzgrabe, U. Investigation of tryptophan-related yellowing in parenteral amino acid solution: Development of a stability-indicating method and assessment of degradation products in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2020, 177, 112839. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A. Cerebrospinal and blood levels of amino acids as potential biomarkers for Parkinson’s disease: Review and meta-analysis. Eur. J. Neurol. 2020, 27, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Lante, A.; Mosca, G. Essential amino acids: Master regulators of nutrition and environmental footprint? Sci. Rep. 2016, 6, 26074. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, F.; Tol, A.J.C.; Gremmels, H.; Wever, K.E.; Paauw, N.D.; Joles, J.A.; Van Der Beek, E.M.; Lely, A.T. Prenatal Amino Acid Supplementation to Improve Fetal Growth: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2535. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef] [PubMed]
- Saldívar-Guerra, E.; Vivaldo-Lima, E. Polymers and polymer types. In Handbook of Polymer Synthesis, Characterization and Processing; Saldívar-Guerra, E., Vivaldo-Lima, E., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; Chapter 1. [Google Scholar]
- Wadhwa, R.; Lagenaur, C.F.; Cui, X.T. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control. Release 2006, 110, 531–541. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Zuliani, C.; Curto, V.; Matzeu, G.; Fraser, K.; Diamond, D. Properties and Customization of Sensor Materials for Biomedical Ap-plications. In Comprehensive Materials Processing; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 221–243. [Google Scholar]
- Yi, N.; Abidian, M.R. Conducting Polymers and Their Biomedical Applications. In Biosynthetic Polymers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 243–276. ISBN 978-1-78242-105-4. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Zaabal, M.; Bakirhan, N.K.; Doulache, M.; Kaddour, S.; Saidat, B.; Ozkan, S.A. A New Approach on Sensitive Assay of Adefovir in Pharmaceutical and Biological Fluid Samples Using Polypyrrole Modified Glassy Carbon Electrode. Sensors Actuators B Chem. 2020, 323, 128657. [Google Scholar] [CrossRef]
- Vernitskaya, T.V.; Efimov, O. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Geană, E.-I.; Artem, V.; Apetrei, C. Discrimination and classification of wines based on polypyrrole modified screen-printed carbon electrodes coupled with multivariate data analysis. J. Food Compos. Anal. 2020, 96, 103704. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Application of voltammetric e-tongue for the detection of ammonia and putrescine in beef products. Sensors Actuators B Chem. 2016, 234, 371–379. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Zhang, Z.-H.; Zhang, H.-B.; Luo, L.-J.; Yao, S.-Z. Electrochemical determination of l-phenylalanine at polyaniline modified carbon electrode based on β-cyclodextrin incorporated carbon nanotube composite material and imprinted sol–gel film. Talanta 2011, 84, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Beskan, U. An amperometric biosensor for glucose detection from glucose oxidase immobilized in polyaniline–polyvinylsulfonate–potassium ferricyanide film. Artif. Cells, Nanomedicine, Biotechnol. 2013, 42, 284–288. [Google Scholar] [CrossRef]
- Siddiq, A.; Ansari, M.O.; Mohammad, A.; Mohammad, F.; El-Desoky, G.E. Synergistic Effect of Polyaniline Modified Silica Gel for Highly Efficient Separation of Non Resolvable Amino Acids. Int. J. Polym. Mater. 2013, 63, 277–281. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Li, H.; Shi, Q.; Wang, K. Chiral voltammetric sensor for tryptophan enantiomers by using a self-assembled multiwalled carbon nanotubes/polyaniline/sodium alginate composite. Chirality 2021, 33, 248–260. [Google Scholar] [CrossRef]
- Niu, J.-L.; Chai, K.-K.; Zeng, M.-X.; Wang, T.-T.; Zhang, C.-Y.; Chen, S.; Xu, J.-K.; Duan, X.-M. Boc-phenylalanine Grafted Poly(3,4-propylenedioxythiophene) Film for Electrochemically Chiral Recognition of 3,4-Dihydroxyphenylalanine Enantiomers. Chin. J. Polym. Sci. 2019, 37, 451–461. [Google Scholar] [CrossRef]
- Dong, L.-Q.; Hu, D.-F.; Duan, X.-M.; Wang, Z.-P.; Zhang, K.-X.; Zhu, X.-F.; Sun, H.; Zhang, Y.-S.; Xu, J.-K. Synthesis and characterization of D-/L-methionine grafted PEDOTs for selective recognition of 3,4-dihydroxyphenylalanine enantiomers. Chin. J. Polym. Sci. 2016, 34, 563–577. [Google Scholar] [CrossRef]
- Hu, D.; Lu, B.; Duan, X.; Xu, J.; Zhang, L.; Zhang, K.; Zhang, S.; Zhen, S. Synthesis of novel chiral l-leucine grafted PEDOT derivatives with excellent electrochromic performances. RSC Adv. 2014, 4, 35597–35608. [Google Scholar] [CrossRef]
- Moral-Vico, J.; Carretero, N.; Pérez, E.; Suñol, C.; Lichtenstein, M.; Casañ-Pastor, N. Dynamic electrodeposition of aminoacid-polypyrrole on aminoacid-PEDOT substrates: Conducting polymer bilayers as electrodes in neural systems. Electrochim. Acta 2013, 111, 250–260. [Google Scholar] [CrossRef]
- GunaVathana, S.D.; Thivya, P.; Wilson, J.; Peter, A.C. Sensitive voltammetric sensor based on silver dendrites decorated polythiophene nanocomposite: Selective determination of L-Tryptophan. J. Mol. Struct. 2020, 1205, 127649. [Google Scholar] [CrossRef]
- GunaVathana, S.D.; Wilson, J.; Prashanthi, R.; Peter, A.C. CuO nanoflakes anchored polythiophene nanocomposite: Voltammetric detection of L-Tryptophan. Inorg. Chem. Commun. 2020, 124, 108398. [Google Scholar] [CrossRef]
- Mishra, A.K.; Agrawal, N.R.; Das, I. Synthesis of water dispersible dendritic amino acid modified polythiophenes as highly effective adsorbent for removal of methylene blue. J. Environ. Chem. Eng. 2017, 5, 4923–4936. [Google Scholar] [CrossRef]
- Guler, E.; Akbulut, H.; Bozokalfa, G.; Demir, B.; Eyrilmez, G.O.; Yavuz, M.; Demirkol, D.O.; Coskunol, H.; Endo, T.; Yamada, S.; et al. Bioapplications of Polythiophene-g-Polyphenylalanine-Covered Surfaces. Macromol. Chem. Phys. 2015, 216, 1868–1878. [Google Scholar] [CrossRef]
- Yin, L.; Duan, H.; Chen, T.; Qi, D.; Deng, J. Amino-acid-substituted polyacetylene-based chiral core–shell microspheres: Helix structure induction and application for chiral resolution and adsorption. Polym. Chem. 2021, 12, 6404–6416. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, W.; Ma, X.; Chen, L.; Liu, L.; Zhang, C. Synthesis of novel hyper-cross-linked chiral porous polymers and their applications in enantioselective adsorption of amino acids. Microporous Mesoporous Mater. 2019, 294, 109892. [Google Scholar] [CrossRef]
- Krupp, A.; Reggelin, M. Phenylalanine-based polyarylacetylenes as enantiomer-differentiating alignment media. Magn. Reson. Chem. 2012, 50, S45–S52. [Google Scholar] [CrossRef]
- Green, R.; Goding, J. Biosynthetic conductive polymer composites for tissue-engineering biomedical devices. In Biosynthetic Polymers for Medical Applications; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 277–298. [Google Scholar]
- Karoyo, A.H.; Wilson, L.D. Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review. Nanomaterials 2015, 5, 981–1003. [Google Scholar] [CrossRef] [PubMed]
- Lamaoui, A.; García-Guzmán, J.J.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Synthesis techniques of molecularly imprinted polymer composites. In Molecularly Imprinted Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–91. [Google Scholar]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for solid-phase microextraction. J. Sep. Sci. 2009, 32, 3278–3284. [Google Scholar] [CrossRef] [PubMed]
- Derz, W.; Fleischmann, M.; Elsinghorst, P.W. Guiding Molecularly Imprinted Polymer Design by Pharmacophore Modeling. Molecules 2021, 26, 5101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Masai, H.; Huang, X.; Tsuda, S.; Terao, J.; Yang, J.; Guo, X. A single-molecule electrical approach for amino acid detection and chirality recognition. Sci. Adv. 2021, 7, eabe4365. [Google Scholar] [CrossRef] [PubMed]
- Pettiwala, A.M.; Singh, P.K. Optical Sensors for Detection of Amino Acids. Curr. Med. Chem. 2018, 25, 2272–2290. [Google Scholar] [CrossRef]
- Matthews, M.E.; Atkinson, I.; Presswala, L.; Najjar, O.; Gerhardstein, N.; Wei, R.; Rye, E.; Riga, A.T. Dielectric classification of D-and L-amino acids by thermal and analytical methods. J. Therm. Anal. Calorim. 2008, 93, 281–287. [Google Scholar] [CrossRef]
- Xu, W.; Zhong, C.; Zou, C.; Wang, B.; Zhang, N. Analytical methods for amino acid determination in organisms. Amino Acids 2020, 52, 1071–1088. [Google Scholar] [CrossRef]
- Luo, Y.; Matejic, T.; Ng, C.-K.; Nunnally, B.; Porter, T.; Raso, S.; Rouse, J.; Shang, T.; Steckert, J. Characterization and Analysis of Biophar-Maceutical Proteins; Elsevier BV: Amsterdam, The Netherlands, 2011; Volume 10, pp. 283–359. [Google Scholar]
- Pundir, C.S.; Lata, S.; Narwal, V. Biosensors for determination of D and L-amino acids: A review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Simões, F.R.; Xavier, M.G. Electrochemical Sensors. In Nanoscience and its Applications; William Andrew: Sao Paulo, Brazil, 2017; pp. 155–178. ISBN 978-0-323-49780-0. [Google Scholar] [CrossRef]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef]
- Chianella, I.; Piletsky, S.; Tothill, I.; Chen, B.; Turner, A. MIP-based solid phase extraction cartridges combined with MIP-based sensors for the detection of microcystin-LR. Biosens. Bioelectron. 2003, 18, 119–127. [Google Scholar] [CrossRef]
- Madhura, T.R.; Devi, K.S.; Ramaraj, R. Introduction to electrochemical sensors for the detection of toxic chemicals. In Metal Oxides in Nanocomposite-Based Electrochemical Sensors for Toxic Chemicals; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 1–18. [Google Scholar]
- Alışık, F.; Burç, M.; Duran, S.T.; Güngör, Ö.; Cengiz, M.A.; Köytepe, S. Development of Gum-Arabic-based polyurethane membrane-modified electrodes as voltammetric sensor for the detection of phenylalanine. Polym. Bull. 2021, 78, 4699–4719. [Google Scholar] [CrossRef]
- Alışık, F.; Burç, M.; Köytepe, S.; Duran, S.T. Preparation of Molecularly Imprinted Electrochemical L-Phenylalanine Sensor with p-Toluene Sulfonic Acid Modified Pt Electrode. J. Electrochem. Soc. 2020, 167, 167508. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Habanová, N.; Řezanka, M.; Broncová, G.; Fitl, P.; Vrňata, M.; Matějka, P. Molecular Recognition of Phenylalanine Enantiomers onto a Solid Surface Modified with Electropolymerized Pyrrole-β-Cyclodextrin Conjugate. Electroanalysis 2019, 32, 767–774. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Ishii, N. Improved gate effect enantioselectivity of phenylalanine-imprinted polymers in water by blending crosslinkers. Anal. Chim. Acta 2015, 862, 77–85. [Google Scholar] [CrossRef]
- Ramya, R.; Muthukumaran, P.; Wilson, J. Electron beam-irradiated polypyrrole decorated with Bovine serum albumin pores: Simultaneous determination of epinephrine and L-tyrosine. Biosens. Bioelectron. 2018, 108, 53–61. [Google Scholar] [CrossRef]
- Saumya, V.; Prathish, K.P.; Rao, T.P. In situ copper oxide modified molecularly imprinted polypyrrole film based voltammetric sensor for selective recognition of tyrosine. Talanta 2011, 85, 1056–1062. [Google Scholar] [CrossRef]
- Dhananjayan, N.; Jeyaraj, W.; Karuppasamy, G. Interactive Studies on Synthetic Nanopolymer decorated with Edible Biopolymer and its Selective Electrochemical determination of L-Tyrosine. Sci. Rep. 2019, 9, 13287. [Google Scholar] [CrossRef]
- Ermis, N. Preparation of Molecularly Imprınted Polypyrrole Modified Gold Electrode for Determination of Tyrosine in Biological Samples. Int. J. Electrochem. Sci. 2018, 13, 2286–2298. [Google Scholar] [CrossRef]
- Wang, C.; Zou, X.; Zhao, X.; Wang, Q.; Tan, J.; Yuan, R. Cu-nanoparticles incorporated overoxidized-poly(3-amino-5-mercapto-1,2,4-triazole) film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. J. Electroanal. Chem. 2015, 741, 36–41. [Google Scholar] [CrossRef]
- Gong, L.; Li, S.; Yin, Z.; Li, K.; Gu, J.; Wu, D.; Kong, Y. Enantioselective recognition of tryptophan isomers with molecularly imprinted overoxidized polypyrrole/poly(p-aminobenzene sulfonic acid) modified electrode. Chirality 2021, 33, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, K.; Bonyadi, S. An electrochemical sensor based on reduced graphene oxide decorated with polypyrrole nanofibers and zinc oxide–copper oxide p–n junction heterostructures for the simultaneous voltammetric determination of ascorbic acid, dopamine, paracetamol, and tryptophan. New J. Chem. 2018, 42, 8512–8523. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2019, 131, 107393. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Tang, H.-P.; Li, X.-D.; Hua, X. Investigations on the Mechanical Properties of Conducting Polymer Coating-Substrate Structures and Their Influencing Factors. Int. J. Mol. Sci. 2009, 10, 5257–5284. [Google Scholar] [CrossRef]
- Liu, C. Recent Developments in Polymer MEMS. Adv. Mater. 2007, 19, 3783–3790. [Google Scholar] [CrossRef]
- Dashtian, K.; Hajati, S.; Ghaedi, M. L-phenylalanine-imprinted polydopamine-coated CdS/CdSe n-n type II heterojunction as an ultrasensitive photoelectrochemical biosensor for the PKU monitoring. Biosens. Bioelectron. 2020, 165, 112346. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G.; Ravishankar, D.K.; Fattepur, S.; Siddaraju, G.; Nanjundaswamy, L. Validated Electrochemical Method for Simultaneous Resolution of Tyrosine, Uric Acid, and Ascorbic Acid at Polymer Modified Nano-Composite Paste Electrode. Surf. Eng. Appl. Electrochem. 2020, 56, 415–426. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, C.; Zheng, L.; He, J.-B.; Ding, Y.; Lu, H.; Zhang, G.; Cho, K.; Qiu, L. Chirality detection of amino acid enantiomers by organic electrochemical transistor. Biosens. Bioelectron. 2018, 105, 121–128. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, I. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical —Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Muguruma, H. Biosensors: Enzyme Immobilization Chemistry. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 64–71. [Google Scholar] [CrossRef]
- Narayan, R.J. Medical Biosensors for Point of Care (POC) Applications; Narayan, R., Ed.; Woodhead Publishing: Tokyo, Japan, 2016; ISBN 0-08-100078-2. [Google Scholar] [CrossRef]
- Cooper, J.C.; Schuber, F. A biosensor for L-amino acids using polytyramine for enzyme immobilization. Electroanalysis 1994, 6, 957–961. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Shojaei, M.; Farbodi, M. Application of a quartz crystal nanobalance to the molecularly imprinted recognition of phenylalanine in solution. Biotechnol. Bioprocess Eng. 2008, 13, 592–597. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, M.; Lu, H.; Fan, X.; Wang, H.; Zhang, Y.; Wang, H. Novel potential and current type chiral amino acids biosensor based on L/D-handed double helix carbon nanotubes@polypyrrole@Au nanoparticles@L/D-cysteine. Sensors Actuators B Chem. 2019, 296, 126667. [Google Scholar] [CrossRef]
- Ning, G.; Wang, H.; Fu, M.; Liu, J.; Sun, Y.; Lu, H.; Fan, X.; Zhang, Y.; Wang, H. Dual Signals Electrochemical Biosensor for Point-of-care Testing of Amino Acids Enantiomers. Electroanalysis 2021. [Google Scholar] [CrossRef]
- Prabakaran, K.; Jandas, P.; Luo, J.; Fu, C.; Wei, Q. Molecularly imprinted poly(methacrylic acid) based QCM biosensor for selective determination of L-tryptophan. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 611, 125859. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A Simple and Efficient Molecularly Imprinted Electro-chemical Sensor for the Selective Determination of Tryptophan. Biomolecules 2019, 9, 294. [Google Scholar] [CrossRef]
- Park, J.K.; Khan, H.; Lee, J.W. Preparation of phenylalanine imprinted polymer by the sol–gel transition method. Enzym. Microb. Technol. 2004, 35, 688–693. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Gerard, M. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Wang, X.; Uchiyam, S. Polymers for Biosensors Construction. In State of the Art in Biosensors—General Aspects; Rinken, T., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1004-0. [Google Scholar]













| Amino Acid | Chemical Structure | Chemical Formula | Chemical and Physical Properties | References |
|---|---|---|---|---|
| Phenylalanine |  | C9H11NO2 | Aromatic Non-polar Mw = 165.19 g/mol Hydrophobic substance | [54] |
| Tyrosine |  | C9H11NO3 | Aromatic Non-Polar Mw = 181.19 g/mol Hydrophobic substance | [55] |
| Tryptophan |  | C11H12N22 | Aromatic Non-polar Mw = 204.22 g/mol Hydrophobic substance | [56] |
| Amino Acid | Pharmaceutical Products | Concentration/Capsule | Producer/Country |
|---|---|---|---|
| Phenylalanine | Amino 75 | 75 mg | SOLGAR/USA 2 |
| L-Phenylalanine 500 | 500 mg | SOLARAY/USA | |
| DLPA 1 500 | 500 mg | SOLGAR/USA | |
| Best D-Phenylalanine | 500 mg | DOCTOR’S BEST/USA | |
| Tyrosine | L-Tyrosine 500 | 500 mg | SOLARAY/USA |
| Tiroidin | 90 mg | PARAPHARM/ROMANIA | |
| Cebrium | 4.12 mg | NEUROPHARMA/GERMANY | |
| Thyroid Caps | 100 mg | SOLARAY/USA | |
| Tryptophan | Sleep Optimizer | 150 mg | SOLARAY/USA |
| Cebrium | 0.2 mg | NEUROPHARMA/GERMANY | |
| L-Tryptophan | 500 mg | SOLARAY/USA | |
| Tonico Vita | 18 mg | TERAPIA/ROMANIA | |
| MaxiMag Women | 150 mg | ZDROVIT/ROMANIA |
| Domain of Use | Uses | References | ||
|---|---|---|---|---|
| Phe | Tyr | Trypt | ||
| Chemistry Medicinal | Depression, ADHD 1, Parkinson’s disease, chronic pain, osteoarthritis, rheumatoid arthritis, alcohol withdrawal symptoms, and vitiligo skin disease | Phenylketonuria mental performance, alertness or memory, depression, or ADHD | Premenstrual dysphoric disorder syndrome, sleep problems (insomnia), anxiety, depression, and ADHD | [76,77,78,79,80,81] |
| Pharmacology Pharmacy | Is part of medicinal supplements under various forms: capsules, creams, vials, and syrups. | [26,28,82,83,84,85,86] | ||
| RDA 1 | Egg 100 g | Milk 100 mL | Beef 100 g | Pig 100 g | Chicken 100 g | Sea Bass 100 g | |
|---|---|---|---|---|---|---|---|
| Protein content (g) | 12.1 | 3.3 | 22 | 20.7 | 23.3 | 21.3 | |
| Essential amino acids | |||||||
| 2100 | Lysine | 1001 | 272 | 2002 | 1737 | 2246 | 2021 |
| 700 | Histidine | 322 | 93 | 849 | 647 | 937 | 552 |
| 1050 | Threonine | 674 | 164 | 898 | 919 | 1160 | 967 |
| 1050 | Cysteine + Methionine | 740 | 118 | 871 | 780 | 974 | 897 |
| 1820 | Valine | 896 | 233 | 1063 | 1243 | 1384 | 1044 |
| 1400 | Isoleucine | 741 | 192 | 950 | 1080 | 1153 | 914 |
| 2730 | Leucine | 748 | 355 | 1892 | 1624 | 1955 | 1655 |
| 1750 | Phenylalanine + Tyrosine | 1247 | 318 | 1677 | 1166 | 1776 | 1531 |
| 280 | Tryptophan | 228 | 50 | 246 | 183 | 273 | 249 |
| 12,880 | Total EAAs (mg) | 6597 | 1795 | 10,448 | 9379 | 11,858 | 9830 |
| RDA 1 | Soybeans 100 g | Beans 100 g | Peas 100 g | Wheat 100 g | Maize 100 g | Rice 100 g | Potato 100 g | Cauliflower 100 g | Quinoa 100 g | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prot. content (g) | 38.9 | 10.2 | 5.5 | 11 | 8.7 | 6.7 | 2.1 | 3.2 | 19.6 | |
| Essential amino acids | ||||||||||
| 2100 | Lysine | 3047 | 714 | 348 | 239 | 258 | 257 | 92 | 120 | 1025 |
| 700 | Histidine | 1170 | 303 | 85 | 228 | 251 | 165 | 28 | 37 | 478 |
| 1050 | Threonine | 1843 | 428 | 310 | 310 | 334 | 246 | 59 | 74 | 849 |
| 1050 | Cyst + Meth | 1183 | 238 | 95 | 454 | 307 | 257 | 51 | 63 | 565 |
| 1820 | Valine | 2176 | 616 | 226 | 452 | 472 | 438 | 99 | 104 | 961 |
| 1400 | Isoleucine | 2222 | 556 | 201 | 403 | 350 | 306 | 68 | 73 | 808 |
| 2730 | Leucine | 3689 | 885 | 342 | 741 | 1028 | 590 | 96 | 126 | 1399 |
| 1750 | Phe + Tyr | 3970 | 963 | 345 | 855 | 761 | 588 | 132 | 129 | 1542 |
| 280 | Tryptophan | 618 | 113 | 54 | 116 | 61 | 84 | / | / | 726 |
| 12,880 | Total EAAs (mg) | 19,918 | 4816 | 2006 | 3798 | 3822 | 2931 | 624 | 726 | 8353 |
| Precision | Selectivity | Accuracy | Detection limit | Cost and Duration | |
|---|---|---|---|---|---|
| High | Electrochemical methods based on achieving sensors and biosensors [15,37,49,92] | ||||
| Medium | Instrumental (electrical methods [122], optical methods [123], thermal methods [124], magnetic methods, and radiochemical methods [125] | ||||
| Low | Chemical methods (volumetry, gravimetry, precipitation methods) [126] | ||||
| AA 1 | CPs 2 | MIPs 3 | ||||
|---|---|---|---|---|---|---|
| Electrode Architecture | Detection Technique | LOD 4 (M)/Sensitivity/Linear Range | Electrode Architecture | Detection Technique | LOD (M)/Sensitivity/Linear Range | |
| Phenylalanine | Sensor with Gum Arabic based polyurethane modification [133] | DPV 5 | 48.01 × 10−6/0.002 × 10−6/ 200–1000 × 10−6 | Sensor with p-Toluene Sulfonic Acid Modified Pt Electrode [134] | DPV | 0.59 × 10−6/ 0.003 × 10−6/ 2–2000 × 10−6 |
| PPy-β-CD/GCE (polypyrrole-β-cyclodextrin conjugate)/glassy carbon electrode [135] | CV 6, LSV 7 | D-Phe (138 ± 15) × 10−3 and for L-Phe (6 ± 1) × 10−3/ 0.1–0.75 ×10−3 | MIP/TP3C-Trp (molecularly imprinted polymer/Thiophen-3- carbonyl tryptophan) [43] | SWV 8, CV | 1.0 × 10−9/ 2.7532 × 10−9/ 1.0 × 10−8–1.0 × 10−7 | |
| β-CD–MWNTs/PAN/CE (polyaniline modified carbon electrode based on cyclodextrin incorporated carbon nanotube composite material and imprinted sol–gel film) [102] | CV, DPV | 1.0 × 10−9/56.283 × 10−9/ 5.0 × 10−7–1.0 × 10−4 | MIP-grafted ITO/EDMA/MBAA (electrode grafted with a molecularly imprinted polymer crosslinked via a combination of hydrophobic ethyleneglycol dimethacrylate and hydrophilic methylene bisacrylamide) [136] | CV | 0.5 × 10−6/ 3–5 × 10−3 | |
| Tyrosine | GC/CNT/PEDOT/NF/Crown (glassy carbon/multi-walled carbon nanotubes/poly (3-4-ethylene dioxythiophene/Nafion/Crown) [45] | CV | 0.429 × 10−9/ 963.1 × 10−9/ 0.06–20 × 10−9 | MIP/pTH/Au@ZIF-67 (molecularly imprinted polyaniline/ polythionine/gold nanoparticles@zeolitic imidazolate framework-67 composite) [47] | DPV | 7.9 × 10−10/ 0.0005 × 10−10/ 1 × 10−8–4 × 10−6 |
| EB-Ppy-BSA/GCE (Electron beam irradiated polypyrrole nanospheres embedded over bovine serum albumin) [137] | SWV | 8.8 × 10−9/ 1.04 × 10−9/ 100 × 10−9–800 × 10−6 | In situ copper oxide modified MIPPy (molecularly imprinted polypyrrole) coated GCE (glassy carbon electrode) [138] | LSV | 4.0 × 10−9/ 0.47 × 10−9/ 1 × 10−8–1 × 10−6 and 2 × 10−6–8 × 10−6 | |
| EB-PPy/MGA (Electron Beam-polypyrrole/Modified Gum Acacia) [139] | CV, SWV | 85 × 10−9/ 18.944 × 10−9/ 0.4–600 × 10−6 | MIP-PPy/AuE (molecularly imprinted polymer-polypyrrole/gold electrode) [140] | CV, SWV | 2.5 × 10−9/ 0.6567 × 10−9/ 5.0 × 10−9–2.5 × 10−8 | |
| Tryptophan | CuNPs/p-TAOX/GCE (copper nanoparticles/poly(3-amino-5-mercapto-1,2,4-triazole)/glassy carbon electrode) [141] | DPV, CV | 0.16 × 10−6/ 8.2058 × 10−6/ 4.0–144.0 × 10−6 | MIOPPy/pABSA/GCE (molecularly imprinted overoxidized Polypyrrole (OPPy)/Poly (p-aminobenzene sulfonic acid) modified glassy carbon electrode [142] | CV | 1.2–4 × 10−6 |
| 3DCu(x)O-ZnO NPs/PPy/RGO A three-dimensional porous nanocomposite of reduced graphene oxide decorated with polypyrrole nanofibers and zinc oxide-copper oxide p-n junction heterostructures [143] | DPV, CV | 0.016 × 10−6/ 0.1345 × 10−6/ 0.053–480 × 10−6 | Nafion-MIP-MWCNTs@IL/GCE (Nafion-molecularly imprinted copolymer-ionic liquid (i.e., 1-butyl-3-methylimidazolium hexafluorophosphate) functionalized multi-walled carbon nanotubes/glassy carbon electrode) [49] | DPV, LSV | 6 × 10−9/ 5.09 × 10−9/ 8 × 10−9–26 × 10−6 | |
| PPy/FeCN/SPCE (polypyrrole/potassium hexacyanoferrate (II))/carbon screen-printed electrode) [28] | CV | 1.05 × 10−7/ 0.87268 × 10−7/ 3.3 × 10−7–1.06 × 10−5 | MIP -MWCNT s/GCE (molecularly imprinted polymer-modified modified with multi -walled carbon nanotubes/glassy carbon electrode) [144] | CV | 1.0 × 10−9/35.863 2 × 10−6, 1.114 2 × 10−6, 0.1635 2 × 10−6/ 2.0 × 10−9−0.2 × 10−6, 0.2 × 10−6−10 × 10−6 and 10 × 10−6−100 × 10−6 | |
| AA | CPs | MIPs | ||||
|---|---|---|---|---|---|---|
| Electrode Architecture | Detection Technique | LOD (M)/Sensitivity/Linear Range | Electrode Architecture | Detection Technique | LOD (M)/Sensitivity/Linear Range | |
| Phenylalanine | L-AAOD-polytyramine electrode (L-amino acid oxidase) [154] | CV | 0.07 × 10−6/ 0.07–3 × 10−3 | MIP/acid (poly(AN-co-AA)/QCN electrode (quartz crystal nanobalance electrode imprinted polyacrylonitrile and acrylic) [155] | 45 mgL−1/ 0.5839 Hz/mgL−1/ 50~500 mgL−1 | |
| L-Phe-IPDA-CdS-CdSe-Zn/Ti PEC (L-Phe-imprinted polydopamine-coated Zn/CdS/CdSe/heterojunction) [147] | CV, CA 1 | 0.9 × 10−9/ 0.005–2.5 and 2.5–130 × 10−6 | ||||
| Tyrosine | Polythreonine-modified graphite-carbon nanotube paste electrode [148] | CV, DPV | 2.9 × 10−7/ 9.92 × 10−7 2 × 10–6 to 2.5 × 10–5 and 3 × 10–5 to 1.2 × 10–4 | MIP-OECTs (molecularly imprinted polymer-organic electrochemical transistors) [149] | CV | 30 × 10−9/ 14.5 and 12.5/ 300 × 10−9 to 10 × 10−6 |
| L/D-DHCNT@PPy@AuNPs@L/D-Cys (left-/right-handed double helix carbon nanotubes/Polypyrrole@Au nanoparticles nanocomposites/L/D-cysteine) [156] | DPV | 1.88 × 10−1 L-Tyr and 5.72 × 10−1 D-Tyr/−0.004 | ||||
| D-CNT@PPy@Pt NPs@beta-CD (polypyrrole-coated chiral carbon nanotubes with Pt nanoparticles and beta-cyclodextrin) [157] | CV | 0.107 × 10−9/ 3–30 × 10−6 | ||||
| Tryptophan | PT-Ag/L-Try/GCE (polythiophene with silver dendrites composite/L-Tryptophan/glassy carbon electrode) [110] | CV, SWV | 20 × 10−9/ 200 × 10−9–400 × 10−3 | MIP-QCM biosensor (molecularly imprinted polymer poly(methacrylic acid)-based quartz crystal microbalance) [158] | DPV | 0.73 ng/mL/ 15.2–750 ng/mL |
| D-CNT@PPy@Pt NPs@beta-CD (polypyrrole-coated chiral carbon nanotubes with Pt nanoparticles and beta-cyclodextrin) [157] | CV | 0.133 × 10−9/ 19.6–196 × 10−6 | MIP-OECTs (molecularly imprinted polymer-organic electrochemical transistors) [149] | CV | 2 × 10−9/ 11.6 and 3.5/ 300 × 10−9 to 10 × 10−6 | |
| L/D-DHCNT@PPy@AuNPs@L/D-Cys (left/right-handed double helix carbon nanotubes/Polypyrrole@Au nanoparticles [156] | DPV | 0.012 L-Trp% and 0.14 D-Trp%/ 0.659 and 0.02 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, A.; Apetrei, C. A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan. Int. J. Mol. Sci. 2022, 23, 1218. https://doi.org/10.3390/ijms23031218
Dinu A, Apetrei C. A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan. International Journal of Molecular Sciences. 2022; 23(3):1218. https://doi.org/10.3390/ijms23031218
Chicago/Turabian StyleDinu, Ancuța, and Constantin Apetrei. 2022. "A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan" International Journal of Molecular Sciences 23, no. 3: 1218. https://doi.org/10.3390/ijms23031218
APA StyleDinu, A., & Apetrei, C. (2022). A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan. International Journal of Molecular Sciences, 23(3), 1218. https://doi.org/10.3390/ijms23031218







