Glutaredoxin Interacts with GR and AhpC to Enhance Low-Temperature Tolerance of Antarctic Psychrophile Psychrobacter sp. ANT206
Abstract
:1. Introduction
2. Results and Discussion
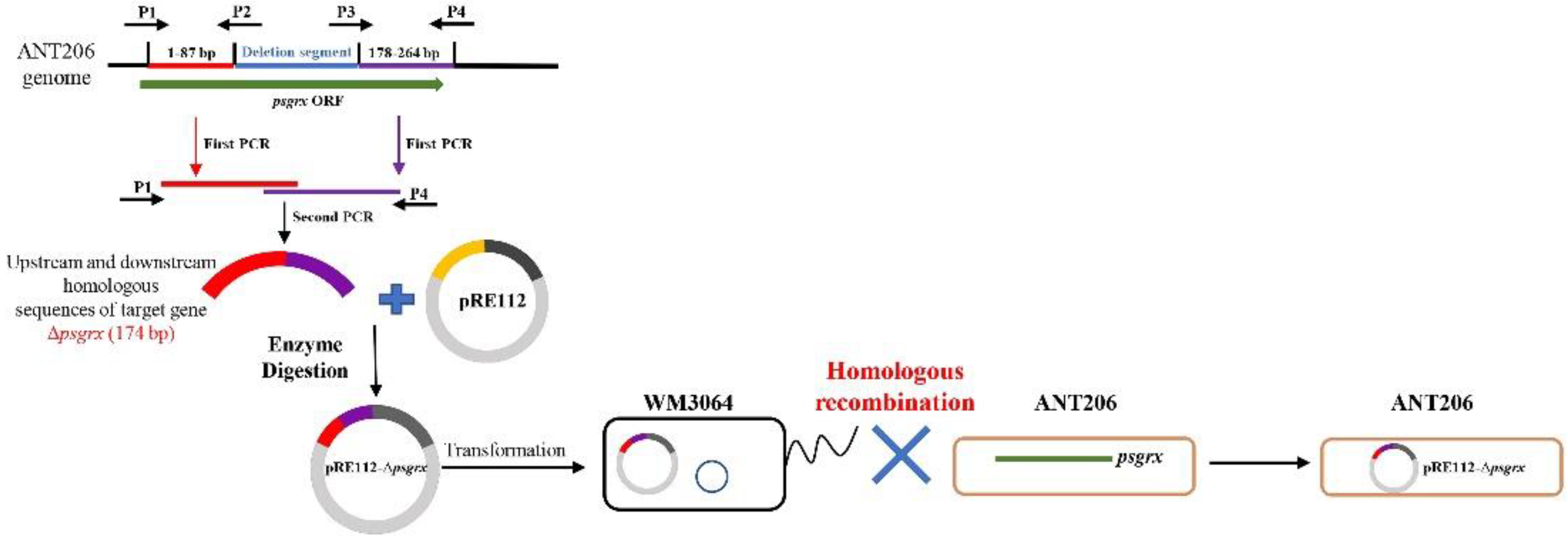
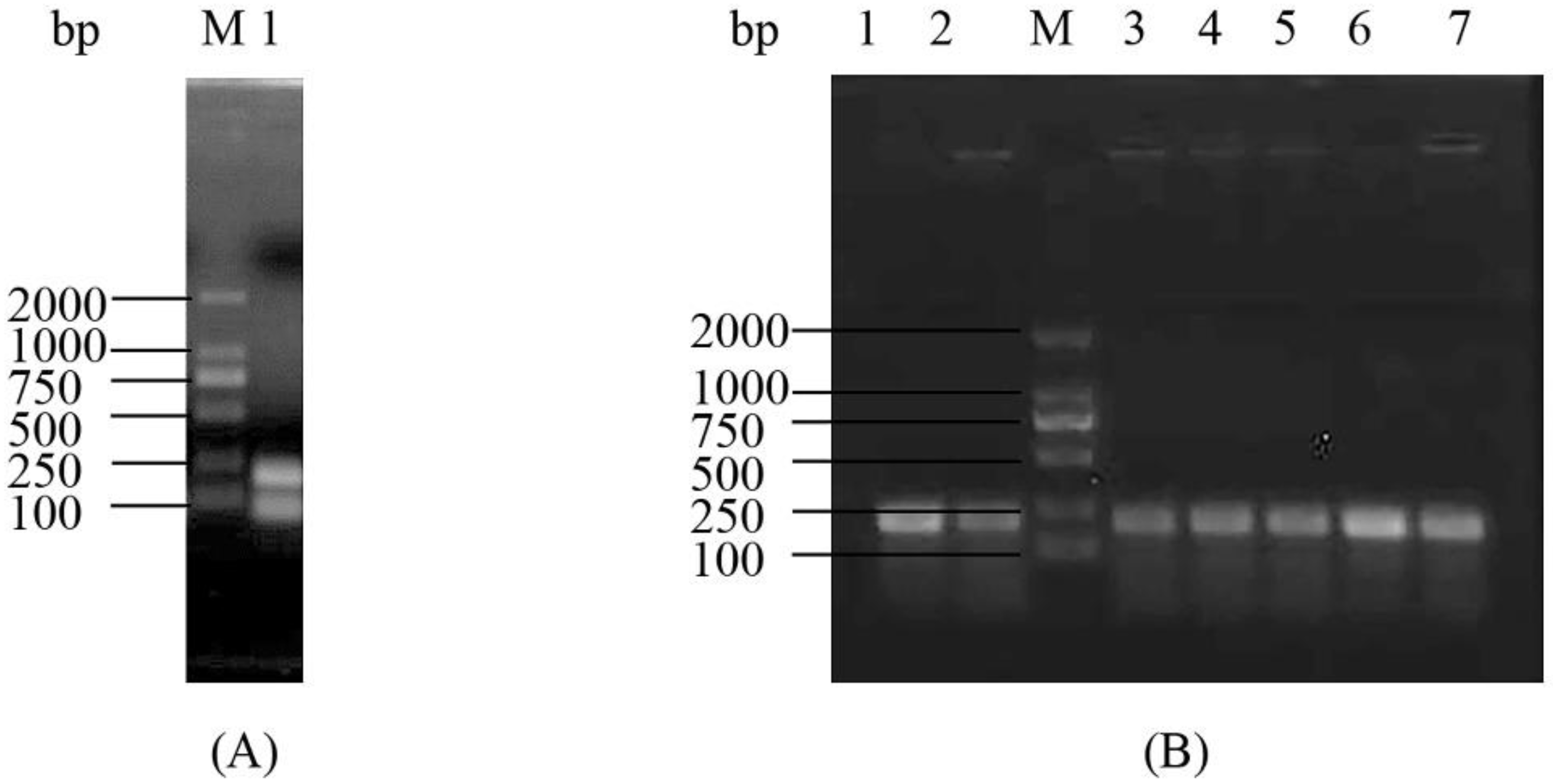
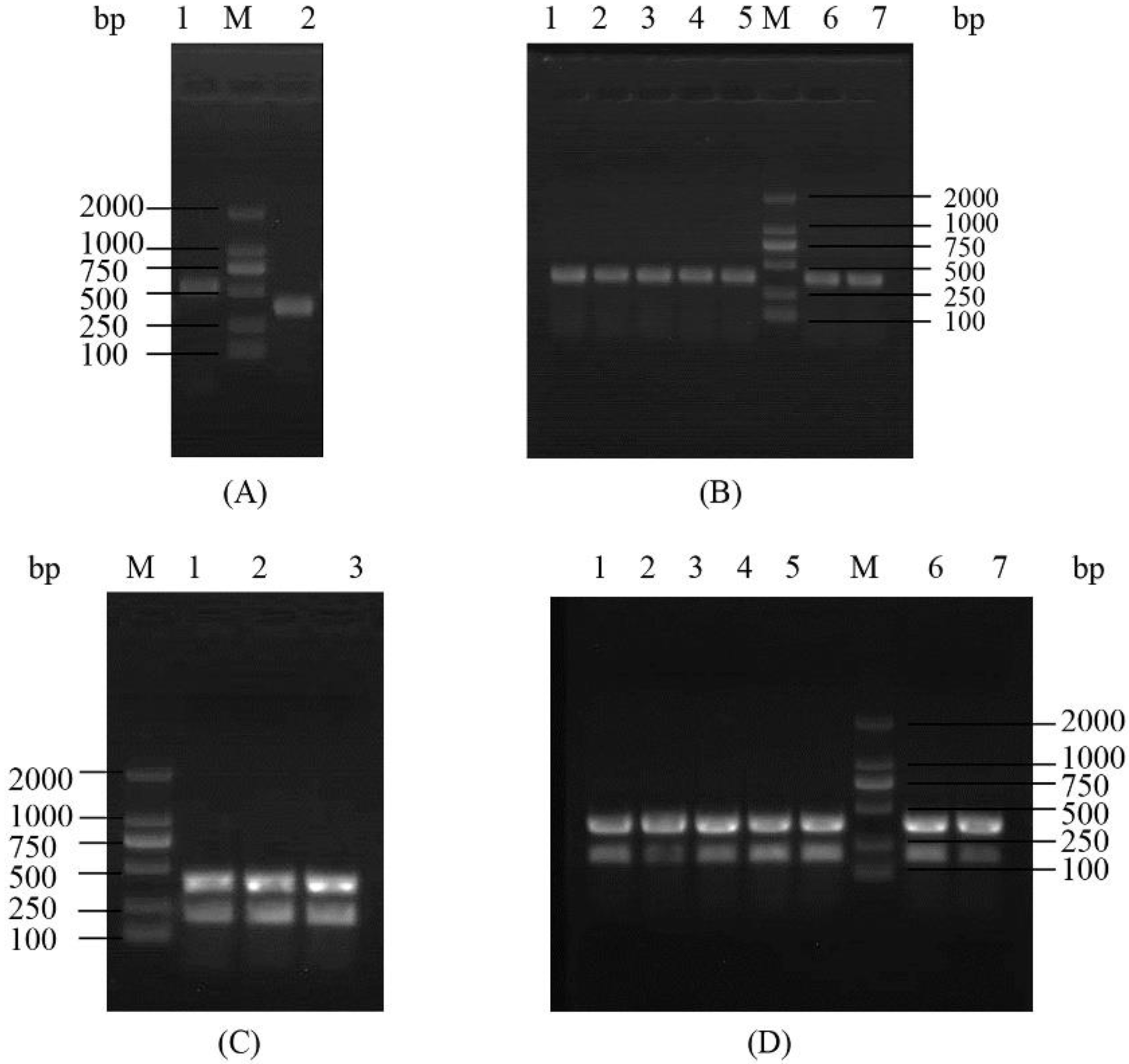
2.1. Construction and Analysis of the Deletion Mutant Δpsgrx
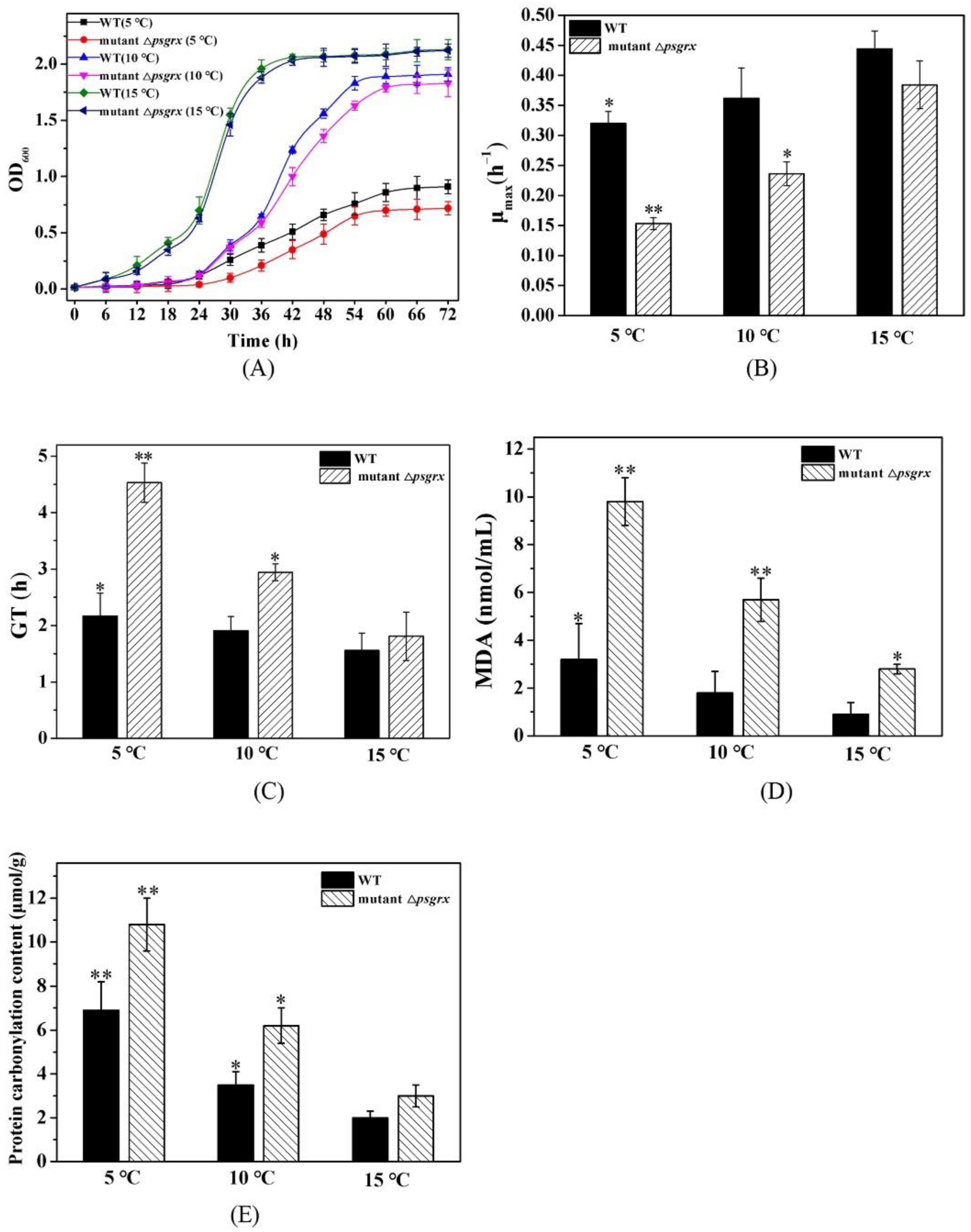
2.2. PsGrx Positively Regulates the Response to Low Temperature
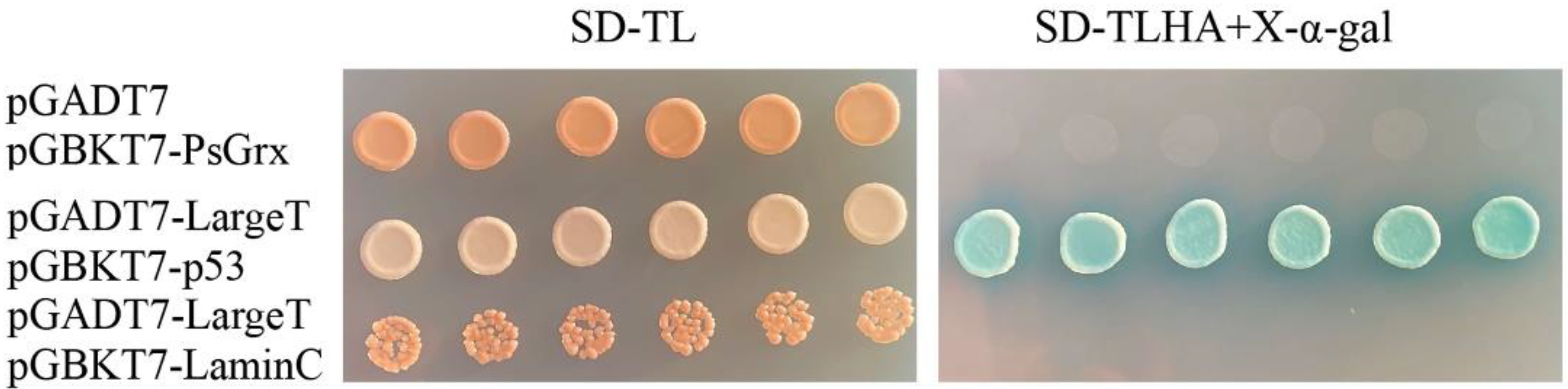
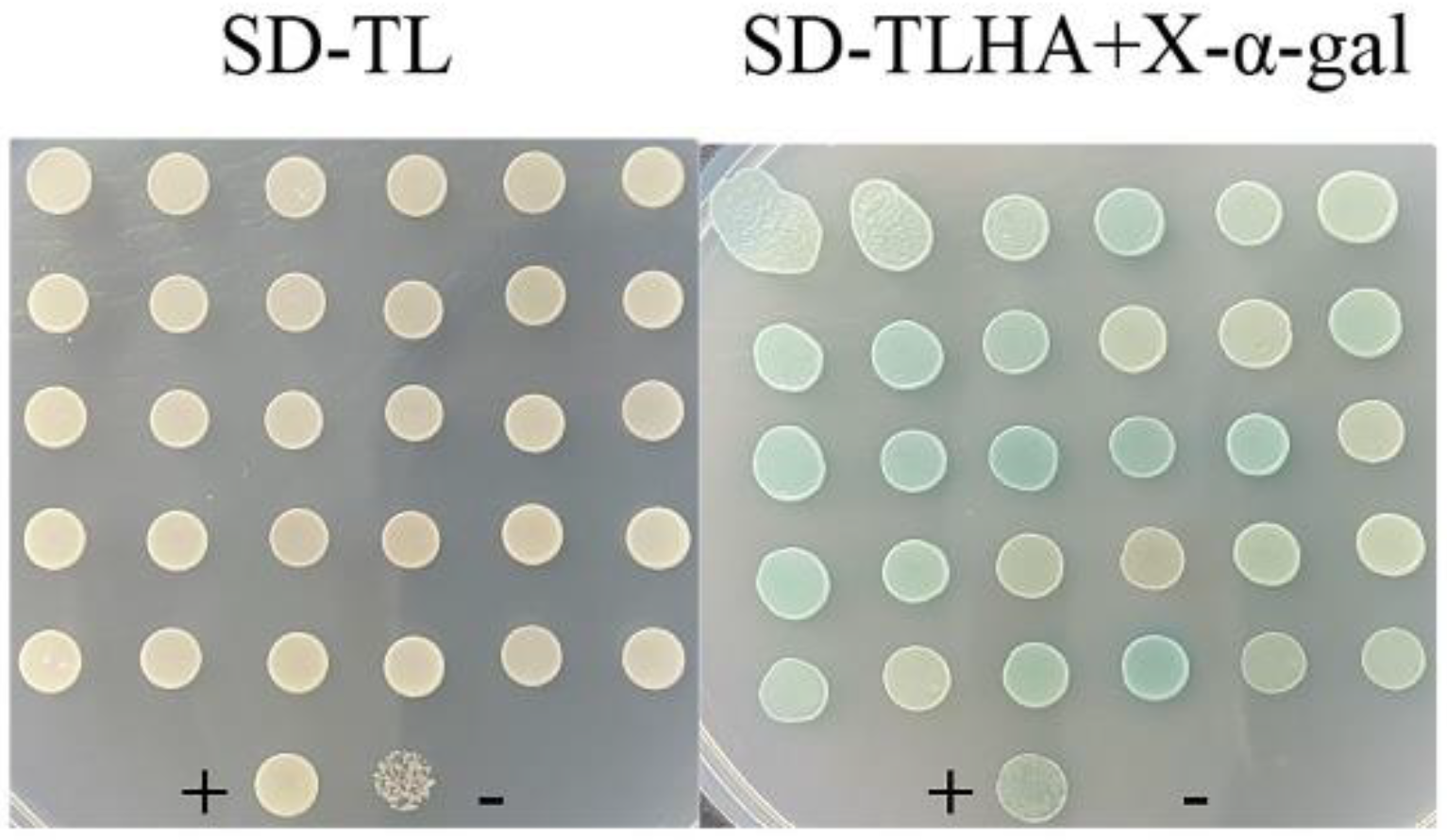
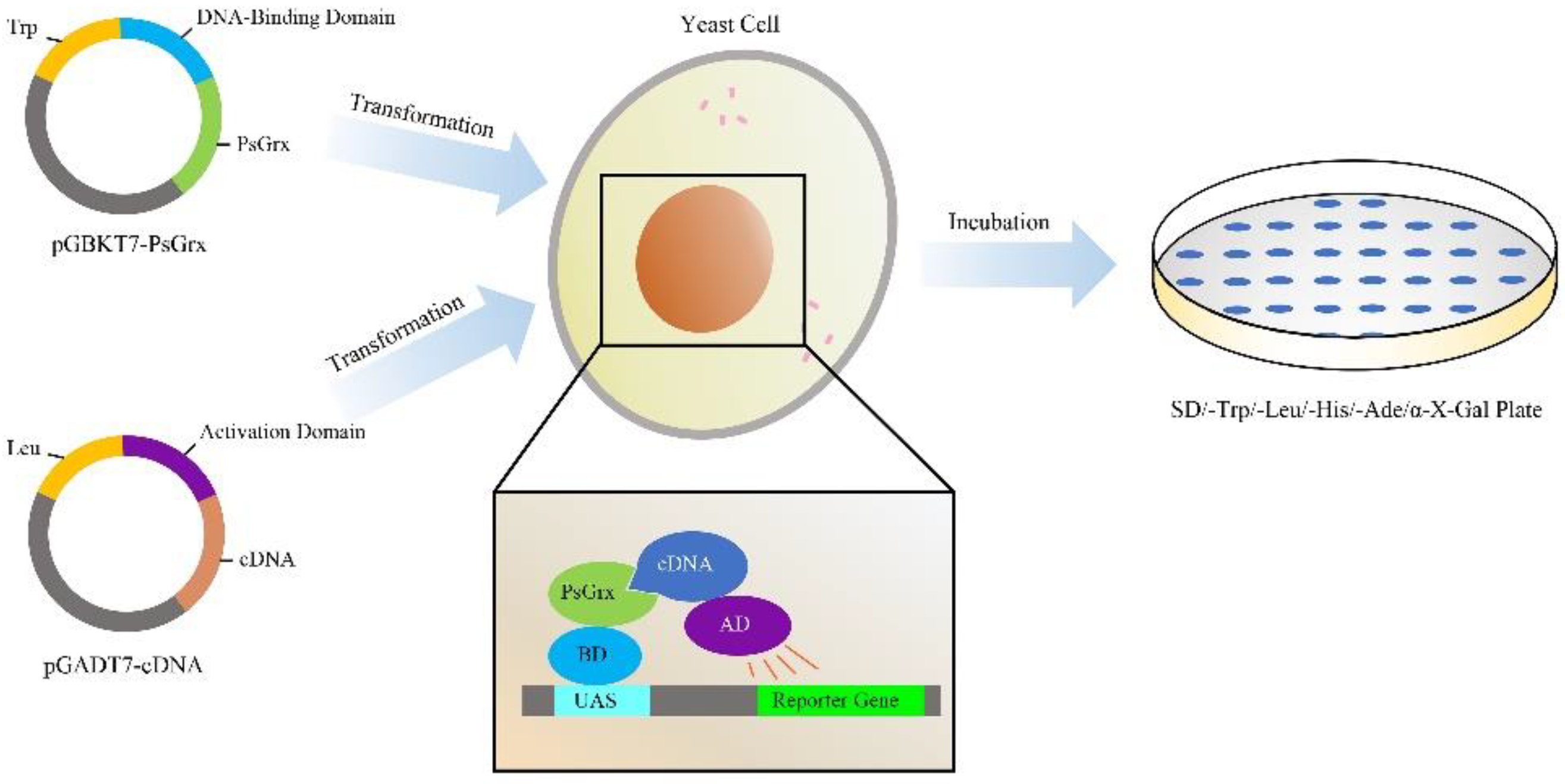
2.3. Screening for Proteins That Interact with PsGrx
2.4. PsGrx Interacts with GR and AhpC
2.5. PsGrx Is Participated in Glutathione Metabolism by Enhancing GR Activity
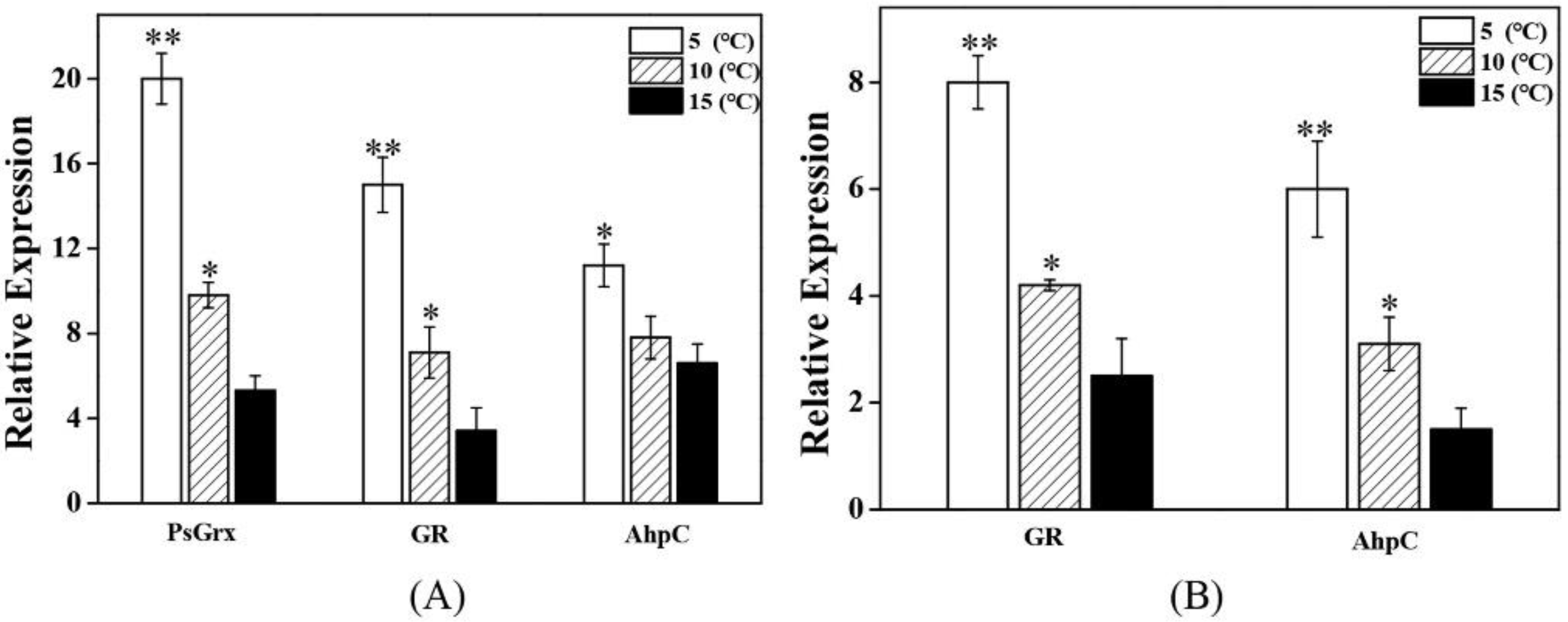
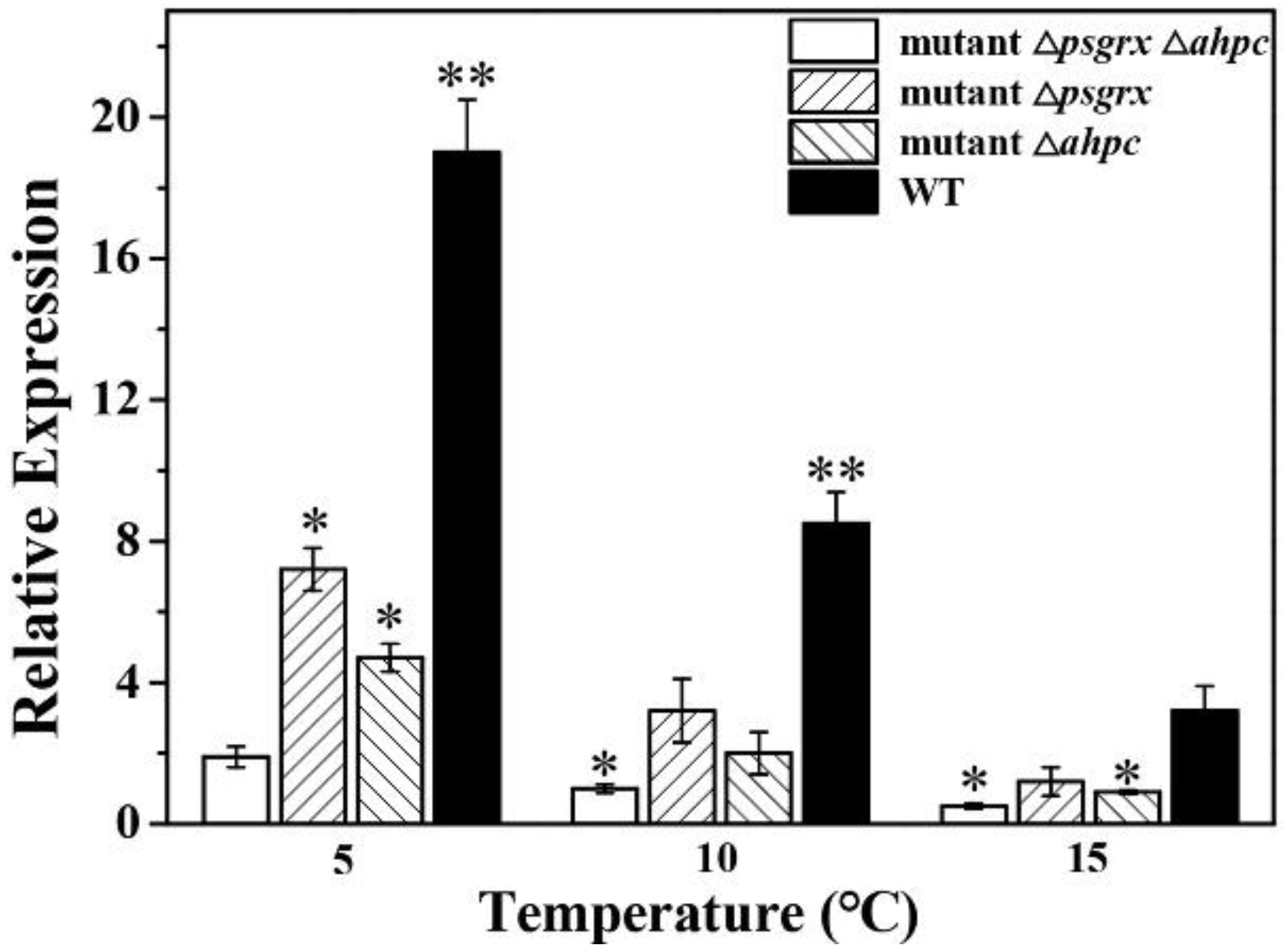
2.6. PsGrx Is Involved in ROS-Elimination Pathway by Regulating AhpC
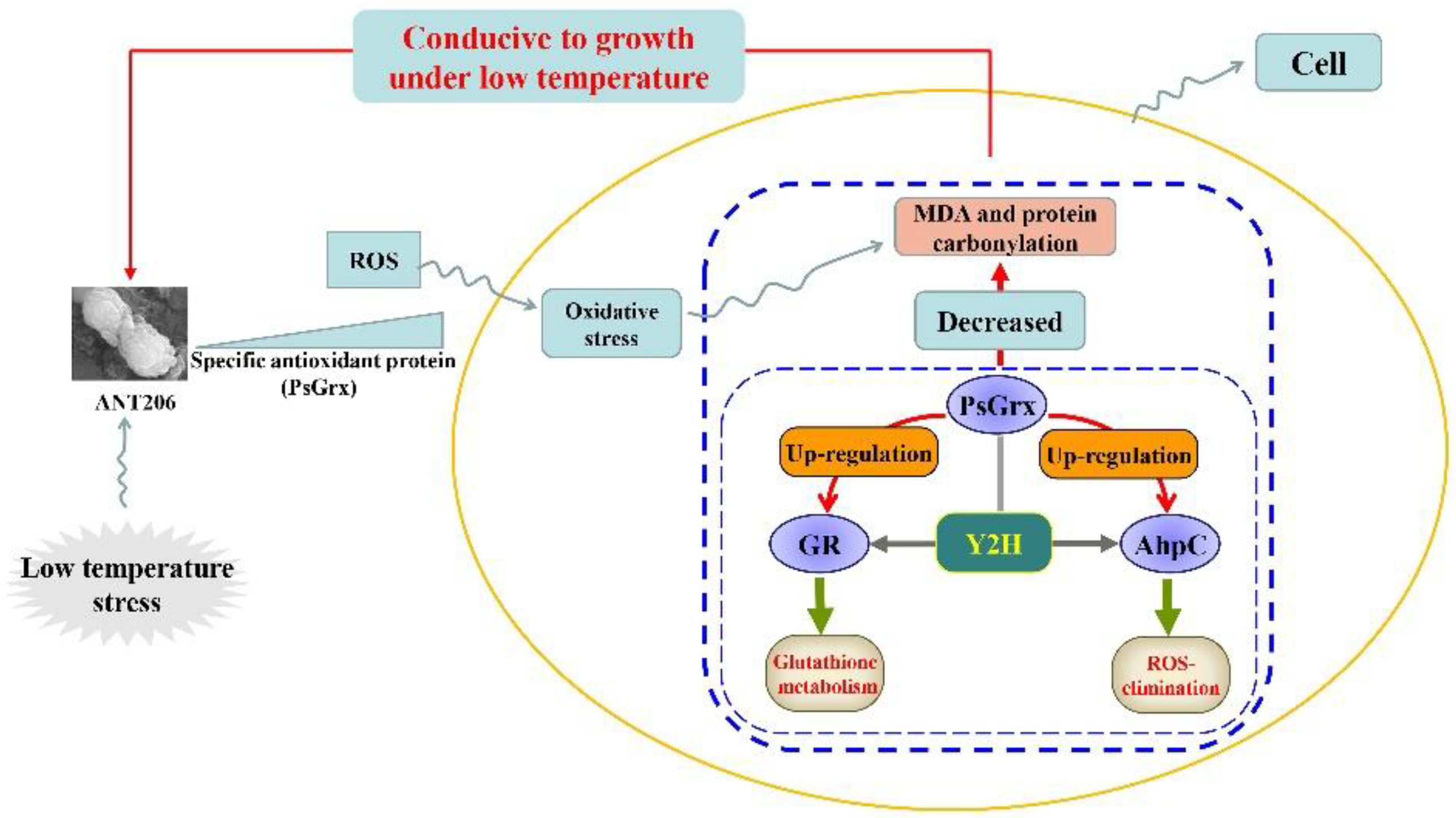
3. Conclusions
4. Materials and Methods
4.1. Strains and Material
4.2. Construction of Mutant Strain Δpsgrx, Δahpc and Δpsgrx Δahpc
4.3. Low-Temperature Treatment, MDA Activity and Protein Carbonylation Assay
4.4. cDNA Library of Strain ANT206
4.5. Yeast Two-Hybrid Analysis
4.6. Bimolecular Fluorescence Complementation (BiFC) and Co-Immunoprecipitation (Co-IP) Assay
4.7. RNA Extraction and Quantitative Real-Time PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. BBA-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [Green Version]
- Couturier, J.; Przybyla-Toscano, J.; Roret, T.; Didierjean, C.; Rouhier, N. The roles of glutaredoxins ligating Fe-S clusters: Sensing, transfer or repair functions? BBA-Mol. Cell Res. 2015, 1853, 1513–1527. [Google Scholar] [CrossRef]
- Outten, C.E.; Albetel, A.N. Iron sensing and regulation in Saccharomyces cerevisiae: Ironing out the mechanistic details. Curr. Opin. Microbiol. 2013, 16, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.L.; Song, J.Q.; Wang, C.; Zuo, J.R.; Dong, X.N. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Herrero, E.; Belli, E.G.; Casas, C. Structural and functional diversity of glutaredoxins in yeast. Curr. Protein Pept. Sci. 2010, 11, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, X.; Sun, Y.; Wang, C.C.; Zhang, W.L.; Sun, M.H.; Hu, H.T.; Liu, J.Z.; Yang, L. A rice CPYC-type glutaredoxin OsGRX20 in protection against bacterial blight, methyl viologen and salt stresses. Front. Plant Sci. 2018, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.J.; Fang, P.P.; Guo, X.; Qian, X.J.; Zhou, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid-mediated apoplastic H2O2-glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef]
- Kang, B.C.; Wu, Q.Y.; Sprague, S.; Park, S.; White, F.F.; Bae, S.J.; Han, J.S. Ectopic overexpression of an Arabidopsis monothiol glutaredoxin AtGRXS17 affects floral development and improves response to heat stress in chrysanthemum (Chrysanthemum morifolium Ramat.). Environ. Exp. Bot. 2019, 167, 103864. [Google Scholar] [CrossRef]
- Martins, L.; Knuesting, J.; Bariat, L.; Dard, A.; Freibert, S.A.; Marchand, C.H.; Young, D.; Dung, N.H.T.; Voth, W.; Debures, A.; et al. Redox modification of the Iron-sulfur glutaredoxin GRXS17 activates holdase activity and protects plants from heat stress. Plant Physiol. 2020, 184, 676–692. [Google Scholar] [CrossRef]
- Li, M.; Huang, W.; Yang, Q.; Liu, X.G.; Wu, Q.Y. Expression and oxidative stress tolerance studies of glutaredoxin from cyanobacterium Synechocystis sp. PCC 6803 in Escherichia coli. Protein Expres. Purif. 2005, 42, 85–91. [Google Scholar] [CrossRef]
- Luikenhuis, S.; Perrone, G.; Dawes, I.W.; Grant, C.M. The yeast Saccharomyces cerevisiae contains two glutaredoxin genes that are required for protection against reactive oxygen species. Mol. Biol. Cell 1998, 9, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.M.; Luikenhuis, S.; Beckhouse, A.; Soderbergh, M.; Dawes, I.W. Differential regulation of glutaredoxin gene expression in response to stress conditions in the yeast Saccharomyces cerevisiae. BBA-Gene Struct. Expr. 2000, 1490, 33–42. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, Y.J.; Yu, Q.L.; Kai, Z.; Zhang, M.; Jia, C.; Xiao, C.P.; Zhang, B.; Zhang, B.; Li, M.C. Function of glutaredoxin 3 (Grx3) in oxidative stress response caused by iron homeostasis disorder in Candida albicans. Future Microbiol. 2017, 12, 1397–1412. [Google Scholar] [CrossRef]
- Xia, H.Y.; Li, B.H.; Zhang, Z.; Wang, Q.; Qiao, T.; Li, K.Y. Human glutaredoxin 3 can bind and effectively transfer [4Fe-4S] cluster to apo-iron regulatory protein 1. Biochem. Biophys. Res. Commun. 2015, 465, 620–624. [Google Scholar] [CrossRef]
- Giordano, D. Bioactive molecules from extreme environments. Mar. Drugs 2020, 18, 640. [Google Scholar] [CrossRef]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- King, M.D.; France, J.L.; Fisher, F.N.; Beine, H.J. Measurement and modelling of UV radiation penetration and photolysis rates of nitrate and hydrogen peroxide in Antarctic sea ice: An estimate of the production rate of hydroxyl radicals in first-year sea ice. J. Photochem. Photobiol. 2005, 176, 39–49. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Raghu, G.; Sharma, Y.V.R.K.; Biju, A.R.; Rajasekharan, M.V.; Shivaji, S. Increase in oxidative stress at low temperature in an Antarctic bacterium. Curr. Opin. Microbiol. 2011, 62, 544–546. [Google Scholar] [CrossRef]
- Hou, Y.H.; Qiao, C.H.; Wang, Y.F.; Wang, Y.T.; Ren, X.L.; Wei, Q.F.; Wang, Q.F. Cold-adapted glutathione S-transferases from Antarctic psychrophilic bacterium Halomonas sp. ANT108: Heterologous expression, characterization, and oxidative resistance. Mar. Drugs 2019, 17, 147. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.L.; Wang, X.L. Superoxide dismutase and ascorbate peroxidase genes in Antarctic endemic brown alga Ascoseira mirabilis (Ascoseirales, Phaeophyceae): Data mining of a de novo transcriptome. Bot. Mar. 2020, 63, 541–549. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hou, Y.H.; Wang, Y.T.; Zheng, L.; Wang, Q.F. A novel cold-adapted nitroreductase from Psychrobacter sp. ANT206: Heterologous expression, characterization and nitrobenzene reduction capacity. Enzym. Microb. Technol. 2019, 131, 109434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Wang, Q.F.; Hou, Y.H. A new cold-adapted and salt-tolerant glutathione reductase from Antarctic Psychrophilic bacterium Psychrobacter sp. and its resistance to oxidation. Int. J. Mol. Sci. 2020, 21, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.T.; Hou, Y.H.; Wang, Q.F. Cloning, expression, characterization, and antioxidant protection of glutaredoxin3 from psychrophilic bacterium Psychrobacter sp. ANT206. Front. Microbiol. 2021, 12, 633362. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rios, E.; Ramos-Alonso, L.; Guillamon, J.M. Correlation between low temperature adaptation and oxidative stress in Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 1199. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rios, E.; Guillen, A.; de la Cerda, R.; Perez-Traves, L.; Querol, A.; Guillamon, J.M. Improving the cryotolerance of wine yeast by interspecification hybridization in the genus Saccharomyces. Front. Microbiol. 2019, 9, 3232. [Google Scholar] [CrossRef] [Green Version]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid-peroxidation. Method Enzymol. 1990, 186, 421–431. [Google Scholar]
- Jing, L.L.; Wu, N.Z.; He, L.; Shao, J.; Ma, H.P. Establishment of an experimental rat model of high altitude cerebral edema by hypobaric hypoxia combined with temperature fluctuation. Brain Res. Bull. 2020, 165, 253–262. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal. 2010, 12, 323–325. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, X.; Zhang, X.Y.; Wang, Y.; Huo, Z.Y.; Huang, M.; Cai, J.; Zhou, Q.; Jiang, D. Involvement of salicylic acid in cold priming-induced freezing tolerance in wheat plants. Plant Growth Regul. 2020, 93, 117–130. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, C.; Guo, H.Y.; Wang, Y.C. BplMYB46 from Betula platyphylla can form homodimers and heterodimers and is involved in salt and osmotic stresses. Int. J. Mol. Sci. 2019, 20, 1171. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.S.; Cai, W.W.; Huang, X.Y.; Yang, S.; Wen, J.Y.; Xia, X.Q.; Yang, F.; Shi, Y.Y.; Guan, D.Y.; He, S.L. Ca14-3-3 interacts with cawrky58 to positively modulate pepper response to low-phosphorus starvation. Front. Plant Sci. 2021, 11, 607878. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Barnwell, C.V.; Grunden, A.M. Characterization of recombinant glutathione reductase from the psychrophilic Antarctic bacterium Colwellia psychrerythraea. Extremophiles 2015, 19, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-nhu, N.T.; Knoops, B. Alkyl hydroperoxide reductase 1 protects Saccharomyces cerevisiae against metal ion toxicity and glutathione depletion. Toxicol. Lett. 2002, 135, 219–228. [Google Scholar] [CrossRef]
- Rouhier, N.; Villarejo, A.; Srivastava, M.; Gelhaye, E.; Keech, O.; Droux, M. Identification of plant glutaredoxin targets. Antioxid. Redox Signal. 2005, 7, 919–929. [Google Scholar] [CrossRef]
- Li, M.; Yang, Q.; Zhang, L.W.; Li, H.; Cui, Y.L.; Wu, Q.Y. Identification of novel targets of cyanobacterial glutaredoxin. Arch. Biochem. Biophys. 2007, 458, 220–228. [Google Scholar] [CrossRef]
- Sandkvist, M. Type II secretion and pathogenesis. Infect. Immun. 2001, 69, 3523–3535. [Google Scholar] [CrossRef] [Green Version]
- Marujo, P.E.; Braun, F.; Haugel-Nielsen, J.; Le Derout, J.; Arraiano, C.M.; Regnier, P. Inactivation of the decay pathway initiated at an internal site by RNase E promotes poly(A)-dependent degradation of the rpsO mRNA in Escherichia coli. Mol. Microbiol. 2003, 50, 1283–1294. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, Y.H. RNases in ColE1 DNA metabolism. Mol. Biol. Rep. 1996, 22, 195–200. [Google Scholar] [CrossRef]
- Bouchara, N.; Senejoux, F.; Fraisse, D.; Felgines, C.; Caldefie-Chezet, F.; Vasson, M.P. Anti-inflammatory and prolonged protective effects of Artemisia herbaalba extracts via glutathione metabolism reinforcement. S. Afr. J. Bot. 2021, 142, 206–215. [Google Scholar] [CrossRef]
- Chen, W.L.; Ko, Y.T. Exogenous hydrogen peroxide induces chilling tolerance in Phalaenopsis seedlings through glutathione-related antioxidant system. Sci. Hortic. 2021, 289, 110421. [Google Scholar] [CrossRef]
- Zuo, F.L.; Yu, R.; Khaskheli, G.B.; Ma, H.Q.; Chen, L.L.; Zeng, Z.; Mao, A.J.; Chen, S.W. Homologous overexpression of alkyl hydroperoxide reductase subunit C (ahpC) protects Bifidobacterium longum strain NCC2705 from oxidative stress. Res. Microbiol. 2014, 165, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.N.; Lee, S.G.; Yun, S.H.; Kim, H.K.; Choi, Y.S.; Kim, G.H. Comparative analyses of Cu-Zn superoxide dismutase (SOD1) and thioredoxin reductase (TrxR) at the mRNA level between Apis mellifera L. and Apis cerana F. (Hymenoptera: Apidae) under stress conditions. J. Insect. Sci. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Wang, Q.Y.; Liu, Q.; Ma, Y.; Cao, X.D.; Zhang, Y.X. Role of RpoS in stress survival, synthesis of extracellular autoinducer 2, and virulence in Vibrio alginolyticus. Arch. Microbiol. 2008, 190, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Hou, T.H.; Wang, Q.F.; Wang, Y.T. The elucidation of the biodegradation of nitrobenzene and p-nitrophenol of nitroreductase from Antarctic psychrophile Psychrobacter sp. ANT206 under low temperature. J. Hazard. Mater. 2021, 413, 125377. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Vantriet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rios, E.; Lopez-Malo, M.; Guillamon, J.M. Global phenotypic and genomic comparison of two Saccharomyces cerevisiae wine strains reveals a novel role of the sulfur assimilation pathway in adaptation at low temperature fermentations. BMC Genom. 2014, 15, 1059. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.X.; Jing, J.; Yu, C.J.; Chen, J. Construction of a high-quality yeast two-hybrid library and its application in identification of interacting proteins with Brn1 in Curvularia lunata. Plant Pathol. J. 2015, 31, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Tang, X.R.; Tian, G.; Wang, F.; Liu, K.D.; Nguyen, V.; Kohalmi, S.E.; Keller, W.A.; Tsang, E.W.T.; Harada, J.J.; et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: Components and anchoring nucleoporin. Plant J. 2010, 61, 259–270. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene Number | Gene Name | Functional Class |
|---|---|---|
| 1 | Glutathione reductase | Stress-related reactions |
| 2 | Glutathione peroxidase | Stress-related reactions |
| 3 | Alkyl hydroperoxide reductase | Stress-related reactions |
| 4 | DNA photolyase | DNA modification |
| 5 | Aldehyde dehydrogenase | Biosynthesis |
| 6 | Translation elongation factor | Translation |
| 7 | Nucleoside diphosphate kinase | Translation |
| 8 | Glyceraldehyde 3-phosphate dehydrogenase | Glycolysis |
| 9 | Transketolase | Calvin cycle; associated reactions |
| 10 | Fructose-1,6-bisphosphatase | Calvin cycle; associated reactions |
| 11 | Triosephosphate isomerase | Calvin cycle; associated reactions |
| 12 | Fructose-bisphosphate aldolase | Calvin cycle; associated reactions |
| 13 | Phosphoglycerate kinase | Calvin cycle; associated reactions |
| 14 | Carbonic anhydrase | Calvin cycle; associated reactions |
| 15 | Methionine synthase | Sulfur metabolism |
| 16 | Cysteine synthase | Sulfur metabolism |
| 17 | Aminotransferase | Nitrogen metabolism |
| 18 | GspI | Protein secretion |
| 19 | DNA translocase FtsK | DNA transportation; cell division |
| 20 | RNase E | RNA metabolism; transcription |
| 21 | AarF/Abc1/UbiB kinase family protein | Fatty acid metabolism; protein synthesis |
| 22 | peptidoglycan-binding protein LysM | Cell separation |
| 23 | ATP synthase α chain | ATP metabolism |
| 24 | Methyltransferase small domain | Hypothetical protein |
| 25 | Thioesterase | Hypothetical protein |
| 26 | Transglutaminase-like domain | Hypothetical protein |
| Name | Primer Sequences | Restriction Enzyme Cutting Sites |
|---|---|---|
| Δpsgrx-P1 | 5′-GCTCTAGACGATGACTGTATCTGTTAAAG-3′ | XbaI |
| Δpsgrx-P2 | 5′-CGGTGCGATAGTTATTCTCTTCATAATC-3′ | |
| Δpsgrx-P3 | 5′-GAAGAGAATAACTATCGCACCGTGC-3′ | |
| Δpsgrx-P4 | 5′-TAGAGCTCTTAACCCGCTAATAGCTC-3′ | SacI |
| Δahpc-P1 | 5′-ATGTCTAGAATGACGACTGATAGCG-3′ | XbaI |
| Δahpc-P2 | 5′-GTTCTCAATGTGACCCCAAAAATAG-3′ | |
| Δahpc-P3 | 5′-CAAGAGTTACACAGATAAAAACCCC-3′ | |
| Δahpc-P4 | 5′-TCAGAGCTCTAAAAACTGACGACAG-3′ | SacI |
| Name | Primer Sequences |
|---|---|
| psgrx-F | 5′-ACAAGTTTGTACAAAAAAATGACTGTATCTGTTAAAGTTTATAC-3′ |
| psgrx-R | 5′-CACCACTTTGTACAAGAAACCCGCTAATAGCTCGTCAAG-3′ |
| gr-F | 5′-ATGACAAAACATTATGATTATATTT TCCATTGGCGGC-3′ |
| gr-R | 5′-CTAACGCATCGTCACAAACTCTTCTGAGCCAGTTGGATGAAT-3′ |
| ahpc-F | 5′-ATGACGACTGATAGCGACAAGACGACTGAGAGATCTAAAAAG-3′ |
| ahpc-R | 5′-AAAAACTGACGACAGCCACAATCTTAATTTCAATGACCATAAC-3′ |
| glutathione peroxidase-F | 5′-ATGACTACTATTTATGATTTTAGTGCTGAGCGTATGGCAT-3′ |
| glutathione peroxidase-R | 5′-TTTGCACGCCTCCTTAACTTGGTCAAGATCAGGGCTGAAC-3′ |
| gspI-F | 5′-ATGATAAATAATGACAGAGCCAAACCTAACCATGTAAACCGA-3′ |
| gspI-R | 5′-GTTTGGCTCTGTCATTATTTATCATTTCGGTTTACATGGTTAG-3′ |
| ftsK-F | 5′-GTGATATCAGCACCAATTATTGATTACTTAAAAAAGGGCATA-3′ |
| ftsK-R | 5′-AATCAATAATTGGTGCTGATATCACATATGCCCTTT-3′ |
| Rnase E-F | 5′-ATGAAACGCATTTTAATCAACGCCACCCAAAACGAAGAAATTC-3′ |
| Rnase E-R | 5′-GCTCTCTCTATCTGAGTTATCTGAATCATCTGACTCTAGTT-3′ |
| Name | Primer Sequences |
|---|---|
| psgrx-F | 5′-GGCGTTGATTATGAAGAGATTGGCATG-3′ |
| psgrx-R | 5′-TGTGGCACGGTACGATAGTTATTAGTC-3′ |
| gr-F | 5′-TGTATGTCCGTCAGCACTCG-3′ |
| gr-R | 5′-TCGCCCAAATCAAGCAGTCT-3′ |
| ahpc-F | 5′-CAAGTCCGGCTCTGACCAAG-3′ |
| ahpc-R | 5′-CTTGGCTCATCTCGCCATCT-3′ |
| trxR-F | 5′-CTGATCGTCAACAGCGGTCT-3′ |
| trxR-R | 5′-CAGCAGAGGTGATCGCTTGA-3′ |
| 16S-F | 5′-CCTTCGCCATCGGTATTCCTCCAG-3′ |
| 16S-R | 5′-GAGCTAGAGTATGTGAGAGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Q.; Hou, Y.; Liu, J. Glutaredoxin Interacts with GR and AhpC to Enhance Low-Temperature Tolerance of Antarctic Psychrophile Psychrobacter sp. ANT206. Int. J. Mol. Sci. 2022, 23, 1313. https://doi.org/10.3390/ijms23031313
Wang Y, Wang Q, Hou Y, Liu J. Glutaredoxin Interacts with GR and AhpC to Enhance Low-Temperature Tolerance of Antarctic Psychrophile Psychrobacter sp. ANT206. International Journal of Molecular Sciences. 2022; 23(3):1313. https://doi.org/10.3390/ijms23031313
Chicago/Turabian StyleWang, Yatong, Quanfu Wang, Yanhua Hou, and Jianan Liu. 2022. "Glutaredoxin Interacts with GR and AhpC to Enhance Low-Temperature Tolerance of Antarctic Psychrophile Psychrobacter sp. ANT206" International Journal of Molecular Sciences 23, no. 3: 1313. https://doi.org/10.3390/ijms23031313





