Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats
Abstract
:1. Introduction
2. Results
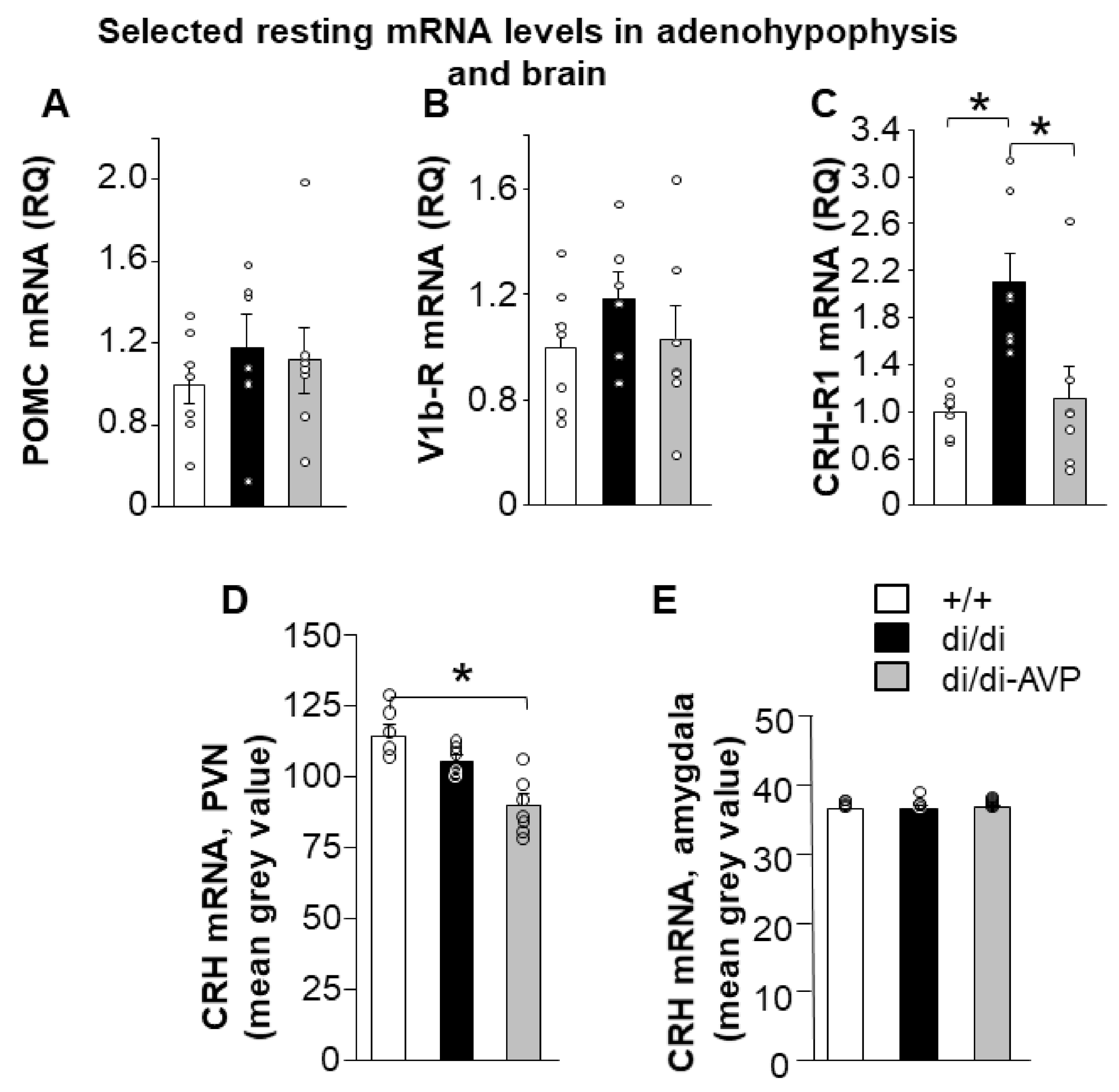
2.1. Resting HPA Axis Levels: Restoration of Adenohypophyseal Corticotropin-Releasing Hormone Receptor 1 Elevation by AVP Synthesis Rescue in SON
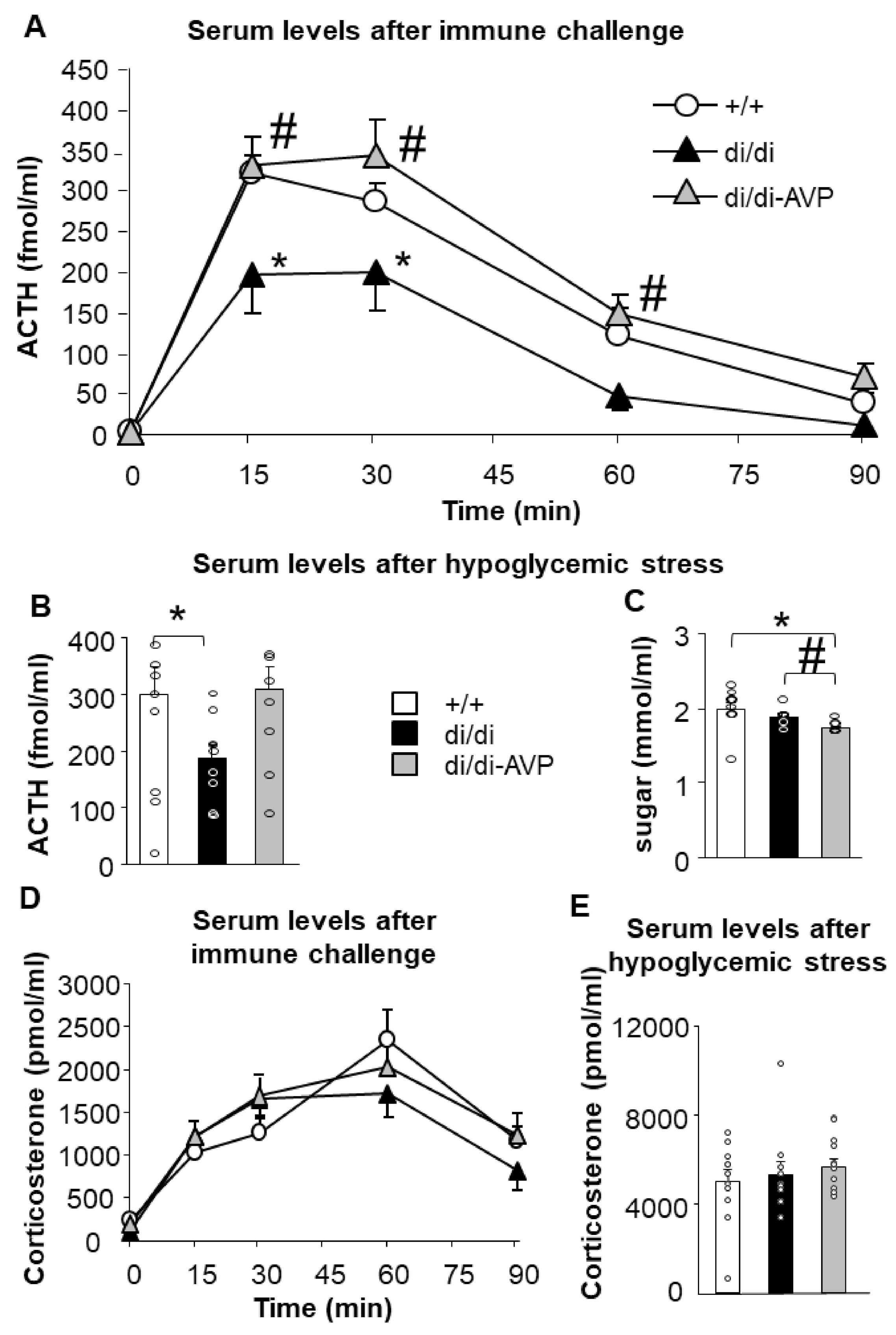
2.2. Restoration of Acute Stress-Induced Lower ACTH Levels in AVP-AAV-Treated Brattleboro Rats (Series 1)
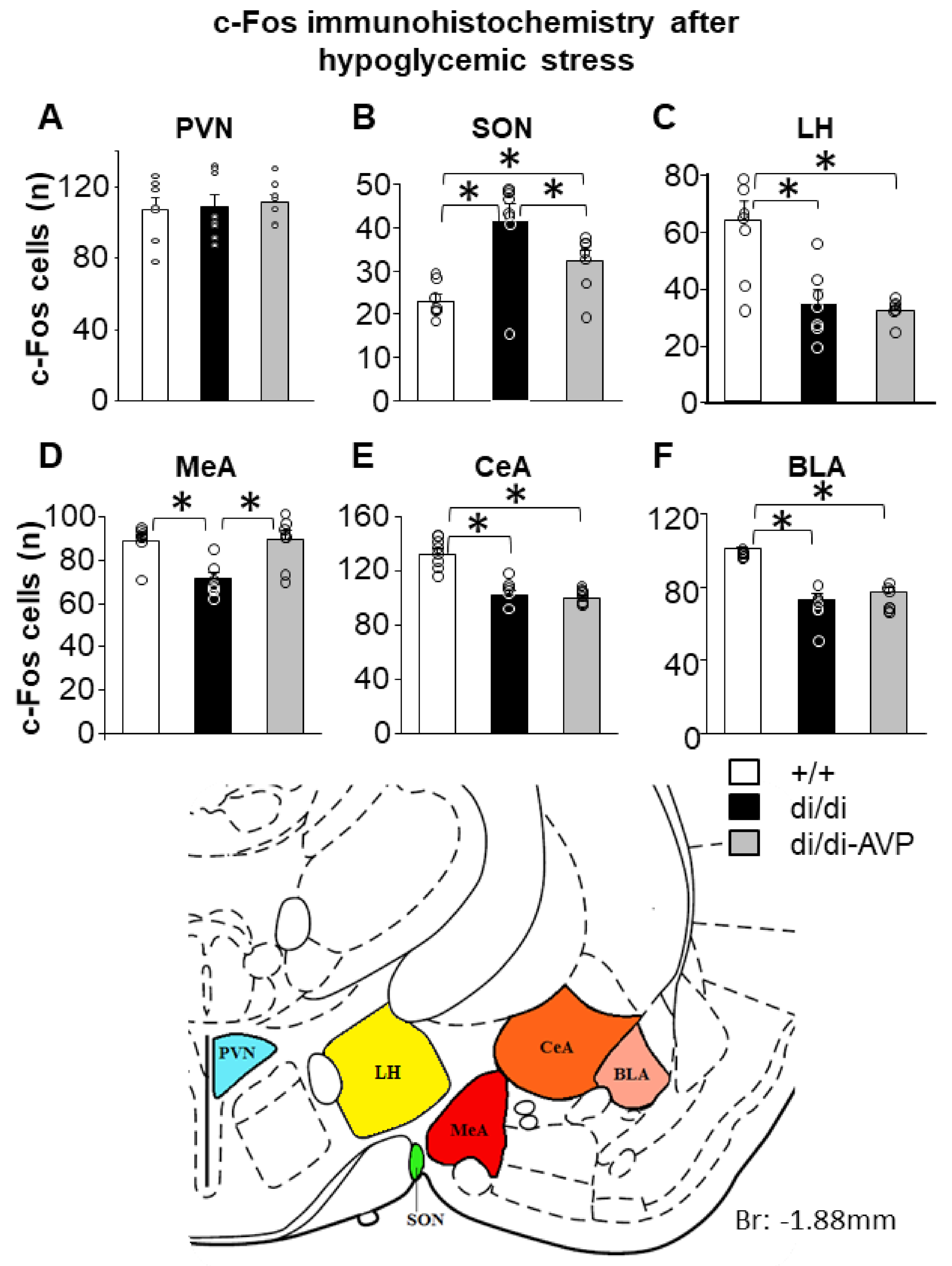
2.3. Altered Hypoglycaemic Stress-Induced c-Fos Signal in the SON and MeA of di/di Rats Is “Normalised” by AVP-AAV Treatment (Series 1)
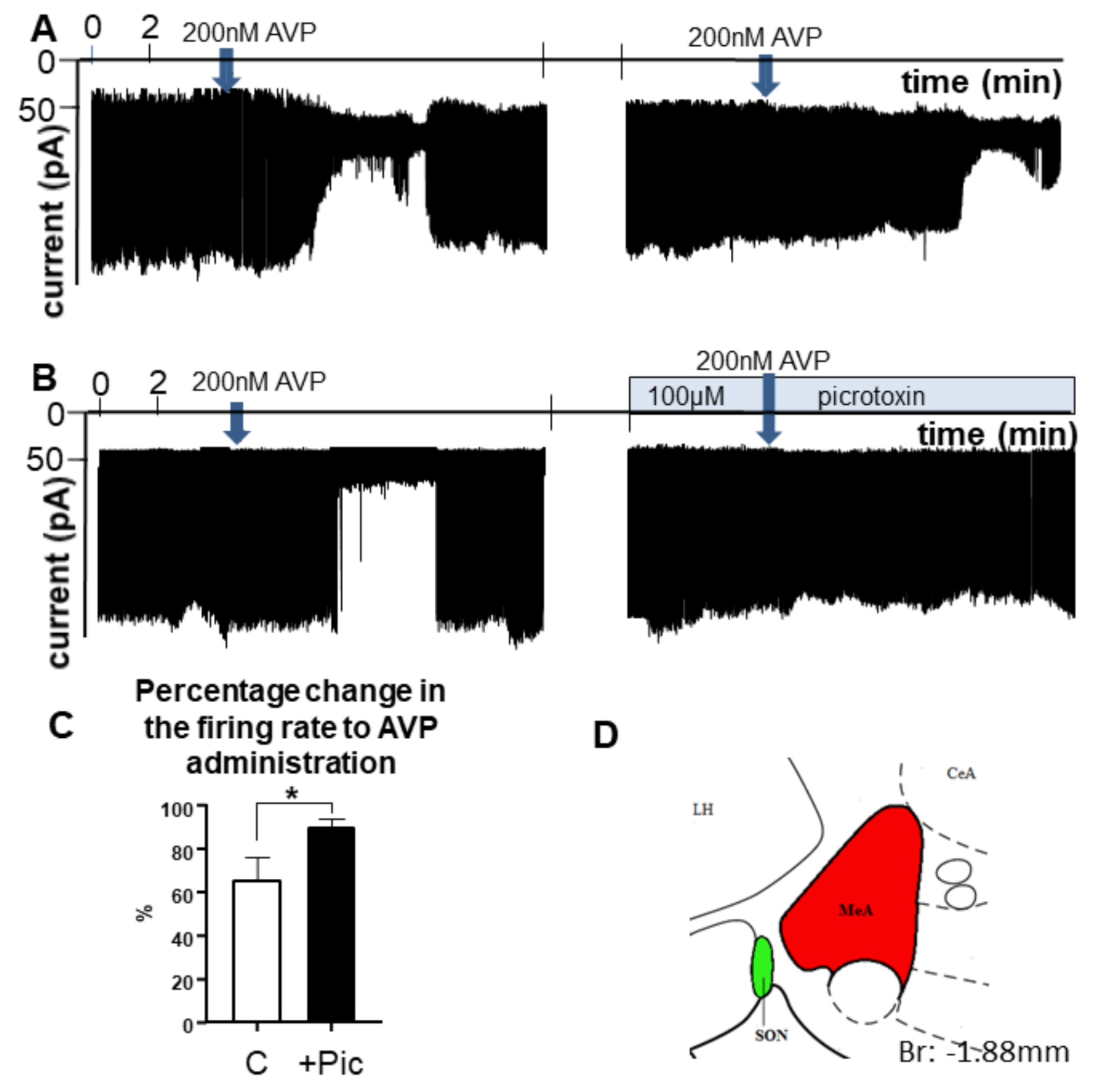
2.4. AVP Inhibited MeA Neurons through Involvement of GABAA Receptors-Electrophysiological Recordings from Brain Slides
2.5. AVP-AAV Rescue in the SON Had No Effect on NOR but Altered the Social Behaviour (Series 1 and 2)
3. Discussion
3.1. AVP of SON Origin and Stress Regulation
3.1.1. Resting Levels
3.1.2. Acute Stress
3.2. Possible Brain Targets of AVP Originating in the Magnocellular SON
3.2.1. c-Fos Data
3.2.2. MeA and Vasopressin
3.2.3. In Vitro AVP Sensitivity of MeA Neurons
3.3. Behavioural Influence of AVP Originating in the Magnocellular SON
3.3.1. Effect on Object Recognition Memory
3.3.2. Effects on Social Behaviour
4. Materials and Methods
4.1. Animals
4.2. Stereotaxic AVP-AAV Injection
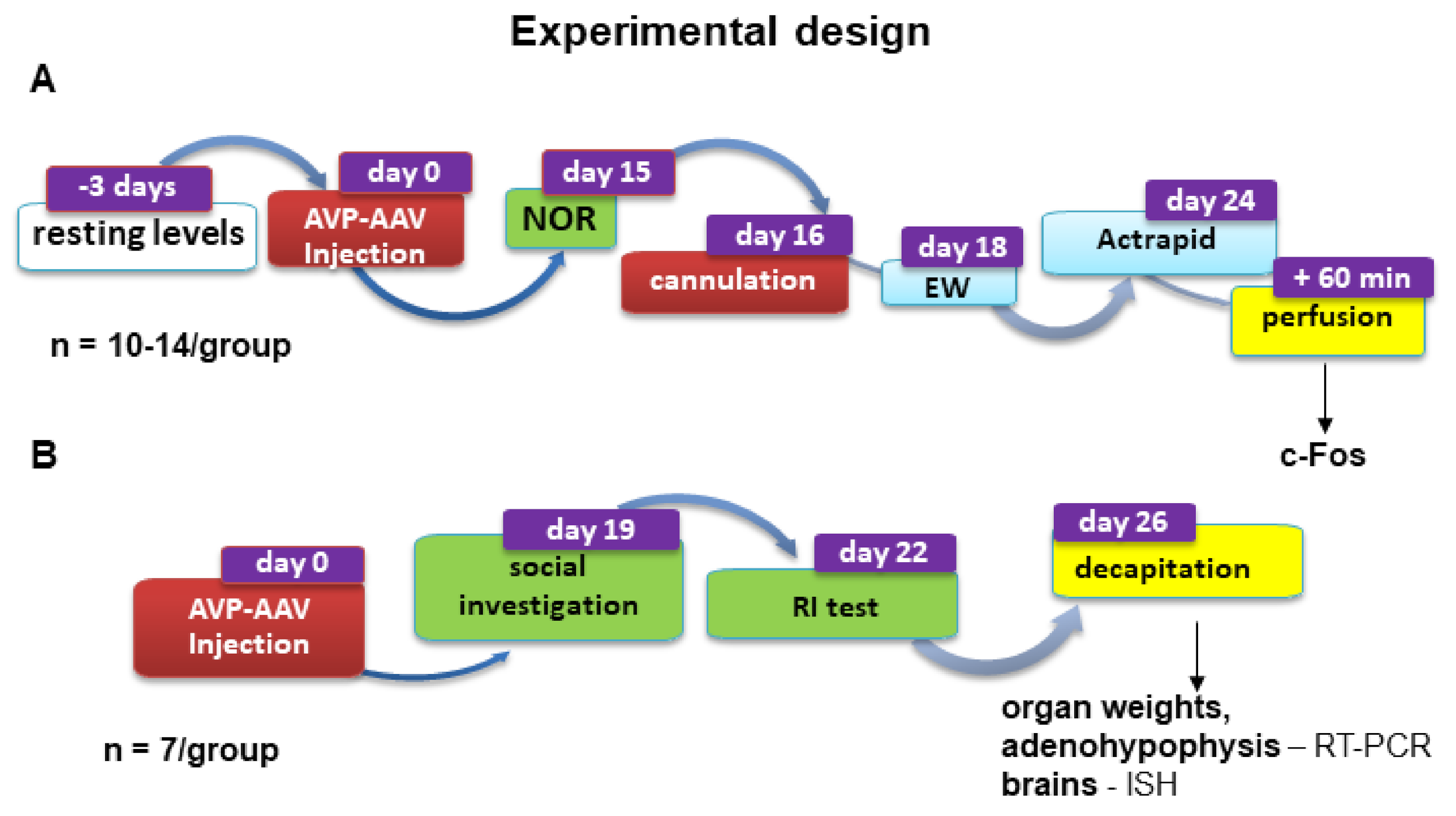
4.3. Experimental Design for Stress and Behavioural Experiments
4.3.1. Series 1
4.3.2. Series 2
4.4. Immunohistochemistry
4.4.1. AVP Immunohistochemistry
4.4.2. c-Fos
4.5. Quantitative Real-Time PCR Measurements
4.6. In-Situ Hybridization
4.7. Stress Experiments
4.7.1. Immune Challenge
4.7.2. Metabolic Stressor
4.8. Hormone Measurements
4.9. Electrophysiological Recordings
4.10. Behavioural Experiments
4.10.1. Novel Object Recognition Test (NOR)
4.10.2. Social Investigation
4.10.3. Resident-Intruder Test (RI)
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szabo, S.; Tache, Y.; Somogyi, A. The legacy of Hans Selye and the origins of stress research: A retrospective 75 years after his landmark brief “letter” to the editor# of nature. Stress 2012, 15, 472–478. [Google Scholar]
- Antoni, F.A. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front. Neuroendocrinol. 1993, 14, 76–122. [Google Scholar] [CrossRef]
- Scott, L.V.; Dinan, T.G. Vasopressin as a target for antidepressant development: An assessment of the available evidence. J. Affect. Disord. 2002, 72, 113–124. [Google Scholar] [CrossRef]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, M.; Landgraf, R.; Wotjak, C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Front. Neuroendocrinol. 2004, 25, 132–149. [Google Scholar] [CrossRef]
- Sivukhina, E.V.; Jirikowski, G.F. Magnocellular hypothalamic system and its interaction with the hypothalamo-pituitary-adrenal axis. Steroids 2016, 111, 21–28. [Google Scholar] [CrossRef]
- Antoni, F.A. Magnocellular Vasopressin and the Mechanism of “Glucocorticoid Escape”. Front. Endocrinol. 2019, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Zelena, D.; Pinter, O.; Balazsfi, D.G.; Langnaese, K.; Richter, K.; Landgraf, R.; Makara, G.B.; Engelmann, M. Vasopressin signaling at brain level controls stress hormone release: The vasopressin-deficient Brattleboro rat as a model. Amino Acids 2015, 47, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Csikota, P.; Fodor, A.; Balázsfi, D.; Pintér, O.; Mizukami, H.; Weger, S.; Heilbronn, R.; Engelmann, M.; Zelena, D. Vasopressinergic control of stress-related behavior: Studies in Brattleboro rats. Stress 2016, 19, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Zelena, D.; Engelmann, M. The Brattleboro Rat: The First and Still Up-to-Date Mutant Rodent Model for Neuroendocrine Research. In Model Animals in Neuroendocrinology: From Worm to Mouse to Man; Ludwig, M., Levkowitz, G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; Chapter 12; pp. 279–296. [Google Scholar]
- Zelena, D.; Domokos, A.; Jain, S.K.; Jankord, R.; Filaretova, L. The stimuli-specific role of vasopressin in the hypothalamus-pituitary-adrenal axis response to stress. J. Endocrinol. 2009, 202, 263–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balázsfi, D.; Pintér, O.; Klausz, B.; Kovács, K.B.; Fodor, A.; Török, B.; Engelmann, M.; Zelena, D. Restoration of peripheral V2 receptor vasopressin signaling fails to correct behavioral changes in Brattleboro rats. Psychoneuroendocrinology 2015, 51, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Fodor, A.; Klausz, B.; Zelena, D. Anxiogenic role of vasopressin during the early postnatal period: Maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids 2015, 47, 2409–2418. [Google Scholar] [CrossRef]
- Mlynarik, M.; Zelena, D.; Bagdy, G.; Makara, G.B.; Jezova, D. Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm. Behav. 2007, 51, 395–405. [Google Scholar] [CrossRef]
- Fodor, A.; Klausz, B.; Pintér, O.; Daviu, N.; Rabasa, C.; Rotllant, D.; Balazsfi, D.; Kovacs, K.B.; Nadal, R.; Zelena, D. Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Horm. Behav. 2012, 62, 539–551. [Google Scholar] [CrossRef]
- Varga, J.; Klausz, B.; Domokos, A.; Kalman, S.; Pakaski, M.; Szucs, S.; Zelena, D. Increase in Alzheimer’s related markers preceeds memory disturbances: Studies in vasopressin-deficient Brattleboro rat. Brain Res. Bull. 2014, 100, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Demeter, K.; Torok, B.; Fodor, A.; Varga, J.; Ferenczi, S.; Kovacs, K.J.; Zelena, D. Possible contribution of epigenetic changes in the development of schizophrenia-like behavior in vasopressin-deficient Brattleboro rats. Behav. Brain Res. 2016, 300, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R.; Koob, G.F.; Bluthe, R.M.; Le Moal, M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988, 457, 143–147. [Google Scholar] [CrossRef]
- Koob, G.F.; Lebrun, C.; Bluthe, R.M.; Dantzer, R.; Le Moal, M. Role of neuropeptides in learning versus performance: Focus on vasopressin. Brain Res. Bull. 1989, 23, 359–364. [Google Scholar] [CrossRef]
- McEwen, B.B. De Wied and colleagues I: Evidence for a VP and an OT influence on MP: Launching the “VP/OT central memory theory”. Adv. Pharmacol. 2004, 50, 655–708. [Google Scholar]
- Engelmann, M. Vasopressin in the septum: Not important versus causally involved in learning and memory—Two faces of the same coin? Prog. Brain Res. 2008, 170, 389–395. [Google Scholar]
- Schatz, K.C.; Kyne, R.F.; Parmeter, S.L.; Paul, M.J. Investigation of social, affective, and locomotor behavior of adolescent Brattleboro rats reveals a link between vasopressin’s actions on arousal and social behavior. Horm. Behav. 2018, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Di Benedictis, B.T.; Nussbaum, E.R.; Cheung, H.K.; Veenema, A.H. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol. 2017, 525, 2549–2570. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Poehlmann, M.L.; Li, S.; Ratnaseelan, A.M.; Bredewold, R.; Veenema, A.H. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: Focus on the social decision-making network. Brain Struct. Funct. 2017, 222, 981–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, B.J.; Harding, T.C.; Lightman, S.L.; Uney, J.B. Long-term gene therapy in the CNS: Reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nat. Med. 1997, 3, 1402–1404. [Google Scholar] [CrossRef]
- Ideno, J.; Mizukami, H.; Honda, K.; Okada, T.; Hanazono, Y.; Kume, A.; Saito, T.; Ishibashi, S.; Ozawa, K. Persistent phenotypic correction of central diabetes insipidus using adeno-associated virus vector expressing arginine-vasopressin in Brattleboro rats. Molecular therapy. J. Am. Soc. Gene Ther. 2003, 8, 895–902. [Google Scholar] [CrossRef]
- Noack, J.; Murau, R.; Engelmann, M. Consequences of temporary inhibition of the medial amygdala on social recognition memory performance in mice. Front. Neurosci. 2015, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Zelena, D.; Langnaese, K.; Domokos, A.; Pinter, O.; Landgraf, R.; Makara, G.B.; Engelmann, M. Vasopressin administration into the paraventricular nucleus normalizes plasma oxytocin and corticosterone levels in Brattleboro rats. Endocrinology 2009, 150, 2791–2798. [Google Scholar] [CrossRef] [Green Version]
- Reichel, J.M.; Bedenk, B.T.; Gassen, N.C.; Hafner, K.; Bura, S.A.; Almeida-Correa, S.; Wotjak, C.T. Beware of your Cre-Ation: LacZ expression impairs neuronal integrity and hippocampus-dependent memory. Hippocampus 2016, 26, 1250–1264. [Google Scholar] [CrossRef]
- Zelena, D.; Demeter, K.; Haller, J.; Balazsfi, D. Considerations for the use of virally delivered genetic tools for in-vivo circuit analysis and behavior in mutant mice: A practical guide to optogenetics. Behav. Pharmacol. 2017, 28, 598–609. [Google Scholar] [CrossRef]
- Bienemann, A.S.; Martin-Rendon, E.; Cosgrave, A.S.; Glover, C.P.J.; Wong, L.-F.; Kingsman, S.M.; A Mitrophanous, K.; Mazarakis, N.D.; Uney, J.B. Long-term replacement of a mutated nonfunctional CNS gene: Reversal of hypothalamic diabetes insipidus using an EIAV-based lentiviral vector expressing arginine vasopressin. Mol. Ther. J. Am. Soc. Gene Ther. 2003, 7, 588–596. [Google Scholar] [CrossRef]
- Nagy, G.M.; Gorcs, T.J.; Halasz, B. Attenuation of the suckling-induced prolactin release and the high afternoon oscillations of plasma prolactin secretion of lactating rats by antiserum to vasopressin. Neuroendocrinology 1991, 54, 566–570. [Google Scholar] [CrossRef]
- Kocsis, K.; Kiss, J.; Gorcs, T.; Halasz, B. Metabotropic glutamate receptor in vasopressin, CRF and VIP hypothalamic neurones. NeuroReport 1998, 9, 4029–4033. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Kovács, K.B.; Balázsfi, D.; Klausz, B.; Pintér, O.; Demeter, K.; Daviu, N.; Rabasa, C.; Rotllant, D.; Nadal, R.; et al. Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol. Behav. 2016, 158, 100–111. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998; pp. 92101–94495. [Google Scholar]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef] [Green Version]
- Balazsfi, D.; Fodor, A.; Torok, B.; Ferenczi, S.; Kovacs, K.J.; Haller, J.; Zelena, D. Enhanced innate fear and altered stress axis regulation in VGluT3 knockout mice. Stress 2018, 21, 151–161. [Google Scholar] [CrossRef]
- Zelena, D.; Mergl, Z.; Foldes, A.; Kovacs, K.J.; Toth, Z.; Makara, G.B. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am. J. Physiol. 2003, 285, E1110–E1117. [Google Scholar] [CrossRef] [Green Version]
- Vas, S.; Adori, C.; Konczol, K.; Katai, Z.; Pap, D.; Papp, R.S.; Tóth, Z.E. Nesfatin-1/NUCB2 as a potential new element of sleep regulation in rats. PLoS ONE 2013, 8, e59809. [Google Scholar]
- Zelena, D.; Stocker, B.; Barna, I.; Toth, Z.E.; Makara, G.B. Vasopressin deficiency diminishes acute and long-term consequences of maternal deprivation in male rat pups. Psychoneuroendocrinology 2015, 51, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Van Zwieten, E.J.; Ravid, R.; Swaab, D.F.; Van de Woude, T. Immunocytochemically stained vasopressin binding sites on blood vessels in the rat brain. Brain Res. 1988, 474, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Raggenbass, M. Overview of cellular electrophysiological actions of vasopressin. Eur. J. Pharmacol. 2008, 583, 243–254. [Google Scholar] [CrossRef]
- Dogterom, J.; Van Greidanus, T.B.W.; Swabb, D.F. Evidence for the release of vasopressin and oxytocin into cerebrospinal fluid: Measurements in plasma and CSF of intact and hypophysectomized rats. Neuroendocrinol. 1977, 24, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.F.; Moriwaki, A.; Tomizawa, K.; Onuma, H.; Cai, X.H.; Matsui, H. Effects of vasopressin and involvement of receptor subtypes in the rat central amygdaloid nucleus in vitro. Brain Res. 1997, 768, 266–272. [Google Scholar] [CrossRef]
- Farkas, I.; Kalló, I.; Deli, L.; Vida, B.; Hrabovszky, E.; Fekete, C.; Moenter, S.M.; Watanabe, M.; Liposits, Z. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 5818–5829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, D.; Veinante, P.; Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Fodor, A.; Barsvari, B.; Aliczki, M.; Balogh, Z.; Zelena, D.; Goldberg, S.R.; Haller, J. The effects of vasopressin deficiency on aggression and impulsiveness in male and female rats. Psychoneuroendocrinology 2014, 47, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Palkovits, M. Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur. J. Pharmacol. 2000, 405, 161–166. [Google Scholar] [CrossRef]
- Engelmann, M.; Hadicke, J.; Noack, J. Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nat. Protoc. 2011, 6, 1152–1162. [Google Scholar] [CrossRef]
- Engelmann, M.; Ludwig, M.; Landgraft, R. Simultaneous monitoring of intracerebral release and behavior: Endogenous vasopressin improves social recognition. J. Neuroendocrinol. 1994, 6, 391–395. [Google Scholar] [CrossRef]
- Hauger, R.L.; Aguilera, G. Regulation of pituitary corticotropin releasing hormone (CRH) receptors by CRH: Interaction with vasopressin. Endocrinology 1993, 133, 1708–1714. [Google Scholar] [CrossRef]
- Bazhan, N.; Zelena, D. Food-intake regulation during stress by the hypothalamo-pituitary-adrenal axis. Brain Res. Bull. 2013, 95, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, G.; Lightman, S.L.; Kiss, A. Regulation of the hypothalamic-pituitary-adrenal axis during water deprivation. Endocrinology 1993, 132, 241–248. [Google Scholar] [CrossRef]
- Amaya, F.; Tanaka, M.; Tamada, Y.; Tanaka, Y.; Nilaver, G.; Ibata, Y. The influence of salt loading on vasopressin gene expression in magno- and parvocellular hypothalamic neurons: An immunocytochemical and in situ hybridization analysis. Neuroscience 1999, 89, 515–523. [Google Scholar] [CrossRef]
- Ueta, Y.; Dayanithi, G.; Fujihara, H. Hypothalamic vasopressin response to stress and various physiological stimuli: Visualization in transgenic animal models. Horm. Behav. 2011, 59, 221–226. [Google Scholar] [CrossRef]
- Wotjak, C.T.; Naruo, T.; Muraoka, S.; Simchen, R.; Landgraf, R.; Engelmann, M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 2001, 13, 2273–2281. [Google Scholar] [CrossRef]
- Rivest, S.; Laflamme, N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J. Neuroendocrinol. 1995, 7, 501–525. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Ai, H.B. Oxytocin and vasopressin involved in restraint water-immersion stress mediated by oxytocin receptor and vasopressin 1b receptor in rat brain. PLoS ONE 2011, 6, e23362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelena, D.; Pinter, O.; Langnaese, K.; Richter, K.; Landgraf, R.; Makara, G.B.; Engelmann, M. Oxytocin in Brattleboro rats: Increased synthesis is contrasted by blunted intrahypothalamic release from supraoptic nucleus neurones. J. Neuroendocrinol. 2013, 25, 711–718. [Google Scholar] [CrossRef]
- Holmes, M.C.; Antoni, F.A.; Aguilera, G.; Catt, K.J. Magnocellular axons in passage through the median eminence release vasopressin. Nature 1986, 319, 326–329. [Google Scholar] [CrossRef]
- Tannahill, L.A.; Sheward, W.J.; Robinson, I.C.; Fink, G. Corticotrophin-releasing factor-41, vasopressin and oxytocin release into hypophysial portal blood in the rat: Effects of electrical stimulation of the hypothalamus, amygdala and hippocampus. J. Endocrinol. 1991, 129, 99–107. [Google Scholar] [CrossRef]
- Antoni, F.A.; Fink, G.; Sheward, W.J. Corticotrophin-releasing peptides in rat hypophysial portal blood after paraventricular lesions: A marked reduction in the concentration of corticotrophin-releasing factor-41, but no change in vasopressin. J. Endocrinol. 1990, 125, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Wotjak, C.T.; Ludwig, M.; Ebner, K.; Russell, J.A.; Singewald, N.; Landgraf, R.; Engelmann, M. Vasopressin from hypothalamic magnocellular neurons has opposite actions at the adenohypophysis and in the supraoptic nucleus on ACTH secretion. Eur. J. Neurosci. 2002, 16, 477–485. [Google Scholar] [CrossRef]
- Makara, G.B.; Antoni, F.A.; Stark, E. Electrical stimulation in the rat of the supraoptic nucleus: Failure to alter plasma corticosterone after surgical lesioning of the paraventricular nucleus. Neurosci. Lett. 1982, 30, 269–273. [Google Scholar] [CrossRef]
- Klavdieva, M.M. The history of neuropeptides II. Front. Neuroendocrinol. 1996, 17, 126–153. [Google Scholar] [CrossRef]
- Zelena, D.; Barna, I.; Pinter, O.; Klausz, B.; Varga, J.; Makara, G.B. Congenital absence of vasopressin and age-dependent changes in ACTH and corticosterone stress responses in rats. Stress 2011, 14, 420–430. [Google Scholar] [CrossRef]
- Fodor, A.; Pinter, O.; Domokos, A.; Langnaese, K.; Barna, I.; Engelmann, M.; Zelena, D. Blunted HPA axis response in lactating, vasopressin-deficient Brattleboro rats. J. Endocrinol. 2013, 219, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Torres-Aleman, I.; Barasoain, I.; Borrell, J.; Guaza, C. Immune activation and psychoneurogenic stress modulate corticosterone-releasing effects of lymphokines and ACTH. Am. J. Physiol. 1988, 255, R839–R845. [Google Scholar] [CrossRef] [Green Version]
- Guldenaar, S.E.; Noctor, S.C.; McCabe, J.T. Fos-like immunoreactivity in the brain of homozygous diabetes insipidus Brattleboro and normal Long-Evans rats. J. Comp. Neurol. 1992, 322, 439–448. [Google Scholar] [CrossRef]
- Bundzikova, J.; Pirnik, Z.; Mikkelsen, J.D.; Zelena, D.; Kiss, A. Activity of oxytocinergic neurons in the supraoptic nucleus under stimulation of alpha2-adrenoceptors in Brattleboro rats. Ann. N. Y. Acad. Sci. 2008, 1148, 154–160. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Onaka, T. The medial amygdala-medullary PrRP-synthesizing neuron pathway mediates neuroendocrine responses to contextual conditioned fear in male rodents. Endocrinology 2014, 155, 2996–3004. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, M. Dendritic release of vasopressin and oxytocin. J. Neuroendocrinol. 1998, 10, 881–895. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Ludwig, M.; Bull, P.M.; Tobin, V.A.; Sabatier, N.; Landgraf, R.; Dayanithi, G.; Leng, G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J. Physiol. 2005, 564, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Otero-Garcia, M.; Agustin-Pavon, C.; Lanuza, E.; Martinez-Garcia, F. Distribution of oxytocin and co-localization with arginine vasopressin in the brain of mice. Brain Struct. Funct. 2016, 221, 3445–3473. [Google Scholar] [CrossRef]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsa, D.M.; Petracca, F.M.; Baskin, D.G.; Cornett, L.E. Localization and characterization of vasopressin-binding sites in the amygdala of the rat brain. J. Neurosci. Off. J. Soc. Neurosci. 1984, 4, 1764–1770. [Google Scholar] [CrossRef]
- Freund-Mercier, M.J.; Stoeckel, M.E.; Dietl, M.M.; Palacios, J.M.; Richard, P. Quantitative autoradiographic mapping of neurohypophysial hormone binding sites in the rat forebrain and pituitary gland—I. Characterization of different types of binding sites and their distribution in the Long-Evans strain. Neuroscience 1988, 26, 261–272. [Google Scholar] [CrossRef]
- Tribollet, E.; Barberis, C.; Jard, S.; Dubois-Dauphin, M.; Dreifuss, J.J. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988, 442, 105–118. [Google Scholar] [CrossRef]
- Ferris, C.F.; Stolberg, T.; Kulkarni, P.; Murugavel, M.; Blanchard, R.; Blanchard, D.C.; Febo, M.; Brevard, M.; Simon, N.G. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, M.K.; Nephew, B.C.; Febo, M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology 2010, 58, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Vaccari, C.; Lolait, S.J.; Ostrowski, N.L. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 1998, 139, 5015–5033. [Google Scholar] [CrossRef]
- Hernando, F.; Schoots, O.; Lolait, S.J.; Burbach, J.P. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: Anatomical support for its involvement in the central effects of vasopressin. Endocrinology 2001, 142, 1659–1668. [Google Scholar] [CrossRef]
- Corbani, M.; Marir, R.; Trueba, M.; Chafai, M.; Vincent, A.; Borie, A.M.; Desarménien, M.G.; Ueta, Y.; Tomboly, C.; Olma, A.; et al. Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands. Gen. Comp. Endocrinol. 2018, 258, 15–32. [Google Scholar] [CrossRef]
- Guirado, S.; Real, M.A.; Davila, J.C. Distinct immunohistochemically defined areas in the medial amygdala in the developing and adult mouse. Brain Res. Bull. 2008, 75, 214–217. [Google Scholar] [CrossRef]
- Neckel, H.; Quagliotto, E.; Casali, K.R.; Montano, N.; Lago, P.D.; Rasia-Filho, A.A. Glutamate and GABA in the medial amygdala induce selective central sympathetic/parasympathetic cardiovascular responses. Can. J. Physiol. Pharmacol. 2012, 90, 525–536. [Google Scholar] [CrossRef]
- Westberry, J.M.; Meredith, M. GABAergic mechanisms contributing to categorical amygdala responses to chemosensory signals. Neuroscience 2016, 331, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Grimes, J.M.; Ricci, L.A.; Melloni, R.H., Jr. Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm. Behav. 2003, 44, 271–280. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z.; Zhao, C.; Li, L. Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav. Brain Res. 2013, 245, 42–49. [Google Scholar] [CrossRef]
- Alescio-Lautier, B.; Paban, V.; Soumireu-Mourat, B. Neuromodulation of memory in the hippocampus by vasopressin. Eur. J. Pharmacol. 2000, 405, 63–72. [Google Scholar] [CrossRef]
- Tobin, V.A.; Hashimoto, H.; Wacker, D.W.; Takayanagi, Y.; Langnaese, K.; Caquineau, C.; Noack, J.; Landgraf, R.; Onaka, T.; Leng, G.; et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 2010, 464, 413–417. [Google Scholar] [CrossRef]
- Leng, G.; Leng, R.I.; Maclean, S. The vasopressin-memory hypothesis: A citation network analysis of a debate. Ann. N. Y. Acad. Sci. 2019, 1455, 126–140. [Google Scholar] [CrossRef]
- Hume, C.; Allchorne, A.; Grinevich, V.; Leng, G.; Ludwig, M. Effects of optogenetic stimulation of vasopressinergic retinal afferents on suprachiasmatic neurones. J. Neuroendocrinol. 2019, 31, e12806. [Google Scholar] [CrossRef] [PubMed]
- Bredewold, R.; Veenema, A.H. Sex differences in the regulation of social and anxiety-related behaviors: Insights from vasopressin and oxytocin brain systems. Curr. Opin. Neurobiol. 2018, 49, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Beiderbeck, D.I.; Lukas, M.; Neumann, I.D. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010, 58, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Wotjak, C.T.; Holsboer, F.; Landgraf, R.; Engelmann, M. Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. Eur. J. Neurosci. 1999, 11, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Wotjak, C.T.; Landgraf, R.; Engelmann, M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur. J. Neurosci. 2002, 15, 384–388. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Sullivan, R.K.; Ianno, D.J.; Sah, P. Functional properties and projections of neurons in the medial amygdala. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 8699–8715. [Google Scholar] [CrossRef] [Green Version]
- Westberry, J.M.; Meredith, M. Characteristic Response to Chemosensory Signals in GABAergic Cells of Medial Amygdala Is Not Driven by Main Olfactory Input. Chem. Senses 2016, 42, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nephew, B.C.; Bridges, R.S. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol. Behav. 2008, 95, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Terranova, J.I.; Ferris, C.F.; Albers, H.E. Sex Differences in the Regulation of Offensive Aggression and Dominance by Arginine-Vasopressin. Front. Endocrinol. 2017, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Cooke, B.M.; Woolley, C.S. Gonadal hormone modulation of dendrites in the mammalian CNS. J. Neurobiol. 2005, 64, 34–46. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Török, B.; Csikota, P.; Fodor, A.; Balázsfi, D.; Ferenczi, S.; Demeter, K.; Tóth, Z.E.; Könczöl, K.; Perna, J.C.; Farkas, I.; et al. Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats. Int. J. Mol. Sci. 2022, 23, 1357. https://doi.org/10.3390/ijms23031357
Török B, Csikota P, Fodor A, Balázsfi D, Ferenczi S, Demeter K, Tóth ZE, Könczöl K, Perna JC, Farkas I, et al. Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats. International Journal of Molecular Sciences. 2022; 23(3):1357. https://doi.org/10.3390/ijms23031357
Chicago/Turabian StyleTörök, Bibiána, Péter Csikota, Anna Fodor, Diána Balázsfi, Szilamér Ferenczi, Kornél Demeter, Zsuzsanna E. Tóth, Katalin Könczöl, Judith Camats Perna, Imre Farkas, and et al. 2022. "Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats" International Journal of Molecular Sciences 23, no. 3: 1357. https://doi.org/10.3390/ijms23031357
APA StyleTörök, B., Csikota, P., Fodor, A., Balázsfi, D., Ferenczi, S., Demeter, K., Tóth, Z. E., Könczöl, K., Perna, J. C., Farkas, I., Kovács, K. J., Haller, J., Engelmann, M., & Zelena, D. (2022). Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats. International Journal of Molecular Sciences, 23(3), 1357. https://doi.org/10.3390/ijms23031357






