Tadalafil Treatment of Mice with Fetal Growth Restriction and Preeclampsia Improves Placental mTOR Signaling
Abstract
:1. Introduction
2. Results
2.1. Expression of Phosphorylated mTOR (p-mTOR) in Mice Placenta
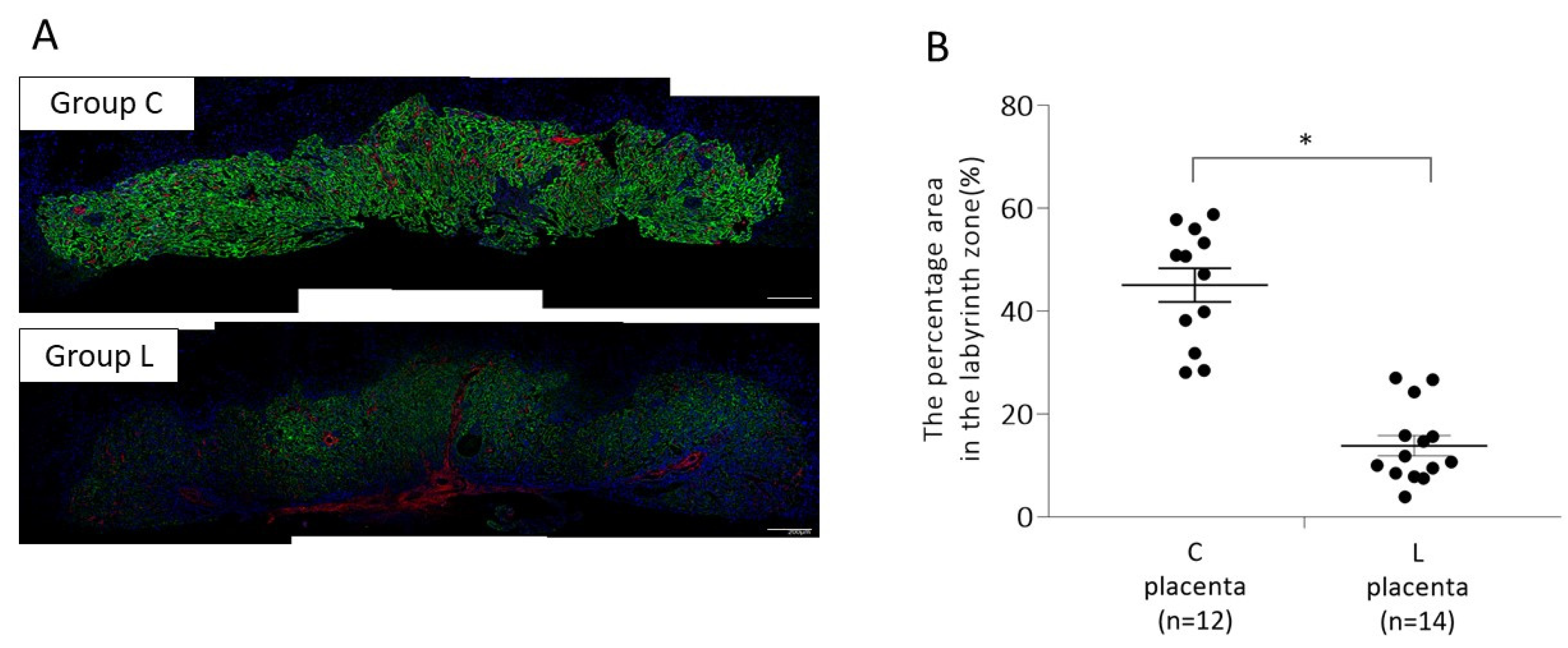
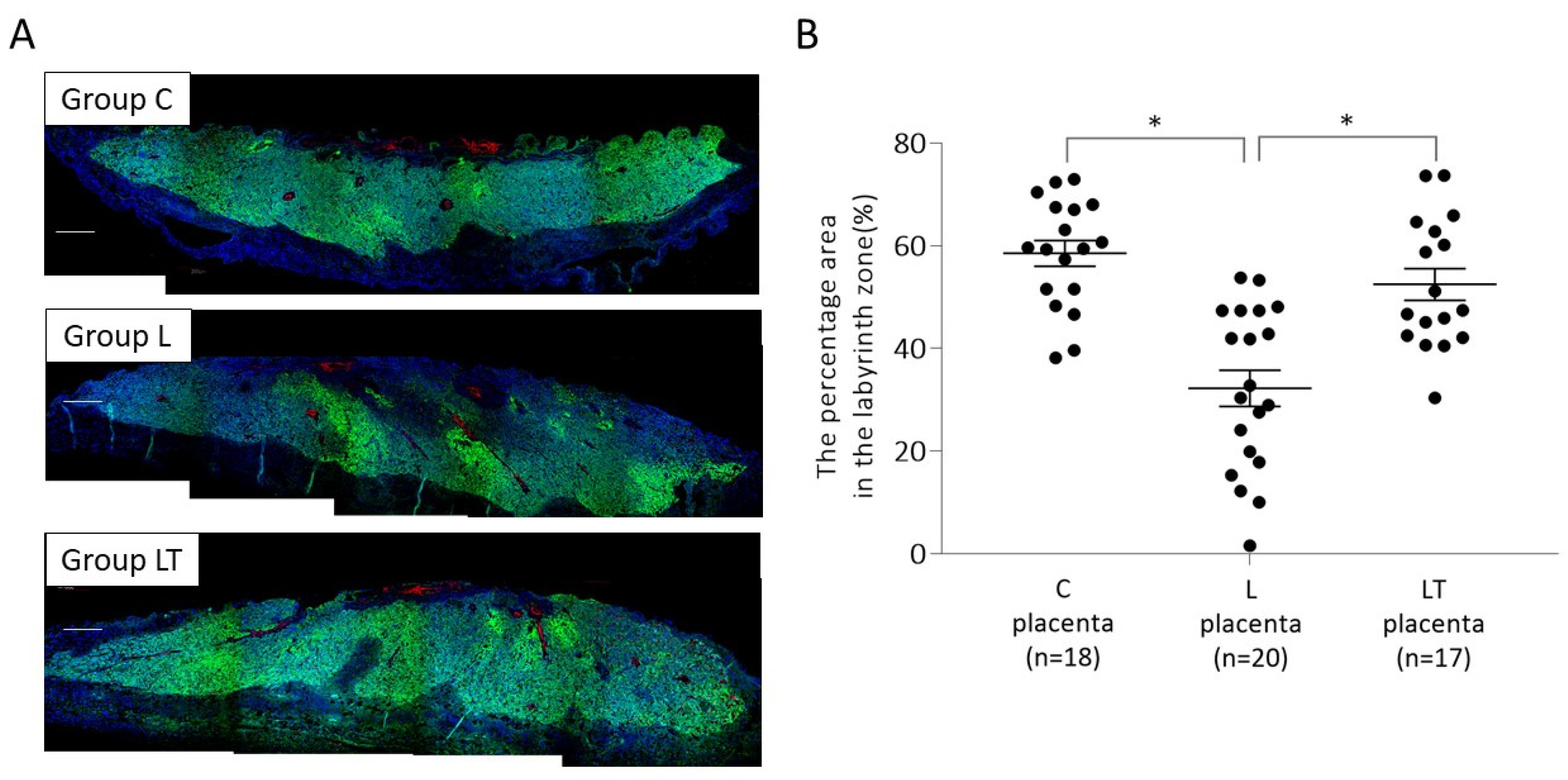
2.1.1. Placental p-mTOR Expression on 13 Days Post Coitum (d.p.c.)
2.1.2. Placental p-mTOR Expression on 17 d.p.c
2.2. Expression Analysis of Phosphorylated 4E (eIF4E)-Binding Protein 1 (p-4E-BP1) and Phosphorylated Ribosomal S6 Protein (p-S6R) in Mouse Placenta
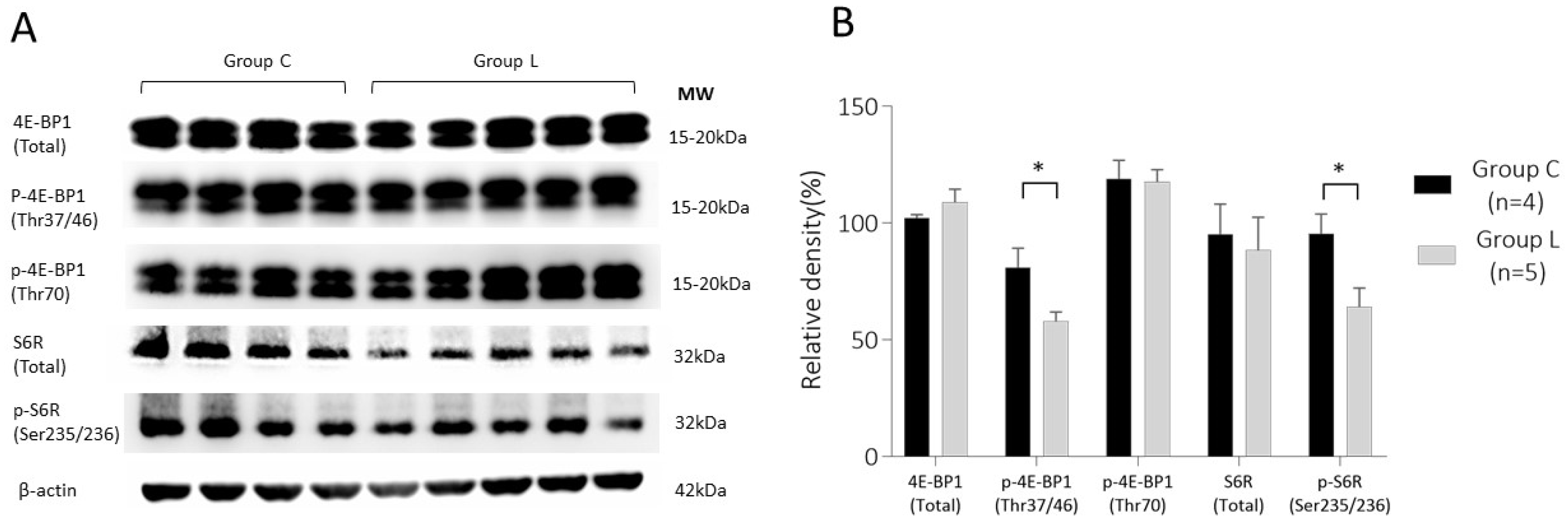
2.2.1. 4E-BP1 and S6R Expression in Placenta on 13 d.p.c.
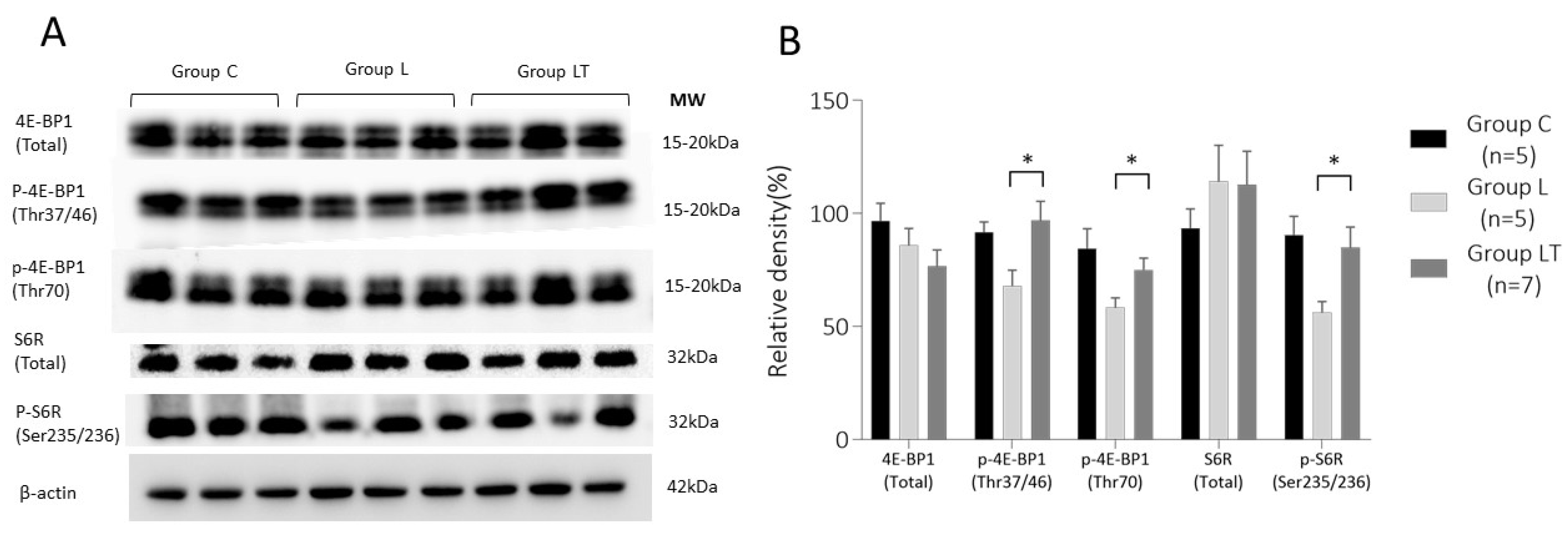
2.2.2. 4E-BP1 and S6R Expression in Placenta on 17 d.p.c.
3. Discussion
4. Materials and Methods
4.1. Establishment of FGR Mouse Model and Experimental Protocol
4.2. Fluorescent Immunohistochemical Staining
4.3. Western Blotting
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pallotto, E.K.; Kilbride, H.W. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, A.; Brunelli, V.; Prefumo, F.; Frusca, T.; Lees, C.C. Early onset fetal growth restriction. Matern Health Neonatol Perinatol. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinonen, K.; Räikkönen, K.; Pesonen, A.K.; Andersson, S.; Kajantie, E.; Eriksson, J.G.; Wolke, D.; Lano, A. Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: A longitudinal study. BMC Pediatr. 2010, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Pearson, R.; Fernandes, M.; Santos, I.S.; Barros, F.C.; Victora, C.G.; Stein, A.; Matijasevich, A. Are fetal growth impairment and preterm birth causally related to child attention problems and ADHD? Evidence from a comparison between high-income and middle-income cohorts. J Epidemiol Community Health. 2016, 70, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.G.; Hornbuckle, J.; Vail, A.; Spiegelhalter, D.J.; Levene, M.; GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): Multicentred randomized controlled trial. Lancet 2004, 364, 513–520. [Google Scholar] [CrossRef]
- Lees, C.C.; Marlow, N.; van Wassenaer-Leemhuis, A.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Calvert, S.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): A randomised trial. Lancet 2015, 385, 2162–2172. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Umekawa, T.; Maki, S.; Kubo, M.; Nii, M.; Tanaka, K.; Tanaka, H.; Osato, K.; Kamimoto, Y.; Kondo, E.; et al. Tadalafil Improves L-NG-Nitroarginine Methyl Ester-Induced Preeclampsia With Fetal Growth Restriction-Like Symptoms in Pregnant Mice. Am J Hypertens. 2017, 31, 89–96. [Google Scholar] [CrossRef]
- Tachibana, R.; Umekawa, T.; Yoshikawa, K.; Owa, T.; Magawa, S.; Furuhashi, F.; Tsuji, M.; Maki, S.; Shimada, K.; Kaneda, M.K.; et al. Tadalafil treatment in mice for preeclampsia with fetal growth restriction has neuro-benefic effects in offspring through modulating prenatal hypoxic conditions. Sci Rep. 2019, 9, 234. [Google Scholar] [CrossRef]
- Sekimoto, A.; Tanaka, K.; Hashizume, Y.; Sato, E.; Sato, H.; Ikeda, T.; Takahashi, N. Tadalafil alleviates preeclampsia and fetal growth restriction in RUPP model of preeclampsia in mice. Biochem Biophys Res Commun. 2020, 521, 769–774. [Google Scholar] [CrossRef]
- Kubo, M.; Tanaka, H.; Maki, S.; Nii, M.; Murabayashi, N.; Osato, K.; Kamimoto, Y.; Umekawa, T.; Kondo, E.; Ikeda, T. Safety and dose-finding trial of tadalafil administered for fetal growth restriction: A phase-1 clinical study. J Obstet Gynaecol Res. 2017, 43, 1159–1168. [Google Scholar] [CrossRef]
- Maki, S.; Tanaka, H.; Tsuji, M.; Furuhashi, F.; Magawa, S.; Kaneda, M.K.; Nii, M.; Tanaka, K.; Kondo, E.; Tamaru, S.; et al. Safety Evaluation of Tadalafil Treatment for Fetuses with Early-Onset Growth Restriction (TADAFER): Results from the Phase II Trial. J. Clin. Med. 2019, 8, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.B.; Jansson, T. Novel roles of mechanistic target of rapamycin signaling in regulating fetal growth†. Biol Reprod. 2019, 100, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jansson, N.; Palmberg, I.; Säljö, K.; Powell, T.L.; Jansson, T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol. 2007, 582, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Golub, T.R.; Sabatini, D.M. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002, 22, 5575–5584. [Google Scholar] [CrossRef] [Green Version]
- Tee, A.R.; Blenis, J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005, 16, 29–37. [Google Scholar] [CrossRef]
- Martin, D.E.; Hall, M.N. The expanding TOR signaling network. Curr Opin Cell Biol. 2005, 17, 158–166. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, E.; Hall, M.N. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003, 4, 117–126, correction appears in Nat Rev Mol Cell Biol. 2003, 4, 249. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Rosario, F.J.; Gupta, M.B.; Myatt, L.; Powell, T.L.; Glenn, J.P.; Cox, L.; Jansson, T. Mechanistic Target of Rapamycin Complex 1 Promotes the Expression of Genes Encoding Electron Transport Chain Proteins and Stimulates Oxidative Phosphorylation in Primary Human Trophoblast Cells by Regulating Mitochondrial Biogenesis. Sci Rep. 2019, 9, 246. [Google Scholar] [CrossRef]
- Roos, S.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol. 2009, 296, C142–C150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, S.; Lagerlöf, O.; Wennergren, M.; Powell, T.L.; Jansson, T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009, 297, C723–C731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 2013, 591, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Dimasuay, K.G.; Kanai, Y.; Powell, T.L.; Jansson, T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2. Clin. Sci (Lond). 2016, 130, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Rosario, F.J.; Powell, T.L.; Jansson, T. Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-α and the RFC. Sci Rep. 2016, 6, 31705. [Google Scholar] [CrossRef] [Green Version]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Rosario, F.J.; Shehab, M.A.; Powell, T.L.; Gupta, M.B.; Jansson, T. Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin. Sci (Lond). 2015, 129, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.H.; Hsieh, T.T.; Wu, C.P.; Li, M.J.; Yeh, Y.L.; Chen, S.F. Mammalian target of rapamycin signaling is a mechanistic link between increased endoplasmic reticulum stress and autophagy in the placentas of pregnancies complicated by growth restriction. Placenta 2017, 60, 9–20. [Google Scholar] [CrossRef]
- Chassen, S.; Jansson, T. Complex, coordinated and highly regulated changes in placental signaling and nutrient transport capacity in IUGR. Biochim Biophys Acta Mol. Basis Dis. 2020, 1866, 165373. [Google Scholar] [CrossRef]
- Kavitha, J.V.; Rosario, F.J.; Nijland, M.J.; McDonald, T.J.; Wu, G.; Kanai, Y.; Powell, T.L.; Nathanielsz, P.W.; Jansson, T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014, 28, 1294–1305. [Google Scholar] [CrossRef] [Green Version]
- Rosario, F.J.; Schumacher, M.A.; Jiang, J.; Kanai, Y.; Powell, T.L.; Jansson, T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J. Physiol. 2012, 590, 1495–1509. [Google Scholar] [CrossRef]
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced Uterine Perfusion Pressure (RUPP) Model of Preeclampsia in Mice. PLoS One 2016, 11, e0155426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakrania, B.A.; George, E.M.; Granger, J.P. Animal models of preeclampsia: Investigating pathophysiology and therapeutic targets. Am. J. Obstet Gynecol. 2020. [Google Scholar] [CrossRef]
- Rotella, D.P. Phosphodiesterase 5 inhibitors: Current status and potential applications. Nat. Rev. Drug Discov. 2002, 1, 674–682. [Google Scholar] [CrossRef] [PubMed]
- França, M.E.R.; Ramos, R.K.L.G.; Oliveira, W.H.; Duarte-Silva, E.; Araújo, S.M.R.; Lós, D.B.; Peixoto, C.A. Tadalafil restores long-term memory and synaptic plasticity in mice with hepatic encephalopathy. Toxicol Appl Pharmacol. 2019, 379, 114673. [Google Scholar] [CrossRef] [PubMed]
- Gresser, U.; Gleiter, C.H. Erectile dysfunction: Comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil-review of the literature. Eur J. Med. Res. 2002, 7, 435–446. [Google Scholar] [PubMed]
- Ghofrani, H.A.; Voswinckel, R.; Reichenberger, F.; Olschewski, H.; Haredza, P.; Karadaş, B.; Schermuly, R.T.; Weissmann, N.; Seeger, W.; Grimminger, F. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: A randomized prospective study. J. Am. Coll Cardiol. 2004, 44, 1488–1496. [Google Scholar] [CrossRef] [Green Version]
- Barroso, F.; Ribeiro, J.C.; Miranda, E.P. Phosphodiesterase Type 5 Inhibitors and Visual Side Effects: A Narrative Review. J. Ophthalmic Vis. Res. 2021, 16, 248–259. [Google Scholar] [CrossRef]
- Saenz de Tejada, I.; Angulo, J.; Cuevas, P.; Fernández, A.; Moncada, I.; Allona, A.; Lledó, E.; Körschen, H.G.; Niewöhner, U.; Haning, H.; et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J. Impot Res. 2001, 13, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Sharp, A.; Cornforth, C.; Jackson, R.; Harrold, J.; Turner, M.A.; Kenny, L.C.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): A multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child. Adolesc Health. 2018, 2, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Groom, K.M.; McCowan, L.M.; Mackay, L.K.; Lee, A.C.; Gardener, G.; Unterscheider, J.; Sekar, R.; Dickinson, J.E.; Muller, P.; Reid, R.A.; et al. STRIDER NZAus: A multicentre randomised controlled trial of sildenafil therapy in early-onset fetal growth restriction. BJOG. 2019, 126, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Pels, A.; Derks, J.; Elvan-Taspinar, A.; van Drongelen, J.; de Boer, M.; Duvekot, H.; van Laar, J.; van Eyck, J.; Al-Nasiry, S.; Sueters, M.; et al. Maternal Sildenafil vs Placebo in Pregnant Women With Severe Early-Onset Fetal Growth Restriction: A Randomized Clinical Trial. JAMA Netw Open. 2020, 3, e205323. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Tanaka, H.; Tachibana, R.; Yoshikawa, K.; Kawamura, T.; Takakura, S.; Takeuchi, H.; Ikeda, T. Tadalafil Treatment of Mice with Fetal Growth Restriction and Preeclampsia Improves Placental mTOR Signaling. Int. J. Mol. Sci. 2022, 23, 1474. https://doi.org/10.3390/ijms23031474
Tanaka K, Tanaka H, Tachibana R, Yoshikawa K, Kawamura T, Takakura S, Takeuchi H, Ikeda T. Tadalafil Treatment of Mice with Fetal Growth Restriction and Preeclampsia Improves Placental mTOR Signaling. International Journal of Molecular Sciences. 2022; 23(3):1474. https://doi.org/10.3390/ijms23031474
Chicago/Turabian StyleTanaka, Kayo, Hiroaki Tanaka, Ryota Tachibana, Kento Yoshikawa, Takuya Kawamura, Sho Takakura, Hiroki Takeuchi, and Tomoaki Ikeda. 2022. "Tadalafil Treatment of Mice with Fetal Growth Restriction and Preeclampsia Improves Placental mTOR Signaling" International Journal of Molecular Sciences 23, no. 3: 1474. https://doi.org/10.3390/ijms23031474
APA StyleTanaka, K., Tanaka, H., Tachibana, R., Yoshikawa, K., Kawamura, T., Takakura, S., Takeuchi, H., & Ikeda, T. (2022). Tadalafil Treatment of Mice with Fetal Growth Restriction and Preeclampsia Improves Placental mTOR Signaling. International Journal of Molecular Sciences, 23(3), 1474. https://doi.org/10.3390/ijms23031474






