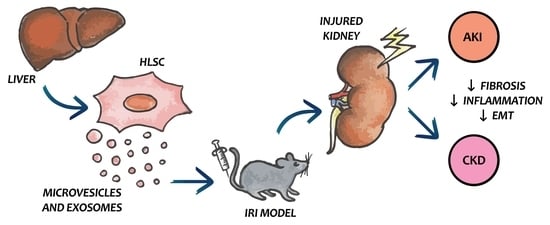
Extracellular Vesicles Derived from Human Liver Stem Cells Attenuate Chronic Kidney Disease Development in an In Vivo Experimental Model of Renal Ischemia and Reperfusion Injury
Abstract
:1. Introduction
2. Results
2.1. HLSC-EVs Improve Kidney Function and Morphology of IRI-CKD Mice
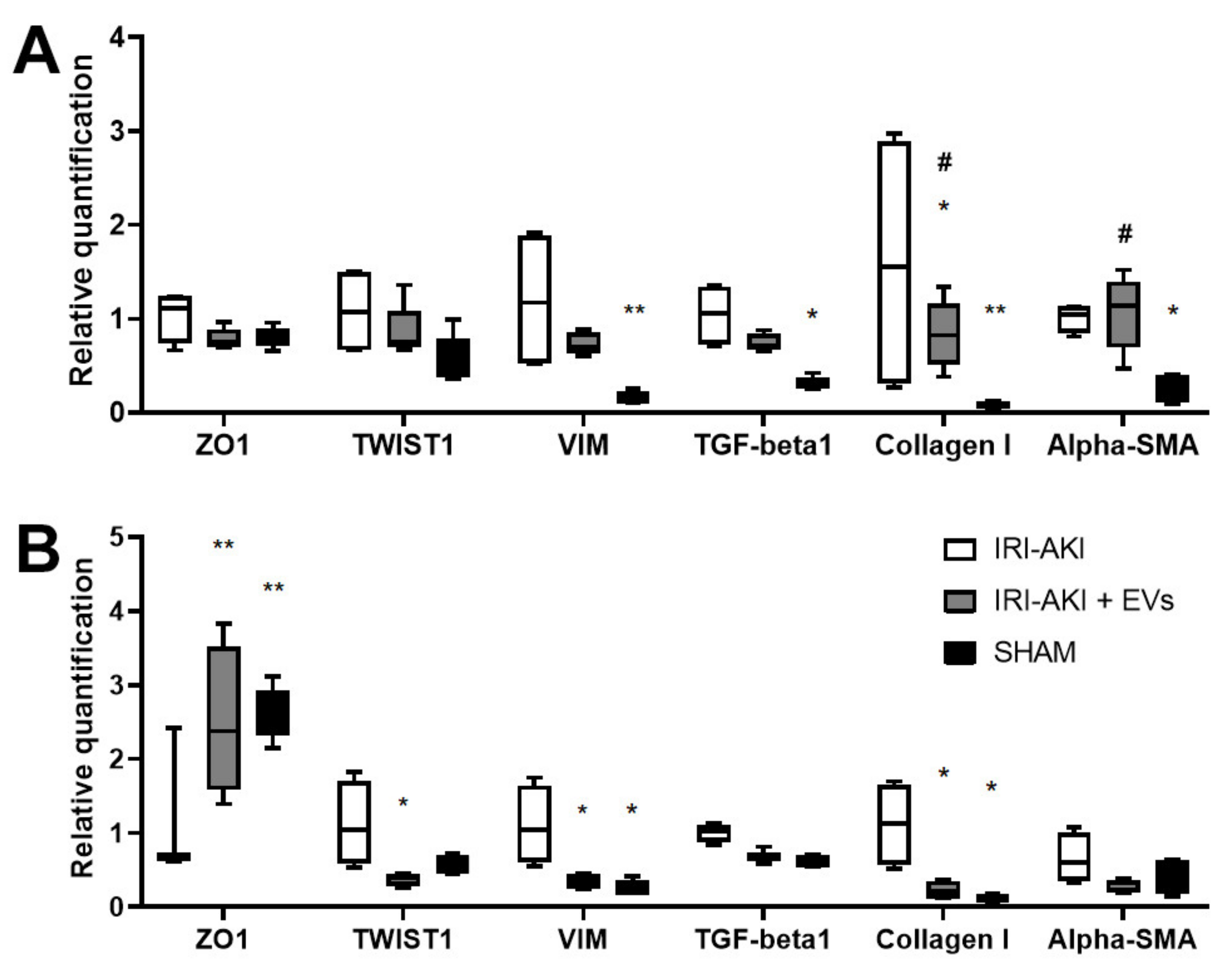
2.2. HLSC-EVs Effects on Renal Function, Morphology and Gene Expression Profile of IRI-Mice Sacrificed at Early Time Points
3. Discussion
4. Materials and Methods
4.1. HLSC Culture
4.2. Purification and Characterization of HLSC-EVs
4.3. Western Blot Analysis
4.4. In Vivo Murine Model
4.5. Renal Function
4.6. Histological Analyses
4.7. Molecular Analyses
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IRI | ischemia and reperfusion injury |
| AKI | acute kidney injury |
| TECs | tubular epithelial cells |
| CKD | chronic kidney disease |
| EMT | epithelial-to-mesenchymal transition |
| TGF-beta | transforming growth factor-beta |
| MSC | mesenchymal stromal cell |
| EV | extracellular vesicle |
| MSC-EVs | EVs derived from MSCs |
| HLSC | human liver stem cell |
| CM | conditioned medium |
| HLSC-EVs | EVs derived from HLSCs |
| alpha-SMA | alpha-smooth muscle actin |
| BUN | blood urea nitrogen |
| ANOVA | analysis of variance |
| IL-6 | interleukin-6 |
| TNF-alpha | tumor necrosis factor-alpha |
| VIM | vimentin |
| ZO1 | zonula occludens 1 |
| PCNA | proliferating cell nuclear antigen |
| TUNEL | terminal transferase-mediated dUTP nick-end labeling |
| HPF | high-power field |
| KIM1 | Kidney injury molecule 1 |
| LCN2 | lipocalin 2/NGAL |
| SELE | E-selectin |
| SELP | P-selectin |
| NF-KB | nuclear factor kappa-light-chain-enhancer of activated B-cells |
| CCNB1 | G2/mitotic-specific cyclin-B1 |
| MFI | median fluorescence intensity |
| qRT-PCR | quantitative Real-Time PCR |
References
- Bonventre, J.V.; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Westenfelder, C. Recent Advances in the Understanding of Acute Kidney Injury. F1000Prime Rep. 2014, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; ADQI XIII Work Group. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Hills, C.E.; Squires, P.E. The Role of TGF-β and Epithelial-to Mesenchymal Transition in Diabetic Nephropathy. Cytokine Growth Factor Rev. 2011, 22, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. New Insights into Epithelial-Mesenchymal Transition in Kidney Fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Menon, M.C.; Ross, M.J. Epithelial-to-Mesenchymal Transition of Tubular Epithelial Cells in Renal Fibrosis: A New Twist on an Old Tale. Kidney Int. 2016, 89, 263–266. [Google Scholar] [CrossRef]
- Gregorini, M.; Bosio, F.; Rocca, C.; Corradetti, V.; Valsania, T.; Pattonieri, E.F.; Esposito, P.; Bedino, G.; Collesi, C.; Libetta, C.; et al. Mesenchymal stromal cells reset the scatter factor system and cytokine network in experimental kidney transplantation. BMC Immunol. 2014, 15, 44. [Google Scholar] [CrossRef] [Green Version]
- Trohatou, O.; Roubelakis, M.G. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram. 2017, 19, 217–224. [Google Scholar] [CrossRef]
- Birtwistle, L.; Chen, X.-M.; Pollock, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int. J. Mol. Sci. 2021, 22, 6596. [Google Scholar] [CrossRef]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells 2019, 8, 1240. [Google Scholar] [CrossRef] [Green Version]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal Stem Cell-Derived Microvesicles Protect against Acute Tubular Injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [Green Version]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Soares Lindoso, R.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S.; et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng. Part A 2017, 23, 1262–1273. [Google Scholar] [CrossRef]
- Choi, H.Y.; Moon, S.J.; Ratliff, B.B.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, S.; Lim, B.J.; Kim, B.S.; Plotkin, M.D.; et al. Microparticles from Kidney-Derived Mesenchymal Stem Cells Act as Carriers of Proangiogenic Signals and Contribute to Recovery from Acute Kidney Injury. PLoS ONE 2014, 9, e87853. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Bruno, S.; Incarnato, D.; Dettori, D.; Neri, F.; Provero, P.; Pomatto, M.; Oliviero, S.; Tetta, C.; Quesenberry, P.J.; et al. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J. Am. Soc. Nephrol. 2015, 26, 2349–2360. [Google Scholar] [CrossRef]
- Collino, F.; Lopes, J.A.; Corrêa, S.; Abdelhay, E.; Takiya, C.M.; Wendt, C.H.C.; de Miranda, K.R.; Vieyra, A.; Lindoso, R.S. Adipose-Derived Mesenchymal Stromal Cells Under Hypoxia: Changes in Extracellular Vesicles Secretion and Improvement of Renal Recovery after Ischemic Injury. Cell Physiol. Biochem. 2019, 52, 1463–1483. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through MiR-30. Stem Cells Int. 2016, 2016, 2093940. [Google Scholar] [CrossRef] [Green Version]
- Herrera Sanchez, M.B.; Bruno, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human Liver Stem Cells and Derived Extracellular Vesicles Improve Recovery in a Murine Model of Acute Kidney Injury. Stem Cell Res. Ther. 2014, 5, 124. [Google Scholar] [CrossRef] [Green Version]
- Ju, G.; Cheng, J.; Zhong, L.; Wu, S.; Zou, X.; Zhang, G.; Gu, D.; Miao, S.; Zhu, Y.; Sun, J.; et al. Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Tubular Epithelial Cell Dedifferentiation and Growth via Hepatocyte Growth Factor Induction. PLoS ONE 2015, 10, e0121534. [Google Scholar] [CrossRef] [Green Version]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; Dimuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The Effects of Glomerular and Tubular Renal Progenitors and Derived Extracellular Vesicles on Recovery from Acute Kidney Injury. Stem Cell Res. Ther. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Reis, L.A.; Borges, F.T.; Simões, M.J.; Borges, A.A.; Sinigaglia-Coimbra, R.; Schor, N. Bone Marrow-Derived Mesenchymal Stem Cells Repaired but Did Not Prevent Gentamicin-Induced Acute Kidney Injury through Paracrine Effects in Rats. PLoS ONE 2012, 7, e44092. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016, 2016, e1240301. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes Released by Human Umbilical Cord Mesenchymal Stem Cells Protect against Cisplatin-Induced Renal Oxidative Stress and Apoptosis In Vivo and In Vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles Derived from Human Wharton’s Jelly Mesenchymal Stromal Cells Ameliorate Renal Ischemia-Reperfusion Injury in Rats by Suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal Stem Cell–Derived Extracellular Vesicles Attenuate Kidney Inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem Cell-Derived Extracellular Vesicles Inhibit and Revert Fibrosis Progression in a Mouse Model of Diabetic Nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone Marrow Stem Cells-Derived Microvesicles Protect against Renal Injury in the Mouse Remnant Kidney Model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-Vesicles Derived from Bone Marrow Stem Cells Protect the Kidney Both In Vivo and In Vitro by MicroRNA-Dependent Repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Niu, X.; Yin, J.; Hu, B.; Guo, S.; Fan, Y.; Wang, Y.; Wang, N. Exosomes Secreted by Human Urine-Derived Stem Cells Could Prevent Kidney Complications from Type I Diabetes in Rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front. Immunol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal Stem Cell Therapy Ameliorates Diabetic Nephropathy via the Paracrine Effect of Renal Trophic Factors Including Exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.B.; Bruno, S.; Buttiglieri, S.; Tetta, C.; Gatti, S.; Deregibus, M.C.; Bussolati, B.; Camussi, G. Isolation and Characterization of a Stem Cell Population from Adult Human Liver. Stem Cells 2006, 24, 2840–2850. [Google Scholar] [CrossRef]
- Bruno, S.; Herrera Sanchez, M.B.; Chiabotto, G.; Fonsato, V.; Navarro-Tableros, V.; Pasquino, C.; Tapparo, M.; Camussi, G. Human Liver Stem Cells: A Liver-Derived Mesenchymal Stromal Cell-Like Population with Pro-Regenerative Properties. Front. Cell Dev. Biol. 2021, 9, 644088. [Google Scholar] [CrossRef]
- Bruno, S.; Pasquino, C.; Herrera Sanchez, M.B.; Tapparo, M.; Figliolini, F.; Grange, C.; Chiabotto, G.; Cedrino, M.; Deregibus, M.C.; Tetta, C.; et al. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-Alcoholic Steatohepatitis. Mol. Ther. 2020, 28, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Chiabotto, G.; Ceccotti, E.; Tapparo, M.; Camussi, G.; Bruno, S. Human liver stem cell-derived extracellular vesicles target hepatic stellate cells and attenuate their pro-fibrotic phenotype. Front. Cell Dev. Biol. 2021, 9, 777462. [Google Scholar] [CrossRef]
- Kholia, S.; Herrera Sanchez, M.B.; Deregibus, M.C.; Sassoè-Pognetto, M.; Camussi, G.; Brizzi, M.F. Human Liver Stem Cell Derived Extracellular Vesicles Alleviate Kidney Fibrosis by Interfering with the β-Catenin Pathway through miR29b. Int. J. Mol. Sci. 2021, 22, 10780. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y. Dissection of Key Events in Tubular Epithelial to Myofibroblast Transition and Its Implications in Renal Interstitial Fibrosis. Am. J. Pathol. 2001, 159, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Carew, R.M.; Wang, B.; Kantharidis, P. The Role of EMT in Renal Fibrosis. Cell Tissue Res. 2012, 347, 103–116. [Google Scholar] [CrossRef]
- Boutet, A.; De Frutos, C.A.; Maxwell, P.H.; Mayol, M.J.; Romero, J.; Nieto, M.A. Snail Activation Disrupts Tissue Homeostasis and Induces Fibrosis in the Adult Kidney. EMBO J. 2006, 25, 5603–5613. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Kamara, H.; Svoboda, K.K.H. The Role of Twist during Palate Development. Dev. Dyn. 2008, 237, 2716–2725. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Marker of Kidney Damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Ozkok, A.; Ravichandran, K.; Wang, Q.; Ljubanovic, D.; Edelstein, C.L. NF-ΚB Transcriptional Inhibition Ameliorates Cisplatin-Induced Acute Kidney Injury (AKI). Toxicol. Lett. 2016, 240, 105–113. [Google Scholar] [CrossRef]
- Witzgall, R.; Brown, D.; Schwarz, C.; Bonventre, J.V. Localization of Proliferating Cell Nuclear Antigen, Vimentin, c-Fos, and Clusterin in the Postischemic Kidney. Evidence for a Heterogenous Genetic Response among Nephron Segments, and a Large Pool of Mitotically Active and Dedifferentiated Cells. J. Clin. Investig. 1994, 93, 2175–2188. [Google Scholar] [CrossRef] [Green Version]
- Calleri, A.; Roggio, D.; Navarro-Tableros, V.; De Stefano, N.; Pasquino, C.; David, E.; Frigatti, G.; Rigo, F.; Antico, F.; Caropreso, P.; et al. Protective Effects of Human Liver Stem Cell-Derived Extracellular Vesicles in a Mouse Model of Hepatic Ischemia-Reperfusion Injury. Stem Cell Rev. Rep. 2021, 17, 459–470. [Google Scholar] [CrossRef]
- Bruno, S.; Herrera Sanchez, M.B.; Pasquino, C.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G. Human Liver-Derived Stem Cells Improve Fibrosis and Inflammation Associated with Nonalcoholic Steatohepatitis. Stem Cells Int. 2019, 2019, 6351091. [Google Scholar] [CrossRef]
- Spada, M.; Porta, F.; Righi, D.; Gazzera, C.; Tandoi, F.; Ferrero, I.; Fagioli, F.; Sanchez, M.B.H.; Calvo, P.L.; Biamino, E.; et al. Intrahepatic Administration of Human Liver Stem Cells in Infants with Inherited Neonatal-Onset Hyperammonemia: A Phase I Study. Stem Cell Rev. Rep. 2020, 16, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-Based Precipitation of Extracellular Vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Koliha, N.; Wiencek, Y.; Heider, U.; Jüngst, C.; Kladt, N.; Krauthäuser, S.; Johnston, I.C.D.; Bosio, A.; Schauss, A.; Wild, S. A Novel Multiplex Bead-Based Platform Highlights the Diversity of Extracellular Vesicles. J. Extracell. Vesicles 2016, 5, 29975. [Google Scholar] [CrossRef]







| Gene | Forward (5′ → 3′) | Reverse (3′ → 5′) |
|---|---|---|
| m-Alpha-SMA | CATCTCCGAAGTCCAGCACA | GACGCACCACTGAACCCTAA |
| m-CCNB1 | GGATTCAAGTGCATTCTCTCAGTG | TCTGGCTGTCAGAATTCAAAGC |
| m-Collagen I | ACCTTGTTTGCCAGGTTCAC | ATCTCCCTGGTGCTGATGGAC |
| m-GAPDH | TGTCAAGCTCATTTCCTGGTA | TCTTACTCCTTGGAGGCCATGT |
| m-IL-1beta | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| m-IL-6 | ACCAGAGGAAATTTTCAATAGGC | TGATGCACTTGCAGAAAACA |
| m-KIM1 | ATGAATCAGATTCAAGTCTTC | TCTGGTTTGTGAGTCCATGTG |
| m-LCN2 | TGCACAGGTATCCTCAGGTACAGA | GGAAAAATACCATGGCGAACTG |
| m-NF-KB | ACAGGTCAAAATTTGCAACTATGTG | TGCATACCCCGTCCTCACA |
| m-SELE | GTCTAGCGCTGGATGAAAG | ATCGCCACCAGATGTGTGTA |
| m-SELP | GGGCTCAACTCATCTGGTTC | CATTGAGGTGAGCGATTTCA |
| m-SNAI1 | CTGCTTCGAGCCATAGAACTAAAG | GAGGGGAACTATTGCATAGTCTGT |
| m-TGF-beta | GCAACAATTCCTGGCGTTACC | CGAAAGCCCTGTATTCCGTCT |
| m-TNF-alpha | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| m-TWIST1 | AGCGGGTCATGGCTAACG | GGACCTGGTACAGGAAGTCGA |
| m-VIM | GAAATTGCAGGAGGAGATGC | TCCACTTTCCGTTCAAGGTC |
| m-ZO1 | GATCCCTGTAAGTCACCCAGA | CGCTCATCTCTTTGCACTACCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, S.; Chiabotto, G.; Cedrino, M.; Ceccotti, E.; Pasquino, C.; De Rosa, S.; Grange, C.; Tritta, S.; Camussi, G. Extracellular Vesicles Derived from Human Liver Stem Cells Attenuate Chronic Kidney Disease Development in an In Vivo Experimental Model of Renal Ischemia and Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 1485. https://doi.org/10.3390/ijms23031485
Bruno S, Chiabotto G, Cedrino M, Ceccotti E, Pasquino C, De Rosa S, Grange C, Tritta S, Camussi G. Extracellular Vesicles Derived from Human Liver Stem Cells Attenuate Chronic Kidney Disease Development in an In Vivo Experimental Model of Renal Ischemia and Reperfusion Injury. International Journal of Molecular Sciences. 2022; 23(3):1485. https://doi.org/10.3390/ijms23031485
Chicago/Turabian StyleBruno, Stefania, Giulia Chiabotto, Massimo Cedrino, Elena Ceccotti, Chiara Pasquino, Samuela De Rosa, Cristina Grange, Stefania Tritta, and Giovanni Camussi. 2022. "Extracellular Vesicles Derived from Human Liver Stem Cells Attenuate Chronic Kidney Disease Development in an In Vivo Experimental Model of Renal Ischemia and Reperfusion Injury" International Journal of Molecular Sciences 23, no. 3: 1485. https://doi.org/10.3390/ijms23031485