Diabetic Retinopathy: From Animal Models to Cellular Signaling
Abstract
1. Introduction
2. Experimental Diabetic Retinopathy
Induction of Hyperglycemia
3. Rodent Models of Diabetic Retinopathy
3.1. Pathological Signs in Rodent Models of Diabetic Retinopathy
3.1.1. Neovascularization and Microvascular Changes in Diabetic Rodents
3.1.2. The Detection of Functional Changes of the Neural Retina in Diabetic Rodents
3.2. Cellular Signaling Changes in the Diabetic Rodent Retina
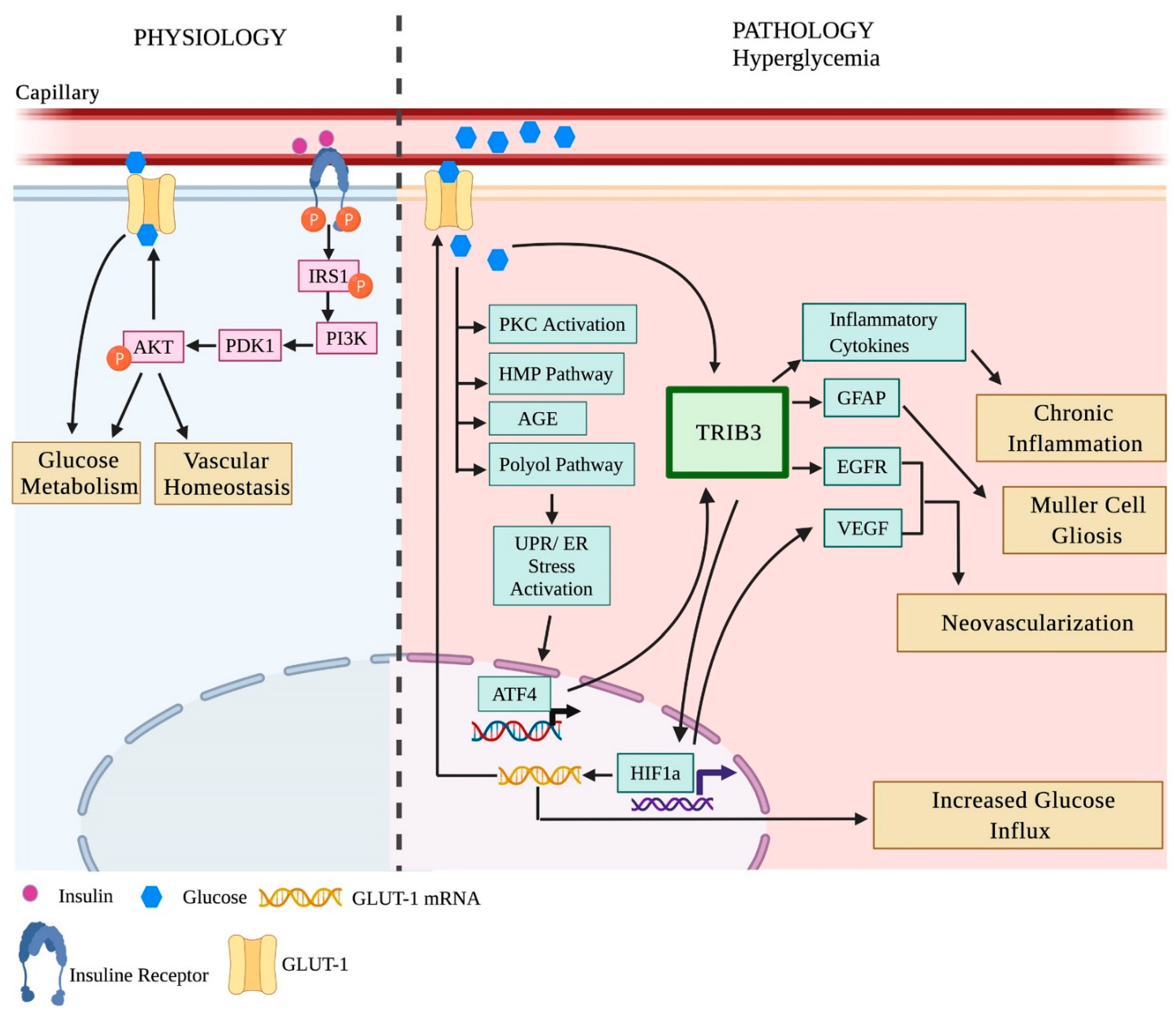
3.2.1. Insulin Signaling in the Diabetic Retina
3.2.2. Unfolded Protein Response (UPR) and Inflammation in the Diabetic Retina
4. Non-Rodent Models of Diabetic Retinopathy
4.1. Pathological Changes in Non-Rodent Animal Models of Diabetic Retinopathy
4.2. Cellular Signaling Changes in the Diabetic Retina of Non-Rodent Models
5. The Limitation of Animal Models of Diabetic Retinopathy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J. Diabetes Res. 2013, 2013, 905058. [Google Scholar] [CrossRef] [PubMed]
- Baget-Bernaldiz, M.; Romero-Aroca, P.; Bautista-Perez, A.; Mercado, J. Multifocal electroretinography changes at the 1-year follow-up in a cohort of diabetic macular edema patients treated with ranibizumab. Doc. Ophthalmol. 2017, 135, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, N.M.; Riazi-Esfahani, H.; Jafarzadehpur, E.; Mirzajani, A.; Talebi, H.; Amini, A.; Mazloumi, M.; Roohipoor, R.; Riazi-Esfahani, M. Multifocal Electroretinogram in Diabetic Macular Edema; Correlation with Visual Acuity and Optical Coherence Tomography. J. Ophthalmic. Vis. Res. 2015, 10, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.E.; DuPont, J.; Riva, C.E. Retinal haemodynamics in patients with early diabetes mellitus. Br. J. Ophthalmol. 1996, 80, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.H.; Schwartz, S.S. Diabetic Retinopathy-An Underdiagnosed and Undertreated Inflammatory, Neuro-Vascular Complication of Diabetes. Front. Endocrinol. 2019, 10, 843. [Google Scholar] [CrossRef]
- Nork, T.M.; Wallow, I.H.; Sramek, S.J.; Anderson, G. Muller’s cell involvement in proliferative diabetic retinopathy. Arch. Ophthalmol. 1987, 105, 1424–1429. [Google Scholar] [CrossRef]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef]
- Rungger-Brandle, E.; Dosso, A.A.; Leuenberger, P.M. Glial reactivity, an early feature of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Barber, A.J.; Antonetti, D.A.; Gardner, T.W. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Abu-El-Asrar, A.M.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2760–2766. [Google Scholar] [CrossRef]
- Muramatsu, D.; Wakabayashi, Y.; Usui, Y.; Okunuki, Y.; Kezuka, T.; Goto, H. Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Struyf, S.; Kangave, D.; Geboes, K.; Van Damme, J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur. Cytokine Netw. 2006, 17, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Freyberger, H.; Brocker, M.; Yakut, H.; Hammer, J.; Effert, R.; Schifferdecker, E.; Schatz, H.; Derwahl, M. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp. Clin. Endocrinol. Diabetes 2000, 108, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Elner, S.G.; Elner, V.M.; Jaffe, G.J.; Stuart, A.; Kunkel, S.L.; Strieter, R.M. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr. Eye Res. 1995, 14, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Yoshida, S.; Kobayashi, Y.; Zhou, Y.; Nakama, T.; Nakao, S.; Sassa, Y.; Oshima, Y.; Niiro, H.; Akashi, K.; et al. Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; McLeod, D.S.; Merges, C.; Diggs, A.; Plouet, J. Localization of vascular endothelial growth factor in human retina and choroid. Arch. Ophthalmol. 1996, 114, 971–977. [Google Scholar] [CrossRef]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Investig. 1996, 97, 2883–2890. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. Glucose metabolism and hyperglycemia. Am. J. Clin. Nutr. 2008, 87, 217S–222S. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Sakata, N.; Yoshimatsu, G.; Tsuchiya, H.; Egawa, S.; Unno, M. Animal models of diabetes mellitus for islet transplantation. Exp. Diabetes Res. 2012, 2012, 256707. [Google Scholar] [CrossRef]
- McLetchie, N.G. Alloxan diabetes: A discovery, albeit a minor one. J. R. Coll. Physicians Edinb. 2002, 32, 134–142. [Google Scholar] [PubMed]
- Engerman, R.L.; Kern, T.S. Experimental galactosemia produces diabetic-like retinopathy. Diabetes 1984, 33, 97–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rakieten, N.; Rakieten, M.L.; Nadkarni, M.V. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother. Rep. 1963, 29, 91–98. [Google Scholar] [PubMed]
- Lai, A.K.; Lo, A.C. Animal models of diabetic retinopathy: Summary and comparison. J. Diabetes Res. 2013, 2013, 106594. [Google Scholar] [CrossRef]
- Serra, A.M.; Waddell, J.; Manivannan, A.; Xu, H.; Cotter, M.; Forrester, J.V. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am. J. Pathol. 2012, 181, 719–727. [Google Scholar] [CrossRef]
- Wright, W.S.; Harris, N.R. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Exp. Eye Res. 2008, 86, 528–536. [Google Scholar] [CrossRef]
- Mostafavinia, A.; Amini, A.; Ghorishi, S.K.; Pouriran, R.; Bayat, M. The effects of dosage and the routes of administrations of streptozotocin and alloxan on induction rate of type1 diabetes mellitus and mortality rate in rats. Lab. Anim. Res. 2016, 32, 160–165. [Google Scholar] [CrossRef]
- Allen, R.S.; Hanif, A.M.; Gogniat, M.A.; Prall, B.C.; Haider, R.; Aung, M.H.; Prunty, M.C.; Mees, L.M.; Coulter, M.M.; Motz, C.T.; et al. TrkB signalling pathway mediates the protective effects of exercise in the diabetic rat retina. Eur. J. Neurosci. 2018, 47, 1254–1265. [Google Scholar] [CrossRef]
- Drago, F.; La Manna, C.; Emmi, I.; Marino, A. Effects of sulfinpyrazone on retinal damage induced by experimental diabetes mellitus in rabbits. Pharmacol. Res. 1998, 38, 97–100. [Google Scholar] [CrossRef]
- Johnson, M.A.; Lutty, G.A.; McLeod, D.S.; Otsuji, T.; Flower, R.W.; Sandagar, G.; Alexander, T.; Steidl, S.M.; Hansen, B.C. Ocular structure and function in an aged monkey with spontaneous diabetes mellitus. Exp. Eye Res. 2005, 80, 37–42. [Google Scholar] [CrossRef]
- Gorbatyuk, O.S.; Pitale, P.M.; Saltykova, I.V.; Dorofeeva, I.B.; Zhylkibayev, A.A.; Athar, M.; Fuchs, P.A.; Samuels, B.C.; Gorbatyuk, M.S. A Novel Tree Shrew Model of Diabetic Retinopathy. Front. Endocrinol. 2021, 12, 799711. [Google Scholar] [CrossRef] [PubMed]
- Black, H.E.; Rosenblum, I.Y.; Capen, C.C. Chemically induced (streptozotocin-alloxan) diabetes mellitus in the dog. Biochemical and ultrastructural studies. Am. J. Pathol. 1980, 98, 295–310. [Google Scholar]
- Cusick, M.; Chew, E.Y.; Ferris, F., 3rd; Cox, T.A.; Chan, C.C.; Kador, P.F. Effects of aldose reductase inhibitors and galactose withdrawal on fluorescein angiographic lesions in galactose-fed dogs. Arch. Ophthalmol. 2003, 121, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hayden, M.R.; Sowers, S.; Bagree, S.V.; Sowers, J.R. Retinal redox stress and remodeling in cardiometabolic syndrome and diabetes. Oxid. Med. Cell. Longev. 2010, 3, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Seita, M.; Noguchi, H.; Kubota, Y.; Kawamoto, H.; Nakaji, S.; Kobayashi, N.; Fujiwara, T. Development of Canine Models of Type 1 Diabetes with Partial Pancreatectomy and the Administration of Streptozotocin. Cell. Med. 2013, 6, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hatchell, D.L.; Toth, C.A.; Barden, C.A.; Saloupis, P. Diabetic retinopathy in a cat. Exp. Eye Res. 1995, 60, 591–593. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Roy, S.; Beglova, E.; Mansfield, K.; Wachtman, L.; Roy, S. Hyperhexosemia-Induced Retinal Vascular Pathology in a Novel Primate Model of Diabetic Retinopathy. Diabetes 2015, 64, 2603–2608. [Google Scholar] [CrossRef]
- Rajagopal, R.; Bligard, G.W.; Zhang, S.; Yin, L.; Lukasiewicz, P.; Semenkovich, C.F. Functional Deficits Precede Structural Lesions in Mice with High-Fat Diet-Induced Diabetic Retinopathy. Diabetes 2016, 65, 1072–1084. [Google Scholar] [CrossRef]
- Buchi, E.R.; Kurosawa, A.; Tso, M.O. Retinopathy in diabetic hypertensive monkeys: A pathologic study. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 388–398. [Google Scholar] [CrossRef]
- Yoshioka, M.; Kayo, T.; Ikeda, T.; Koizumi, A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997, 46, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Wicker, L.S.; Todd, J.A.; Peterson, L.B. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 1995, 13, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Kunimoto, K.; Muraoka, Y.; Mizushima, Y.; Katagiri, K.; Tochino, Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 1980, 29, 1–13. [Google Scholar]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a new mutation in the mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef]
- van Eeden, P.E.; Tee, L.B.; Lukehurst, S.; Lai, C.M.; Rakoczy, E.P.; Beazley, L.D.; Dunlop, S.A. Early vascular and neuronal changes in a VEGF transgenic mouse model of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4638–4645. [Google Scholar] [CrossRef]
- Rakoczy, E.P.; Ali Rahman, I.S.; Binz, N.; Li, C.R.; Vagaja, N.N.; de Pinho, M.; Lai, C.M. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am. J. Pathol. 2010, 177, 2659–2670. [Google Scholar] [CrossRef]
- Sima, A.A.; Chakrabarti, S.; Garcia-Salinas, R.; Basu, P.K. The BB-rat—An authentic model of human diabetic retinopathy. Curr. Eye Res. 1985, 4, 1087–1092. [Google Scholar] [CrossRef]
- Sima, A.A.; Garcia-Salinas, R.; Basu, P.K. The BB Wistar rat: An experimental model for the study of diabetic retinopathy. Metabolism 1983, 32, 136–140. [Google Scholar] [CrossRef]
- Miyamura, N.; Amemiya, T. Lens and retinal changes in the WBN/Kob rat (spontaneously diabetic strain). Electron-microscopic study. Ophthalmic. Res. 1998, 30, 221–232. [Google Scholar] [CrossRef]
- Yokoi, N.; Hoshino, M.; Hidaka, S.; Yoshida, E.; Beppu, M.; Hoshikawa, R.; Sudo, K.; Kawada, A.; Takagi, S.; Seino, S. A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. J. Diabetes Res. 2013, 2013, 103731. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.G.; Shaw, W.N.; Neel, M.A.; Little, L.A.; Eichberg, J. Zucker Diabetic Fatty Rat as a Model for Non-insulin-dependent Diabetes Mellitus. ILAR J. 1990, 32, 16–19. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Bhutto, I.A.; Amemiya, T. Retinal changes in Otsuka long-evans Tokushima Fatty rats (spontaneously diabetic rat)—Possibility of a new experimental model for diabetic retinopathy. Jpn. J. Ophthalmol. 2003, 47, 28–35. [Google Scholar] [CrossRef][Green Version]
- Shinohara, M.; Masuyama, T.; Shoda, T.; Takahashi, T.; Katsuda, Y.; Komeda, K.; Kuroki, M.; Kakehashi, A.; Kanazawa, Y. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int. J. Exp. Diabetes Res. 2000, 1, 89–100. [Google Scholar] [CrossRef]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Patz, A.; Eastham, A.; Higginbotham, D.H.; Kleh, T. Oxygen studies in retrolental fibroplasia. II. The production of the microscopic changes of retrolental fibroplasia in experimental animals. Am. J. Ophthalmol. 1953, 36, 1511–1522. [Google Scholar] [CrossRef]
- McLeod, D.S.; Lutty, G.A. Targeting VEGF in canine oxygen-induced retinopathy—A model for human retinopathy of prematurity. Eye Brain 2016, 8, 55–65. [Google Scholar] [CrossRef]
- Zhang, S.X.; Ma, J.X.; Sima, J.; Chen, Y.; Hu, M.S.; Ottlecz, A.; Lambrou, G.N. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am. J. Pathol. 2005, 166, 313–321. [Google Scholar] [CrossRef][Green Version]
- Ozaki, H.; Hayashi, H.; Vinores, S.A.; Moromizato, Y.; Campochiaro, P.A.; Oshima, K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp. Eye Res. 1997, 64, 505–517. [Google Scholar] [CrossRef]
- Cao, R.; Jensen, L.D.; Soll, I.; Hauptmann, G.; Cao, Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS ONE 2008, 3, e2748. [Google Scholar] [CrossRef]
- van Rooijen, E.; Voest, E.E.; Logister, I.; Bussmann, J.; Korving, J.; van Eeden, F.J.; Giles, R.H.; Schulte-Merker, S. Von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis. Model. Mech. 2010, 3, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Z.Z.; Morris, D.L.; Moss, D.R.; Sims, E.K.; Chiong, Y.; Kono, T.; Evans-Molina, C. Streptozotocin is equally diabetogenic whether administered to fed or fasted mice. Lab. Anim. 2013, 47, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.S.; Aiello, L.P.; Ferris, F.L., 3rd; Klein, R. Diabetic retinopathy. Diabetes Care 2004, 27, 2540–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, J.J.; Gao, G.; Shao, C.; Mott, R.; Ma, J.X. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006, 20, 323–325. [Google Scholar] [CrossRef]
- Kim, C.B.; D’Amore, P.A.; Connor, K.M. Revisiting the mouse model of oxygen-induced retinopathy. Eye Brain 2016, 8, 67–79. [Google Scholar] [CrossRef]
- Vessey, K.A.; Wilkinson-Berka, J.L.; Fletcher, E.L. Characterization of retinal function and glial cell response in a mouse model of oxygen-induced retinopathy. J. Comp. Neurol. 2011, 519, 506–527. [Google Scholar] [CrossRef]
- Downie, L.E.; Pianta, M.J.; Vingrys, A.J.; Wilkinson-Berka, J.L.; Fletcher, E.L. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia 2008, 56, 1076–1090. [Google Scholar] [CrossRef]
- Shen, W.Y.; Lai, C.M.; Graham, C.E.; Binz, N.; Lai, Y.K.; Eade, J.; Guidolin, D.; Ribatti, D.; Dunlop, S.A.; Rakoczy, P.E. Long-term global retinal microvascular changes in a transgenic vascular endothelial growth factor mouse model. Diabetologia 2006, 49, 1690–1701. [Google Scholar] [CrossRef][Green Version]
- Han, Z.; Guo, J.; Conley, S.M.; Naash, M.I. Retinal angiogenesis in the Ins2(Akita) mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 574–584. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Cho, C.S.; Kim, K.W. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J. Cereb. Blood Flow Metab. 2009, 29, 621–628. [Google Scholar] [CrossRef]
- Feit-Leichman, R.A.; Kinouchi, R.; Takeda, M.; Fan, Z.; Mohr, S.; Kern, T.S.; Chen, D.F. Vascular damage in a mouse model of diabetic retinopathy: Relation to neuronal and glial changes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4281–4287. [Google Scholar] [CrossRef] [PubMed]
- Jariyapongskul, A.; Rungjaroen, T.; Kasetsuwan, N.; Patumraj, S.; Seki, J.; Niimi, H. Long-term effects of oral vitamin C supplementation on the endothelial dysfunction in the iris microvessels of diabetic rats. Microvasc. Res. 2007, 74, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.R.; Stitt, A.W.; Gardiner, T.A.; Archer, D.B. Diabetic retinopathy: Morphometric analysis of basement membrane thickening of capillaries in different retinal layers within arterial and venous environments. Br. J. Ophthalmol. 1995, 79, 1120–1123. [Google Scholar] [CrossRef]
- Gong, C.Y.; Lu, B.; Hu, Q.W.; Ji, L.L. Streptozotocin induced diabetic retinopathy in rat and the expression of vascular endothelial growth factor and its receptor. Int. J. Ophthalmol. 2013, 6, 573–577. [Google Scholar] [CrossRef]
- Schroder, S.; Palinski, W.; Schmid-Schonbein, G.W. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol. 1991, 139, 81–100. [Google Scholar] [PubMed]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef]
- Blair, N.P.; Tso, M.O.; Dodge, J.T. Pathologic studies of the blood—Retinal barrier in the spontaneously diabetic BB rat. Investig. Ophthalmol. Vis. Sci. 1984, 25, 302–311. [Google Scholar]
- Behl, Y.; Krothapalli, P.; Desta, T.; DiPiazza, A.; Roy, S.; Graves, D.T. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am. J. Pathol. 2008, 172, 1411–1418. [Google Scholar] [CrossRef]
- Cheung, A.K.; Fung, M.K.; Lo, A.C.; Lam, T.T.; So, K.F.; Chung, S.S.; Chung, S.K. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes 2005, 54, 3119–3125. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Robison, W.G., Jr.; Cohen, M.P. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J. Diabetes Complicat. 1998, 12, 28–33. [Google Scholar] [CrossRef]
- Li, Q.; Zemel, E.; Miller, B.; Perlman, I. Early retinal damage in experimental diabetes: Electroretinographical and morphological observations. Exp. Eye Res. 2002, 74, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, K.; Reddy, S.S.; Reddy, G.B. Ubiquitin-proteasome system and ER stress in the retina of diabetic rats. Arch. Biochem. Biophys. 2017, 627, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.W.; Park, S.J.; Kim, K.Y.; Chung, J.W.; Chun, M.H.; Oh, S.J. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 2003, 46, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Pitale, P.M.; Saltykova, I.V.; Adu-Agyeiwaah, Y.; Calzi, S.L.; Satoh, T.; Akira, S.; Gorbatyuk, O.; Boulton, M.E.; Pardue, M.T.; Garvey, W.T.; et al. Tribbles Homolog 3 Mediates the Development and Progression of Diabetic Retinopathy. Diabetes 2021, 70, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Robison, W.G., Jr.; Tillis, T.N.; Laver, N.; Kinoshita, J.H. Diabetes-related histopathologies of the rat retina prevented with an aldose reductase inhibitor. Exp. Eye Res. 1990, 50, 355–366. [Google Scholar] [CrossRef]
- Szabo, K.; Enzsoly, A.; Dekany, B.; Szabo, A.; Hajdu, R.I.; Radovits, T.; Matyas, C.; Olah, A.; Laurik, L.K.; Somfai, G.M.; et al. Histological Evaluation of Diabetic Neurodegeneration in the Retina of Zucker Diabetic Fatty (ZDF) Rats. Sci. Rep. 2017, 7, 8891. [Google Scholar] [CrossRef]
- Downie, L.E.; Pianta, M.J.; Vingrys, A.J.; Wilkinson-Berka, J.L.; Fletcher, E.L. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J. Comp. Neurol. 2007, 504, 404–417. [Google Scholar] [CrossRef]
- Liu, K.; Akula, J.D.; Falk, C.; Hansen, R.M.; Fulton, A.B. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2639–2647. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, D.; Chen, X.; Zhao, L.; Tian, Q.; Liu, C.; Zhou, B.L. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Mol. Vis. 2012, 18, 1411–1420. [Google Scholar]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Hombrebueno, J.R.; Chen, M.; Penalva, R.G.; Xu, H. Loss of synaptic connectivity, particularly in second order neurons is a key feature of diabetic retinal neuropathy in the Ins2Akita mouse. PLoS ONE 2014, 9, e97970. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Y.; Xie, P.; Cheng, H.; Song, Q.; Su, T.; Yuan, S.; Liu, Q. Retinal Neurodegeneration in db/db Mice at the Early Period of Diabetes. J. Ophthalmol. 2015, 2015, 757412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Zhuo, L. Longitudinal in vivo imaging of retinal gliosis in a diabetic mouse model. Exp. Eye Res. 2010, 91, 530–536. [Google Scholar] [CrossRef]
- Pardue, M.T.; Barnes, C.S.; Kim, M.K.; Aung, M.H.; Amarnath, R.; Olson, D.E.; Thule, P.M. Rodent Hyperglycemia-Induced Inner Retinal Deficits are Mirrored in Human Diabetes. Transl. Vis. Sci. Technol. 2014, 3, 6. [Google Scholar] [CrossRef]
- Aung, M.H.; Kim, M.K.; Olson, D.E.; Thule, P.M.; Pardue, M.T. Early visual deficits in streptozotocin-induced diabetic long evans rats. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1370–1377. [Google Scholar] [CrossRef]
- Hancock, H.A.; Kraft, T.W. Oscillatory potential analysis and ERGs of normal and diabetic rats. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1002–1008. [Google Scholar] [CrossRef]
- Okuno, T.; Oku, H.; Sugiyama, T.; Ikeda, T. Electroretinographic study of spontaneously diabetic Torii rats. Doc. Ophthalmol. 2008, 117, 191–196. [Google Scholar] [CrossRef]
- Sasase, T.; Ohta, T.; Masuyama, T.; Yokoi, N.; Kakehashi, A.; Shinohara, M. The spontaneously diabetic torii rat: An animal model of nonobese type 2 diabetes with severe diabetic complications. J. Diabetes Res. 2013, 2013, 976209. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Moore-Dotson, J.M.; Beckman, J.J.; Mazade, R.E.; Hoon, M.; Bernstein, A.S.; Romero-Aleshire, M.J.; Brooks, H.L.; Eggers, E.D. Early Retinal Neuronal Dysfunction in Diabetic Mice: Reduced Light-Evoked Inhibition Increases Rod Pathway Signaling. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Du, Y.; Miller, C.; Gubitosi-Klug, R.A.; Kern, T.S.; Ball, S.; Berkowitz, B.A. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 2007, 50, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumi, J.; Ramos, D.; Ruberte, J.; Simo, R.; Hernandez, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.E.; Wu, X.; Sandirasegarane, L.; Nakamura, M.; Gilbert, K.A.; Singh, R.S.; Fort, P.E.; Antonetti, D.A.; Gardner, T.W. Diabetes reduces basal retinal insulin receptor signaling: Reversal with systemic and local insulin. Diabetes 2006, 55, 1148–1156. [Google Scholar] [CrossRef]
- Rajala, A.; Tanito, M.; Le, Y.Z.; Kahn, C.R.; Rajala, R.V. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 2008, 283, 19781–19792. [Google Scholar] [CrossRef]
- Rajala, R.V.; Wiskur, B.; Tanito, M.; Callegan, M.; Rajala, A. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1033–1040. [Google Scholar] [CrossRef]
- He, K.; Lv, W.; Zhang, Q.; Wang, Y.; Tao, L.; Liu, D. Gene set enrichment analysis of pathways and transcription factors associated with diabetic retinopathy using a microarray dataset. Int. J. Mol. Med. 2015, 36, 103–112. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Li, H.; Wang, J.M.; Steinle, J.J. Insulin Signal Transduction is Impaired in the Type 2 Diabetic Retina. J. Diabetes Clin. Res. 2020, 2, 12–15. [Google Scholar]
- Zhu, H.; Zhang, W.; Zhao, Y.; Shu, X.; Wang, W.; Wang, D.; Yang, Y.; He, Z.; Wang, X.; Ying, Y. GSK3beta-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions. Mol. Neurodegener. 2018, 13, 62. [Google Scholar] [CrossRef]
- Ma, J.H.; Wang, J.J.; Zhang, S.X. The unfolded protein response and diabetic retinopathy. J. Diabetes Res. 2014, 2014, 160140. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Wang, D.; Li, Y.; Dou, H.; Tso, M.O.; Ma, Z. Role of endoplasmic reticulum stress in the loss of retinal ganglion cells in diabetic retinopathy. Neural Regen. Res. 2013, 8, 3148–3158. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, H.S.; Li, G.G.; Zhao, M.J.; Zhao, M.H. The role of endoplasmic reticulum stress in the early stage of diabetic retinopathy. Acta Diabetol. 2011, 48, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. KIOM-79 protects AGE-induced retinal pericyte apoptosis via inhibition of NF-kappaB activation in vitro and in vivo. PLoS ONE 2012, 7, e43591. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Choi, J.A.; Koh, J.Y.; Yoon, Y.H. Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice. J. Diabetes Res. 2017, 2017, 1763292. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, J.; Chen, Y.; Wang, J.J.; Ratan, R.; Zhang, S.X. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes 2012, 61, 492–504. [Google Scholar] [CrossRef]
- Kondo, T.; Kahn, C.R. Altered insulin signaling in retinal tissue in diabetic states. J. Biol. Chem. 2004, 279, 37997–38006. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Wang, M.; Zhang, S.X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009, 583, 1521–1527. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Kunte, M.; Shinde, V.; Gorbatyuk, M. Modulation of angiogenesis by genetic manipulation of ATF4 in mouse model of oxygen-induced retinopathy [corrected]. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5995–6002. [Google Scholar] [CrossRef]
- Bhatta, M.; Ma, J.H.; Wang, J.J.; Sakowski, J.; Zhang, S.X. Enhanced endoplasmic reticulum stress in bone marrow angiogenic progenitor cells in a mouse model of long-term experimental type 2 diabetes. Diabetologia 2015, 58, 2181–2190. [Google Scholar] [CrossRef]
- Rana, T.; Shinde, V.M.; Starr, C.R.; Kruglov, A.A.; Boitet, E.R.; Kotla, P.; Zolotukhin, S.; Gross, A.K.; Gorbatyuk, M.S. An activated unfolded protein response promotes retinal degeneration and triggers an inflammatory response in the mouse retina. Cell Death Dis. 2014, 5, e1578. [Google Scholar] [CrossRef]
- Kirwin, S.J.; Kanaly, S.T.; Linke, N.A.; Edelman, J.L. Strain-dependent increases in retinal inflammatory proteins and photoreceptor FGF-2 expression in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5396–5404. [Google Scholar] [CrossRef] [PubMed]
- Kador, P.F.; Takahashi, Y.; Wyman, M.; Ferris, F., 3rd. Diabeteslike proliferative retinal changes in galactose-fed dogs. Arch. Ophthalmol. 1995, 113, 352–354. [Google Scholar] [CrossRef] [PubMed]
- King, J.L.; Mason, J.O., 3rd; Cartner, S.C.; Guidry, C. The influence of alloxan-induced diabetes on Muller cell contraction-promoting activities in vitreous. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7485–7491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acharya, N.K.; Qi, X.; Goldwaser, E.L.; Godsey, G.A.; Wu, H.; Kosciuk, M.C.; Freeman, T.A.; Macphee, C.H.; Wilensky, R.L.; Venkataraman, V.; et al. Retinal pathology is associated with increased blood-retina barrier permeability in a diabetic and hypercholesterolaemic pig model: Beneficial effects of the LpPLA2 inhibitor Darapladib. Diab. Vasc. Dis. Res. 2017, 14, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Kleinwort, K.J.H.; Amann, B.; Hauck, S.M.; Hirmer, S.; Blutke, A.; Renner, S.; Uhl, P.B.; Lutterberg, K.; Sekundo, W.; Wolf, E.; et al. Retinopathy with central oedema in an INS (C94Y) transgenic pig model of long-term diabetes. Diabetologia 2017, 60, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.R.; Grant, D.G.; Olver, T.D.; Padilla, J.; Czajkowski, A.M.; Schnurbusch, T.R.; Mohan, R.R.; Hainsworth, D.P.; Walters, E.M.; Chaurasia, S.S. Young Ossabaw Pigs Fed a Western Diet Exhibit Early Signs of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Tso, M.O.; Kurosawa, A.; Benhamou, E.; Bauman, A.; Jeffrey, J.; Jonasson, O. Microangiopathic retinopathy in experimental diabetic monkeys. Trans. Am. Ophthalmol. Soc. 1988, 86, 389–421. [Google Scholar]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef]
- Alvarez, Y.; Chen, K.; Reynolds, A.L.; Waghorne, N.; O’Connor, J.J.; Kennedy, B.N. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis. Model. Mech. 2010, 3, 236–245. [Google Scholar] [CrossRef]
- Kim, S.Y.; Johnson, M.A.; McLeod, D.S.; Alexander, T.; Otsuji, T.; Steidl, S.M.; Hansen, B.C.; Lutty, G.A. Retinopathy in monkeys with spontaneous type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4543–4553. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.; Yang, J. Development of a zebrafish screening model for diabetic retinopathy induced by hyperglycemia: Reproducibility verification in animal model. Biomed. Pharmacother. 2021, 135, 111201. [Google Scholar] [CrossRef] [PubMed]
- Millan, I.; Desco, M.D.C.; Torres-Cuevas, I.; Perez, S.; Pulido, I.; Mena-Molla, S.; Mataix, J.; Asensi, M.; Ortega, A.L. Pterostilbene Prevents Early Diabetic Retinopathy Alterations in a Rabbit Experimental Model. Nutrients 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.G.; Michelis, M.A.; Kim, Y.T.; Shin, S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes 1982, 31, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Saadane, A.; Lessieur, E.M.; Du, Y.; Liu, H.; Kern, T.S. Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS ONE 2020, 15, e0238727. [Google Scholar] [CrossRef]
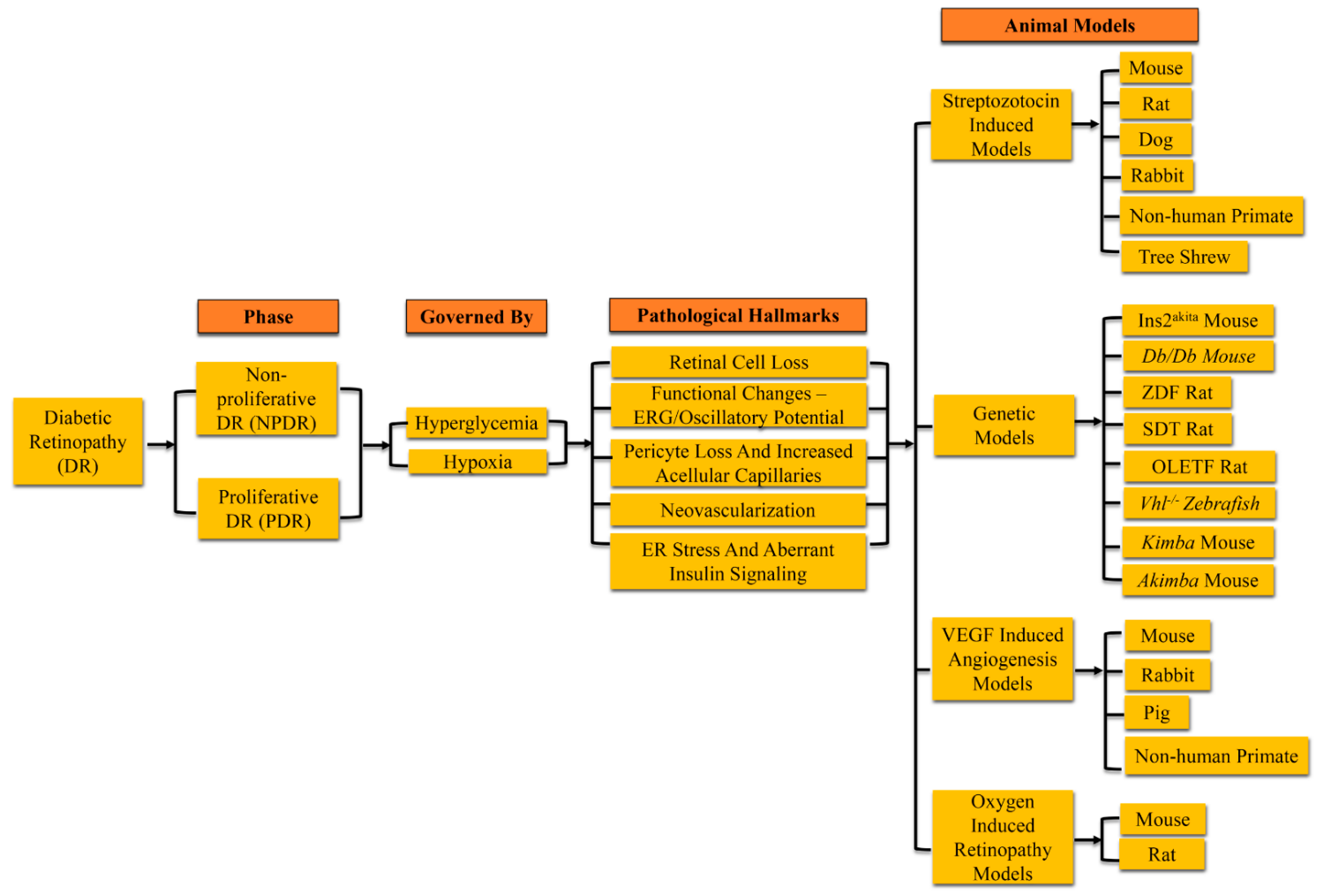
| Hyperglycemia Induction | |||||
|---|---|---|---|---|---|
| Method | Species | Dosage | Hyperglycemia | References | |
| 1. | Streptozotocin (STZ) | mouse, rat, rabbit, tree shrew, monkey, cat | mouse and rat—intraperitoneal (IP) 40–80 mg/kg (5 days), mouse—IP 150–200 mg/kg (single dose), rat—IP 30–80 mg/kg (single dose), rabbit—intravenous (IV) 110 mg/kg (single dose), tree shrew—IP 80 mg twice a week apart and IP 175 mg/kg (single dose). | mouse and rat approx. 1-week post-STZ | [24,25,26,27,28,29,30,31] |
| 2. | Alloxan | mouse, rat, rabbit, swine, dog | rat-IP 80–140 mg/kg (single dose), rat-subcutaneous (SC) 80–120 mg/kg (single dose), dogs-IV 50 mg/kg (single dose). | [27,32,33,34] | |
| 3. | Pancreatectomy | cat, dog | [35,36,37] | ||
| 4. | High galactose /fat type 2 diet | mouse, rat, dog, swine, zebrafish, monkey | [22,35,38,39,40] | ||
| Spontaneous Hyperglycemia | |||||
| Mouse | Hyperglycemia | ||||
| 1. | Ins2Akita mouse: Type I Diabetes Mellitus (DM), mutation in insulin | 4 weeks | [41,42] | ||
| 2. | Non-obese mouse (NOD): Type I DM, autoimmune model | 12 weeks | [43,44] | ||
| 3. | db/db (Leprdb) mouse: Type II DM | 8–10 weeks | [45] | ||
| 4. | Kimba mouse:Transgenic mouse (tr029VEGF) | [46] | |||
| 5. | Akimba mouse: Ins2Akita /VEGF (+/−) | 4 weeks | [47] | ||
| Rat | Hyperglycemia | ||||
| 1. | Biobreeding rats: Type I DM, autoimmune model | 3 months | [48,49] | ||
| 2. | Wistar Bonn/Kobori (WBN/Kob) rats: Type II DM | 9 months | [50] | ||
| 3. | Zuker diabetic fatty (ZDF) rats: Type II DM | 5–10 weeks | [51,52] | ||
| 4. | Otsuka Long-Evans Tokushima fatty (OLETF) rats: Type II DM | 5 months | [53] | ||
| 5. | Spontaneous diabetic torii (SDT) rats: Type II DM | 5 months | [54] | ||
| Neovascularization | |||||
| Mouse | |||||
| 1. | Oxygen induced retinopathy (OIR) | [55] | |||
| 2. | Kimba mouse | [46] | |||
| 3. | Akimba mouse | [47] | |||
| Rat, Canine | |||||
| 1. | Oxygen induced retinopathy (OIR) | [56,57,58] | |||
| Rabbit | |||||
| 1. | Implantation of human recombinant VEGF in the vitreous | [59] | |||
| Zebrafish | |||||
| 1. | Angiogenesis | [60,61] | |||
| Monkey | |||||
| 1. | Implantation of human recombinant VEGF in the vitreous | [59] | |||
| Molecular Signaling | ||||
|---|---|---|---|---|
| Model | Changes | Duration of Hyperglycemia | References | |
| 1. | STZ Rat | Elevated CHOP, Caspase 12, MAPK retinal cytokines | 8 weeks | [82,104,111,121] |
| Reduced IR kinase activity | 8 weeks | |||
| Elevated retinal cytokines | 3 months | |||
| Reduced IR kinase activity and autophosphorylation and downregulation of IRS-2 & PI3K | 3 months | |||
| Upregulation of HIF-A, ATF-6, XBP1 | 4 months | |||
| 2. | ZFD Rat | Elevated Bax, TNF-α and NF-kappaB | 6 weeks | [113] |
| 3. | OIR Rat | Elevated VEGF, PDEG and TNF-α | P16 | [58,64] |
| 4. | STZ Mouse | Upregulation of GRP78, pPERK, CHOP, VEGF, pEIF2α, retinal cytokine and TNF-α | 4 weeks | [84,105,106,114,115,116,117] |
| Elevated IR expression and tyrosine phosphorylation; upregulated IRS-2 and reduced PDK1/ AKT protein levels and phosphorylation | 1 week | |||
| Reduced IR phosphorylation | 1 week | |||
| Upregulation of TRIB3 and inflammatory cytokines (Icam1, Nf-kb1, Rc3h1, Zc3h12a, VEGF, COX2, and AIF1) | 4 weeks | |||
| 5. | Ins2Akita Mouse | VEGF and TNF-α elevation, increased mRNA expression; protein expression of GRP78 and elevated peIF2α and ATF4 and reduced IR kinase activity | 12 weeks | [42,116,117] |
| 6. | Leprdb (db/db) Mouse | Increased IRS-2 expression and reduced PDK1/ AKT protein levels and phosphorylation | 10 weeks | [116,119] |
| GFAP activation, increased expression of HIF-A, VEGF, GRP78, p-IRE-1, CHOP, Casapase-3 and ATF4 | 15 months | |||
| Microangiopathy | ||||
| Model | Changes | Duration of Hyperglycemia | References | |
| 1. | STZ Rat | Blood retinal barrier disruption | 2 weeks | [8,72,74] |
| Adherent leukocytes | 8 weeks | |||
| Thickened Basement Membrane (BM) | 12 weeks | |||
| Neovascularization | 3–4 months | |||
| 2. | Alloxan Rat | Leukocytosis | 2 months | [75,76] |
| Neovascularization | 9 months | |||
| Pericyte loss, acellular capillaries, and BM thickening | 12 months | |||
| 3. | BB Rat | Basement membrane thickening | 4 months | [48,49,77] |
| Blood retinal barrier breakdown | 6 months | |||
| Pericyte loss | 8 months | |||
| 4. | ZDF Rat | BM thickening, pericyte loss and acellular capillaries | 6 months | [51,52] |
| 5. | OLETF Rat | BM thickening, pericyte loss and acellular capillaries | 9 months | [53,78] |
| 6. | OIR SD Rat | Increased extra retinal neovascularization and impaired pericyte distribution | P18 | [67] |
| 7. | STZ Mouse | Increased vascular permeability | 8 days | [26,70,71,84] |
| Decreased arteriolar diameter and velocity | 8 weeks | |||
| BM thickening | 4–15 months | |||
| Pericyte loss, acellular capillaries and pericyte ghost | 6–9 months | |||
| 8. | Ins2Akita Mouse | Leukocytosis | 8 weeks | [42,69] |
| Increased vascular permeability | 12 weeks | |||
| Blood vessels in the outer plexiform layer (OPL) and microaneurysms | 6 months | |||
| Acellular capillaries, BM thickening and neovascularization. | 9 months | |||
| 9. | Kimba Mouse | Abnormal blood vessel development around photoreceptor | P28 | [46,68] |
| Increased vascular permeability and adherent leukocytes | 6 weeks | |||
| Loss of retinal capillaries, neovascularization, increased avascular area and alteration in the vessel length | 9 weeks | |||
| Pericyte loss | 24 weeks | |||
| 10. | Akimba Mouse | Microaneurysms, neovascularization, blood vessel constriction, beading, vessel edema, capillary dropout, and new vessel formation it the ONL | 8 weeks | [47] |
| 11. | OIR Mouse | Irregular blood vessel development and reduced inner retinal plexus and deep plexus | P18 | [66] |
| 12. | Db/db Mouse | Increased vascular permeability and BM thickening | 13–14 weeks | [79,80] |
| Pericyte loss | 18 weeks | |||
| Acellular capillaries | 26 weeks | |||
| 13. | High-fat diet Mouse | Pericyte loss, blood retinal barrier disruption and vascular leakage | 12 months | [39] |
| Retinal Integrity | ||||
| Model | Changes | Duration of Hyperglycemia | References | |
| 1. | STZ Rat | Decreased pre- and post-synaptic photoreceptor ribbon synapses | 4 weeks | [81,82,83] |
| Increased GFAP reactivity | 6–7 weeks | |||
| Loss of ONL, INL, GCL | 12–16 weeks | |||
| Severe photoreceptor cell loss | 24 weeks | |||
| 2. | WBN/Kob Rat | Photoreceptor degeneration | 4 weeks | [50] |
| Severe OS and ONL degeneration | 5–14 months | |||
| 3. | BB Rat | RPE degeneration | 4 months | [85] |
| 4. | ZDF Rat | Decreased OS, damage to amacrine cells and RPE with gliosis | 32 weeks | [86] |
| 5. | OLETF Rat | Decreased INL and photoreceptor cells | 9 months | [53] |
| 6. | OIR Rat | Reduction in OS, INL, IPL, total retinal thickness, astrocytes and increased muller activity | P18 | [67,87] |
| 7. | High galactose Rat | Increased gliosis and reduced INL and OPL | 28 months | [85] |
| 8. | STZ Mouse | GFAP hyperactivity | 5 weeks | [71,84,89,91,94] |
| Reduced ONL, INL thickness | 6–14 weeks | |||
| Total retinal thickness reduced | 20 weeks | |||
| No retinal cell loss and gliosis | 8–12 months | |||
| Reduced RGCs | 8 months | |||
| 9. | Ins2Akita Mouse | GFAP hyperactivity | 8 weeks | [42,92] |
| Reduced IPL, INL and cone photoreceptors | 3 months | |||
| Reduced RGCs | 22 weeks | |||
| Decreased presynaptic and post-synaptic photoreceptor ribbons | 36 weeks | |||
| 10. | db/db Mouse | Reduced NFL and RGCs | 16-28 weeks | [91,93] |
| Reduced total retinal thickness | 28 weeks | |||
| 11. | Akimba Mouse | Photoreceptor cell death | 28 weeks | [47] |
| 12. | OIR Mouse | Total retinal thickness reduction, distorted photoreceptor OS, neuronal loss, hyperactivity of Müller cells, microglial activation and disrupted INL and IPL | P17-188 | [66,84] |
| Retinal Electrophysiology | ||||
| Model | Changes | Duration of Hyperglycemia | References | |
| 1. | STZ Rat | Decrease in OP amplitude | 2–7 weeks | [81,98,99] |
| Decrease in OP implicit time | 7 weeks | |||
| Decreased a- and b-wave amplitude | 10–12 weeks and at 44 weeks | |||
| 2. | OIR Rat | Decreased a- and b-wave amplitude | P18 | [67,87,88] |
| 3. | STZ Mouse | Reduced OP amplitude and implicit time | 4–6 weeks | [84,100,101,102] |
| Reduced a- and b-wave amplitude | 6 months | |||
| Reduced PhNR amplitude | 8 months | |||
| 4. | Ins2Akita Mouse | Decreased OP amplitude, delay in the OP and decreased b-wave | 9 months | [42,92] |
| 5. | Db/db Mouse | Delay in the b-wave, delay in the OP implicit time and decreased amplitude of both photopic and scotopic b-wave | 16–24 weeks | [93,103] |
| 6. | OIR Mouse | Significant decrease in the amplitude of a- and b-wave | P18 | [66] |
| 7. | High-fat diet Mouse | Decreased OP amplitude | 12 months | [39] |
| Model | Pathological Changes | Induction of DR | References | |
|---|---|---|---|---|
| 1. | VEGF-induced angiogenesis Rabbit | Tortuous blood vessels | 2 weeks | [59] |
| Vascular leakage | ||||
| Neovascularization | 3 weeks | |||
| 2. | Alloxan Rabbit | Increase in the oxidated proteins and lipids | 6 weeks | [132] |
| Decline in p-PI3K/PI3K, p-AKT/AKT and p-GSK3/GSK3 ratios | ||||
| 3. | STZ Rabbit | Retinal hemorrhages and venous thrombosis | 19 weeks | [29] |
| Vascular lesions | ||||
| 4. | Hypoxic Zebrafish/vhl-mutant Zebrafish | Aberrant blood vessel formation | 2-days-post-fertilization (dpf) | [60,61] |
| Formation of capillary tips and sprouts in optic capillary plexus | 12 dpf | |||
| Increased mRNA VEGF | ||||
| 5. | Hyperglycemic Zebrafish | Increased Vegf, Il-6, Il-1β, Stat3, and Tnfα mRNA expression | 3-6 dpf | [131] |
| Reduction in the IPL and INL | 30 dpf | [128,129] | ||
| Thickening of the blood vessels | ||||
| BM thickening | ||||
| 6. | VEGF induced angiogenesis-Primate | Vascular permeability | 2–3 weeks | [59] |
| Breakdown of BRB | ||||
| Tortuosity of the blood vessels | ||||
| 7. | STZ Primate | Presence of cotton-wool spots | 6 years | [127] |
| Macular atrophy | ||||
| Arteriolar occlusion | ||||
| Focal intraretinal capillary leakage | ||||
| Capillary dilatation | ||||
| 8. | Obese Primate | Decreased a-wave of the scotopic ERG | 5 years | [130] |
| Reduced oscillatory potential | ||||
| 9. | Diet-induced DR Marmoset | Excess vascular permeability. | 2.5 years | [38] |
| Increased acellular capillaries and pericyte loss | ||||
| BM thickening and vessel tortuosity | ||||
| Thickening of the retinal foveal | ||||
| Microaneurysms | ||||
| 10. | STZ Tree shrew | Upregulation of TRIB3 | 16 weeks | [31] |
| Upregulation of p-AKT/AKT→ p-mTOR/mTOR | ||||
| Increased IRS | ||||
| RGC function loss and cell death | ||||
| 11. | STZ Dog | Pericyte loss | 9 months | [33,122] |
| Hemorrhages and microaneurysms | 28–68 months | |||
| BM thickening | ||||
| Vitreous detachment | ||||
| Neovascularization | ||||
| 12. | Pancreatectomy Cat | BM thickening | 3 months | [37] |
| Microaneurysm | 5 years | |||
| Neovascularization | 6.5–8 years | |||
| 13. | Alloxan Pig | Pericyte loss and BRB breakdown | 20 weeks | [34] |
| 14. | STZ Pig | BRB permeability | 24 weeks | [124] |
| Gliosis and microglial activation | ||||
| Decrease in retinal thickness | ||||
| 15. | High-fat diet Pig | INL disruption | 6 months | [126] |
| BM thickening | ||||
| Pericyte ghosts and acellular capillaries | ||||
| Increase in fibronectin expression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitale, P.M.; Gorbatyuk, M.S. Diabetic Retinopathy: From Animal Models to Cellular Signaling. Int. J. Mol. Sci. 2022, 23, 1487. https://doi.org/10.3390/ijms23031487
Pitale PM, Gorbatyuk MS. Diabetic Retinopathy: From Animal Models to Cellular Signaling. International Journal of Molecular Sciences. 2022; 23(3):1487. https://doi.org/10.3390/ijms23031487
Chicago/Turabian StylePitale, Priyamvada M., and Marina S. Gorbatyuk. 2022. "Diabetic Retinopathy: From Animal Models to Cellular Signaling" International Journal of Molecular Sciences 23, no. 3: 1487. https://doi.org/10.3390/ijms23031487
APA StylePitale, P. M., & Gorbatyuk, M. S. (2022). Diabetic Retinopathy: From Animal Models to Cellular Signaling. International Journal of Molecular Sciences, 23(3), 1487. https://doi.org/10.3390/ijms23031487






