Resolution of Inflammation in Retinal Disorders: Briefly the State
Abstract
:1. Inflammation in Retinopathies
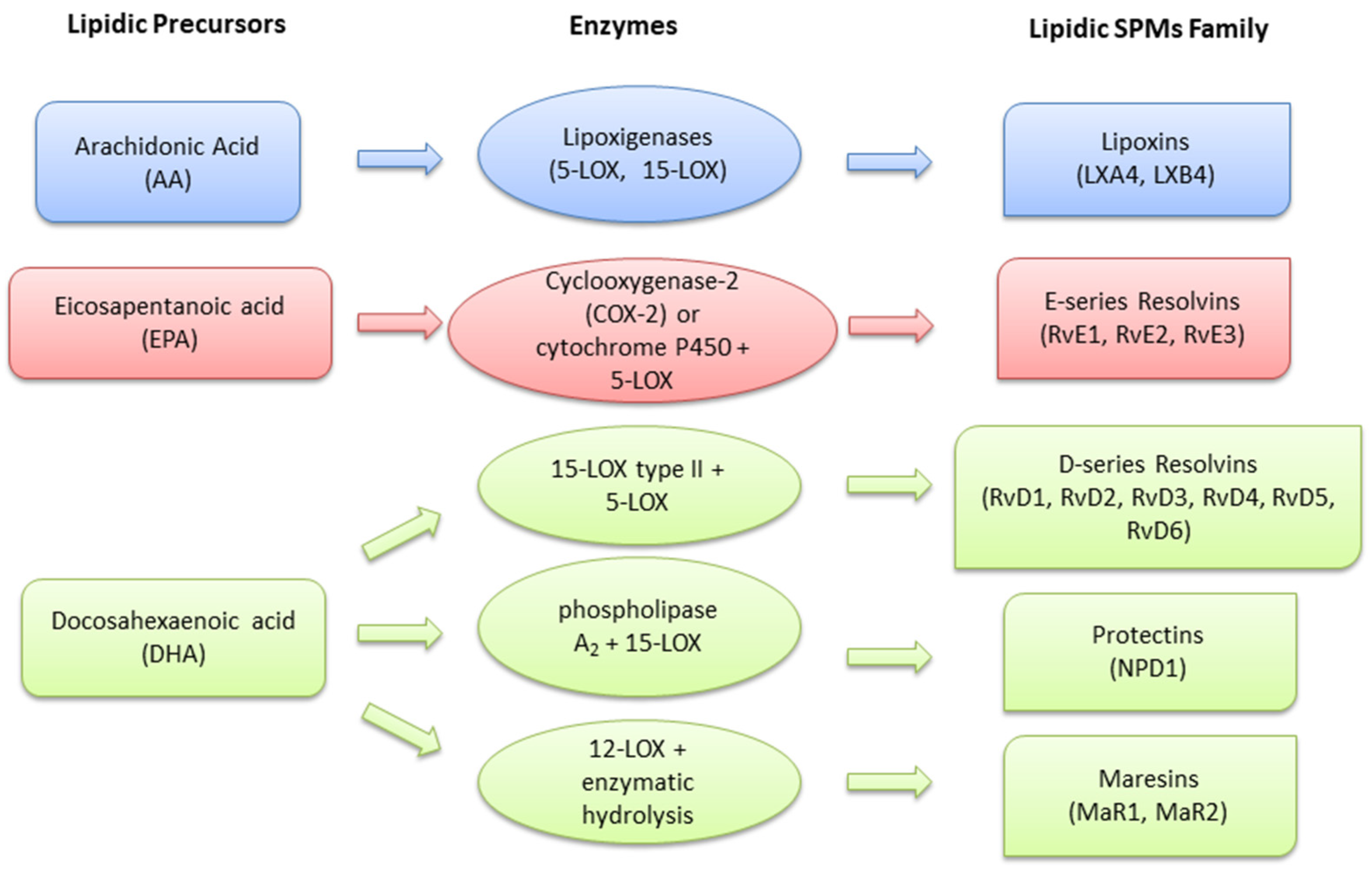
2. Pro-Resolving Mediators in Retinopathies
2.1. Lipoxins
2.2. Resolvins
| Author, Year | Model | Treatment | Main Results |
|---|---|---|---|
| Connor et al., 2007 [46] | In vivo | C57BL/6J mice fed with diet containing 2% ω-6-PUFAs (AA) and no ω-3-PUFAs (DHA and EPA), or 2% ω-3-PUFAs and no ω-6-PUFAs in 10% (w/w) safflower oil | Among the bioactive ω-3-PUFA-derived mediators, RvD1 and RvE1 potently protected against neovascularization |
| C57BL/6J mice exposed to 75% O2 from postnatal day 7 (P7) to postnatal day 12 (P12), then returned to room air for 5 days as a model of hypoxia-induced ischemic retinopathy | |||
| Tian et al., 2008 [44] | In vivo | Intraperitoneal injections of RvE1 (18.7 µg/kg) and RX-10008 (RvE1 analog, 14.3 µg/kg) on days 1, 2, 4, 6, and 8 after CNV induction. Evaluation after 14 days | RvE1 reduced leakage and choroid lesion starting from day 7, while RvE1 analog was efficient at day 14 |
| Laser rupture of Bruch’s membrane, as a CNV model | |||
| Tian et al., 2009 [45] | In vitro | Cells stimulated with 50 nM RvE1 or RvD1 for 8 h before IL-1β exposure | RvD1 and RvE1 biosynthesis was stimulated in CRECs co-cultured with lymphocytes under inflammatory stimulus. RvD1 and RvE1 reduced pro-inflammatory mediators such as VCAM, IL-8, MIP-1β and TNF-α. Moreover, RvD1 and RvE1 reduced PMN transmigration across the CREC barrier |
| CRECs cultured alone, in co-culture with lymphocytes or PMN. Cells exposed to 2 ng/mL IL-1β for 4, 12 and 24 h | |||
| Rossi et al., 2015 [41] | In vivo | Intravitreal injections (5 µL) of RvD1 (10–100–1000 ng/kg) into the right eye 1 h following LPS treatment. Evaluation after 24 h | The EIU score was significantly and dose-dependently decreased by all 3 doses of RvD1. Retinal layers showed a reduced presence of B and T lymphocytes. Neuroretinal layers exhibited a decreased staining of pro-inflammatory M1 macrophages and a concomitant increment of anti-inflammatory M2 macrophages |
| Rat EIU with retinal involvement, induced with 200 μg of LPS into the footpad of Sprague-Dawley rats | |||
| Rossi et al., 2015 [42] | In vivo | Intravitreal injections of RvD1 (10–100–1000 ng/kg) into the right eye 1 h following LPS treatment; intravitreal injections of the ALX/FPR2 inhibitor Boc-2 (0.4mg/kg/4 μL) 30 min before RvD1 1000 ng/kg. Evaluation after 24 h. Daily intraperitoneal injections of SIRT1 inhibitor EX527 (10mg/kg/day) for 7 days prior EIU, followed by RvD1 (1000 ng/kg), intravitreally injected 1 h following LPS. Evaluation after 24 h. | SIRT1 expression was dose-dependently increased by RvD1. The inhibition of ALX/FPR2 receptor decreased SIRT1 expression. The effects of RvD1 (1000 ng/kg) were partly abolished by the inhibition of SIRT-1 activity |
| Rat EIU with retinal involvement, induced with 200 μg of LPS into the footpad of Sprague-Dawley rats | |||
| Maisto et al., 2020 [39] | In vivo | RvD1 (50 nM) alone or combined with the ALX/FPR2 inhibitor Boc-2 (20 µM) added to primary retinal photoreceptor cells at the time of adding high glucose | High glucose increased VEGF levels in photoreceptors and their released exosomes, with a decrement of cellular and exosomal anti-angiogenic miR-20a-3p, miR-20a-5p, miR-106a-5p and miR-20b. By activating ALX/FPR2 receptor, RvD1 reverted the effects of high glucose on both primary retinal photoreceptor and their released exosomes |
| Primary retinal photoreceptor cells isolated from C57BL/6J mice and exposed to high glucose (30 mM) for 96 h, as a model of DR | |||
| Trotta et al., 2020 [40] | In vivo | RvD1 (50 nM) alone or combined with the ALX/FPR2 inhibitor Boc-2 (20 µM) added to primary retinal photoreceptor cells at the time of adding high glucose | Primary retinal photoreceptor cells exhibited short and small mitochondria (fragmentation, aggregation), along with high cytosolic MMP-9, MMP-2 activity. RvD1 (50 nM) restored mitochondrial morphology and function, improved mitochondrial DNA repair and cell survival, by decreasing MMP-9 and MMP-2 activity |
| Primary retinal photoreceptor cells isolated from C57BL/6J mice and exposed to high glucose (30 mM) for 96 h, as a model of DR | |||
| Trotta et al., 2021 [38] | In vivo | - | Endogenous retinal levels of RvD1 were reduced in aged mice, with a higher decrement in aged males. RvD1 levels negatively correlated with retinal levels of Iba-1 (microglia activation), GFAP (astrocyte activation), NF-kB and TNF-α (neuroinflammation), caspase 3 (apoptosis), and nitrosative stress. |
| Balb-c mice aged 3 months (control group) and 24 months (aged group; approximately 75–85 years for humans), as a model of aged retina |
2.3. Protectins
2.4. Maresins
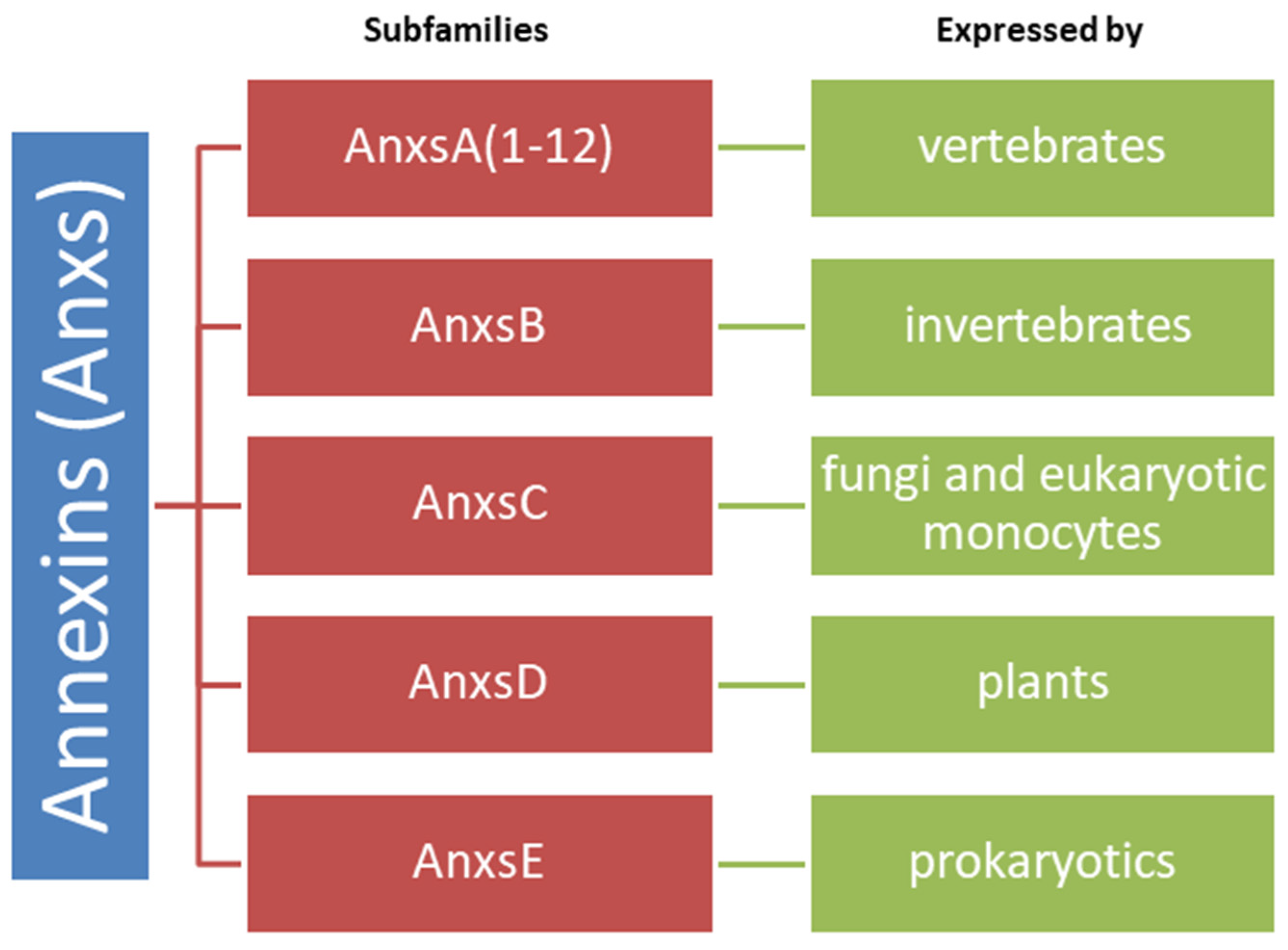
2.5. Annexins
3. Perspectives on New Non-Lipidic Mediators in Retinopathies and Specific Tools
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Rao, N.A. Grand Challenges in Ocular Inflammatory Diseases. Front. Ophthalmol. 2022, 2, 756689. [Google Scholar] [CrossRef]
- Gesualdo, C.; Balta, C.; Platania, C.B.M.; Trotta, M.C.; Herman, H.; Gharbia, S.; Rosu, M.; Petrillo, F.; Giunta, S.; Della Corte, A.; et al. Fingolimod and Diabetic Retinopathy: A Drug Repurposing Study. Front. Pharmacol. 2021, 12, 718902. [Google Scholar] [CrossRef] [PubMed]
- Whitcup, S.M.; Nussenblatt, R.B.; Lightman, S.L.; Hollander, D.A. Inflammation in Retinal Disease. Int. J. Inflamm. 2013, 2013, 724648. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Amin, S.; Roy, S. Retinal Fibrosis in Diabetic Retinopathy. Exp. Eye Res. 2016, 142, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, R.P.; Feeney-Burns, L. Clinico-Morphologic Correlations of Drusen of Bruch’s Membrane. Trans. Am. Ophthalmol. Soc. 1980, 78, 206–225. [Google Scholar] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A Role for Local Inflammation in the Formation of Drusen in the Aging Eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen Proteome Analysis: An Approach to the Etiology of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [Green Version]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Sève, P.; Cacoub, P.; Bodaghi, B.; Trad, S.; Sellam, J.; Bellocq, D.; Bielefeld, P.; Sène, D.; Kaplanski, G.; Monnet, D.; et al. Uveitis: Diagnostic Work-up. A Literature Review and Recommendations from an Expert Committee. Autoimmun. Rev. 2017, 16, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.E.; Pepple, K.L. Cytokines in Uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Kim, S.J. Nonsteroidal Anti-Inflammatory Drugs for Retinal Disease. Int. J. Inflamm. 2013, 2013, 281981. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Titos, E.; Clària, J. Pro-Resolving Mediators Produced from EPA and DHA: Overview of the Pathways Involved and Their Mechanisms in Metabolic Syndrome and Related Liver Diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma-walia, N.; Chandrasekharan, J. Lipoxins: Nature’s Way to Resolve Inflammation. JIR 2015, 30, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohli, P.; Levy, B.D. Resolvins and Protectins: Mediating Solutions to Inflammation: Chemical Mediators of Inflammation Resolution. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Hansen, T.V.; Vik, A.; Serhan, C.N. The Protectin Family of Specialized Pro-Resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Front. Pharmacol. 2019, 9, 1582. [Google Scholar] [CrossRef]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 Activates LGR6 Receptor Promoting Phagocyte Immunoresolvent Functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef] [Green Version]
- Marcheselli, V.L.; Mukherjee, P.K.; Arita, M.; Hong, S.; Antony, R.; Sheets, K.; Winkler, J.W.; Petasis, N.A.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1/Protectin D1 Stereoselective and Specific Binding with Human Retinal Pigment Epithelial Cells and Neutrophils. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalli, J.; Consalvo, A.P.; Ray, V.; Di Filippo, C.; D’Amico, M.; Mehta, N.; Perretti, M. Proresolving and Tissue-Protective Actions of Annexin A1–Based Cleavage-Resistant Peptides Are Mediated by Formyl Peptide Receptor 2/Lipoxin A4 Receptor. J. Immunol. 2013, 190, 6478–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.L.; Flower, R.J.; Perretti, M. Ligand-Specific Conformational Change of the G-Protein-Coupled Receptor ALX/FPR2 Determines Proresolving Functional Responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerke, V.; Moss, S.E. Annexins: From Structure to Function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Liclican, E.L.; Gronert, K. Molecular Circuits of Resolution in the Eye. Sci. World J. 2010, 10, 1029–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livne-Bar, I.; Wei, J.; Liu, H.-H.; Alqawlaq, S.; Won, G.-J.; Tuccitto, A.; Gronert, K.; Flanagan, J.G.; Sivak, J.M. Astrocyte-Derived Lipoxins A4 and B4 Promote Neuroprotection from Acute and Chronic Injury. J. Clin. Investig. 2017, 127, 4403–4414. [Google Scholar] [CrossRef] [Green Version]
- Kaviarasan, K.; Jithu, M.; Arif Mulla, M.; Sharma, T.; Sivasankar, S.; Das, U.N.; Angayarkanni, N. Low Blood and Vitreal BDNF, LXA4 and Altered Th1/Th2 Cytokine Balance Are Potential Risk Factors for Diabetic Retinopathy. Metabolism 2015, 64, 958–966. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Zhang, X.; Gao, Y.; Yin, Z.Q. Lipoxin A4 Delays the Progression of Retinal Degeneration via the Inhibition of Microglial Overactivation. Biochem. Biophys. Res. Commun. 2019, 516, 900–906. [Google Scholar] [CrossRef]
- Xie, T.; Cai, J.; Yao, Y.; Sun, C.; Yang, Q.; Wu, M.; Xu, Z.; Sun, X.; Wang, X. LXA4 Protects against Blue-Light Induced Retinal Degeneration in Human A2E-Laden RPE Cells and Balb-c Mice. Ann. Transl. Med. 2021, 9, 1249. [Google Scholar] [CrossRef]
- Medeiros, R.; Rodrigues, G.B.; Figueiredo, C.P.; Rodrigues, E.B.; Grumman, A.; Menezes-de-Lima, O.; Passos, G.F.; Calixto, J.B. Molecular Mechanisms of Topical Anti-Inflammatory Effects of Lipoxin A4 in Endotoxin-Induced Uveitis. Mol. Pharmacol. 2008, 74, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. From The Cover: Neuroprotectin D1: A Docosahexaenoic Acid-Derived Docosatriene Protects Human Retinal Pigment Epithelial Cells from Oxidative Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Xu, Z.-Z.; Strichartz, G.; Serhan, C.N. Emerging Roles of Resolvins in the Resolution of Inflammation and Pain. Trends Neurosci. 2011, 34, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdolmaleki, F.; Kovanen, P.T.; Mardani, R.; Gheibi-hayat, S.M.; Bo, S.; Sahebkar, A. Resolvins: Emerging Players in Autoimmune and Inflammatory Diseases. Clin. Rev. Allerg. Immunol. 2020, 58, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Use of Docosatrienes, Resolvins, and Their Stable Analogs in the Treatment of Airway Diseases and Asthma. U.S. Patent US7759395B2, 20 July 2013. [Google Scholar]
- Rashid, S. Topical Omega-3 and Omega-6 Fatty Acids for Treatment of Dry Eye. Arch. Ophthalmol. 2008, 126, 219. [Google Scholar] [CrossRef] [Green Version]
- Aleo, D.; Barabino, S.; Mangiafico, S.; Rolando, M.; Saita, M.G. Ophthalmic Compositions Based on Polyunsaturated Omega-3 and Omega-6 Fatty Acids. U.S. Patent US20120010280A1, 17 February 2015. [Google Scholar]
- Trotta, M.C.; Gharbia, S.; Herman, H.; Mladin, B.; Hermenean, A.; Balta, C.; Cotoraci, C.; Peteu, V.E.; Gesualdo, C.; Petrillo, F.; et al. Sex and Age-Related Differences in Neuroinflammation and Apoptosis in Balb/c Mice Retina Involve Resolvin D1. Int. J. Mol. Sci. 2021, 22, 6280. [Google Scholar] [CrossRef]
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M.; et al. Resolvin D1 Modulates the Intracellular VEGF-Related MiRNAs of Retinal Photoreceptors Challenged with High Glucose. Front. Pharmacol. 2020, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Trotta, M.C.; Pieretti, G.; Petrillo, F.; Alessio, N.; Hermenean, A.; Maisto, R.; D’Amico, M. Resolvin D1 Reduces Mitochondrial Damage to Photoreceptors of Primary Retinal Cells Exposed to High Glucose. J. Cell Physiol. 2020, 235, 4256–4267. [Google Scholar] [CrossRef]
- Rossi, S.; Di Filippo, C.; Gesualdo, C.; Potenza, N.; Russo, A.; Trotta, M.C.; Zippo, M.V.; Maisto, R.; Ferraraccio, F.; Simonelli, F.; et al. Protection from Endotoxic Uveitis by Intravitreal Resolvin D1: Involvement of Lymphocytes, MiRNAs, Ubiquitin-Proteasome, and M1/M2 Macrophages. Mediat. Inflamm. 2015, 2015, 149381. [Google Scholar] [CrossRef]
- Rossi, S.; Di Filippo, C.; Gesualdo, C.; Testa, F.; Trotta, M.C.; Maisto, R.; Ferraro, B.; Ferraraccio, F.; Accardo, M.; Simonelli, F.; et al. Interplay between Intravitreal RvD1 and Local Endogenous Sirtuin-1 in the Protection from Endotoxin-Induced Uveitis in Rats. Mediat. Inflamm. 2015, 2015, 126408. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, J.; Zhang, H. Role of Sirtuin 1 in the Pathogenesis of Ocular Disease (Review). Int. J. Mol. Med. 2018, 42, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Zhou, Y.; Elison, J.; Gordon, W.C.; Gjorstrup, P.; Bazan, N.G. Resolvin E1 or a Resolvin E1 Analog Inhibits Vascular Leakage in Experimental Choroidal Neovascularization (CNV). Investig. Ophthalmol. Vis. Sci. 2008, 49, 5414. [Google Scholar]
- Tian, H.; Lu, Y.; Sherwood, A.M.; Hongqian, D.; Hong, S. Resolvins E1 and D1 in Choroid-Retinal Endothelial Cells and Leukocytes: Biosynthesis and Mechanisms of Anti-Inflammatory Actions. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3613. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased Dietary Intake of ω-3-Polyunsaturated Fatty Acids Reduces Pathological Retinal Angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Pelaez, R.; Lukiw, W.J.; Bazan, N.G. Omega-3 Essential Fatty Acids Modulate Initiation and Progression of Neurodegenerative Disease. Mol. Neurobiol. 2010, 41, 367–374. [Google Scholar] [CrossRef]
- Bazan, N.G. Homeostatic Regulation of Photoreceptor Cell Integrity: Significance of the Potent Mediator Neuroprotectin D1 Biosynthesized from Docosahexaenoic Acid The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4866. [Google Scholar] [CrossRef]
- Calandria, J.M.; Marcheselli, V.L.; Mukherjee, P.K.; Uddin, J.; Winkler, J.W.; Petasis, N.A.; Bazan, N.G. Selective Survival Rescue in 15-Lipoxygenase-1-Deficient Retinal Pigment Epithelial Cells by the Novel Docosahexaenoic Acid-Derived Mediator, Neuroprotectin D1. J. Biol. Chem. 2009, 284, 17877–17882. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G. Neuroprotectin D1 (NPD1): A DHA-Derived Mediator That Protects Brain and Retina Against Cell Injury-Induced Oxidative Stress. Brain Pathol. 2006, 15, 159–166. [Google Scholar] [CrossRef]
- Bazan, N.G.; Calandria, J.M.; Serhan, C.N. Rescue and Repair during Photoreceptor Cell Renewal Mediated by Docosahexaenoic Acid-Derived Neuroprotectin D1. J. Lipid Res. 2010, 51, 2018–2031. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and Maresins: New pro-Resolving Families of Mediators in Acute Inflammation and Resolution Bioactive Metabolome. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Marcheselli, V.L.; Barreiro, S.; Hu, J.; Bok, D.; Bazan, N.G. Neurotrophins Enhance Retinal Pigment Epithelial Cell Survival through Neuroprotectin D1 Signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 13152–13157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazan, N.G. Cell Survival Matters: Docosahexaenoic Acid Signaling, Neuroprotection and Photoreceptors. Trends Neurosci. 2006, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Molecular Principles for Decoding Homeostasis Disruptions in the Retinal Pigment Epithelium: Significance of Lipid Mediators to Retinal Degenerative Diseases. In Retinal Degenerative Diseases; Bowes Rickman, C., LaVail, M.M., Anderson, R.E., Grimm, C., Hollyfield, J., Ash, J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 854, pp. 385–391. ISBN 978-3-319-17120-3. [Google Scholar]
- Sheets, K.G.; Zhou, Y.; Ertel, M.K.; Knott, E.J.; Regan, C.E.; Elison, J.R.; Gordon, W.C.; Gjorstrup, P.; Bazan, N.G. Neuroprotectin D1 Attenuates Laser-Induced Choroidal Neovascularization in Mouse. Mol. Vis. 2010, 16, 320–329. [Google Scholar] [PubMed]
- Shaw, L.C.; Li Calzi, S.; Li, N.; Moldovan, L.; Sengupta-Caballero, N.; Quigley, J.L.; Ivan, M.; Jun, B.; Bazan, N.G.; Boulton, M.E.; et al. Enteral Arg-Gln Dipeptide Administration Increases Retinal Docosahexaenoic Acid and Neuroprotectin D1 in a Murine Model of Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2018, 59, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Marcheselli, V.L.; de Rivero Vaccari, J.C.; Gordon, W.C.; Jackson, F.E.; Bazan, N.G. Photoreceptor Outer Segment Phagocytosis Attenuates Oxidative Stress-Induced Apoptosis with Concomitant Neuroprotectin D1 Synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13158–13163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, M.V.; Lyngstadaas, A.V.; Bair, J.A.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Maresin 1, a Specialized Proresolving Mediator, Stimulates Intracellular [Ca2+] and Secretion in Conjunctival Goblet Cells. J. Cell Physiol. 2021, 236, 340–353. [Google Scholar] [CrossRef]
- Christensen, M.; Høgdall, C.; Jochumsen, K.; Høgdall, E. Annexin A2 and Cancer: A Systematic Review. Int. J. Oncol. 2017, 52, 5–18. [Google Scholar] [CrossRef]
- Xi, Y.; Ju, R.; Wang, Y. Roles of Annexin A Protein Family in Autophagy Regulation and Therapy. Biomed. Pharmacother. 2020, 130, 110591. [Google Scholar] [CrossRef]
- Gardner, P.J.; Yazid, S.; Ribeiro, J.; Ali, R.R.; Dick, A.D. Augmenting Endogenous Levels of Retinal Annexin A1 Suppresses Uveitis in Mice. Trans. Vis. Sci. Tech. 2017, 6, 10. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Zhu, L.; Du, S.; Wang, Z.; Zhang, Y.; Guo, Y.; Tu, Y.; Song, E. Crosstalk between RPE Cells and Choroidal Endothelial Cells via the ANXA1/FPR2/SHP2/NLRP3 Inflammasome/Pyroptosis Axis Promotes Choroidal Neovascularization. Inflammation 2022, 45, 414–427. [Google Scholar] [CrossRef]
- Hajjar, K.A. The Biology of Annexin A2: From Vascular Fibrinolysis to Innate Immunity. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 144–155. [Google Scholar] [PubMed]
- Ling, Q.; Jacovina, A.T.; Deora, A.; Febbraio, M.; Simantov, R.; Silverstein, R.L.; Hempstead, B.; Mark, W.H.; Hajjar, K.A. Annexin II Regulates Fibrin Homeostasis and Neoangiogenesis in Vivo. J. Clin. Investig. 2004, 113, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yang, X.; Dong, X. The expression and function of annexin A2 in the course of retinal angiogenesis of mouse. Zhonghua Yan Ke Za Zhi 2013, 49, 642–648. [Google Scholar] [PubMed]
- Zhao, S.; Huang, L.; Wu, J.; Zhang, Y.; Pan, D.; Liu, X. Vascular Endothelial Growth Factor Upregulates Expression of Annexin A2 in Vitro and in a Mouse Model of Ischemic Retinopathy. Mol. Vis. 2009, 15, 1231–1242. [Google Scholar]
- Zhao, S.; Pan, D.; Zhang, Y.; Wu, J.; Liu, X.; Xu, Y. Annexin A2 Promotes Choroidal Neovascularization by Increasing Vascular Endothelial Growth Factor Expression in a Rat Model of Argon Laser Coagulation-Induced Choroidal Neovascularization. Chin. Med. J. 2010, 123, 713–721. [Google Scholar]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical Coenzyme Q10 Demonstrates Mitochondrial-Mediated Neuroprotection in a Rodent Model of Ocular Hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Rostoker, R.; Yaseen, H.; Schif-Zuck, S.; Lichtenstein, R.G.; Rabinovich, G.A.; Ariel, A. Galectin-1 Induces 12/15-Lipoxygenase Expression in Murine Macrophages and Favors Their Conversion toward a pro-Resolving Phenotype. Prostaglandins Other Lipid Mediat. 2013, 107, 85–94. [Google Scholar] [CrossRef]
- Caridi, B.; Doncheva, D.; Sivaprasad, S.; Turowski, P. Galectins in the Pathogenesis of Common Retinal Disease. Front. Pharmacol. 2021, 12, 687495. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Chen, Y.; Zuo, J.; Gupta, N.; Chang, Y.; Fang, F. Proteomics-Based Identification of Differentially-Expressed Proteins Including Galectin-1 in the Blood Plasma of Type 2 Diabetic Patients. J. Proteome Res. 2009, 8, 1255–1262. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The Db/Db Mouse: A Useful Model for the Study of Diabetic Retinal Neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [Green Version]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The Therapeutic Potential of Galectin-3 Inhibition in Fibrotic Disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Lou, Y.; Li, T.; Chen, H.; Liu, Q.; He, X. Serum Galectin-3: A Risk Factor for Vascular Complications in Type 2 Diabetes Mellitus. Chin. Med. J. 2013, 126, 2109–2115. [Google Scholar] [PubMed]
- Canning, P.; Glenn, J.V.; Hsu, D.K.; Liu, F.-T.; Gardiner, T.A.; Stitt, A.W. Inhibition of Advanced Glycation and Absence of Galectin-3 Prevent Blood-Retinal Barrier Dysfunction during Short-Term Diabetes. Exp. Diabetes Res. 2007, 2007, 51837. [Google Scholar] [CrossRef] [PubMed]
- Galecto Biotech, A.B.; Syneos Health bioRASI, LLC. A Study to Test the Efficacy and Safety of Inhaled GB0139 in Subjects with Idiopathic Pulmonary Fibrosis (IPF). Available online: https://clinicaltrials.gov/ct2/show/NCT03832946 (accessed on 22 March 2022).
- University of Edinburgh; University of Oxford; Latus Therapeutics. DEFINE—Evaluating Therapies for COVID-19 (DEFINE). Available online: https://clinicaltrials.gov/ct2/show/NCT04473053 (accessed on 22 March 2022).
- Al-Obaidi, N.; Mohan, S.; Liang, S.; Zhao, Z.; Nayak, B.K.; Li, B.; Sriramarao, P.; Habib, S.L. Galectin-1 Is a New Fibrosis Protein in Type 1 and Type 2 Diabetes. FASEB J. 2019, 33, 373–387. [Google Scholar] [CrossRef] [Green Version]
| Author, Year | Model | Treatment | Main Results |
|---|---|---|---|
| Medeiros et al., 2008 [31] | In vivo | Topical administration (20 µL eye drops) of LXA4 1, 5, or 10 ng/eye to both eyes, 1 h before LPS and 6–12–18 h after LPS | Topical LXA4 (1–10 ng/eye) pre-treatment and 10 ng/eye post-treatment reduced inflammatory cell number and the protein leakage into the aqueous humor. IL-1, PGE2, VEGF, COX-2 and TNF-α in LXA4 treated eyes were reduced |
| EIU in male Wistar rats, induced with 200 µg of LPS into one rat hind paw | |||
| Kaviarasan et al., 2015 [28] | Case control study | - | LXA4 levels were significantly decreased in NPDR and PDR patients |
| 27 healthy controls, 27 diabetic patients without retinopathy, 30 NPDR and 30 PDR patients | |||
| In vitro | LXA4 (10, 25, 50 nmol/L) treatment 30 min before LPS | LXA4 reduced IL-6 at lower concentrations | |
| ARPE-19 cells exposed to LPS (500 ng/mL) for 48 h | |||
| Lu et al., 2019 [29] | In vivo | Intravitreal injection of LXA4 (100 ng/μL) at 6, 9 and 12 postnatal days | LXA4 intravitreal injections delay the loss of visual function, by reducing POS apoptosis and inhibiting microglial activation |
| RD1 mice as a model of rapid retinal degeneration | |||
| Xie et al., 2021 [30] | In vivo | Mice were orally administered with LXA4 once a day for 3 days before blue-light exposure | LXA4 treatment reduces ONL degeneration and improved the number of cells positive to zonula occludens-1 staining |
| Male Balb-c mice exposed to blue-light (10,000 lux, 430 nm) for 1 h/day for 14 days, as a model of retinal degeneration | |||
| In vitro | ARPE-19 cells were treated with LXA4 (50, 100 nM) 30 min before blue-light exposure | LXA4 treatment reduced cell death and ROS content in RPE cells by activating NRF2/HO1 pathway | |
| ARPE-19 cells exposed to blue-light illumination (1000 lux, 430 nm) for 15 h |
| Author, Year | Model | Treatment | Main Results |
|---|---|---|---|
| Mukherjee et al., 2004 [32] | In vitro | NPD1 (50 nM) added to ARPE-19 cells at the time of adding TNF-α/H2O | NPD1 inhibited ARPE-19 apoptosis induced by oxidative stress |
| RPE-19 cells serum- starved and exposed to oxidative stress for 14 h (400–800 µM H2O2 and 10 ng/mL TNF-α) | |||
| ARPE-19 cells transfected with COX-2 promoter | NPD1 (0.05–0.5–5–50 100 nM) added to ARPE-19 cells | NPD1 reduces IL-1β content induced by COX2 | |
| Connor et al., 2007 [46] | In vivo | C57BL/6J mice fed with diet containing 2% ω-6-PUFAs (AA) and no ω-3-PUFAs (DHA and EPA), or 2% ω-3-PUFAs and no ω-6-PUFAs in 10% (w/w) safflower oil | Among the bioactive ω-3-PUFA-derived mediators, NPD1 potently protected against neovascularization |
| C57BL/6J mice exposed to 75% O2 from postnatal day 7 to postnatal day 12, then returned to room air for 5 days as a model of hypoxia-induced ischemic retinopathy | |||
| Mukherjee et al., 2007 [58] | In vitro | ||
| ARPE-19 cells serum-starved and exposed to: - A2E, a lipofuscin component (20 μM) in the presence of 430 nM light and O2 for 15 min, followed by 60 min of incubation in the dark, as a model of oxidative stress; - 600 µM H2O2 and 10 ng/mL TNF-α) for 15 h, as a second model of oxidative stress | NPD1 (50 nM) added 5 min before oxidative stress and at different time points after oxidative stress | NPD1 decreased ARPE-19 damage in both models of oxidative stress | |
| DHA (30 nM) and PEDF (20 ng/mL) added to ARPE-19 cells at the time of adding TNF-α/H2O2 | PEDF increased NPD1 synthesis and release through the apical surface of ARPE-19 cells. PEDF-induced NPD1 synthesis and release is selectively potentiated by DHA | ||
| Mukherjee et al., 2007 [53] | In vitro | ARPE-19 cells incubated with bovine POS (10 million per well) | NPD1 increased in POS-mediated ARPE-19 protection against oxidative stress |
| ARPE-19 cells serum-starved and exposed to oxidative stress (H2O2 and 10 ng/mL TNF-α) for 16 h | |||
| Calandria et al., 2009 [49] | In vitro | ||
| ARPE-19 cells silenced for 15-LOX, then serum-starved and exposed to oxidative stress (30% H2O2 and 10 ng/mL TNF-α) at different time points | ARPE-19 cells exposed to 50 nM NPD1 after oxidative stress | NPD1 not detectable in 15-LOX silenced-ARPE-19 cells | |
| NPD1 promoted cell survival in 15-LOX silenced cells, by reducing oxidative stress | |||
| Marcheselli et al., 2010 [22] | In vitro | NPD1 50 nM added to ARPE-19 cells at the time of adding TNF-α/H2O2 | NPD1 showed a specific and stereoselective binding capacity to ARPE-19 cells and inhibited cell apoptosis induced by stress induction |
| ARPE-19 cells serum- starved and exposed to oxidative stress (600 μM H2O2 and 10 ng/mL TNF-α) for 15 h | |||
| Sheets et al., 2010 [56] | In vivo | Intraperitoneal injections of NPD1 (1 mg/mL) 1 h before laser treatment and 1, 3, 5 and 7 days after laser treatment | NPD1 reduces CNV lesions and cell proliferations at lesion sites |
| Male C57BL/6J mice receiving laser rupture of Bruch’s membrane as a model of CNV | |||
| Shaw et al., 2018 [57] | In vivo | Arg-Gln dipeptide (1, 2.5 or 5 g/kg body weight per day on postnatal day 12 and 17 | Arg-Gln dipeptide restored retinal DHA and NPD1 levels in OIR model, by reducing preretinal neovascularization and vaso-obliteration |
| 7-day-old C57BL/6J mice exposed to 5% oxygen atmosphere for 5 days as a model of OIR |
| Author, Year | Model | Treatment | Main Results |
|---|---|---|---|
| Ling et al., 2004 [65] | In vivo | - | AnxA2-null mice showed an impaired angiogenic response to O2 stimulation (-50%), with endothelial cells failing to react to O2 by not migrating to the ILM and penetrating in the same retinal layer |
| AnxA2-null C57BL/6 mice, exposed at postnatal day 7 to continuous-flow 75% O2/25% N2 for 5 days, then returned to room air | |||
| Zhao et al., 2009 [67] | In vivo | Intravitreous injection of AnxA2 (1 μg) or AnxA2 sirna (1 μg) at postnatal day 7 and 12 in mice returned to room air. | AnxA2 increased in endothelial cells of ischemic retina. More neovascularization in eyes injected with AnxA2 than in eyes injected with AnxA2 sirna. |
| C57BL/6J mice exposed to 75% O2 from postnatal day 7 to postnatal day 12, then returned to room air for 5 days as a model of hypoxia-induced ischemic retinopathy | |||
| In vitro | 25 ng/mL VEGF for 2 h | AnxA2 upregulated by VEGF; high expression of VEGFR2 consequent to AnxA1 increase | |
| CRECs exposed to hypoxic conditions (1% O2, 94% N2, 5% CO2) for 2 and 4 h | |||
| Zhao et al., 2020 [68] | In vivo | - | AnxA2 and VEGF showed the maximum expression after 14 days from CNV in vascular endothelial cells, RGCs, INL and RPE cells |
| Norvegian rats exposed to argon laser coagulation-induced CNV | |||
| In vitro | VEGF expression was reduced by the silenced expression of AnxA2 | ||
| RPE-J cells silenced for AnxA2 and exposed to photocoagulation by laser ablation | |||
| Wang et al., 2013 [66] | In vivo | AnxA2 siRNA | AnxA2 overexpressed in OIR mice, paralleled by high neovessel growth. VEGF, metalloproteinases 2 and 9 (MMP-2 and MMP-9) reduced by AnxA2 siRNA |
| C57BL/6 mice, exposed to OIR | |||
| Davis et al., 2017 [69] | In vivo | - | RGCs showing apoptosis in OHT animals were positive to Anx5 staining |
| Adult male Dark Agouti rats injected with hypertonic saline solution (1.80 M) into two episcleral veins as a model of OHT for 21 days | |||
| Gardner et al., 2017 [62] | In vivo | Intravitreal injections (2 µL) of hrAnxA1 (50 and 500 ng) in EIU mice | AnxA1 and ALX/FPR2 receptor are expressed in healthy mice and human retina (AnxA1 in RGCs and RPEs cells; ALX/FPR2 in GLC, ONL and POS) AnxA1 predominantly colocalizes with cells positive to CD45, GFAP and Iba-1 |
| EIU in C57BL/6 mice, induced with 1 ng LPS intreavitreally injected (2 µL) | |||
| Post-mortem human retinae from healthy patients or patients with uveitis | - | hrAnxA1 (500 ng) attenuated the EIU severity in mice by reducing the expression of pro-inflammatory cytokines and chemokines | |
| Zhu et al., 2022 [63] | In vitro | HCECs exposed to AnxA1 recombinant protein | ALX/FPR2 expression, along with AnxA1 expression and secretion are upregulated by hypoxia in ARPE-19 cells. AnxA1 promoted HCECs proliferation, migration and tube formation. Moreover, AnxA1 inhibited NLRP3-inflammosome activation and NLRP3-inflammasome mediated pyrocitosis in HCECs |
| ARPE-19 cells and HCECs exposed to hypoxic conditions (1% O2, 5% CO2 and 94% N2) for 24 h as a model of CNV | |||
| In vivo | AnxA1 secreted from RPE cells resulted in an overall reduction of CNV volume | ||
| male C57BL/6J mice were subjected to laser photocoagulation (rupture of Bruch’s membrane) as a model of CNV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotta, M.C.; Gesualdo, C.; Petrillo, F.; Lepre, C.C.; Della Corte, A.; Cavasso, G.; Maggiore, G.; Hermenean, A.; Simonelli, F.; D’Amico, M.; et al. Resolution of Inflammation in Retinal Disorders: Briefly the State. Int. J. Mol. Sci. 2022, 23, 4501. https://doi.org/10.3390/ijms23094501
Trotta MC, Gesualdo C, Petrillo F, Lepre CC, Della Corte A, Cavasso G, Maggiore G, Hermenean A, Simonelli F, D’Amico M, et al. Resolution of Inflammation in Retinal Disorders: Briefly the State. International Journal of Molecular Sciences. 2022; 23(9):4501. https://doi.org/10.3390/ijms23094501
Chicago/Turabian StyleTrotta, Maria Consiglia, Carlo Gesualdo, Francesco Petrillo, Caterina Claudia Lepre, Alberto Della Corte, Giancuomo Cavasso, Giulia Maggiore, Anca Hermenean, Francesca Simonelli, Michele D’Amico, and et al. 2022. "Resolution of Inflammation in Retinal Disorders: Briefly the State" International Journal of Molecular Sciences 23, no. 9: 4501. https://doi.org/10.3390/ijms23094501
APA StyleTrotta, M. C., Gesualdo, C., Petrillo, F., Lepre, C. C., Della Corte, A., Cavasso, G., Maggiore, G., Hermenean, A., Simonelli, F., D’Amico, M., & Rossi, S. (2022). Resolution of Inflammation in Retinal Disorders: Briefly the State. International Journal of Molecular Sciences, 23(9), 4501. https://doi.org/10.3390/ijms23094501










