Repolarization of Unbalanced Macrophages: Unmet Medical Need in Chronic Inflammation and Cancer
Abstract
1. Introduction
1.1. Cytokine Receptors
- -
- Hematopoietin (i.e., class I cytokines) receptors have a common chain that accounts for the ligand specificity (the γ-chain for interleukin-2 (IL2), the β-chain for the granulocyte macrophage-colony stimulating factor (GM-CSF)). They recognized GM-CSF and IL6, among others, and they signal via the JAK-STAT (Janus kinases-signal transducer and activator of transcription) pathway [2];
- -
- Interferon (i.e., class II cytokines) receptors are heterodimeric receptors that also signal via the JAK-STAT pathway [3];
- -
- Tumor necrosis factor (TNF) family receptors recognize TNF, and also Fas-ligand, CD40-ligand. The binding of the ligand triggers the trimerization of the receptor [4], leading to the activation of the trimer receptor at the membrane of MP. However, these receptors can be cleaved, and are therefore present as soluble receptors in the microenvironment. These receptors are characterized by a death domain (such as TRADD (tumor necrosis factor receptor type 1-associated death domain), and FADD (Fas-associated protein with death domain)) at their cytoplasmic tail, and signal due to adaptors associated therewith;
- -
- Immunoglobulins superfamily receptors recognize cytokines of the IL1 family (IL1α/β), the interleukin-1 receptor antagonist (IL1-ra), IL18, and IL33, and growth factors such as macrophage-colony stimulating factor (M-CSF) [5]. They are characterized by an “immunoglobulin-like” extracellular domain and signal after dimerization via Toll/interleukin-1 receptor (TIR) of their cytoplasmic tail, involving signaling proteins such as interleukin-1 receptor-associated kinase (IRAK) and myeloid differentiation primary response protein 88 (Myd88);
- -
- Transforming growth factor (TGF) receptors recognize TGFβ, among others. They have a serine/threonine kinase activity and signal via either a hetero-tetrameric complex that induces Smad complexes, or a Smad-independent pathway such as mitogen-activated protein kinases (MAPK), phosphoinositide 3 kinase (PI3K), and Rho-GTPases [6];
- -
- Chemokine and formyl-methionyl-leucyl-phenylalanine (fMLP) receptors recognize in particular IL8, C-C chemokine ligand 2 (CCL2) (also called monocyte chemoattractant protein 1 (MCP1)). These receptors harbor seven transmembrane domains and are coupled to hetero-trimeric G proteins that mediate the signaling cascade [7].
1.2. Pattern-Recognition Receptors (PRR)
- -
- Toll-like receptors (TLR) recognize intracellular (TLR3, TLR7, TLR8, TLR9) or extracellular (TLR1, TLR2, TLR4, TLR5, TLR6) danger signals [8];
- -
- Nucleotide oligomerization domain (NOD)-like receptors (NLR), such as NOD-like receptor family pyrin domain containing 3 (NLRP3) and NOD1, recognize cytoplasmic patterns mainly of bacterial origin, and induce the formation of inflammasome [9];
- -
- Retinoic acid-inducible gene I-like receptors (RLR), such as melanoma differentiation-associated protein 5 (MDA5) and RIG-I, recognize cytoplasmic patterns from viral origin and induce an interferon (IFN) type 1 response [10].
1.3. Phagocytosis Receptors
- -
- Scavenger receptors (such as CD36 and CD163) that in particular recognize modified lipoproteins [11]. Scavenger receptors are able to associate with co-receptors. This ability broadens the variety of ligands they recognize and the associated functions they carry out, including the clearance of pathogens, and the transport of lipids and cargos within the cells. Therefore, scavenger receptors are involved in the immune response, in particular in the polarization of MP, and thereby in the pathogeny of inflammatory disorders;
- -
- Lectin receptors such as mannose receptor (CD206), Dectin-1, and dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN, also named CD209) that have a binding domain for carbohydrates [12]. Lectin receptors are crucial for adapting the immune response to pathogens. Their activation leads to the secretion of cytokines that will shape the immune response of T lymphocytes. This property can be advantageously taken into account to promote new vaccination approaches;
- -
- The γ receptors for the constant fragment (Fc) of immunoglobulins, such as CD16 (FcγRIII), CD32 (FcγRII), and CD64 (FcγRI), that bind immunoglobulins, in particular those from opsonized particles [13]. The recognition of immunoglobulins and immune complexes by activating FcγR influences the uptake processing, and presentation of antigens by monocyte-derived DC and MP, both in the steady state and during inflammation.
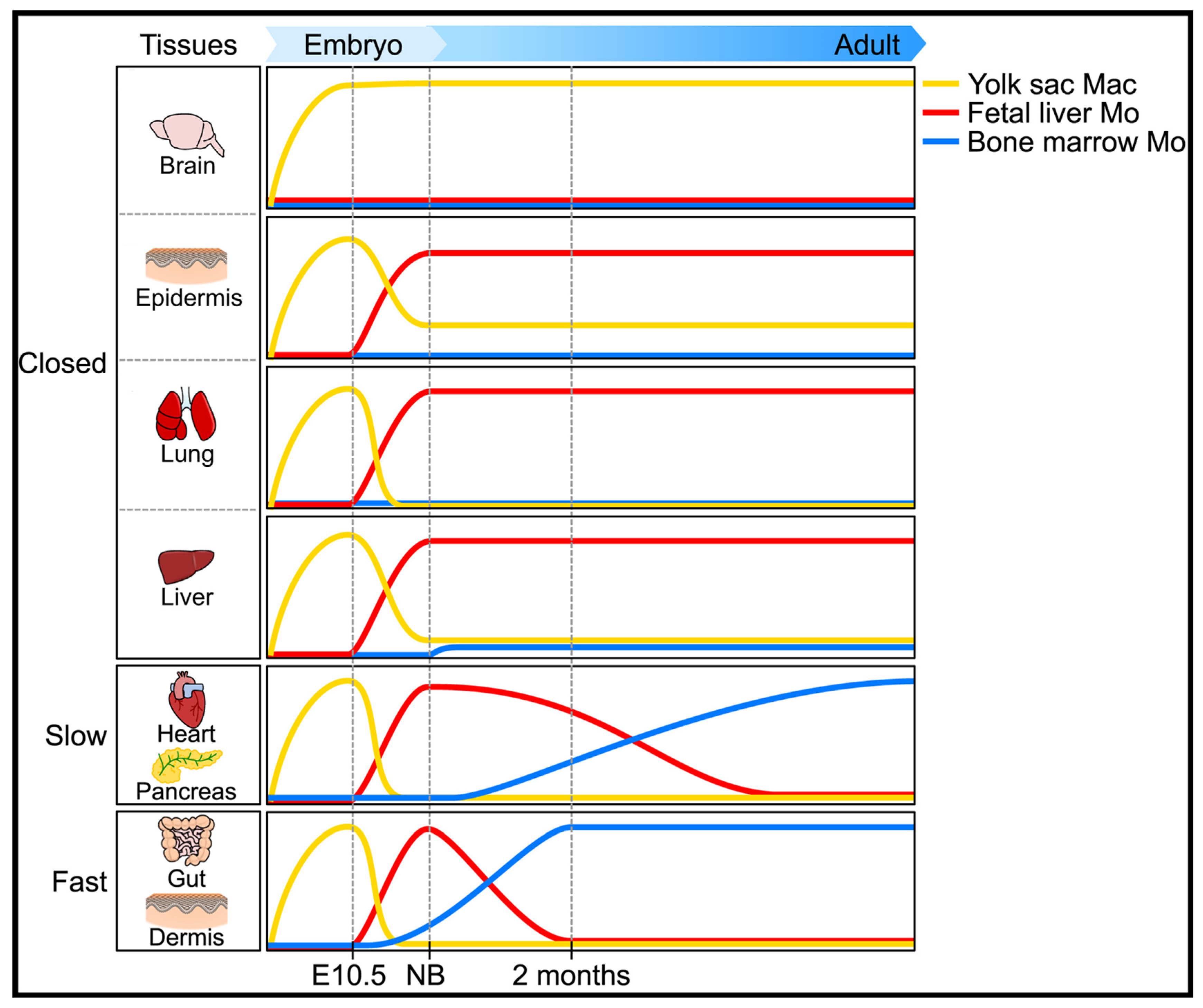
1.4. The Ontogeny of Macrophages
- (i)
- a primitive wave of erythro-myeloid precursors from the yolk sac at day E7.5. These cells express the receptor for M-CSF (CSF-1R), but not the transcription factor c-Myb;
- (ii)
- a second wave called “transient definitive” that comes from the hemogenic endothelium of the yolk sac. This wave generates erythromyeloid precursors (c-Myb+) that will migrate to the fetal liver thanks to the development of the blood vasculature at day E8.5;
- (iii)
- a third wave called “definitive” that also comes from the hemogenic endothelium of the yolk sac. It produces hematopoietic stem cells (c-Kit+ Sca-1+) at day E10.5 in aorta, gonads, and mesonephros. These precursors will set up the definitive hematopoiesis of both fetal liver and fetal bone marrow.
- (i)
- In “closed” tissue, such as brain, skin, and lungs, “resident” MP originate from fetal hematopoiesis and are self-renewed. Of note, the particular status of the liver in which Kupfer cells may have a minor contribution of the neonate homeostasis;
- (ii)
- In open tissue with slow kinetics (heart, pancreas), MP originating from adult hematopoiesis rapidly replace the ones originating from embryonic hematopoiesis;
- (iii)
- In open tissue with fast kinetics (lamina propria, dermis), MP derive from circulating monocytes and depend constantly on blood for their renewal.
1.5. The Polarization of Macrophages
2. Managing Macrophages in Inflammatory Disorders
2.1. Macrophages in Rheumatoid Arthritis
2.1.1. Subpopulations of Synovial MP
2.1.2. Ontogeny of Synovial MP
2.1.3. Roles of MP in the Synovium
2.1.4. (Re)programming Synovial MP
2.2. Therapeutic Modulation of Macrophages in Arthritis
2.2.1. Impact of RA Treatment on Synovitis and MP
2.2.2. Theranostics and MP in Arthritis
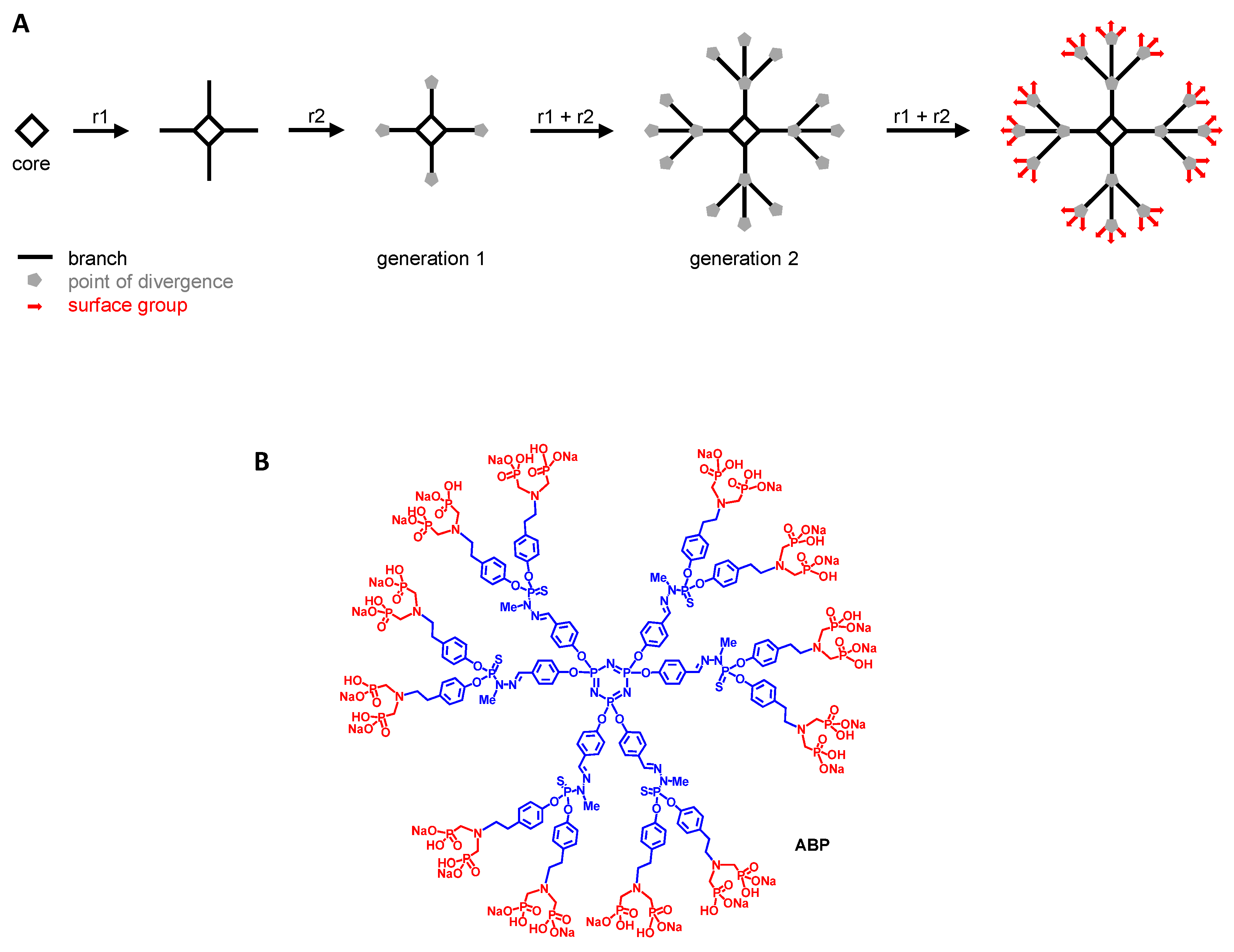
2.2.3. Reprogramming MP with Poly(phosphorhydrazone) (PPH) Dendrimers
3. Managing Tumor-Associated Macrophages (TAM)
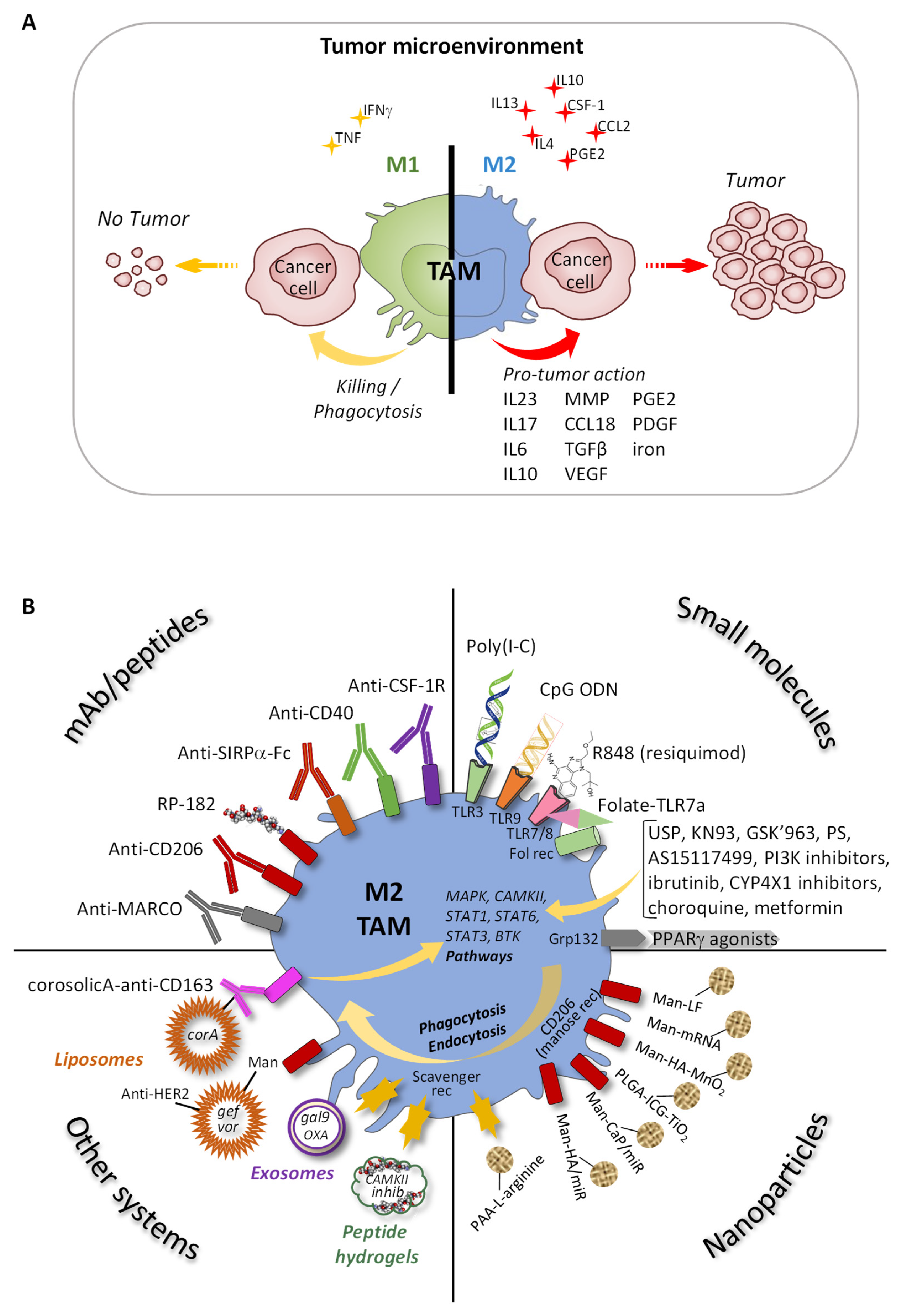
3.1. Origin and Functions of TAM Phagocytosis Receptors
3.2. Reprogramming TAM
3.2.1. Specific Antibodies and Peptides
3.2.2. Small Molecules
3.2.3. Functionalized Nanoparticles
3.2.4. Other Systems
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Underhill, D.M.; Gordon, S.; Imhof, B.A.; Núñez, G.; Bousso, P. Élie Metchnikoff (1845–1916): Celebrating 100 Years of Cellular Immunology and Beyond. Nat. Rev. Immunol. 2016, 16, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Robb, L. Cytokine Receptors and Hematopoietic Differentiation. Oncogene 2007, 26, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- Renauld, J.-C. Class II Cytokine Receptors and Their Ligands: Key Antiviral and Inflammatory Modulators. Nat. Rev. Immunol. 2003, 3, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling Pathways of the TNF Superfamily: A Double-Edged Sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Sims, J.E.; Smith, D.E. The IL-1 Family: Regulators of Immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-Dependent and Smad-Independent Pathways in TGF-Beta Family Signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Kufareva, I.; Salanga, C.L.; Handel, T.M. Chemokine and Chemokine Receptor Structure and Interactions: Implications for Therapeutic Strategies. Immunol. Cell Biol. 2015, 93, 372–383. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of Inflammasome Signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef]
- Loo, Y.-M.; Gale, M. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger Receptors in Homeostasis and Immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-Type Lectin Receptors: Shaping Immune Responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The Function of Fcγ Receptors in Dendritic Cells and Macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans Cells Derive Predominantly from Embryonic Fetal Liver Monocytes with a Minor Contribution of Yolk Sac-Derived Macrophages. J. Exp. Med. 2012, 209, 1167–1181. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Mackaness, G.B. Cellular Resistance to Infection. J. Exp. Med. 1962, 116, 381–406. [Google Scholar] [CrossRef]
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of Interferon-Gamma as the Lymphokine That Activates Human Macrophage Oxidative Metabolism and Antimicrobial Activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 Potently Enhances Murine Macrophage Mannose Receptor Activity: A Marker of Alternative Immunologic Macrophage Activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M. TH1/TH2 Paradigm Extended: Macrophage Polarization as an Unappreciated Pathogen-Driven Escape Mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Mosser, D.M. A Novel Phenotype for an Activated Macrophage: The Type 2 Activated Macrophage. J. Leukoc. Biol. 2002, 72, 101–106. [Google Scholar] [PubMed]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and Functional Characterization of Three Activated Macrophage Populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Schroder, K.; Irvine, K.M.; Taylor, M.S.; Bokil, N.J.; Le Cao, K.-A.; Masterman, K.-A.; Labzin, L.I.; Semple, C.A.; Kapetanovic, R.; Fairbairn, L.; et al. Conservation and Divergence in Toll-like Receptor 4-Regulated Gene Expression in Primary Human versus Mouse Macrophages. Proc. Natl. Acad. Sci. USA 2012, 109, E944–E953. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Sander, J.; Schmidt, S.V.; Cirovic, B.; McGovern, N.; Papantonopoulou, O.; Hardt, A.-L.; Aschenbrenner, A.C.; Kreer, C.; Quast, T.; Xu, A.M.; et al. Cellular Differentiation of Human Monocytes Is Regulated by Time-Dependent Interleukin-4 Signaling and the Transcriptional Regulator NCOR2. Immunity 2017, 47, 1051–1066.e12. [Google Scholar] [CrossRef]
- Shapiro, E.; Biezuner, T.; Linnarsson, S. Single-Cell Sequencing-Based Technologies Will Revolutionize Whole-Organism Science. Nat. Rev. Genet. 2013, 14, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Barg, E.; Weedon, H.; Papengelis, V.; Smeets, T.; Tak, P.P.; Kraan, M.; Coleman, M.; Ahern, M.J. Microarchitecture and Protective Mechanisms in Synovial Tissue from Clinically and Arthroscopically Normal Knee Joints. Ann. Rheum. Dis. 2003, 62, 303–307. [Google Scholar] [CrossRef]
- Henderson, B.; Revell, P.A.; Edwards, J.C. Synovial Lining Cell Hyperplasia in Rheumatoid Arthritis: Dogma and Fact. Ann. Rheum. Dis. 1988, 47, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Barnes, M.R.; Blighe, K.; Goldmann, K.; Rana, S.; Hackney, J.A.; Ramamoorthi, N.; John, C.R.; Watson, D.S.; Kummerfeld, S.K.; et al. Molecular Portraits of Early Rheumatoid Arthritis Identify Clinical and Treatment Response Phenotypes. Cell Rep. 2019, 28, 2455–2470.e5. [Google Scholar] [CrossRef] [PubMed]
- Culemann, S.; Grüneboom, A.; Nicolás-Ávila, J.Á.; Weidner, D.; Lämmle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally Renewing Resident Synovial Macrophages Provide a Protective Barrier for the Joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct Synovial Tissue Macrophage Subsets Regulate Inflammation and Remission in Rheumatoid Arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef]
- Tu, J.; Hong, W.; Guo, Y.; Zhang, P.; Fang, Y.; Wang, X.; Chen, X.; Lu, S.; Wei, W. Ontogeny of Synovial Macrophages and the Roles of Synovial Macrophages From Different Origins in Arthritis. Front. Immunol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Thurlings, R.M.; Wijbrandts, C.A.; Bennink, R.J.; Dohmen, S.E.; Voermans, C.; Wouters, D.; Izmailova, E.S.; Gerlag, D.M.; van Eck-Smit, B.L.F.; Tak, P.P. Monocyte Scintigraphy in Rheumatoid Arthritis: The Dynamics of Monocyte Migration in Immune-Mediated Inflammatory Disease. PLoS ONE 2009, 4, e7865. [Google Scholar] [CrossRef]
- Misharin, A.V.; Cuda, C.M.; Saber, R.; Turner, J.D.; Gierut, A.K.; Haines, G.K.; Berdnikovs, S.; Filer, A.; Clark, A.R.; Buckley, C.D.; et al. Nonclassical Ly6C(-) Monocytes Drive the Development of Inflammatory Arthritis in Mice. Cell Rep. 2014, 9, 591–604. [Google Scholar] [CrossRef]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef] [PubMed]
- Waterborg, C.E.J.; Beermann, S.; Broeren, M.G.A.; Bennink, M.B.; Koenders, M.I.; van Lent, P.L.E.M.; van den Berg, W.B.; van der Kraan, P.M.; van de Loo, F.A.J. Protective Role of the MER Tyrosine Kinase via Efferocytosis in Rheumatoid Arthritis Models. Front. Immunol. 2018, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, F.; Gauthier, T.; Vallion, R.; Martin-Rodriguez, O.; Missey, A.; Daoui, A.; Valmary-Degano, S.; Saas, P.; Couturier, M.; Perruche, S. Factors Produced by Macrophages Eliminating Apoptotic Cells Demonstrate Pro-Resolutive Properties and Terminate Ongoing Inflammation. Front. Immunol. 2018, 9, 2586. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial Tissue Macrophages: Friend or Foe? RMD Open 2017, 3, e000527. [Google Scholar] [CrossRef]
- Antoniv, T.T.; Ivashkiv, L.B. Dysregulation of Interleukin-10-Dependent Gene Expression in Rheumatoid Arthritis Synovial Macrophages. Arthritis Rheum. 2006, 54, 2711–2721. [Google Scholar] [CrossRef]
- Haringman, J.J.; Gerlag, D.M.; Zwinderman, A.H.; Smeets, T.J.M.; Kraan, M.C.; Baeten, D.; McInnes, I.B.; Bresnihan, B.; Tak, P.P. Synovial Tissue Macrophages: A Sensitive Biomarker for Response to Treatment in Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2005, 64, 834–838. [Google Scholar] [CrossRef]
- Wijbrandts, C.A.; Vergunst, C.E.; Haringman, J.J.; Gerlag, D.M.; Smeets, T.J.M.; Tak, P.P. Absence of Changes in the Number of Synovial Sublining Macrophages after Ineffective Treatment for Rheumatoid Arthritis: Implications for Use of Synovial Sublining Macrophages as a Biomarker. Arthritis Rheum. 2007, 56, 3869–3871. [Google Scholar] [CrossRef]
- Bresnihan, B.; Pontifex, E.; Thurlings, R.M.; Vinkenoog, M.; El-Gabalawy, H.; Fearon, U.; Fitzgerald, O.; Gerlag, D.M.; Rooney, T.; van de Sande, M.G.; et al. Synovial Tissue Sublining CD68 Expression Is a Biomarker of Therapeutic Response in Rheumatoid Arthritis Clinical Trials: Consistency across Centers. J. Rheumatol. 2009, 36, 1800–1802. [Google Scholar] [CrossRef]
- Mulherin, D.; Fitzgerald, O.; Bresnihan, B. Synovial Tissue Macrophage Populations and Articular Damage in Rheumatoid Arthritis. Arthritis Rheum. 1996, 39, 115–124. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Q.; He, P.; Zhou, B.; He, K.; Sun, X.; Lei, G.; Gong, T.; Zhang, Z. Targeted Apoptosis of Macrophages and Osteoclasts in Arthritic Joints Is Effective against Advanced Inflammatory Arthritis. Nat. Commun. 2021, 12, 2174. [Google Scholar] [CrossRef]
- Chu, C.Q.; Field, M.; Feldmann, M.; Maini, R.N. Localization of Tumor Necrosis Factor Alpha in Synovial Tissues and at the Cartilage-Pannus Junction in Patients with Rheumatoid Arthritis. Arthritis Rheum. 1991, 34, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Leung, B.P.; Field, M.; Wei, X.Q.; Huang, F.P.; Sturrock, R.D.; Kinninmonth, A.; Weidner, J.; Mumford, R.; Liew, F.Y. Production of Nitric Oxide in the Synovial Membrane of Rheumatoid and Osteoarthritis Patients. J. Exp. Med. 1996, 184, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The Pathogenic Role of Angiogenesis in Rheumatoid Arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Kiener, H.P.; Agarwal, S.K.; Noss, E.H.; Watts, G.F.M.; Chisaka, O.; Takeichi, M.; Brenner, M.B. Cadherin-11 in Synovial Lining Formation and Pathology in Arthritis. Science 2007, 315, 1006–1010. [Google Scholar] [CrossRef]
- Kuo, D.; Ding, J.; Cohn, I.S.; Zhang, F.; Wei, K.; Rao, D.A.; Rozo, C.; Sokhi, U.K.; Shanaj, S.; Oliver, D.J.; et al. HBEGF+ Macrophages in Rheumatoid Arthritis Induce Fibroblast Invasiveness. Sci. Transl. Med. 2019, 11, eaau8587. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving Concepts in Bone-Immune Interactions in Health and Disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Danks, L.; Sabokbar, A.; Gundle, R.; Athanasou, N.A. Synovial Macrophage-Osteoclast Differentiation in Inflammatory Arthritis. Ann. Rheum. Dis. 2002, 61, 916–921. [Google Scholar] [CrossRef]
- Madel, M.-B.; Ibáñez, L.; Ciucci, T.; Halper, J.; Rouleau, M.; Boutin, A.; Hue, C.; Duroux-Richard, I.; Apparailly, F.; Garchon, H.-J.; et al. Dissecting the Phenotypic and Functional Heterogeneity of Mouse Inflammatory Osteoclasts by the Expression of Cx3cr1. Elife 2020, 9, e54493. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Joosten, L.A.B.; Koenders, M.I.; Devesa, I.; Roelofs, M.F.; Radstake, T.R.D.J.; Heuvelmans-Jacobs, M.; Akira, S.; Nicklin, M.J.H.; Ribeiro-Dias, F.; et al. Stimulation of TLR2 and TLR4 Differentially Skews the Balance of T Cells in a Mouse Model of Arthritis. J. Clin. Investig. 2008, 118, 205–216. [Google Scholar] [CrossRef]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K.-A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine Deiminase from Porphyromonas Gingivalis Citrullinates Human Fibrinogen and α-Enolase: Implications for Autoimmunity in Rheumatoid Arthritis. Arthritis Rheum. 2010, 62, 2662–2672. [Google Scholar] [CrossRef]
- Nativel, B.; Couret, D.; Giraud, P.; Meilhac, O.; d’Hellencourt, C.L.; Viranaïcken, W.; Da Silva, C.R. Porphyromonas Gingivalis Lipopolysaccharides Act Exclusively through TLR4 with a Resilience between Mouse and Human. Sci. Rep. 2017, 7, 15789. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.R.; Gelani, V.; Fernandes, A.P.; Gasparoto, T.H.; Torres, S.A.; Santos, C.F.; Garlet, G.P.; da Silva, J.S.; Campanelli, A.P. The Essential Role of Toll like Receptor-4 in the Control of Aggregatibacter Actinomycetemcomitans Infection in Mice. J. Clin. Periodontol. 2010, 37, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Zhao, X.; Chandra, P.E.; Robinson, W.H. Immune Complexes Containing Citrullinated Fibrinogen Costimulate Macrophages via Toll-like Receptor 4 and Fcγ Receptor. Arthritis Rheum. 2011, 63, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Laurent, L.; Clavel, C.; Lemaire, O.; Anquetil, F.; Cornillet, M.; Zabraniecki, L.; Nogueira, L.; Fournié, B.; Serre, G.; Sebbag, M. Fcγ Receptor Profile of Monocytes and Macrophages from Rheumatoid Arthritis Patients and Their Response to Immune Complexes Formed with Autoantibodies to Citrullinated Proteins. Ann. Rheum. Dis. 2011, 70, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Laurent, L.; Anquetil, F.; Clavel, C.; Ndongo-Thiam, N.; Offer, G.; Miossec, P.; Pasquali, J.-L.; Sebbag, M.; Serre, G. IgM Rheumatoid Factor Amplifies the Inflammatory Response of Macrophages Induced by the Rheumatoid Arthritis-Specific Immune Complexes Containing Anticitrullinated Protein Antibodies. Ann. Rheum. Dis. 2015, 74, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Ceccato, L.; Anquetil, F.; Serre, G.; Sebbag, M. Among Human Macrophages Polarised to Different Phenotypes, the M-CSF-Oriented Cells Present the Highest pro-Inflammatory Response to the Rheumatoid Arthritis-Specific Immune Complexes Containing ACPA. Ann. Rheum. Dis. 2016, 75, 2184–2191. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring Synovial Homeostasis in Rheumatoid Arthritis by Targeting Fibroblast-like Synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P.; Eisenberg, R.A.; Fournié, J.-J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Garcia, S.; Hartkamp, L.M.; Malvar-Fernandez, B.; van Es, I.E.; Lin, H.; Wong, J.; Long, L.; Zanghi, J.A.; Rankin, A.L.; Masteller, E.L.; et al. Colony-Stimulating Factor (CSF) 1 Receptor Blockade Reduces Inflammation in Human and Murine Models of Rheumatoid Arthritis. Arthritis Res. Ther. 2016, 18, 75. [Google Scholar] [CrossRef]
- Nakano, K.; Okada, Y.; Saito, K.; Tanikawa, R.; Sawamukai, N.; Sasaguri, Y.; Kohro, T.; Wada, Y.; Kodama, T.; Tanaka, Y. Rheumatoid Synovial Endothelial Cells Produce Macrophage Colony-Stimulating Factor Leading to Osteoclastogenesis in Rheumatoid Arthritis. Rheumatology 2007, 46, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Fuentelsaz-Romero, S.; Cuervo, A.; Estrada-Capetillo, L.; Celis, R.; García-Campos, R.; Ramírez, J.; Sastre, S.; Samaniego, R.; Puig-Kröger, A.; Cañete, J.D. GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis. Front. Immunol. 2020, 11, 613975. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovsky, J.; Guo, X.; White, W.I.; Ryan, P.C.; Godwood, A.; Albulescu, M.; et al. A Randomized Phase IIb Study of Mavrilimumab and Golimumab in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.A.; Grigoriev, G.; Lee, A.; Kalliolias, G.D.; Ivashkiv, L.B. The Interferon Signature and STAT1 Expression in Rheumatoid Arthritis Synovial Fluid Macrophages Are Induced by Tumor Necrosis Factor α and Counter-Regulated by the Synovial Fluid Microenvironment. Arthritis Rheum. 2012, 64, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Park, S.H.; Chen, J.; Qiao, Y.; Giannopoulou, E.; Berg, K.; Hanidu, A.; Li, J.; Nabozny, G.; Kang, K.; et al. Interferon-γ Represses M2 Gene Expression in Human Macrophages by Disassembling Enhancers Bound by the Transcription Factor MAF. Immunity 2017, 47, 235–250.e4. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a Proinflammatory Regulator in Clinical and Experimental Arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Elmesmari, A.; Fraser, A.R.; Wood, C.; Gilchrist, D.; Vaughan, D.; Stewart, L.; McSharry, C.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155 Regulates Monocyte Chemokine and Chemokine Receptor Expression in Rheumatoid Arthritis. Rheumatology 2016, 55, 2056–2065. [Google Scholar] [CrossRef]
- Paoletti, A.; Rohmer, J.; Ly, B.; Pascaud, J.; Rivière, E.; Seror, R.; Le Goff, B.; Nocturne, G.; Mariette, X. Monocyte/Macrophage Abnormalities Specific to Rheumatoid Arthritis Are Linked to MiR-155 and Are Differentially Modulated by Different TNF Inhibitors. J. Immunol. 2019, 203, 1766–1775. [Google Scholar] [CrossRef]
- Ammari, M.; Presumey, J.; Ponsolles, C.; Roussignol, G.; Roubert, C.; Escriou, V.; Toupet, K.; Mausset-Bonnefont, A.-L.; Cren, M.; Robin, M.; et al. Delivery of MiR-146a to Ly6Chigh Monocytes Inhibits Pathogenic Bone Erosion in Inflammatory Arthritis. Theranostics 2018, 8, 5972–5985. [Google Scholar] [CrossRef]
- Saeki, N.; Imai, Y. Reprogramming of Synovial Macrophage Metabolism by Synovial Fibroblasts under Inflammatory Conditions. Cell Commun. Signal. 2020, 18, 188. [Google Scholar] [CrossRef]
- Papathanassiu, A.E.; Ko, J.-H.; Imprialou, M.; Bagnati, M.; Srivastava, P.K.; Vu, H.A.; Cucchi, D.; McAdoo, S.P.; Ananieva, E.A.; Mauro, C.; et al. BCAT1 Controls Metabolic Reprogramming in Activated Human Macrophages and Is Associated with Inflammatory Diseases. Nat. Commun. 2017, 8, 16040. [Google Scholar] [CrossRef] [PubMed]
- Boutet, M.-A.; Courties, G.; Nerviani, A.; Le Goff, B.; Apparailly, F.; Pitzalis, C.; Blanchard, F. Novel Insights into Macrophage Diversity in Rheumatoid Arthritis Synovium. Autoimmun. Rev. 2021, 20, 102758. [Google Scholar] [CrossRef] [PubMed]
- Municio, C.; Dominguez-Soto, Á.; Fuentelsaz-Romero, S.; Lamana, A.; Montes, N.; Cuevas, V.D.; Campos, R.G.; Pablos, J.L.; González-Álvaro, I.; Puig-Kröger, A. Methotrexate Limits Inflammation through an A20-Dependent Cross-Tolerance Mechanism. Ann. Rheum. Dis. 2018, 77, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Blackburn, G.; Best, C.; Goodyear, C.S.; Mudaliar, M.; Burgess, K.; Stirling, A.; Porter, D.; McInnes, I.B.; Barrett, M.P.; et al. Changes in Plasma Itaconate Elevation in Early Rheumatoid Arthritis Patients Elucidates Disease Activity Associated Macrophage Activation. Metabolites 2020, 10, 241. [Google Scholar] [CrossRef]
- Cutolo, M.; Montecucco, C.M.; Cavagna, L.; Caporali, R.; Capellino, S.; Montagna, P.; Fazzuoli, L.; Villaggio, B.; Seriolo, B.; Sulli, A. Serum Cytokines and Steroidal Hormones in Polymyalgia Rheumatica and Elderly-Onset Rheumatoid Arthritis. Ann. Rheum. Dis. 2006, 65, 1438–1443. [Google Scholar] [CrossRef]
- Badot, V.; Galant, C.; Nzeusseu Toukap, A.; Theate, I.; Maudoux, A.-L.; Van den Eynde, B.J.; Durez, P.; Houssiau, F.A.; Lauwerys, B.R. Gene Expression Profiling in the Synovium Identifies a Predictive Signature of Absence of Response to Adalimumab Therapy in Rheumatoid Arthritis. Arthritis Res. Ther. 2009, 11, R57. [Google Scholar] [CrossRef]
- Degboé, Y.; Rauwel, B.; Baron, M.; Boyer, J.-F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.-L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Diallo, K.; Simons, N.; Sayegh, S.; Baron, M.; Degboé, Y.; Boyer, J.-F.; Kruglov, A.; Nedospasov, S.; Novarino, J.; Aloulou, M.; et al. Evidence for TmTNF Reverse Signaling in Vivo: Implications for an Arginase-1-Mediated Therapeutic Effect of TNF Inhibitors during Inflammation. iScience 2021, 24, 102331. [Google Scholar] [CrossRef]
- Boyer, J.F.; Baron, M.; Constantin, A.; Degboé, Y.; Cantagrel, A.; Davignon, J.-L. Anti-TNF Certolizumab Pegol Induces Antioxidant Response in Human Monocytes via Reverse Signaling. Arthritis Res. Ther. 2016, 18, 56. [Google Scholar] [CrossRef][Green Version]
- Ducreux, J.; Durez, P.; Galant, C.; Nzeusseu Toukap, A.; Van den Eynde, B.; Houssiau, F.A.; Lauwerys, B.R. Global Molecular Effects of Tocilizumab Therapy in Rheumatoid Arthritis Synovium. Arthritis Rheumatol. 2014, 66, 15–23. [Google Scholar] [CrossRef]
- Chatzidionysiou, K.; Circiumaru, A.; Rethi, B.; Joshua, V.; Engstrom, M.; Hensvold, A.; Af Klint, E.; Catrina, A. Tocilizumab Decreases T Cells but Not Macrophages in the Synovium of Patients with Rheumatoid Arthritis While It Increases the Levels of Serum Interleukin-6 and RANKL. RMD Open 2021, 7, e001662. [Google Scholar] [CrossRef]
- Triaille, C.; Durez, P.; Sokolova, T.; Tilman, G.; Méric de Bellefon, L.; Galant, C.; Coulie, P.; Lauwerys, B.R.; Limaye, N. Common Transcriptomic Effects of Abatacept and Other DMARDs on Rheumatoid Arthritis Synovial Tissue. Front. Immunol. 2021, 12, 724895. [Google Scholar] [CrossRef]
- Cutolo, M.; Soldano, S.; Montagna, P.; Sulli, A.; Seriolo, B.; Villaggio, B.; Triolo, P.; Clerico, P.; Felli, L.; Brizzolara, R. CTLA4-Ig Interacts with Cultured Synovial Macrophages from Rheumatoid Arthritis Patients and Downregulates Cytokine Production. Arthritis Res. Ther. 2009, 11, R176. [Google Scholar] [CrossRef]
- Wenink, M.H.; Santegoets, K.C.M.; Platt, A.M.; van den Berg, W.B.; van Riel, P.L.C.M.; Garside, P.; Radstake, T.R.D.J.; McInnes, I.B. Abatacept Modulates Proinflammatory Macrophage Responses upon Cytokine-Activated T Cell and Toll-like Receptor Ligand Stimulation. Ann. Rheum. Dis. 2012, 71, 80–83. [Google Scholar] [CrossRef]
- Bozec, A.; Luo, Y.; Engdahl, C.; Figueiredo, C.; Bang, H.; Schett, G. Abatacept Blocks Anti-Citrullinated Protein Antibody and Rheumatoid Factor Mediated Cytokine Production in Human Macrophages in IDO-Dependent Manner. Arthritis Res. Ther. 2018, 20, 24. [Google Scholar] [CrossRef]
- Gutierrez-Roelens, I.; Galant, C.; Theate, I.; Lories, R.J.; Durez, P.; Nzeusseu-Toukap, A.; Van den Eynde, B.; Houssiau, F.A.; Lauwerys, B.R. Rituximab Treatment Induces the Expression of Genes Involved in Healing Processes in the Rheumatoid Arthritis Synovium. Arthritis Rheum. 2011, 63, 1246–1254. [Google Scholar] [CrossRef]
- Toubi, E.; Kessel, A.; Slobodin, G.; Boulman, N.; Pavlotzky, E.; Zisman, D.; Rozenbaum, M.; Rosner, I. Changes in Macrophage Function after Rituximab Treatment in Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2007, 66, 818–820. [Google Scholar] [CrossRef]
- McInnes, I.B.; Byers, N.L.; Higgs, R.E.; Lee, J.; Macias, W.L.; Na, S.; Ortmann, R.A.; Rocha, G.; Rooney, T.P.; Wehrman, T.; et al. Comparison of Baricitinib, Upadacitinib, and Tofacitinib Mediated Regulation of Cytokine Signaling in Human Leukocyte Subpopulations. Arthritis Res. Ther. 2019, 21, 183. [Google Scholar] [CrossRef]
- Traves, P.G.; Murray, B.; Campigotto, F.; Galien, R.; Meng, A.; Di Paolo, J.A. JAK Selectivity and the Implications for Clinical Inhibition of Pharmacodynamic Cytokine Signalling by Filgotinib, Upadacitinib, Tofacitinib and Baricitinib. Ann. Rheum. Dis. 2021, 80, 865–875. [Google Scholar] [CrossRef]
- Choy, E.H. Clinical Significance of Janus Kinase Inhibitor Selectivity. Rheumatology 2019, 58, 953–962. [Google Scholar] [CrossRef]
- Boyle, D.L.; Soma, K.; Hodge, J.; Kavanaugh, A.; Mandel, D.; Mease, P.; Shurmur, R.; Singhal, A.K.; Wei, N.; Rosengren, S.; et al. The JAK Inhibitor Tofacitinib Suppresses Synovial JAK1-STAT Signalling in Rheumatoid Arthritis. Ann. Rheum. Dis. 2015, 74, 1311–1316. [Google Scholar] [CrossRef]
- Yarilina, A.; Xu, K.; Chan, C.; Ivashkiv, L.B. Regulation of Inflammatory Responses in Tumor Necrosis Factor-Activated and Rheumatoid Arthritis Synovial Macrophages by JAK Inhibitors. Arthritis Rheum. 2012, 64, 3856–3866. [Google Scholar] [CrossRef]
- Pattison, M.J.; Mackenzie, K.F.; Arthur, J.S.C. Inhibition of JAKs in Macrophages Increases Lipopolysaccharide-Induced Cytokine Production by Blocking IL-10-Mediated Feedback. J. Immunol. 2012, 189, 2784–2792. [Google Scholar] [CrossRef]
- Maeshima, K.; Yamaoka, K.; Kubo, S.; Nakano, K.; Iwata, S.; Saito, K.; Ohishi, M.; Miyahara, H.; Tanaka, S.; Ishii, K.; et al. The JAK Inhibitor Tofacitinib Regulates Synovitis through Inhibition of Interferon-γ and Interleukin-17 Production by Human CD4+ T Cells. Arthritis Rheum. 2012, 64, 1790–1798. [Google Scholar] [CrossRef]
- McGarry, T.; Orr, C.; Wade, S.; Biniecka, M.; Wade, S.; Gallagher, L.; Low, C.; Veale, D.J.; Fearon, U. JAK/STAT Blockade Alters Synovial Bioenergetics, Mitochondrial Function, and Proinflammatory Mediators in Rheumatoid Arthritis. Arthritis Rheum. 2018, 70, 1959–1970. [Google Scholar] [CrossRef]
- Dennis, G.; Holweg, C.T.J.; Kummerfeld, S.K.; Choy, D.F.; Setiadi, A.F.; Hackney, J.A.; Haverty, P.M.; Gilbert, H.; Lin, W.Y.; Diehl, L.; et al. Synovial Phenotypes in Rheumatoid Arthritis Correlate with Response to Biologic Therapeutics. Arthritis Res. Ther. 2014, 16, R90. [Google Scholar] [CrossRef]
- Nerviani, A.; Di Cicco, M.; Mahto, A.; Lliso-Ribera, G.; Rivellese, F.; Thorborn, G.; Hands, R.; Bellan, M.; Mauro, D.; Boutet, M.-A.; et al. A Pauci-Immune Synovial Pathotype Predicts Inadequate Response to TNFα-Blockade in Rheumatoid Arthritis Patients. Front. Immunol. 2020, 11, 845. [Google Scholar] [CrossRef]
- Lliso-Ribera, G.; Humby, F.; Lewis, M.; Nerviani, A.; Mauro, D.; Rivellese, F.; Kelly, S.; Hands, R.; Bene, F.; Ramamoorthi, N.; et al. Synovial Tissue Signatures Enhance Clinical Classification and Prognostic/Treatment Response Algorithms in Early Inflammatory Arthritis and Predict Requirement for Subsequent Biological Therapy: Results from the Pathobiology of Early Arthritis Cohort (PEAC). Ann. Rheum. Dis. 2019, 78, 1642–1652. [Google Scholar] [CrossRef]
- Hayder, M.; Fruchon, S.; Fournié, J.-J.; Poupot, M.; Poupot, R. Anti-Inflammatory Properties of Dendrimers per Se. Sci. World J. 2011, 11, 1367–1382. [Google Scholar] [CrossRef]
- Poupot, R.; Bergozza, D.; Fruchon, S. Nanoparticle-Based Strategies to Treat Neuro-Inflammation. Materials 2018, 11, 270. [Google Scholar] [CrossRef]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.-O.; Fournié, J.-J.; Majoral, J.-P.; Caminade, A.-M.; Poupot, R. Regulatory Activity of Azabisphosphonate-Capped Dendrimers on Human CD4+ T Cell Proliferation Enhances Ex-Vivo Expansion of NK Cells from PBMCs for Immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef]
- Degboé, Y.; Fruchon, S.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.-M.; Poupot, R.; Cantagrel, A.; Davignon, J.-L. Modulation of Pro-Inflammatory Activation of Monocytes and Dendritic Cells by Aza-Bis-Phosphonate Dendrimer as an Experimental Therapeutic Agent. Arthritis Res. Ther. 2014, 16, R98. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.-O.; Majoral, J.-P.; Fournié, J.-J.; Caminade, A.-M.; Poupot, R. Anti-Inflammatory and Immunosuppressive Activation of Human Monocytes by a Bioactive Dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef]
- Poupot, M.; Turrin, C.-O.; Caminade, A.-M.; Fournié, J.-J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(Phosphorhydrazone) Dendrimers: Yin and Yang of Monocyte Activation for Human NK Cell Amplification Applied to Immunotherapy against Multiple Myeloma. Nanomedicine 2016, 12, 2321–2330. [Google Scholar] [CrossRef]
- Fruchon, S.; Caminade, A.-M.; Abadie, C.; Davignon, J.-L.; Combette, J.-M.; Turrin, C.-O.; Poupot, R. An Azabisphosphonate-Capped Poly(Phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef]
- Hayder, M.; Garzoni, M.; Bochicchio, D.; Caminade, A.-M.; Couderc, F.; Ong-Meang, V.; Davignon, J.-L.; Turrin, C.-O.; Pavan, G.M.; Poupot, R. Three-Dimensional Directionality Is a Pivotal Structural Feature for the Bioactivity of Azabisphosphonate-Capped Poly(PhosphorHydrazone) Nanodrug Dendrimers. Biomacromolecules 2018, 19, 712–720. [Google Scholar] [CrossRef]
- Hayder, M.; Varilh, M.; Turrin, C.-O.; Saoudi, A.; Caminade, A.-M.; Poupot, R.; Liblau, R.S. Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4(+) T Cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef]
- Jebbawi, R.; Oukhrib, A.; Clement, E.; Blanzat, M.; Turrin, C.-O.; Caminade, A.-M.; Lacoste, E.; Fruchon, S.; Poupot, R. An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis. Biomolecules 2020, 10, 949. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells. Molecules 2018, 23, 1272. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. Pro-Inflammatory Versus Anti-Inflammatory Effects of Dendrimers: The Two Faces of Immuno-Modulatory Nanoparticles. Nanomaterials 2017, 7, 251. [Google Scholar] [CrossRef]
- Fruchon, S.; Mouriot, S.; Thiollier, T.; Grandin, C.; Caminade, A.-M.; Turrin, C.-O.; Contamin, H.; Poupot, R. Repeated Intravenous Injections in Non-Human Primates Demonstrate Preclinical Safety of an Anti-Inflammatory Phosphorus-Based Dendrimer. Nanotoxicology 2015, 9, 433–441. [Google Scholar] [CrossRef]
- Fruchon, S.; Bellard, E.; Beton, N.; Goursat, C.; Oukhrib, A.; Caminade, A.-M.; Blanzat, M.; Turrin, C.-O.; Golzio, M.; Poupot, R. Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate. Biomolecules 2019, 9, 475. [Google Scholar] [CrossRef]
- Zhu, Y.; Herndon, J.M.; Sojka, D.K.; Kim, K.-W.; Knolhoff, B.L.; Zuo, C.; Cullinan, D.R.; Luo, J.; Bearden, A.R.; Lavine, K.J.; et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity 2017, 47, 597. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R Inhibition Alters Macrophage Polarization and Blocks Glioma Progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Yamamoto, Y.; Akasaki, Y.; Sasaki, H. Dual Role of Macrophage in Tumor Immunity. Immunotherapy 2018, 10, 899–909. [Google Scholar] [CrossRef]
- Dace, D.S.; Chen, P.W.; Niederkorn, J.Y. CD4+ T-Cell-Dependent Tumour Rejection in an Immune-Privileged Environment Requires Macrophages. Immunology 2008, 123, 367–377. [Google Scholar] [CrossRef]
- van Loveren, H.; de Groot, J.W.; Koten, J.W.; Piersma, A.H.; de Weger, R.A.; den Otter, W. A Macrophage Factor Enhancing the Systemic Anti-Tumour Effect of T Lymphocytes. Immunobiology 1984, 166, 118–130. [Google Scholar] [CrossRef]
- Suzuki, M.; Arika, T.; Amemiya, K.; Fujiwara, M. Cooperative Role of T Lymphocytes and Macrophages in Anti-Tumor Activity of Mice Pretreated with Schizophyllan (SPG). Jpn. J. Exp. Med. 1982, 52, 59–65. [Google Scholar]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic Significance of Tumor-Associated Macrophages in Breast Cancer: A Meta-Analysis of the Literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting Macrophages: Therapeutic Approaches in Cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Goswami, K.K.; Ghosh, T.; Ghosh, S.; Sarkar, M.; Bose, A.; Baral, R. Tumor Promoting Role of Anti-Tumor Macrophages in Tumor Microenvironment. Cell. Immunol. 2017, 316, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.-Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-Linked Barrier Defects and Microbial Products Drive IL-23/IL-17-Mediated Tumour Growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a Central Player and Promising Target in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef]
- Kong, L.; Zhou, Y.; Bu, H.; Lv, T.; Shi, Y.; Yang, J. Deletion of Interleukin-6 in Monocytes/Macrophages Suppresses the Initiation of Hepatocellular Carcinoma in Mice. J. Exp. Clin. Cancer Res. 2016, 35, 131. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Chen, S.; Lu, M.; Luo, X.; Yao, S.; Liu, S.; Qin, Y.; Chen, H. Tumor-Associated Macrophages Provide a Suitable Microenvironment for Non-Small Lung Cancer Invasion and Progression. Lung Cancer 2011, 74, 188–196. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Kato, Y.; Tabata, K.; Kimura, T.; Yachie-Kinoshita, A.; Ozawa, Y.; Yamada, K.; Ito, J.; Tachino, S.; Hori, Y.; Matsuki, M.; et al. Lenvatinib plus Anti-PD-1 Antibody Combination Treatment Activates CD8+ T Cells through Reduction of Tumor-Associated Macrophage and Activation of the Interferon Pathway. PLoS ONE 2019, 14, e0212513. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of Tumor-Associated Macrophages and T Cells in Glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, X.; Wu, Y.; Wang, X. Interaction between Treg Cells and Tumor-Associated Macrophages in the Tumor Microenvironment of Epithelial Ovarian Cancer. Oncol. Rep. 2016, 36, 3472–3478. [Google Scholar] [CrossRef]
- Paulus, P.; Stanley, E.R.; Schäfer, R.; Abraham, D.; Aharinejad, S. Colony-Stimulating Factor-1 Antibody Reverses Chemoresistance in Human MCF-7 Breast Cancer Xenografts. Cancer Res. 2006, 66, 4349–4356. [Google Scholar] [CrossRef]
- Shiao, S.L.; Ruffell, B.; DeNardo, D.G.; Faddegon, B.A.; Park, C.C.; Coussens, L.M. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol. Res. 2015, 3, 518–525. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose Receptor (CD206) Activation in Tumor-Associated Macrophages Enhances Adaptive and Innate Antitumor Immune Responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Dheilly, E.; Moine, V.; Broyer, L.; Salgado-Pires, S.; Johnson, Z.; Papaioannou, A.; Cons, L.; Calloud, S.; Majocchi, S.; Nelson, R.; et al. Selective Blockade of the Ubiquitous Checkpoint Receptor CD47 Is Enabled by Dual-Targeting Bispecific Antibodies. Mol. Ther. 2017, 25, 523–533. [Google Scholar] [CrossRef]
- Long, K.B.; Gladney, W.L.; Tooker, G.M.; Graham, K.; Fraietta, J.A.; Beatty, G.L. IFNγ and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016, 6, 400–413. [Google Scholar] [CrossRef]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef]
- Georgoudaki, A.-M.; Prokopec, K.E.; Boura, V.F.; Hellqvist, E.; Sohn, S.; Östling, J.; Dahan, R.; Harris, R.A.; Rantalainen, M.; Klevebring, D.; et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep. 2016, 15, 2000–2011. [Google Scholar] [CrossRef]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-Cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Digifico, E.; Andon, F.T.; Mantovani, A.; Allavena, P. Poly(I:C) Stimulation Is Superior than Imiquimod to Induce the Antitumoral Functional Profile of Tumor-Conditioned Macrophages. Eur. J. Immunol. 2019, 49, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, M.R.; Richly, H.; von Bergwelt-Baildon, M.S.; Becker, H.J.; Schmidt, M.; Hacker, U.T.; Shimabukuro-Vornhagen, A.; Holtick, U.; Nokay, B.; Schroff, M.; et al. Phase I Clinical Study of the Toll-like Receptor 9 Agonist MGN1703 in Patients with Metastatic Solid Tumours. Eur. J. Cancer 2015, 51, 146–156. [Google Scholar] [CrossRef]
- Sommariva, M.; Le Noci, V.; Storti, C.; Bianchi, F.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Activation of NK Cell Cytotoxicity by Aerosolized CpG-ODN/Poly(I:C) against Lung Melanoma Metastases Is Mediated by Alveolar Macrophages. Cell. Immunol. 2017, 313, 52–58. [Google Scholar] [CrossRef]
- Yin, T.; He, S.; Wang, Y. Toll-like Receptor 7/8 Agonist, R848, Exhibits Antitumoral Effects in a Breast Cancer Model. Mol. Med. Rep. 2015, 12, 3515–3520. [Google Scholar] [CrossRef]
- Lizotte, P.H.; Baird, J.R.; Stevens, C.A.; Lauer, P.; Green, W.R.; Brockstedt, D.G.; Fiering, S.N. Attenuated Listeria Monocytogenes Reprograms M2-Polarized Tumor-Associated Macrophages in Ovarian Cancer Leading to INOS-Mediated Tumor Cell Lysis. Oncoimmunology 2014, 3, e28926. [Google Scholar] [CrossRef]
- Cresswell, G.M.; Wang, B.; Kischuk, E.M.; Broman, M.M.; Alfar, R.A.; Vickman, R.E.; Dimitrov, D.S.; Kularatne, S.A.; Sundaram, C.P.; Singhal, S.; et al. Folate Receptor Beta Designates Immunosuppressive Tumor-Associated Myeloid Cells That Can Be Reprogrammed with Folate-Targeted Drugs. Cancer Res. 2021, 81, 671–684. [Google Scholar] [CrossRef]
- Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G.; et al. USP7 Targeting Modulates Anti-Tumor Immune Response by Reprogramming Tumor-Associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332–9347. [Google Scholar] [CrossRef]
- Bartish, M.; Tong, D.; Pan, Y.; Wallerius, M.; Liu, H.; Ristau, J.; de Souza Ferreira, S.; Wallmann, T.; van Hoef, V.; Masvidal, L.; et al. MNK2 Governs the Macrophage Antiinflammatory Phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 27556–27565. [Google Scholar] [CrossRef]
- Dai, X.; Meng, J.; Deng, S.; Zhang, L.; Wan, C.; Lu, L.; Huang, J.; Hu, Y.; Zhang, Z.; Li, Y.; et al. Targeting CAMKII to Reprogram Tumor-Associated Macrophages and Inhibit Tumor Cells for Cancer Immunotherapy with an Injectable Hybrid Peptide Hydrogel. Theranostics 2020, 10, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Marinis, J.M.; Beal, A.M.; Savadkar, S.; Wu, Y.; Khan, M.; Taunk, P.S.; Wu, N.; Su, W.; Wu, J.; et al. RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer. Cancer Cell 2018, 34, 757–774.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hao, Z.; Hong, Y.; He, W.; Zhao, W. Reprogramming Tumor Associated Macrophage Phenotype by a Polysaccharide from Ilex Asprella for Sarcoma Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3816. [Google Scholar] [CrossRef]
- Zong, S.; Li, J.; Ye, Z.; Zhang, X.; Yang, L.; Chen, X.; Ye, M. Lachnum Polysaccharide Suppresses S180 Sarcoma by Boosting Anti-Tumor Immune Responses and Skewing Tumor-Associated Macrophages toward M1 Phenotype. Int. J. Biol. Macromol. 2020, 144, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 Pathway in Tumor-Associated Macrophages Reduces Tumor Growth and Metastatic Niche Formation in Breast Cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, T.; Shen, X.; Xia, X.; Xu, G.; Bai, X.; Liang, T. Macrophage-Induced Tumor Angiogenesis Is Regulated by the TSC2-MTOR Pathway. Cancer Res. 2012, 72, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. PI3Kγ Is a Molecular Switch That Controls Immune Suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truitt, M.; Olson, P.; et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-Talk Drives Pancreas Cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Chen, H.; Huang, K.; Liu, X.; Qiu, M.; Liu, Y.; Yang, Y.; Yang, J. CYP4X1 Inhibition by Flavonoid CH625 Normalizes Glioma Vasculature through Reprogramming TAMs via CB2 and EGFR-STAT3 Axis. J. Pharmacol. Exp. Ther. 2018, 365, 72–83. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Man, K.; Tsao, S.-W.; Che, C.-M.; Feng, Y. Autophagy-Induced RelB/P52 Activation Mediates Tumour-Associated Macrophage Repolarisation and Suppression of Hepatocellular Carcinoma by Natural Compound Baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine Modulates Antitumor Immune Response by Resetting Tumor-Associated Macrophages toward M1 Phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Cheng, H.; Mu, C.; Geng, G.; Zhao, T.; Luo, Q.; Ma, K.; Chang, R.; Liu, Q.; Gao, R.; et al. The SIAH2-NRF1 Axis Spatially Regulates Tumor Microenvironment Remodeling for Tumor Progression. Nat. Commun. 2019, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 Sensing of Lactate Mediates Tumor-Macrophage Interplay to Promote Breast Cancer Metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Eikawa, S.; Nishida, M.; Kunisada, Y.; Yoshida, A.; Fujiwara, T.; Kunisada, T.; Ozaki, T.; Udono, H. Metformin Induces CD11b+-Cell-Mediated Growth Inhibition of an Osteosarcoma: Implications for Metabolic Reprogramming of Myeloid Cells and Anti-Tumor Effects. Int. Immunol. 2019, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cheng, Y.; Zheng, R.; Xu, K.; Yan, J.; Song, P.; Wang, Y.; Rauf, A.; Pan, Y.; Zhang, H. Immunomodulation of Tumor Microenvironment by Arginine-Loaded Iron Oxide Nanoparticles for Gaseous Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 19825–19835. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Liu, T.; Guo, Z.; Zhuang, R.; Zhang, X.; Chen, X. Reprogramming Tumor-Associated Macrophages by Nanoparticle-Based Reactive Oxygen Species Photogeneration. Nano Lett. 2018, 18, 7330–7342. [Google Scholar] [CrossRef]
- Song, M.; Liu, T.; Shi, C.; Zhang, X.; Chen, X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano 2016, 10, 633–647. [Google Scholar] [CrossRef]
- Ai, X.; Hu, M.; Wang, Z.; Lyu, L.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Enhanced Cellular Ablation by Attenuating Hypoxia Status and Reprogramming Tumor-Associated Macrophages via NIR Light-Responsive Upconversion Nanocrystals. Bioconjug. Chem. 2018, 29, 928–938. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Y.; Fang, Y.; Zhang, M.; Wang, H.; He, Z.; Wang, B.; Xu, Q.; Huang, Y. Reprogramming Tumor Immune Microenvironment (TIME) and Metabolism via Biomimetic Targeting Codelivery of Shikonin/JQ1. Nano Lett. 2019, 19, 2935–2944. [Google Scholar] [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic Programming of Macrophages to Perform Anti-Tumor Functions Using Targeted MRNA Nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhang, X.; Zhao, X.; Hu, H.; Qiao, M.; Deng, Y.; Chen, D. Targeted Delivery of MiRNA 155 to Tumor Associated Macrophages for Tumor Immunotherapy. Mol. Pharm. 2019, 16, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.N.; Etzerodt, A.; Graversen, J.H.; Holthof, L.C.; Moestrup, S.K.; Hokland, M.; Møller, H.J. STAT3 Inhibition Specifically in Human Monocytes and Macrophages by CD163-Targeted Corosolic Acid-Containing Liposomes. Cancer Immunol. Immunother. 2019, 68, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, B.; Huang, W.; Tang, Y.; Jiang, Y.; Zhang, W.; Huang, Y. Reprogramming Tumor-Associated Macrophages To Reverse EGFRT790M Resistance by Dual-Targeting Codelivery of Gefitinib/Vorinostat. Nano Lett. 2017, 17, 7684–7690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic Cancer-Targeting Exosomes for Enhancing Immunotherapy and Reprogramming Tumor Microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degboé, Y.; Poupot, R.; Poupot, M. Repolarization of Unbalanced Macrophages: Unmet Medical Need in Chronic Inflammation and Cancer. Int. J. Mol. Sci. 2022, 23, 1496. https://doi.org/10.3390/ijms23031496
Degboé Y, Poupot R, Poupot M. Repolarization of Unbalanced Macrophages: Unmet Medical Need in Chronic Inflammation and Cancer. International Journal of Molecular Sciences. 2022; 23(3):1496. https://doi.org/10.3390/ijms23031496
Chicago/Turabian StyleDegboé, Yannick, Rémy Poupot, and Mary Poupot. 2022. "Repolarization of Unbalanced Macrophages: Unmet Medical Need in Chronic Inflammation and Cancer" International Journal of Molecular Sciences 23, no. 3: 1496. https://doi.org/10.3390/ijms23031496
APA StyleDegboé, Y., Poupot, R., & Poupot, M. (2022). Repolarization of Unbalanced Macrophages: Unmet Medical Need in Chronic Inflammation and Cancer. International Journal of Molecular Sciences, 23(3), 1496. https://doi.org/10.3390/ijms23031496






