Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nerve Collection
2.2. Experimental Scheme
2.3. Decellularization Protocols
- Experimental method
- (A)
- Nerves were immersed in 40 mL of phosphate-buffered saline (PBS) containing Sulfobetaine-10 (SB-10) 125 mM (SB-10, Soltec Bio Science, Beverly, MA, USA), 0.2% v/v Triton X-100 (Sigma-Aldrich S.r.l., Milan, Italy) (Triton X-200 was no longer available) and 2% v/v Pen Strep (Pen Strep, Thermo Fisher Scientific, Inc., Waltham, MA USA), incubated for 120 h at room temperature in the orbital shaker, and then frozen.
- (B)
- On the day of manipulation, nerves were transferred to a Class A glove box to simulate sterile conditions and thawed.
- (C)
- Nerves were rinsed three times with PBS (total 30 min) and then immersed in sterile PBS containing 0.25% Sodium Dodecyl Sulfate (SDS, Sigma- Aldrich S.r.l., Milan, Italy) for 180 min. During this phase, ultrasounds (40 Hz) were applied for 3 min every 30 min. Sonication cycles were performed with nerves soaked in 30 mL of decellularization solution sealed inside sterile 50 mL tubes, which, in turn, were immersed in a sonicator (Branson 2510 DTH Bath Sonicator; ultrasound frequency 40 Hz). During incubation, tubes were kept in agitation on a radial shaker.
- (D)
- At the end, ANAs were rinsed three times with PBS for 30 min and immersed in 10% v/v Dimethyl Sulfoxide (DMSO) in isotonic saline solution, then frozen at −80 °C for long-term preservation.
- Hudson method (control) (adapted from [11]):
2.4. Microbiology
2.5. Histology and Immunohistochemistry
2.6. Transmission Electron Microscopy
3. Results
3.1. Microbiology
3.2. Transmission Electronic Microscopy
3.3. Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Silva, T.H.; Oliveira, J.M. Peripheral nerve injury: Current challenges, conventional treatment approaches, and new trends in biomaterials-based regenerative strategies. ACS Biomater. Sci. Eng. 2017, 3, 3098–3122. [Google Scholar] [CrossRef] [PubMed]
- Huckhagel, T.; Nüchtern, J.; Regelsberger, J.; Lefering, R. Nerve injury in severe trauma with upper extremity involvement: Evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 76. [Google Scholar] [CrossRef]
- Biglioli, F.; Allevi, F.; Rabbiosi, D.; Cupello, S.; Battista, V.M.A.; Saibene, A.M.; Colletti, G. Triple innervation for re-animation of recent facial paralysis. J. Craniomaxillofac. Surg. 2018, 46, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, P.; Giannaccare, G.; Bolognesi, F.; Digiuni, M.; Tranchina, L.; Rossetti, L.; Dipinto, A.; Allevi, F.; Lozza, A.; Rabbiosi, D.; et al. Direct versus indirect corneal neurotization for the treatment of neurotrophic keratopathy: A multicenter prospective comparative study. Am. J. Ophthalmol. 2020, 220, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Bolognesi, F.; Pellegrini, M.; Spena, R.; Allevi, F.; Marchetti, C.; Scorcia, V.; Biglioli, F. Corneal neurotization: A game-changing surgical procedure for neurotrophic keratopathy. Cornea 2021. [Google Scholar] [CrossRef]
- Biglioli, F.; Allevi, F.; Lozza, A. Surgical treatment of painful lesions of the inferior alveolar nerve. J. Craniomaxillofac. Surg. 2015, 43, 1541–1545. [Google Scholar] [CrossRef]
- Rasulic, L. Current concept in adult peripheral nerve and brachial plexus surgery. J. Brachial Plex. Peripher. Nerve Inj. 2017, 12, e7–e14. [Google Scholar] [CrossRef] [Green Version]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [Green Version]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of using sterilization by gamma radiation: The other side of the coin. Int. J. Med. Sci. 2018, 15, 274. [Google Scholar] [CrossRef] [Green Version]
- Gut, G.; Marowska, J.; Jastrzebska, A.; Olender, E.; Kamiński, A. Structural mechanical properties of radiation-sterilized human bone-tendon-bone grafts preserved by different methods. Cell Tissue Bank. 2016, 17, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Hudson, T.W.; Liu, S.Y.; Schmidt, C.E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004, 10, 1346–1358. [Google Scholar] [CrossRef]
- Boriani, F.F.; Fazio, N.N.; Fotia, C.C.; Savarino, L.L.; Aldini, N.N.N.N.; Martini, L.L.; Zini, N.N.; Bernardini, M.; Baldini, N.N. A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction. J. Biomed. Mater. Res. Part A 2017, 105, 2228–2240. [Google Scholar] [CrossRef]
- Boriani, F.; Savarino, L.; Fazio, N.; Pedrini, F.A.; Fini, M.; Aldini, N.N.; Martini, L.; Zini, N.; Bernardini, M.; Bolognesi, F.; et al. Auto-allo graft parallel juxtaposition for improved neuroregeneration in peripheral nerve reconstruction based on acellular nerve allografts. Ann. Plast. Surg. 2019, 83, 318–325. [Google Scholar] [CrossRef]
- Isaacs, J.; Mallu, S.; Patel, G.; Kite, A.; Shah, S.; Graham, G.P. Implantation of acellular nerve allograft using nerve connectors. Hand 2019, 15, 625–630. [Google Scholar] [CrossRef]
- Hong, T.; Wood, I.; Hunter, D.A.; Yan, Y.; Mackinnon, S.E.; Wood, M.; Moore, A.M. Neuroma management: Capping nerve injuries with an acellular nerve allograft can limit axon regeneration. Hand 2019, 16, 157–163. [Google Scholar] [CrossRef]
- Yampolsky, A.; Ziccardi, V.; Chuang, S.K. Efficacy of acellular nerve allografts in trigeminal nerve reconstruction. J. Oral Maxillofac. Surg. 2017, 75, 2230–2234. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Zheng, C.; Gu, L.; Zhu, Q.; Xiang, J.; He, B.; Zhou, X.; Liu, X. Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: A 3 year follow-up study. J. Tissue Eng. Regen. Med. 2017, 11, 2314–2322. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Qin, B.; Wang, H.; Yang, Y.; Fang, J.; Chen, G.; Liu, X.; Tu, Z.; Gu, L. Analysis of human acellular nerve allograft combined with contralateral C7 nerve root transfer for restoration of shoulder abduction and elbow flexion in brachial plexus injury: A mean 4-year follow-up. J. Neurosurg. 2020, 132, 1914–1924. [Google Scholar] [CrossRef]
- Isaacs, J.; Patel, G.; Mallu, S.; Ugwu-Oju, O.; Desai, A.; Borschel, G.; David, D.; Protzuk, O.; Shah, S.; Semus, R. Effect of reverse end-to-side (supercharging) neurotization in long processed acellular nerve allograft in a rat model. J. Hand Surg. 2019, 44, 419.E1–419.E10. [Google Scholar] [CrossRef]
- Yang, J.T.; Fang, J.T.; Li, L.; Chen, G.; Qin, B.G.; Gu, L.Q. Contralateral C7 transfer combined with acellular nerve allografts seeded with differentiated adipose stem cells for repairing upper brachial plexus injury in rats. Neural Regen. Res. 2019, 14, 1932–1940. [Google Scholar] [CrossRef]
- Philips, C.; Campos, F.; Roosens, A.; Sánchez-Quevedo, M.D.C.; Declercq, H.; Carriel, V. Qualitative and quantitative evaluation of a novel detergent-based method for decellularization of peripheral nerves. Ann. Biomed. Eng. 2018, 46, 1921–1937. [Google Scholar] [CrossRef]
- Azhim, A.; Syazwani, N.; Morimoto, Y.; Furukawa, K.S.; Ushida, T. The use of sonication treatment to decellularize aortic tissues for preparation of bioscaffolds. J. Biomater. Appl. 2014, 29, 130–141. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Cebotari, S.; Tudorache, I.; Jaekel, T.; Hilfiker, A.; Dorfman, S.; Ternes, W.; Haverich, A.; Lichtenberg, A. Detergent decellularization of heart valves for tissue engineering: Toxicological effects of residual detergents on human endothelial cells. Artif. Organs 2010, 34, 206–210. [Google Scholar] [CrossRef]
- Rieder, E.; Kasimir, M.T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [Green Version]
- Karabekmez, F.E.; Duymaz, A.; Moran, S.L. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand 2009, 4, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Szynkaruk, M.; Kemp, S.W.P.; Wood, M.D.; Gordon, T.; Borschel, G.H. Experimental and clinical evidence for use of decellularized nerve allografts in peripheral nerve gap reconstruction. Tissue Eng. Part B Rev. 2013, 19, 83–96. [Google Scholar] [CrossRef]
- Pedrini, F.A.; Boriani, F.; Bolognesi, F.; Fazio, N.; Marchetti, C.; Baldini, N. Cell-enhanced acellular nerve allografts for peripheral nerve reconstruction: A systematic review and a meta-analysis of the literature. Neurosurgery 2019, 85, 575–604. [Google Scholar] [CrossRef]
- Boriani, F.; Fazio, N.; Bolognesi, F.; Pedrini, F.A.; Marchetti, C.; Baldini, N. Noncellular modification of acellular nerve allografts for peripheral nerve reconstruction: A systematic critical review of the animal literature. World Neurosurg. 2019, 122, 692–703. [Google Scholar] [CrossRef]

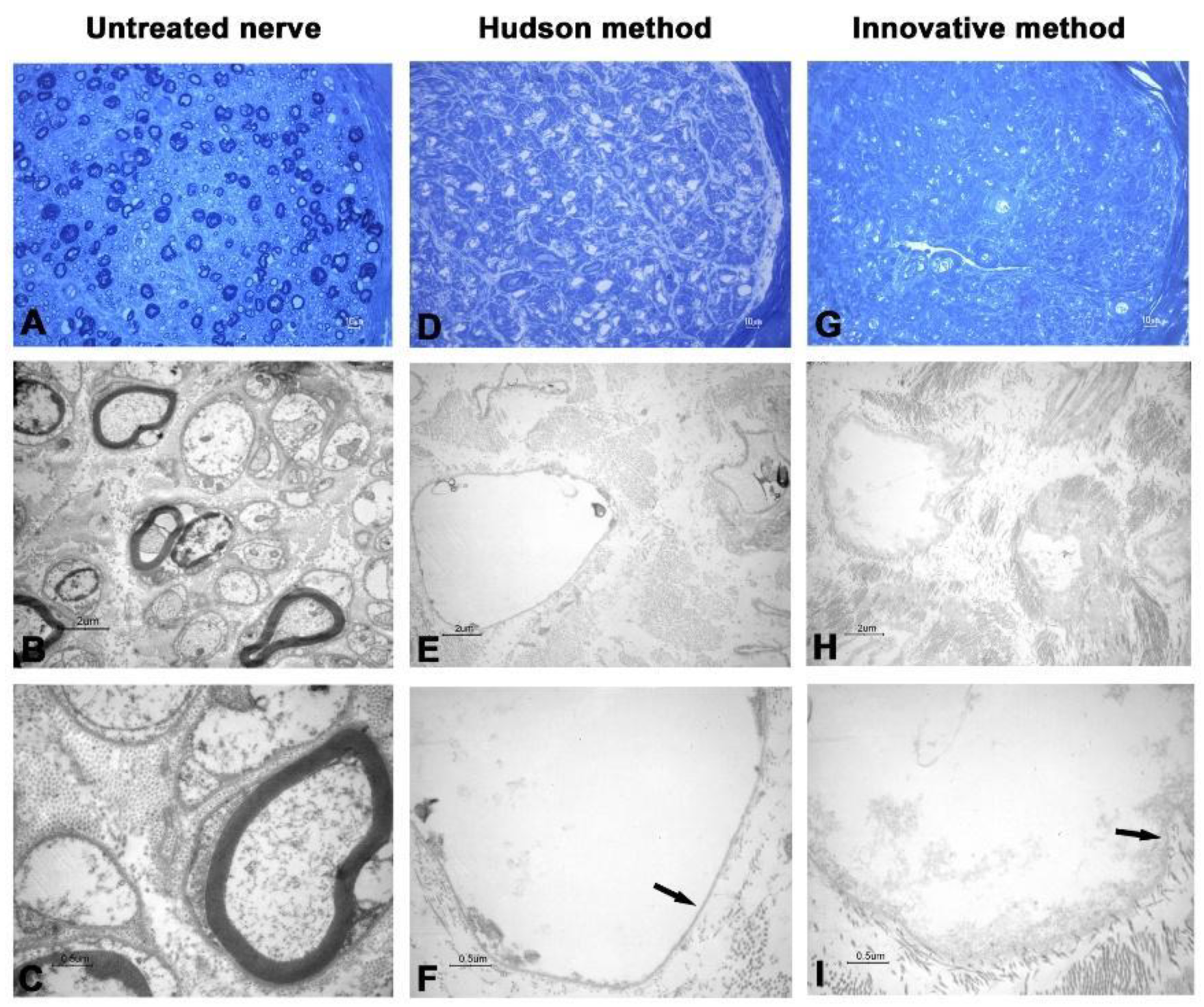
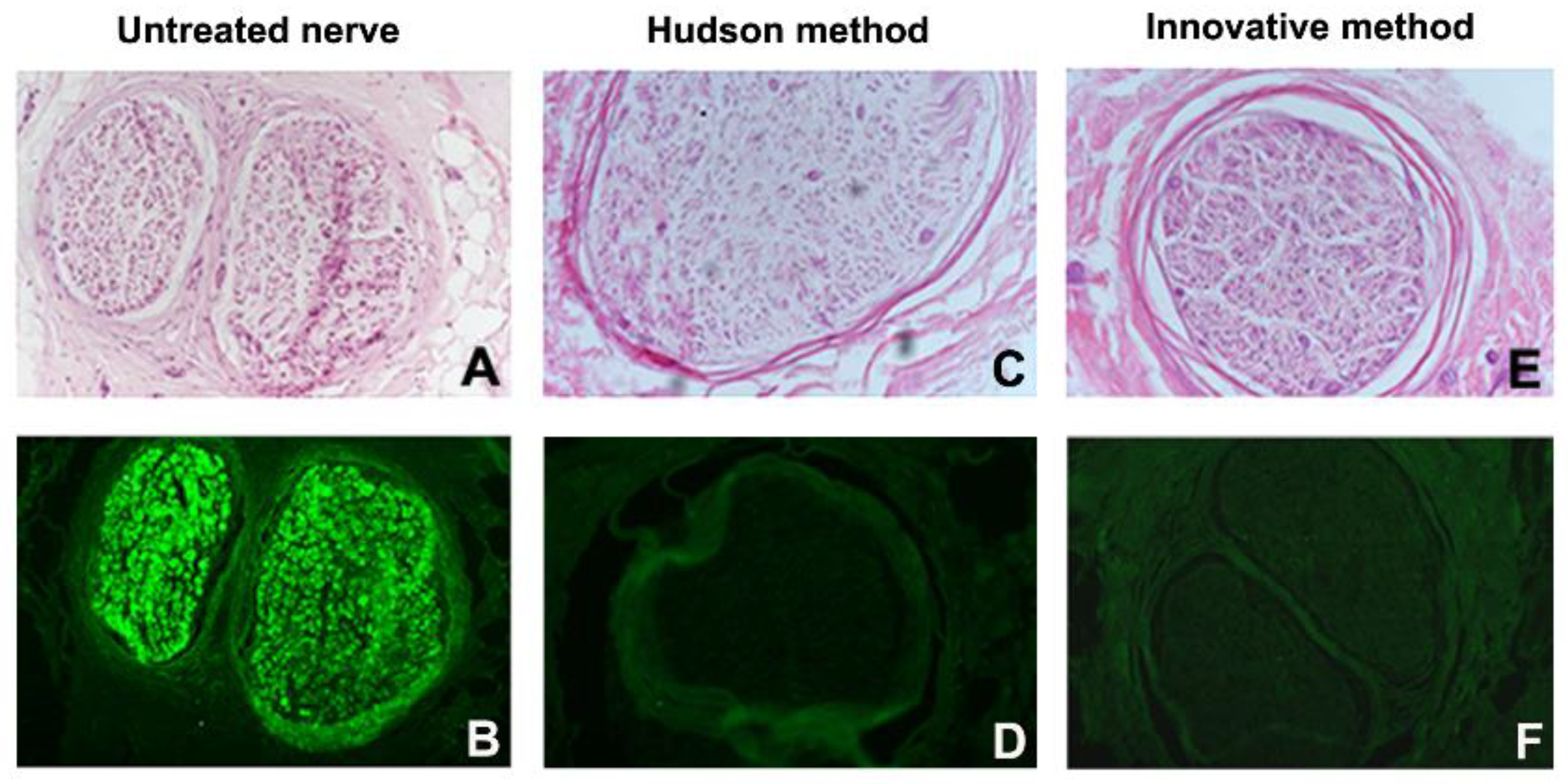
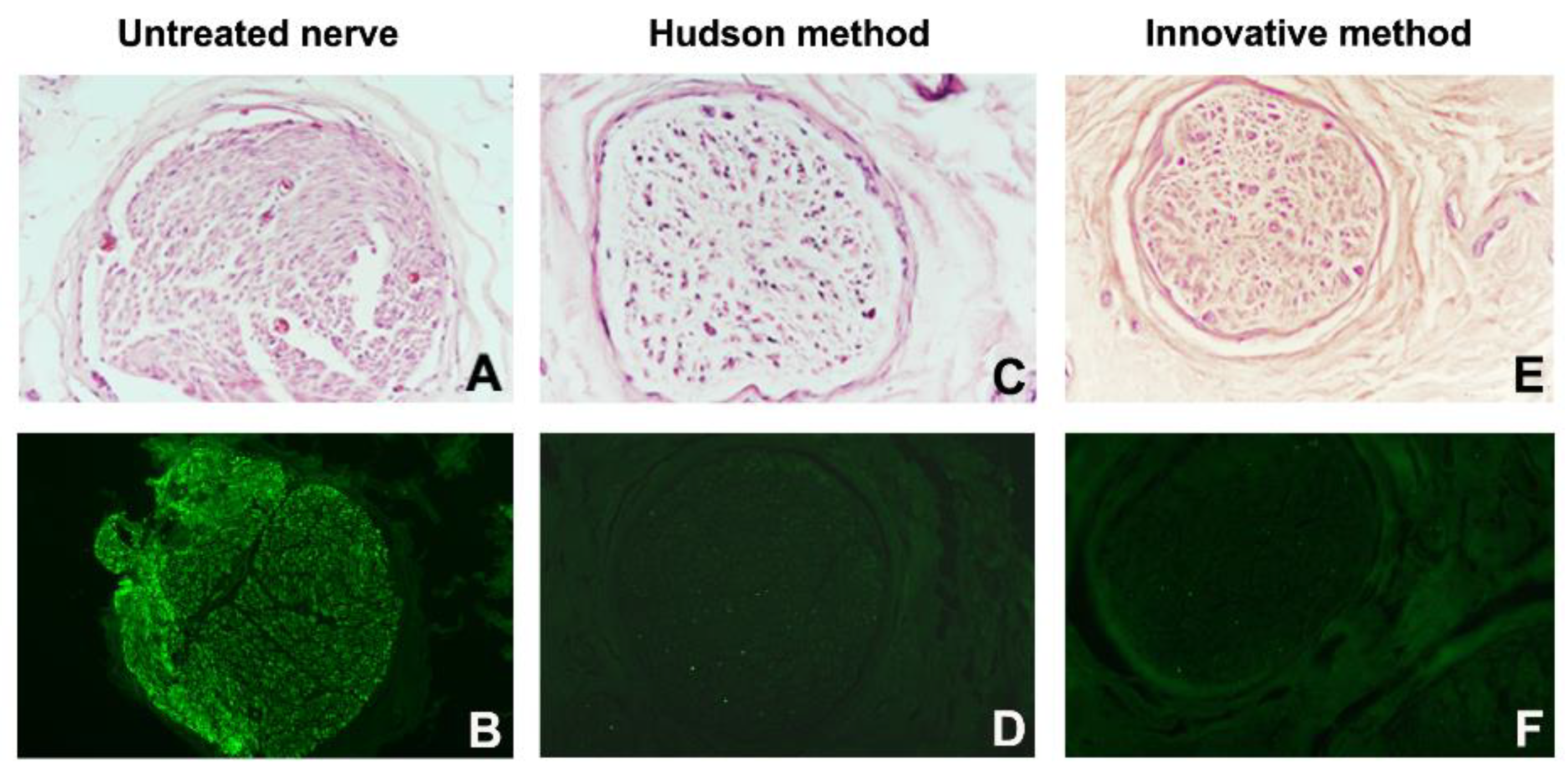

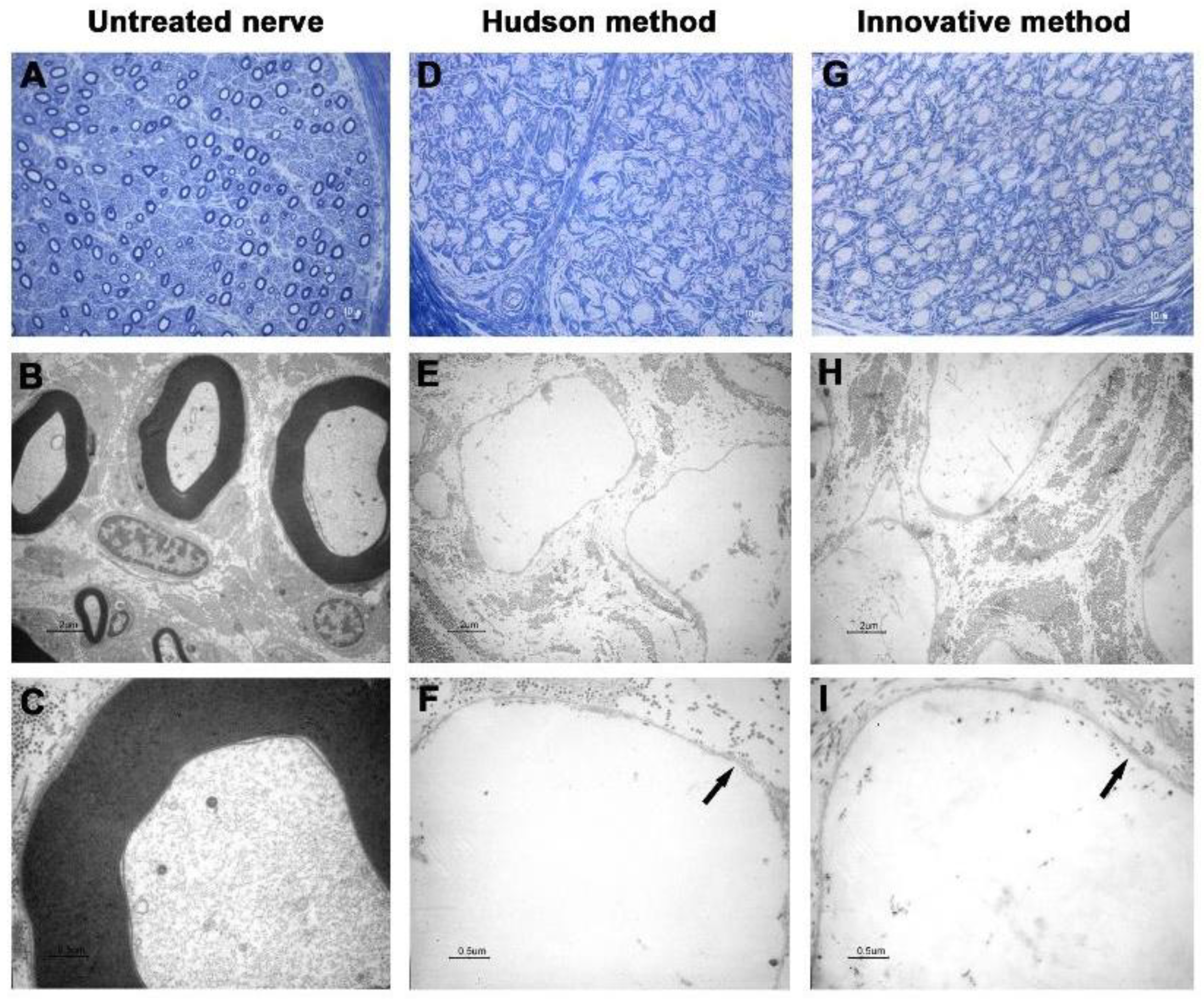
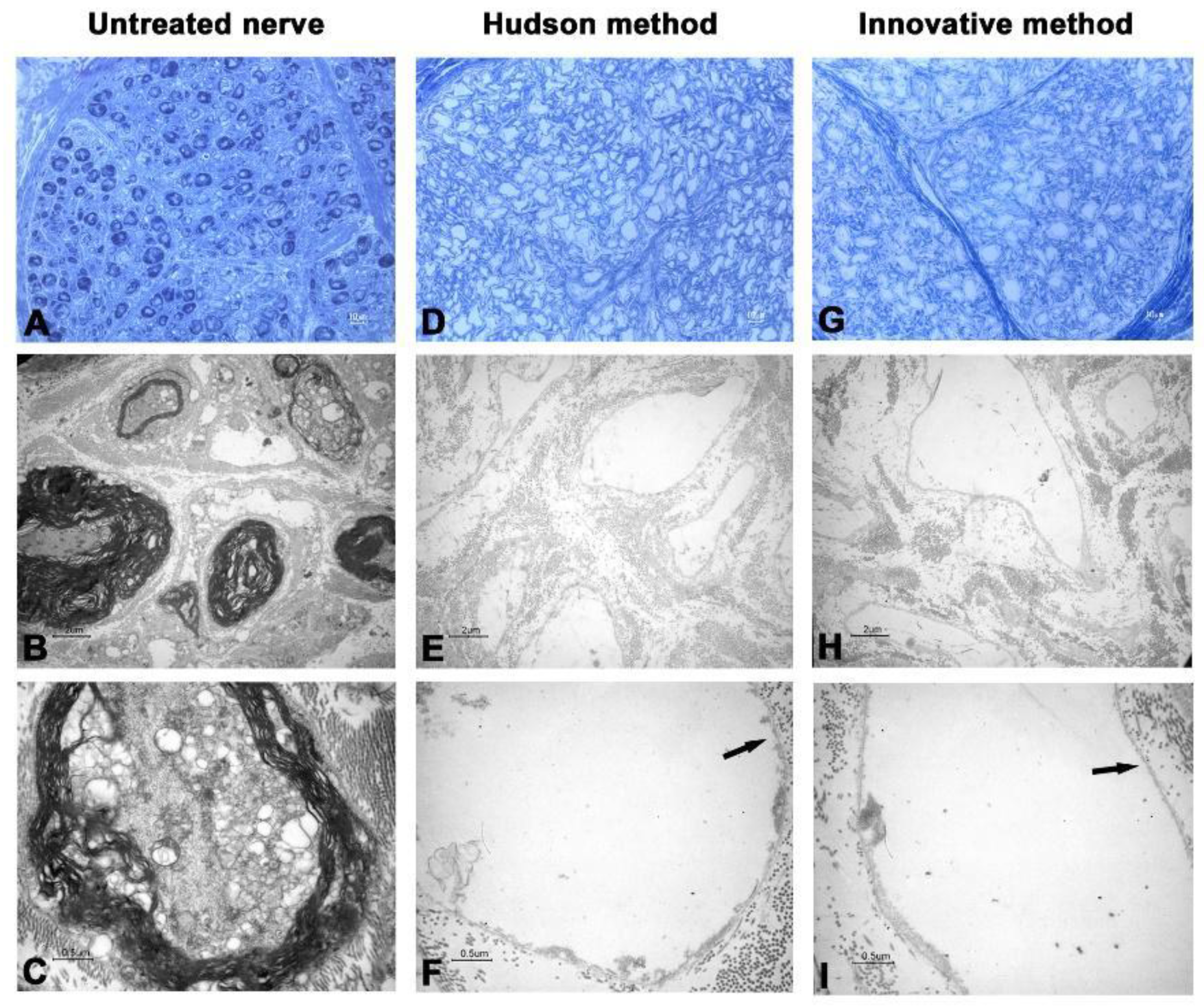
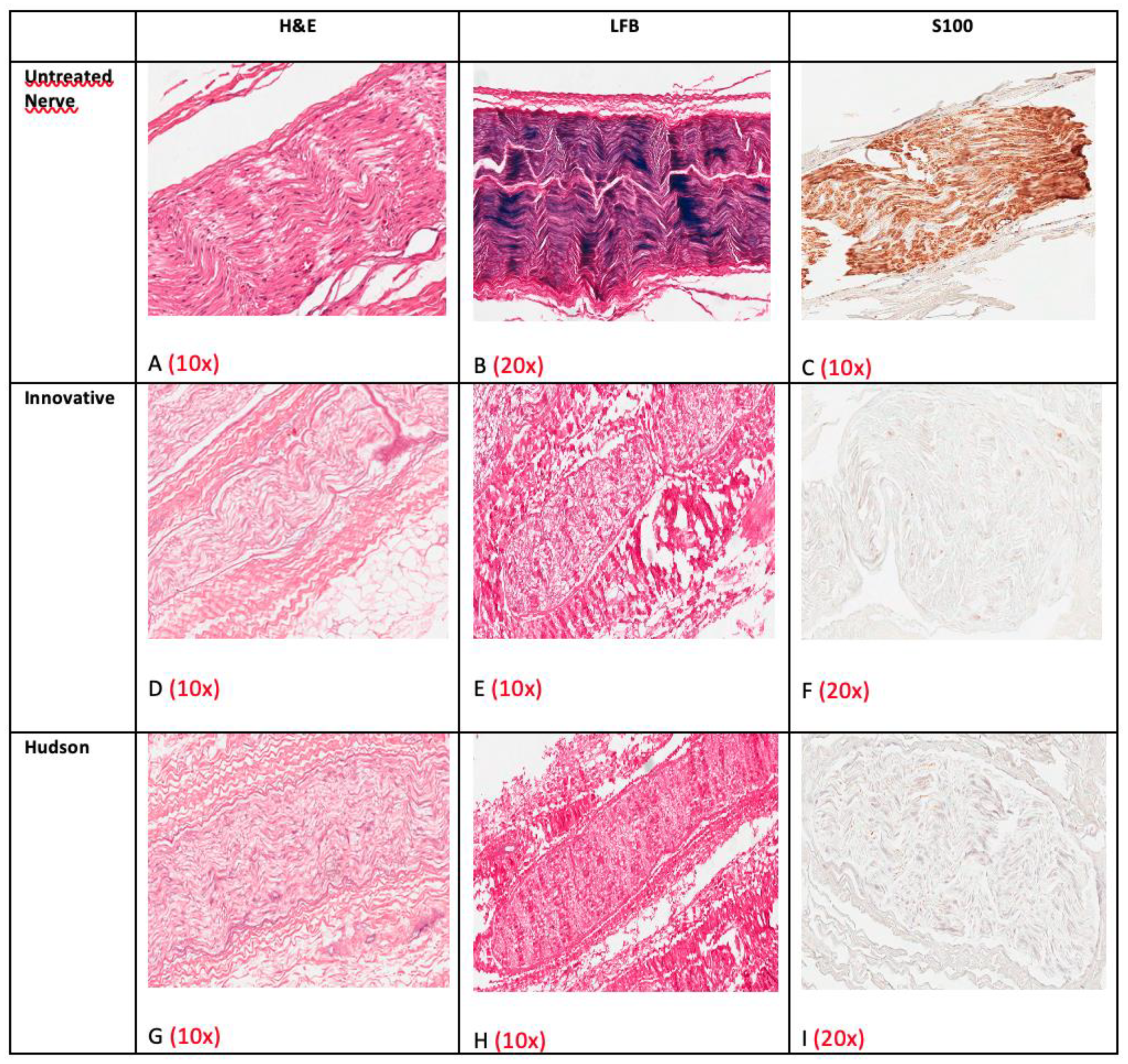

| Cases | Decellularization Techniques | Parameters for the Evaluation of Decellularization Process | Evaluation of Neural Support Structure | Results | ||||
|---|---|---|---|---|---|---|---|---|
| Global Nerve Preservation | Nuclear Density and Status | Schwann Cells (S100) | Perineural Cells (EMA) | Axons Preservation (NF) | ECM Preservation (Laminin and Collagen IV) | |||
| 1 | Native Innovative Hudson | well well well | High, intact Low, degenerated Medium, degenerated | High Absent Absent | High Absent Absent | Well Badly Badly | Well Well Well | Innovative better than Hudson |
| 2 | Native Innovative Hudson | well well well | High, intact Low, degenerated Medium, degenerated | High Absent Absent | High Absent Absent | Badly Badly Badly | Well Well Well | Innovative better than Hudson |
| 3 | Native Innovative Hudson | well moderately moderately | High, intact Absent Low, degenerated | High Absent Absent | High Absent Absent | Well Badly Badly | Well Well Well | Innovative better than Hudson (Innovative with complete decellularization) |
| 4 | Native Innovative Hudson | well well moderately | High, intact Medium, degenerated Low, degenerated | High Absent Absent | High Absent Absent | Badly Badly Badly | Well Well Bad | Hudson better than Innovative but without ECM preservation |
| 5 | Native Innovative Hudson | well well well | High, intact Medium, degenerated Low, degenerated | High Absent Absent | High Absent Absent | Badly Badly Badly | Well Well Well | Hudson better than Innovative |
| 6 | Native Innovative Hudson | well well well | High, intact Low, degenerated Medium, degenerated | Medium Absent Absent | Medium Absent Absent | Well Badly Bad | Well Well Well | Innovative better than Hudson |
| 7 | Native Innovative Hudson | moderately moderately moderately | High, intact Low, degenerated Medium, degenerated | High Absent Absent | High Absent Absent | Well Badly Badly | Well Well Badly | Innovative better than Hudson |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolognesi, F.; Fazio, N.; Boriani, F.; Fabbri, V.P.; Gravina, D.; Pedrini, F.A.; Zini, N.; Greco, M.; Paolucci, M.; Re, M.C.; et al. Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production. Int. J. Mol. Sci. 2022, 23, 1530. https://doi.org/10.3390/ijms23031530
Bolognesi F, Fazio N, Boriani F, Fabbri VP, Gravina D, Pedrini FA, Zini N, Greco M, Paolucci M, Re MC, et al. Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production. International Journal of Molecular Sciences. 2022; 23(3):1530. https://doi.org/10.3390/ijms23031530
Chicago/Turabian StyleBolognesi, Federico, Nicola Fazio, Filippo Boriani, Viscardo Paolo Fabbri, Davide Gravina, Francesca Alice Pedrini, Nicoletta Zini, Michelina Greco, Michela Paolucci, Maria Carla Re, and et al. 2022. "Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production" International Journal of Molecular Sciences 23, no. 3: 1530. https://doi.org/10.3390/ijms23031530
APA StyleBolognesi, F., Fazio, N., Boriani, F., Fabbri, V. P., Gravina, D., Pedrini, F. A., Zini, N., Greco, M., Paolucci, M., Re, M. C., Asioli, S., Foschini, M. P., D’Errico, A., Baldini, N., & Marchetti, C. (2022). Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production. International Journal of Molecular Sciences, 23(3), 1530. https://doi.org/10.3390/ijms23031530








