State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis
Abstract
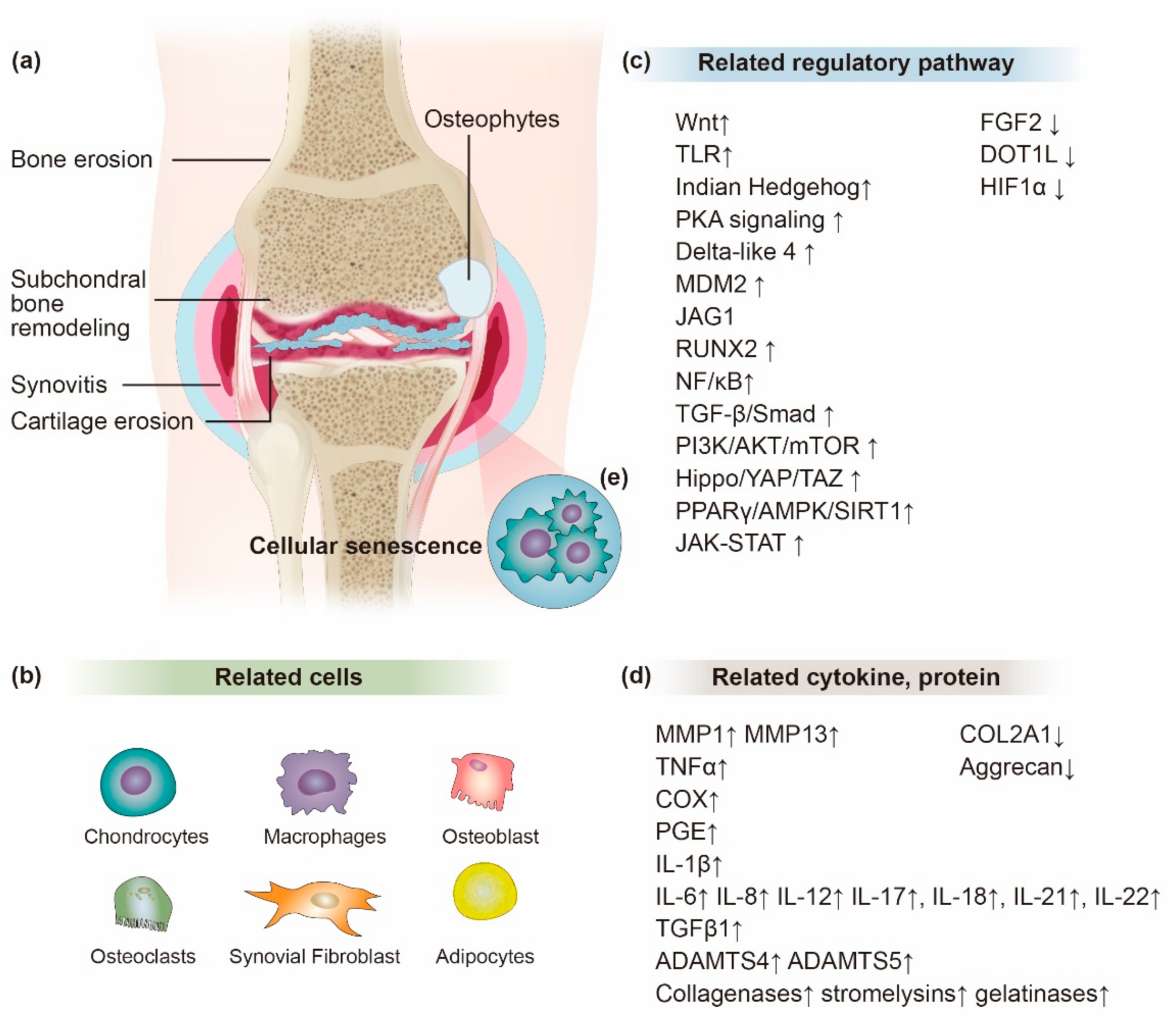
:1. Introduction
2. The Effect of Inflammation in OA Progression
3. The Mesenchymal Stem Cells
3.1. Source of MSCs
3.2. Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs)
3.3. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs)
3.4. Synovial Membrane-Derived Mesenchymal Stem Cells (SDMSCs)
3.5. Human Umbilical Cord Blood/Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJDMSCs)
4. Mode of Anti-Inflammatory and Immunomodulatory Actions of MSCs for OA
Enhanced MSC Function
5. Exosomes
5.1. Therapeutic Carrier Role of Exosomes
5.2. Promoting Cartilage Repair or Regeneration Using Exosomes
6. Exosomal miRNA
Mechanism of miRNA-Mediated Gene Regulation
7. Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aljuhani, W.S.; Qasim, S.S.; Alrasheed, A.; Altwalah, J.; Alsalman, M.J. The effect of gender, age, and body mass index on the medial and lateral posterior tibial slopes: A magnetic resonance imaging study. Knee Surg. Relat. Res. 2021, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Angadi, D.S.; Macdonald, H.; Atwal, N. Autologous cell-free serum preparations in the management of knee osteoarthritis: What is the current clinical evidence? Knee Surg. Relat. Res. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Zingler, C.; Carl, H.D.; Swoboda, B.; Krinner, S.; Hennig, F.; Gelse, K. Limited evidence of chondrocyte outgrowth from adult human articular cartilage. Osteoarthr. Cartil. 2016, 24, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage-Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef]
- Pers, Y.M.; Ruiz, M.; Noël, D.; Jorgensen, C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: State of the art and perspectives. Osteoarthr. Cartil. 2015, 23, 2027–2035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, J.N.; Neogi, T.; Callahan, L.F.; Block, J.A.; Conaghan, P.G.; Simon, L.S.; Kraus, V.B.; Hochberg, M.C. Disease modification in osteoarthritis; pathways to drug approval. Osteoarthr. Cartil. Open 2020, 2, 100059. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Proudman, S.; Kivitz, A.J.; Burch, F.X.; Donohue, J.P.; Burstein, D.; Sun, Y.N.; Banfield, C.; Vincent, M.S.; Ni, L.; et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 2011, 13, R125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, X.; Ravaud, P.; Maheu, E.; Baron, G.; Rialland, A.; Vergnaud, P.; Roux, C.; Maugars, Y.; Mulleman, D.; Lukas, C.; et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2015, 74, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Maheshwer, B.; Polce, E.M.; Paul, K.; Williams, B.T.; Wolfson, T.S.; Yanke, A.; Verma, N.N.; Cole, B.J.; Chahla, J. Regenerative Potential of Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis and Chondral Defects: A Systematic Review and Meta-analysis. Arthrosc.-J. Arthrosc. Relat. Surg. 2021, 37, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Ichiseki, T.; Shimazaki, M.; Ueda, Y.; Ueda, S.; Tsuchiya, M.; Souma, D.; Kaneuji, A.; Kawahara, N. Intraarticularly-injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. Int. J. Mol. Sci. 2018, 19, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, D.J.; Jeon, Y.S.; Park, J.S.; Bae, G.C.; Kim, J.S.; Kim, M.K. Comparison of Bone Marrow Aspirate Concentrate and Allogenic Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Implantation on Chondral Defect of Knee: Assessment of Clinical and Magnetic Resonance Imaging Outcomes at 2-Year Follow-Up. Cell Transplant. 2020, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Suh, Y.J.; Moon, K.H.; Park, J.S.; Roh, T.H.; Park, M.H.; Ryu, D.J. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: A matched pair control study with simple core decompression. Stem Cell Res. Ther. 2018, 9, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsello, T.; Amico, G.; Corrao, S.; Anzalone, R.; Timoneri, F.; Lo Iacono, M.; Russo, E.; Spatola, G.F.; Uzzo, M.L.; Giuffrè, M.; et al. Wharton’s Jelly Mesenchymal Stromal Cells from Human Umbilical Cord: A Close-up on Immunomodulatory Molecules Featured In Situ and In Vitro. Stem Cell Rev. Reports 2019, 15, 900–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zou, R.; Wang, Z.; Wen, C.; Zhang, F.; Lin, F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 2018, 475, 3629–3638. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Nurul, A.A.; Azlan, M.; Ahmad Mohd Zain, M.R.; Sebastian, A.A.; Fan, Y.Z.; Fauzi, M.B. Mesenchymal stem cells: Current concepts in the management of inflammation in osteoarthritis. Biomedicines 2021, 9, 785. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr. Rheumatol. Rep. 2013, 15, 323. [Google Scholar] [CrossRef] [Green Version]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef]
- Goldring, M.B.; Berenbaum, F. The regulation of chondrocyte function by proinflammatory mediators: Prostaglandins and nitric oxide. Clin. Orthop. Relat. Res. 2004, 427, S37–S46. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Archer, C.W.; Francis-West, P. The chondrocyte. Int. J. Biochem. Cell Biol. 2003, 35, 401–404. [Google Scholar] [CrossRef]
- Fosang, A.J.; Beier, F. Emerging Frontiers in cartilage and chondrocyte biology. Best Pract. Res. Clin. Rheumatol. 2011, 25, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Carter, S.D.; Martín-Vasallo, P.; Shakibaei, M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol. Int. 2002, 26, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hudelmaier, M.; Glaser, C.; Hohe, J.; Englmeier, K.H.; Reiser, M.; Putz, R.; Eckstein, F. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001, 44, 2556–2561. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Cimmino, M.A.; Scarpa, R.; Caporali, R.; Parazzini, F.; Zaninelli, A.; Atzeni, F.; Canesi, B. Osteoarthritis: An overview of the disease and its treatment strategies. Semin. Arthritis Rheum. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar]
- Hu, X.; Ji, X.; Yang, M.; Fan, S.; Wang, J.; Lu, M.; Shi, W.; Mei, L.; Xu, C.; Fan, X.; et al. Cdc42 Is Essential for Both Articular Cartilage Degeneration and Subchondral Bone Deterioration in Experimental Osteoarthritis. J. Bone Miner. Res. 2018, 33, 945–958. [Google Scholar] [CrossRef]
- Diekman, B.O.; Sessions, G.A.; Collins, J.A.; Knecht, A.K.; Strum, S.L.; Mitin, N.K.; Carlson, C.S.; Loeser, R.F.; Sharpless, N.E. Expression of p16 INK 4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 2018, 17, e12771. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, M.J.; Valín, Á.; Usategui, A.; Ergueta, S.; Martín, E.; Municio, C.; Cañete, J.D.; Blanco, F.J.; Criado, G.; Pablos, J.L. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun. Ageing 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Looi, Q.H.; How, C.W.; Lee, S.H.; Al-Masawa, M.E.; Chong, P.P.; Law, J.X. Mesenchymal stem cell-derived exosomes and micrornas in cartilage regeneration: Biogenesis, efficacy, mirna enrichment and delivery. Pharmaceuticals 2021, 14, 1093. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Feijt, C.; De Clerck, L.; Verhaar, J.A.N.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Somville, J.; Kloppenburg, M.; Van Osch, G.J.V.M. Cytokine production by infrapatellar fat pad can be stimulated by interleukin 1β and inhibited by peroxisome proliferator activated receptor α agonist. Ann. Rheum. Dis. 2012, 71, 1012–1018. [Google Scholar] [CrossRef]
- Min, S.; Wang, C.; Lu, W.; Xu, Z.; Shi, D.; Chen, D.; Teng, H.; Jiang, Q. Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-α in knee osteoarthritis patients. Clin. Rheumatol. 2017, 36, 2351–2358. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Wu, N.; Wu, Z.; Wang, Y.; Lin, M. ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 2021, 8, 692. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis–looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, A.I.; Beekhuizen, M.; Rutgers, M.; van Osch, G.J.V.M.; Bekkers, J.E.J.; Bot, A.G.J.; Geurts, B.; Dhert, W.J.A.; Saris, D.B.F.; Creemers, L.B. Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model. Arthritis Res. Ther. 2012, 14, R262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; de Andrés, M.C.; Hashimoto, K.; Itoi, E.; Oreffo, R.O.C. Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Qi, Y.; Li, H.; Zhang, R.; Lian, Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp. Ther. Med. 2018, 16, 4737–4744. [Google Scholar] [CrossRef] [Green Version]
- Gosset, M.; Pigenet, A.; Salvat, C.; Berenbaum, F.; Jacques, C. Inhibition of Matrix Metalloproteinase-3 and -13 Synthesis Induced by IL-1β in Chondrocytes from Mice Lacking Microsomal Prostaglandin E Synthase-1. J. Immunol. 2010, 185, 6244–6252. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Xiang, J.S.; DiGrandi, M.J.; Du, X.; Ipek, M.; Laakso, L.M.; Li, J.; Li, W.; Rush, T.S.; Schmid, J.; et al. Potent, selective, and orally bioavailable matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. Bioorg. Med. Chem. 2005, 13, 6629–6644. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 2008, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Saperstein, S.; Chen, L.; Oakes, D.; Pryhuber, G.; Finkelstein, J. IL-1beta augments TNF-alpha-mediated inflammatory responses from lung epithelial cells. J. Interferon Cytokine Res. 2009, 29, 273–284. [Google Scholar] [CrossRef]
- Faust, H.J.; Zhang, H.; Han, J.; Wolf, M.T.; Jeon, O.H.; Sadtler, K.; Peña, A.N.; Chung, L.; Maestas, D.R.; Tam, A.J.; et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Investig. 2020, 130, 5493–5507. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Schelbergen, R.F.P.; Blom, A.B.; Van Den Bosch, M.H.J.; Slöetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, B.W.; Mort, J.S.; Vogl, T.; Roth, J.; Van Den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012, 64, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal. 2019, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, C.; Yi, Z.; Lan, C. Explore the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early stage of osteoarthritis. IUBMB Life 2016, 68, 293–302. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Sarugaser, R.; Hanoun, L.; Keating, A.; Stanford, W.L.; Davies, J.E. Human Mesenchymal Stem Cells Self-Renew and Differentiate According to a Deterministic Hierarchy. PLoS ONE 2009, 4, e6498. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G. Biochemical heterogeneity of mesenchymal stem cell populations: Clues to their therapeutic efficacy. Cell Cycle 2007, 6, 2884–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Ter Huurne, M.; Schelbergen, R.; Blattes, R.; Blom, A.; De Munter, W.; Grevers, L.C.; Jeanson, J.; Noël, D.; Casteilla, L.; Jorgensen, C.; et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012, 64, 3604–3613. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Ha, J.; Lee, M.; Jin, H.J.; Kim, D.H.; Choi, S.J.; Oh, W.; Yang, Y.S.; Kim, J.S.; Kim, B.G.; et al. Autocrine action of thrombospondin-2 determines the chondrogenic differentiation potential and suppresses hypertrophic maturation of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells 2015, 33, 3291–3303. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, A.; Whyte, G.P. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am. J. Sports Med. 2016, 44, 2846–2854. [Google Scholar] [CrossRef]
- Ding, J.; Chen, B.; Lv, T.; Liu, X.; Fu, X.; Wang, Q.; Yan, L.; Kang, N.; Cao, Y.; Xiao, R. Bone Marrow Mesenchymal Stem Cell-Based Engineered Cartilage Ameliorates Polyglycolic Acid/Polylactic Acid Scaffold-Induced Inflammation Through M2 Polarization of Macrophages in a Pig Model. Stem Cells Transl. Med. 2016, 5, 1079–1089. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Zhai, Z.; Dai, K. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics 2019, 9, 6949–6961. [Google Scholar] [CrossRef]
- Chahal, J.; Gómez-Aristizábal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Mirsaidi, A.; Kleinhans, K.N.; Rimann, M.; Tiaden, A.N.; Stauber, M.; Rudolph, K.L.; Richards, P.J. Telomere length, telomerase activity and osteogenic differentiation are maintained in adipose-derived stromal cells from senile osteoporotic SAMP6 mice. J. Tissue Eng. Regen. Med. 2012, 6, 378–390. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, G.I. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: Greater doses of growth factor are necessary. J. Orthop. Res. 2009, 27, 612–619. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE 2018, 13, e0202922. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees With Cartilage Defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rocca, G.; Lo Iacono, M.; Corsello, T.; Corrao, S.; Farina, F.; Anzalone, R. Human Wharton’s jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: New perspectives for cellular therapy. Curr. Stem Cell Res. Ther. 2013, 8, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Hung, S.; Peng, S.; Huang, C.; Wei, H.; Guo, Y.; Fu, Y.; Lai, M.; Chen, C. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulnier, N.; Viguier, E.; Perrier-Groult, E.; Chenu, C.; Pillet, E.; Roger, T.; Maddens, S.; Boulocher, C. Intra-articular administration of xenogeneic neonatal Mesenchymal Stromal Cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Ha, C.; Lee, C.; Yoon, Y.C.; Park, Y. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613. [Google Scholar] [CrossRef]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X.; et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, B.; Tian, Y.; Jiao, H.; Zheng, W.; Wang, J.; Guan, F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell. Immunol. 2011, 272, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadlik, B.; Jaroslawski, G.; Puszkarz, M.; Blasiak, A.; Oldak, T.; Gladysz, D.; Whyte, G.P. Cartilage Repair in the Knee Using Umbilical Cord Wharton’s Jelly–Derived Mesenchymal Stem Cells Embedded Onto Collagen Scaffolding and Implanted Under Dry Arthroscopy. Arthrosc. Tech. 2018, 7, e57–e63. [Google Scholar] [CrossRef] [Green Version]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Xue, Q.; Chen, Y.J.; Yu, G.H.; de Qing, M.; Shen, Y.; Wang, M.Y.; Shi, Q.; Zhang, X.G. Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells. Hum. Immunol. 2013, 74, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Quaedackers, M.E.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur. J. Immunol. 2009, 39, 3436–3446. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, M.; Zhang, L.; Fu, B.; Bai, J.; Zhang, Y.; Cai, G.; Bai, X.; Feng, Z.; Sun, S.; Chen, X. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018, 93, 814–825. [Google Scholar] [CrossRef]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Petri, R.M.; Hackel, A.; Hahnel, K.; Dumitru, C.A.; Bruderek, K.; Flohe, S.B.; Paschen, A.; Lang, S.; Brandau, S. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Rep. 2017, 9, 985–998. [Google Scholar] [CrossRef] [Green Version]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Lozito, T.P.; Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014, 34, 132–143. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [Green Version]
- Toh, W.S.; Foldager, C.B.; Pei, M.; Hui, J.H.P. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev. Rep. 2014, 10, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng. Part B Rev. 2021, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kadir, N.D.; Yang, Z.; Hassan, A.; Denslin, V.; Lee, E.H. Electrospun fibers enhanced the paracrine signaling of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Voskamp, C.; Koevoet, W.J.L.M.; Somoza, R.A.; Caplan, A.I.; Lefebvre, V.; van Osch, G.J.V.M.; Narcisi, R. Enhanced Chondrogenic Capacity of Mesenchymal Stem Cells after TNFα Pre-treatment. Front. Bioeng. Biotechnol. 2020, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.H.P.; Franco-Obregón, A.; Yang, Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2020, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Lee, W.Y.W.; Feng, Q.; Xu, L.; Wang, B.; Man, G.C.W.; Chen, Y.; Jiang, X.; Bian, L.; Cui, L.; et al. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res. Ther. 2017, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Park, Y.B.; Ha, C.W.; Kim, J.A.; Kim, S.; Park, Y.G. Comparison of Undifferentiated Versus Chondrogenic Predifferentiated Mesenchymal Stem Cells Derived From Human Umbilical Cord Blood for Cartilage Repair in a Rat Model. Am. J. Sports Med. 2019, 47, 451–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cai, Y.; Jiang, Y.; Lin, X. Exosomes in osteoarthritis and cartilage injury: Advanced development and potential therapeutic strategies. Int. J. Biol. Sci. 2020, 16, 1811–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liau, L.L.; Al-Masawa, M.E.; Koh, B.; Looi, Q.H.; Foo, J.B.; Lee, S.H.; Cheah, F.C.; Law, J.X. The Potential of Mesenchymal Stromal Cell as Therapy in Neonatal Diseases. Front. Pediatr. 2020, 8, 591693. [Google Scholar] [CrossRef] [PubMed]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xue, K.; Zhang, X.; Zheng, Z.; Liu, K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res. Ther. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.L.; Zhang, S.; Wang, M.; Ren, X.; Afizah, H.; Lai, R.C.; Lim, S.K.; Lee, E.H.; Hui, J.H.P.; Toh, W.S. Intra-Articular Injections of Mesenchymal Stem Cell Exosomes and Hyaluronic Acid Improve Structural and Mechanical Properties of Repaired Cartilage in a Rabbit Model. Arthrosc.-J. Arthrosc. Relat. Surg. 2020, 36, 2215–2228.e2. [Google Scholar] [CrossRef]
- Munir, J.; Yoon, J.K.; Ryu, S. Therapeutic miRNA-Enriched Extracellular Vesicles: Current Approaches and Future Prospects. Cells 2020, 9, 2271. [Google Scholar] [CrossRef]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]
- To, K.; Romain, K.; Mak, C.; Kamaraj, A.; Henson, F.; Khan, W. The Treatment of Cartilage Damage Using Human Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review of in vivo Studies. Front. Bioeng. Biotechnol. 2020, 8, 580. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collino, F.; Pomatto, M.; Bruno, S.; Lindoso, R.S.; Tapparo, M.; Sicheng, W.; Quesenberry, P.; Camussi, G. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem cell Rev. Rep. 2017, 13, 226–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; Da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef]
- Baek, D.; Lee, K.M.; Park, K.W.; Suh, J.W.; Choi, S.M.; Park, K.H.; Lee, J.W.; Kim, S.H. Inhibition of miR-449a Promotes Cartilage Regeneration and Prevents Progression of Osteoarthritis in In Vivo Rat Models. Mol. Ther.-Nucleic Acids 2018, 13, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Lolli, A.; Sivasubramaniyan, K.; Vainieri, M.L.; Oieni, J.; Kops, N.; Yayon, A.; van Osch, G.J.V.M. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J. Control. Release 2019, 309, 220–230. [Google Scholar] [CrossRef]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orang, A.V.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-specific upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef] [Green Version]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Lee, I.; Ajay, S.S.; Jong, I.Y.; Hyun, S.K.; Su, H.H.; Nam, H.K.; Dhanasekaran, S.M.; Chinnaiyan, A.M.; Athey, B.D. New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome Res. 2009, 19, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genomics Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Brümmer, A.; Hausser, J. MicroRNA binding sites in the coding region of mRNAs: Extending the repertoire of post-transcriptional gene regulation. Bioessays 2014, 36, 617–626. [Google Scholar] [CrossRef]
- Carroll, A.P.; Goodall, G.J.; Liu, B. Understanding principles of miRNA target recognition and function through integrated biological and bioinformatics approaches. Wiley Interdiscip. Rev. RNA 2014, 5, 361–379. [Google Scholar] [CrossRef]
- Jin, Z.; Ren, J.; Qi, S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020, 381, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Malizos, K.N.; Oikonomou, P.; Tsezou, A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS ONE 2008, 3, e3740. [Google Scholar] [CrossRef] [PubMed]
- Ham, O.; Song, B.W.; Lee, S.Y.; Choi, E.; Cha, M.J.; Lee, C.Y.; Park, J.H.; Kim, I.K.; Chang, W.; Lim, S.; et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase a signaling. Biomaterials 2012, 33, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.F.; Wang, B.; Qu, Y.X.; Zheng, C.; Xu, J.D.; Xie, Z.K.; Ma, Y. MicroRNA-23c inhibits articular cartilage damage recovery by regulating MSCs differentiation to chondrocytes via reducing FGF2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 941–948. [Google Scholar] [CrossRef]
- Jin, Z.; Ren, J.; Qi, S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 2020, 78, 105946. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, R.; Shi, B.; Chen, L.; Yang, L.; Fu, Q. MicroRNA-30a promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting Delta-like 4 expression. Life Sci. 2016, 148, 220–228. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, Z.; Zhang, Z.; Wu, P.; Zhao, X.; Fu, M.; Sheng, P.; Kang, Y.; Liao, W. Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 4877–4886. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Matsukawa, T.; Sakai, T.; Yonezawa, T.; Hiraiwa, H.; Hamada, T.; Nakashima, M.; Ono, Y.; Ishizuka, S.; Nakahara, H.; Lotz, M.K.; et al. MicroRNA-125b regulates the expression of aggrecanase-1 (ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res. Ther. 2013, 15, R28. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Li, L.; Fang, X.; Zang, M. Exosome-Encapsulated microRNA-127-3p Released from Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Osteoarthritis Through Regulating CDH11-Mediated Wnt/β-Catenin Pathway. J. Pain Res. 2021, 14, 297–310. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, D.; Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021, 269, 118987. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B.; Xu, H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 2018, 17, 2756–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, S.Z.; Sun, H.; Sun, L.; Zhou, D.; Yan, J. miR-199b-5p promoted chondrogenic differentiation of C3H10T1/2 cells by regulating JAG1. J. Tissue Eng. Regen. Med. 2020, 14, 1618–1629. [Google Scholar] [CrossRef]
- Li, Z.; Meng, D.; Li, G.; Xu, J.; Tian, K.; Li, Y. Overexpression of microRNA-210 promotes chondrocyte proliferation and extracellular matrix deposition by targeting HIF-3α in osteoarthritis. Mol. Med. Rep. 2016, 13, 2769–2776. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xu, Z.; Shao, J.; Fu, P.; Wu, H. MicroRNA-218 promotes early chondrogenesis of mesenchymal stem cells and inhibits later chondrocyte maturation. BMC Biotechnol. 2019, 19, 6. [Google Scholar] [CrossRef]
- Kim, D.; Song, J.; Jin, E.J. MicroRNA-221 Regulates Chondrogenic Differentiation through Promoting Proteosomal Degradation of Slug by Targeting Mdm2 *. J. Biol. Chem. 2010, 285, 26900–26907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Zhang, Z.; Chen, W.; Huang, G.; He, A.; Hou, C.; Long, Y.; Yang, Z.; Zhang, Z.; Liao, W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1β-induced chondrocyte responses. Osteoarthr. Cartil. 2016, 24, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhou, J.; Wang, Z.; Tao, H.; Bai, J.; Ge, G.; Li, W.; Zhang, W.; Hao, Y.; Yang, X.; et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg. Chem. 2021, 113, 104978. [Google Scholar] [CrossRef]
- Moghadasi, S.; Elveny, M.; Rahman, H.S.; Suksatan, W.; Jalil, A.T.; Abdelbasset, W.K.; Yumashev, A.V.; Shariatzadeh, S.; Motavalli, R.; Behzad, F.; et al. A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. J. Transl. Med. 2021, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collén, A.; Sunnerhagen, P.; et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.; Ren, Y.; Hu, X.; Mu, J.; Samykutty, A.; Zhuang, X.; Deng, Z.; Kumar, A.; Zhang, L.; Merchant, M.L.; et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Christiansen, G.; Gurevich, L.; Moos, T.; Duroux, M. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 2016, 68, 2125–2138. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, Q.; Zhang, J.; Li, C.; Miao, Y.R.; Lei, Q.; Li, Q.; Guo, A.Y. EVmiRNA: A database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019, 47, D89–D93. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Yao, H.; Yao, Z.; Ho, K.K.W.; Ong, M.T.Y.; Dai, B.; Tong, W.; Xu, J.; Qin, L. Comprehensive Analysis of Key Genes, Signaling Pathways and miRNAs in Human Knee Osteoarthritis: Based on Bioinformatics. Front. Pharmacol. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, Y.; Li, X. Identification of key gene modules and transcription factors for human osteoarthritis by weighted gene co-expression network analysis. Exp. Ther. Med. 2019, 18, 2479–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawitzke, A.D. Personalized medicine for osteoarthritis: Where are we now? Ther. Adv. Musculoskelet. Dis. 2013, 5, 67–75. [Google Scholar] [CrossRef]
- Ingale, D.; Kulkarni, P.; Electricwala, A.; Moghe, A.; Kamyab, S.; Jagtap, S.; Martson, A.; Koks, S.; Harsulkar, A. Synovium-synovial fluid axis in osteoarthritis pathology: A key regulator of the cartilage degradation process. Genes 2021, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| miRNA | Origin | Target | Actions |
|---|---|---|---|
| miR-9-5p | BMSCs | Syndecan-1 | has anti-inflammatory and cartilage protective effects on osteoarthritis [151]. |
| miR-22 | BMSCs | PPARA/BMP-7 | inhibition upregulates BMP-7 and PPARA expression, inhibits IL-1 expression, and suppresses MMP-13 expression in OA chondrocytes [152]. |
| miR-23b | BMSCs | PKA | induces chondrogenic differentiation of human MSCs by inhibiting PKA signaling [153]. |
| miR-23c | BMSCs | FGF2 | inhibits articular cartilage damage recovery by regulating MSCs differentiation to chondrocytes via reducing FGF2 [154]. |
| miR-26a-5p | BMSCs | PTGS2 | promotes the survival of synovial fibroblasts and reduce synovitis damage [155]. |
| miR-30a | BMSCs | DLL4 | promotes chondrogenic differentiation of mesenchymal stem cells through inhibiting DLL4 expression [156]. |
| miR-92a | BMSCs | Noggin3 | targets Noggin3 and activates the PI3K/Akt/mTOR pathway to positively regulate the proliferation and matrix synthesis of chondroprogenitors [157]. |
| miR-92a-3p | BMSCs | Wnt5a | regulates cartilage development and homeostasis by targeting Wnt5a [158]. |
| miR-100-5p | IPFP-MSCs | mTOR | inhibits mTOR autophagy signaling pathway to enhance chondrocyte autophagy [159]. |
| miR-124-3p | BMSCs | circHIPK3/MYH9 | chondrocyte proliferation and migration induction and in chondrocyte apoptosis inhibition via MYH9 axis [132]. |
| miR-125b | BMSCs | ADAMTS-4 | suppresses IL-1-induced upregulation of ADAMTS-4 in human OA chondrocytes [160]. |
| miR-127-3p | BMSCs | Wnt/β-catenin | inhibits CDH11, blocks the Wnt/β-catenin pathway in chondrocytes, and reduces the chondrocyte damage in osteoarthritic joints [161]. |
| miR-129- 5p | SMSCs | HMGB1 | declined the inflammatory response and apoptosis of chondrocytes via HMGB1 upregulation [162]. |
| miR-135b | BMSCs | Sp1a MAPK6 | promotes chondrocyte proliferation and cartilage regeneration in OA by downregulating Sp1a in chondrocytes [163] |
| miR-136-5p | BMSCs | ELF3 | promotes chondrocyte proliferation and inhibits chondrocyte degeneration [164]. |
| miR-140-5p | SMSCs | Wnt/YAP | enhances ECM secretion and induces proliferation and migration of chondrocytes via activating YAP as well as preventing osteoarthritic joint damage [165]. |
| miR-145 | BMSCs ADMSCs | Sox9/MKK | inhibition upregulates Sox9 expression and promotes MSC chondrogenesis [166]. attenuate TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4 [167]. |
| miR-199b-5p | BMSCs | JAG1 | positive regulators to modulate chondrogenic differentiation of C3H10T1/2 cells by targeting JAG1 [168]. |
| miR-210 | BMSCs | HIF-3α | promotes chondrocyte proliferation and extracellular matrix deposition [169]. |
| miR-218 | SDSCs | HPGD | induces chondrogenic differentiation with regulatory role on 15-hydroxyprostaglandin dehydrogenase (HPGD) [170]. |
| miR-221 | BMSCs ADMSCs | MDM2 | downregulates MDM2 to prevent slug protein degradation, which negatively regulates chondroprogenitor proliferation [171]. |
| miR-320 | BMSCs | MMP-13/ SOX9 | downregulates MMP-13 expression / up- regulate SOX9 expression to induce cartilage differentiation [172]. |
| miR-361-5p | BMSCs | DDX20 NF-κB | inhibits the NF-κB signaling pathway via targeting DDX20 [173]. |
| miR-449a | BMSCs | SIRT1 | targets SIRT1 and lymphoid enhancer-binding factor-1 (LEF-1), and increased cartilage regeneration and expression of type II collagen [139]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. https://doi.org/10.3390/ijms23031618
Kwon DG, Kim MK, Jeon YS, Nam YC, Park JS, Ryu DJ. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. International Journal of Molecular Sciences. 2022; 23(3):1618. https://doi.org/10.3390/ijms23031618
Chicago/Turabian StyleKwon, Dae Gyu, Myung Ku Kim, Yoon Sang Jeon, Yoon Cheol Nam, Jin Seong Park, and Dong Jin Ryu. 2022. "State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis" International Journal of Molecular Sciences 23, no. 3: 1618. https://doi.org/10.3390/ijms23031618
APA StyleKwon, D. G., Kim, M. K., Jeon, Y. S., Nam, Y. C., Park, J. S., & Ryu, D. J. (2022). State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. International Journal of Molecular Sciences, 23(3), 1618. https://doi.org/10.3390/ijms23031618







