Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints
Abstract
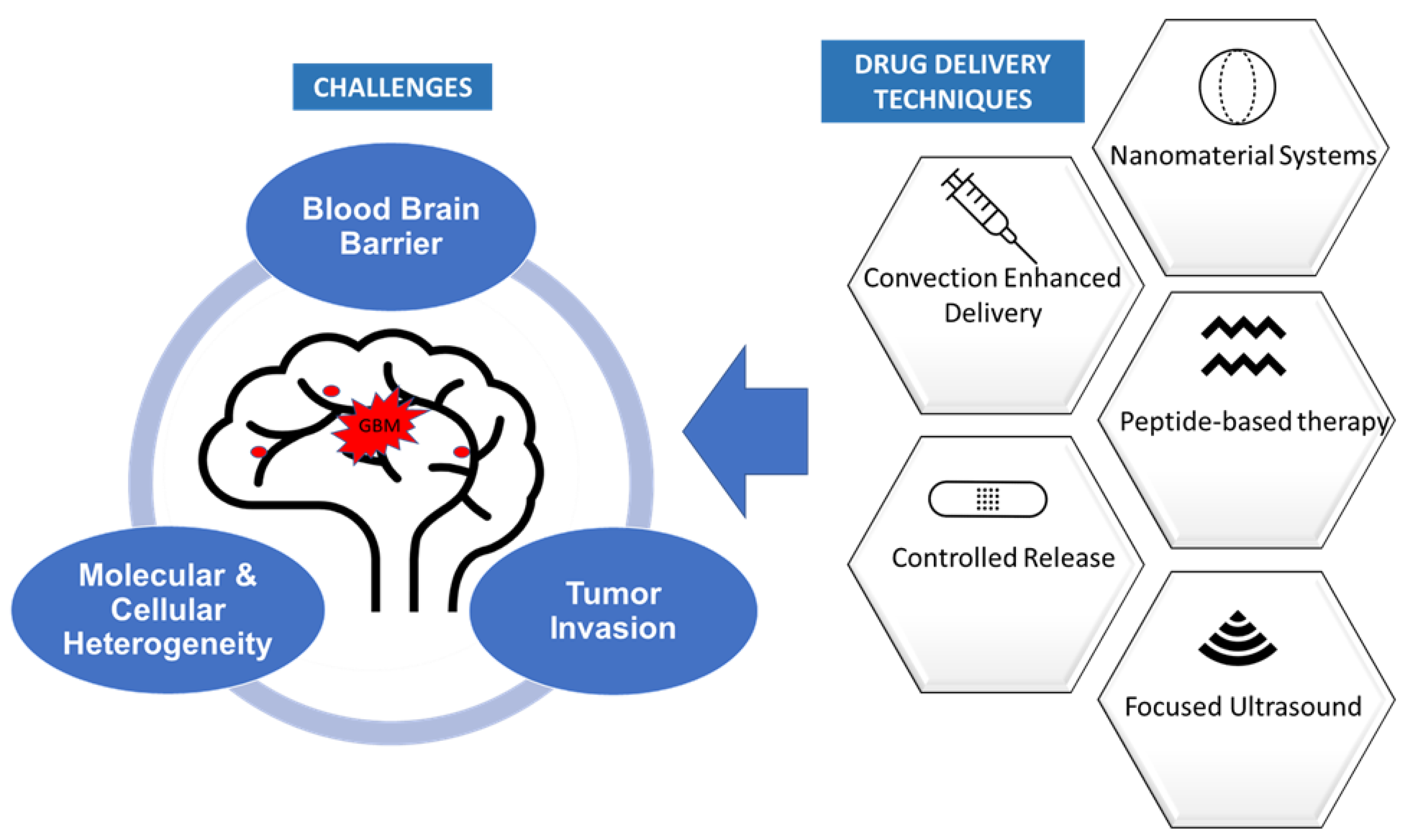
:1. Introduction
1.1. Molecular Heterogeneity
1.2. Blood–Brain Barrier
2. Methods of Drug Delivery to Glioblastoma
2.1. Controlled-Release Systems
2.2. Convection-Enhanced Delivery
2.3. Nanomaterial Systems
2.4. Peptide Based Therapeutics
2.5. Focused Ultrasound
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| GBM | Glioblastoma multiforme |
| WHO | World Health Organization |
| IDH | Isocitrate dehydrogenase |
| EGFR | Epidermal growth factor receptor |
| MGMT | O6-methylguanine DNA methyltransferase |
| PTN | Pleiotrophin |
| CDKN2A | Cyclin Dependent Kinase Inhibitor 2A |
| MDM2 | Mouse double minute 2 homolog |
| NF1 | Neurofibromatosis type 1 |
| PDGFRA | Platelet Derived Growth Factor Receptor Alpha |
| GSC | Glioblastoma stem cells |
| NSC | Neural stem cell |
| BEMC | Brain microvascular endothelial cells |
| ATP | Adenosine triphosphate |
| PDE5 | Phosphodiesterase 5 |
| FUS | Focused ultrasound |
| CED | Convection enhanced delivery |
| EPR | Enhanced permeability and retention |
| ROS | Reactive oxygen species |
| PCL | Poly(ε-caprolactone) |
| GHK | glycyl-L-histidyl-L-lysine |
| CPP | Cell penetrating peptide |
| PDA | Polydopamine |
| MMP | Matrix Metalloproteinase |
| MRI | Magnetic resonance imaging |
| MRgFUS | Magnetic resonance guided focused ultrasound |
| LIFU | Low intensity fluorescent ultrasound |
| HIFU | High intensity fluorescent ultrasound |
References
- Schiffer, D.; Annovazzi, L.; Casalone, C.; Corona, C.; Mellai, M. Glioblastoma: Microenvironment and Niche Concept. Cancers 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Cavenee, W.K.; Ohgaki, H.; Wiestler O., D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SEER*Explorer Application. Available online: https://seer.cancer.gov/explorer/application.html?site=661&data_type=4&graph_type=5&compareBy=sex&chk_sex_3=3&chk_sex_2=2&series=9&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=2 (accessed on 13 October 2021).
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470003/ (accessed on 29 April 2021).
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Prev Biomark. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharm. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [Green Version]
- Orr, B.A.; Clay, M.R.; Pinto, E.M.; Kesserwan, C. An update on the central nervous system manifestations of Li–Fraumeni syndrome. Acta Neuropathol 2020, 139, 669–687. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef]
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef]
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R.; et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv. Drug Deliv Rev. 2021, 171, 108–138. [Google Scholar] [CrossRef]
- Piraino, S.W.; Thomas, V.; O’Donovan, P.; Furney, S.J. Mutations: Driver Versus Passenger. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Academic Press: Salt Lake City, UT, USA, 2019; pp. 551–562. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D. Glioblastoma Heterogeneity and Cancer Cell Plasticity. Crit Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Aum, D.J.; Kim, D.H.; Beaumont, T.L.; Leuthardt, E.C.; Dunn, G.P.; Kim, A.H. Molecular and cellular heterogeneity: The hallmark of glioblastoma. Neurosurg. Focus 2014, 37, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleihues, P.; Ohgaki, H. Primary and secondary glioblastomas: From concept to clinical diagnosis. Neuro-Oncology 1999, 1, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell. 2010, 17, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couturier, C.P.; Ayyadhury, S.; Le, P.U.; Nadaf, J.; Monlong, J.; Riva, G.; Allache, R.; Baig, S.; Yan, X.; Bourgey, M.; et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 2020, 11, 3406. [Google Scholar] [CrossRef]
- Guelfi, S.; Duffau, H.; Bauchet, L.; Rothhut, B.; Hugnot, J.P. Vascular Transdifferentiation in the CNS: A Focus on Neural and Glioblastoma Stem-Like Cells. Stem Cells Int. 2016, 2016, e2759403. [Google Scholar] [CrossRef] [Green Version]
- Matarredona, E.R.; Pastor, A.M. Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications. Front. Oncol. 2019, 9, 779. [Google Scholar] [CrossRef]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev. Neurother. 2015, 15, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Shusta, E.V. Blood-Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics 2020, 12, 1085. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Sundgren, P.C.; Tsien, C.I.; Chenevert, T.T.; Junck, L. Physiologic and metabolic magnetic resonance imaging in gliomas. J. Clin. Oncol. 2006, 24, 1228–1235. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, 2101090. [Google Scholar] [CrossRef]
- Rapoport, S.I. Osmotic opening of the blood-brain barrier: Principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Wala, K.; Szlasa, W.; Saczko, J.; Rudno-Rudzińska, J.; Kulbacka, J. Modulation of Blood–Brain Barrier Permeability by Activating Adenosine A2 Receptors in Oncological Treatment. Biomolecules 2021, 11, 633. [Google Scholar] [CrossRef]
- Sawyer, A.J.; Piepmeier, J.M.; Saltzman, W.M. New Methods for Direct Delivery of Chemotherapy for Treating Brain Tumors. Yale J. Biol. Med. 2006, 79, 141–152. [Google Scholar]
- Zhou, J.; Atsina, K.B.; Himes, B.T.; Strohbehn, G.W.; Saltzman, W.M. Novel Delivery Strategies for Glioblastoma. Cancer J. Sudbury Mass. 2012, 18, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Yeini, E.; Ofek, P.; Albeck, N.; Ajamil, D.R.; Neufeld, L.; Eldar-Boock, A.; Kleiner, R.; Vaskovich, D.; Koshrovski-Michael, S.; Dangoor, S.I.; et al. Targeting Glioblastoma: Advances in Drug Delivery and Novel Therapeutic Approaches. Adv. Ther. 2021, 4, 2000124. [Google Scholar] [CrossRef]
- Shapira-Furman, T.; Serra, R.; Gorelick, N.; Doglioli, M.; Tagliaferri, V.; Cecia, A.; Peters, M.; Kumar, A.; Rottenberg, Y.; Langer, R.; et al. Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer. J. Control. Release 2019, 295, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Wadsworth, S.; Recinos, V.; Mehta, V.; Vellimana, A.; Li, K.; Rosenblatt, J.; Do, H.; Gallia, G.L.; Siu, I.M.; et al. Local delivery of rapamycin: A toxicity and efficacy study in an experimental malignant glioma model in rats. Neuro-Oncology 2011, 13, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Dluska, E.; Markowska-Radomska, A.; Metera, A.; Ordak, M. Multiple Emulsions as a Biomaterial-Based Delivery System for the Controlled Release of an Anti-cancer Drug. J. Phys. Conf. Ser. 2020, 1681, 012021. [Google Scholar] [CrossRef]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Préat, V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J. Control. Release 2019, 309, 72–81. [Google Scholar] [CrossRef]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels 2018, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Enríquez Pérez, J.; Kopecky, J.; Visse, E.; Darabi, A.; Siesjö, P. Convection-enhanced delivery of temozolomide and whole cell tumor immunizations in GL261 and KR158 experimental mouse gliomas. BMC Cancer 2020, 20, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Barth, R.F.; Cavaliere, R.; Puduvalli, V.K.; Giglio, P.; Lonser, R.R.; Elder, J.B. Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas. PLoS ONE 2020, 15, e0244383. [Google Scholar] [CrossRef]
- Muldoon, L.L.; Soussain, C.; Jahnke, K.; Johanson, C.; Siegal, T.; Smith, Q.R.; Hall, W.A.; Hynynen, K.; Senter, P.D.; Peereboom, D.M.; et al. Chemotherapy Delivery Issues in Central Nervous System Malignancy: A Reality Check. J. Clin. Oncol. 2007, 25, 2295–2305. [Google Scholar] [CrossRef] [Green Version]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII Antibody–Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging–Guided Convection-Enhanced Delivery and Targeted Therapy of Glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [Green Version]
- Nwagwu, C.D.; Immidisetti, A.V.; Jiang, M.Y.; Adeagbo, O.; Adamson, D.C.; Carbonell, A.M. Convection Enhanced Delivery in the Setting of High-Grade Gliomas. Pharmaceutics 2021, 13, 561. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Michael, J.S.; Lee, B.S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Intern. Med. 2018, 6, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C. 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. In Cancer Nanotechnology: Methods and Protocols. Methods in Molecular Biology; Grobmyer, S.R., Moudgil, B.M., Eds.; Humana Press: New York, NY, USA, 2010; pp. 25–37. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Alphandéry, E. Nano-Therapies for Glioblastoma Treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef] [Green Version]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Taiarol, L.; Formicola, B.; Magro, R.D.; Sesana, S.; Re, F. An update of nanoparticle-based approaches for glioblastoma multiforme immunotherapy. Nanomedicine 2020, 15, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Ngobili, T.A.; Daniele, M.A. Nanoparticles and direct immunosuppression. Exp. Biol. Med. 2016, 241, 1064–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blank, F.; Gerber, P.; Rothen-Rutishauser, B.; Sakulkhu, U.; Salaklang, J.; De Peyer, K.; Gehr, P.; Nicod, L.P.; Hofmann, H.; Geiser, T.; et al. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology 2011, 5, 606–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budama-Kilinc, Y.; Kecel-Gunduz, S.; Cakir-Koc, R.; Aslan, B.; Bicak, B.; Kokcu, Y.; Ozel, A.E.; Akyuz, S. Structural Characterization and Drug Delivery System of Natural Growth-Modulating Peptide Against Glioblastoma Cancer. Int. J. Pept. Res. Ther. 2021, 27, 2015–2028. [Google Scholar] [CrossRef]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. The Effect of the Human Peptide GHK on Gene Expression Relevant to Nervous System Function and Cognitive Decline. Brain Sci. 2017, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.H.; Chen, W.; Li, C.H.; Fang, C.Y.; Chang, Y.C.; Wei, D.H.; Liu, R.S.; Hsiao, M. An Advanced In Situ Magnetic Resonance Imaging and Ultrasonic Theranostics Nanocomposite Platform: Crossing the Blood–Brain Barrier and Improving the Suppression of Glioblastoma Using Iron-Platinum Nanoparticles in Nanobubbles. ACS Appl. Mater. Interfaces 2021, 13, 26759–26769. [Google Scholar] [CrossRef]
- Pasut, G. Grand Challenges in Nano-Based Drug Delivery. Front. Med. Technol. 2019, 1, 1. Available online: https://www.frontiersin.org/article/10.3389/fmedt.2019.00001 (accessed on 28 January 2022). [CrossRef] [Green Version]
- Sharma, D.; Sharma, N.; Pathak, M.; Agrawala, P.K.; Basu, M.; Ojha, H. Chapter 2—Nanotechnology-based drug delivery systems: Challenges and opportunities. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 39–79. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. Available online: https://www.frontiersin.org/article/10.3389/fbioe.2020.00166 (accessed on 26 January 2022). [CrossRef]
- Raucher, D. Tumor targeting peptides: Novel therapeutic strategies in glioblastoma. Curr. Opin. Pharmacol. 2019, 47, 14–19. [Google Scholar] [CrossRef]
- Polisetty, R.V.; Gautam, P.; Sharma, R.; Harsha, H.C.; Nair, S.C.; Gupta, M.K.; Uppin, M.S.; Challa, S.; Puligopu, A.K.; Ankathi, P.; et al. LC-MS/MS analysis of differentially expressed glioblastoma membrane proteome reveals altered calcium signaling and other protein groups of regulatory functions. Mol. Cell. Proteom. 2012, 11, M111.013565. [Google Scholar] [CrossRef] [Green Version]
- Kondo, E.; Iioka, H.; Saito, K. Tumor-homing peptide and its utility for advanced cancer medicine. Cancer Sci. 2021, 112, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Audrey, G.; Claire, L.C.; Joel, E. Effect of the NFL-TBS.40-63 peptide on canine glioblastoma cells. Int. J. Pharm. 2021, 605, 120811. [Google Scholar] [CrossRef] [PubMed]
- Rahn, J.J.; Lun, X.; Jorch, S.K.; Hao, X.; Venugopal, C.; Vora, P.; Ahn, B.Y.; Babes, L.; Alshehri, M.M.; Cairncross, J.; et al. Development of a peptide-based delivery platform for targeting malignant brain tumors. Biomaterials 2020, 252, 120105. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.F.A.; Shahid, S.; Mu, S.; Zhang, H. Hybridization of tumor homing and mitochondria-targeting peptide domains to design novel dual-imaging self-assembled peptide nanoparticles for theranostic applications. Drug Deliv. Transl. Res. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Liu, W.; Zhang, Z.A.; Han, Y.; Qi, L.-L.; Zhang, Y.-Y.; Zhang, X.-T.; Duan, H.-X.; Chen, L.-Q.; Jin, M.-J.; et al. Efficient Anti-Glioma Therapy Through the Brain-Targeted RVG15-Modified Liposomes Loading Paclitaxel-Cholesterol Complex. Int. J. Nanomed. 2021, 16, 5755–5776. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.S.; Zuhorn, I. Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Shabaninejad, Z.; Pourhanifeh, M.H.; Movahedpour, A.; Mottaghi, R.; Nickdasti, A.; Mortezapour, E.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; Sadeghian, M.; et al. Therapeutic potentials of curcumin in the treatment of glioblstoma. Eur. J. Med. Chem. 2020, 188, 112040. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, e186864. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Tang, L.; Wang, W.; Tang, S.; Yu, L.; Zhou, X.; Wang, Q.; Sun, C.; Shi, C.; Luo, W.; et al. Improved Antiglioblastoma Activity and BBB Permeability by Conjugation of Paclitaxel to a Cell-Penetrative MMP-2-Cleavable Peptide. Adv. Sci. 2021, 8, 2001960. [Google Scholar] [CrossRef] [PubMed]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Currie, J.C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef]
- Hong, H.-Y.; Lee, H.Y.; Kwak, W.; Yoo, J.; Na, M.-H.; So, I.S.; Kwon, T.-H.; Park, H.-S.; Huh, S.; Oh, G.T.; et al. Phage display selection of peptides that home to atherosclerotic plaques: IL-4 receptor as a candidate target in atherosclerosis. J. Cell. Mol. Med. 2008, 12, 2003–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper. Neurosurg. 2020, 19, 9–18. [Google Scholar] [CrossRef]
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Xu, Z.; Sheehan, J.P. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: A therapeutic concept. J. Ther. Ultrasound. 2016, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.C.; Chu, P.C.; Wang, H.Y.J.; Huang, C.Y.; Chen, P.Y.; Tsai, H.C.; Lu, Y.J.; Lee, P.I.; Tseng, I.C.; Feng, L.Y.; et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: A preclinical study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Hua, M.Y.; Chen, P.Y.; Chu, P.C.; Pan, C.H.; Yang, H.W.; Huang, C.Y.; Wang, J.J.; Yen, T.C.; Wei, K.C. Blood-Brain Barrier Disruption with Focused Ultrasound Enhances Delivery of Chemotherapeutic Drugs for Glioblastoma Treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef]
- Papachristodoulou, A.; Signorell, R.D.; Werner, B.; Brambilla, D.; Luciani, P.; Cavusoglu, M.; Grandjean, J.; Silginer, M.; Rudin, M.; Martin, E.; et al. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J. Control. Release 2019, 295, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1137–1148. [Google Scholar] [CrossRef]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; People, C.B.; Davidson, B.; et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef]
- Timbie, K.F.; Mead, B.P.; Price, R.J. Drug and gene delivery across the blood–brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).. Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier Disruption for the Treatment of High Grade Glioma in Patients Undergoing Standard Chemotherapy. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03551249 (accessed on 6 December 2021).
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).. A Study to Evaluate the Safety and Feasibility of Exablate Model 4000 Type-2 to Temporarily Mediate Blood-Brain Barrier Disruption (BBBD) in Patients with Suspected Infiltrating Glioma in the Setting of Planned Surgical Interventions. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03322813 (accessed on 6 December 2021).
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
| Method of Drug Delivery | Specific Examples |
|---|---|
| Controlled release systems | Gliadel [11,31,32,33] |
| Biodegradable wafers for the combined delivery of temozolomide and carmustine [34] | |
| Biodegradable polymer implants releasing rapamycin [35] | |
| Carboxymethylcellulose biopolymer system delivering rhodamine B [36] | |
| Hydrogel based co-delivery of paclitaxel and temozolomide [37] | |
| Convection enhanced delivery | Temozolomide [40] |
| Carboplatin [41] | |
| Iron oxide nanoparticles conjugated to epidermal growth factor receptor deletion mutant III antibody (EG-FRVIIIAb)/MRI-guided [43] | |
| Nanomaterial Systems | Poly(ε-caprolactone) (PCL) based nanoparticle system to deliver the natural growth modulating tripeptide GHK (glycyl-L-histidyl-L-lysine) [58] |
| Nanobubble-based theranostic system consisting of intravenously administered iron-platinum nanoparticles loaded with doxorubicin and surface-functionalized with transferrin [60] | |
| Peptide based therapeutics | Tumor targeting peptides delivering deliver the oncolytic virus VSVΔM51, in combination with gadolinium [68] |
| Self-assembled spherical nanoparticles containing a peptide probe (Cy5.5-SAPD-99mTc) with mitochondria targeting [69] | |
| Peptide derivatives of rabies virus glycoproteins, RVG29 and RVG15-liposome, delivering anticancer chemotherapeutic docetaxel nanoparticles and paclitaxel-cholesterol [70,71] | |
| WSW (also called PhrCACET1) peptide fused to paclitaxel nanosuspensions [72] | |
| Use of polydopamine (PDA)-coated zein-curcumin nanoparticles functionalized with the peptide G23 [73] | |
| Dual peptide nanocomplex created by combining SynB3 (a cell penetration peptide) with PVGLIG (an MMP-2 sensitive peptide) and paclitaxel [77] | |
| Focused ultrasound | Temozolomide [84] |
| BCNU [85] | |
| Liposomal O6-(4-bromothenyl)guanine (O6BTG) [86] | |
| Liposome-encapsulated doxorubicin [88] | |
| Cisplatin conjugated gold nanoparticles [89] | |
| Trastzumab [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. https://doi.org/10.3390/ijms23031711
Mathew EN, Berry BC, Yang HW, Carroll RS, Johnson MD. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. International Journal of Molecular Sciences. 2022; 23(3):1711. https://doi.org/10.3390/ijms23031711
Chicago/Turabian StyleMathew, Elza N., Bethany C. Berry, Hong Wei Yang, Rona S. Carroll, and Mark D. Johnson. 2022. "Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints" International Journal of Molecular Sciences 23, no. 3: 1711. https://doi.org/10.3390/ijms23031711
APA StyleMathew, E. N., Berry, B. C., Yang, H. W., Carroll, R. S., & Johnson, M. D. (2022). Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. International Journal of Molecular Sciences, 23(3), 1711. https://doi.org/10.3390/ijms23031711






