The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin
Abstract
:1. Introduction
2. Results
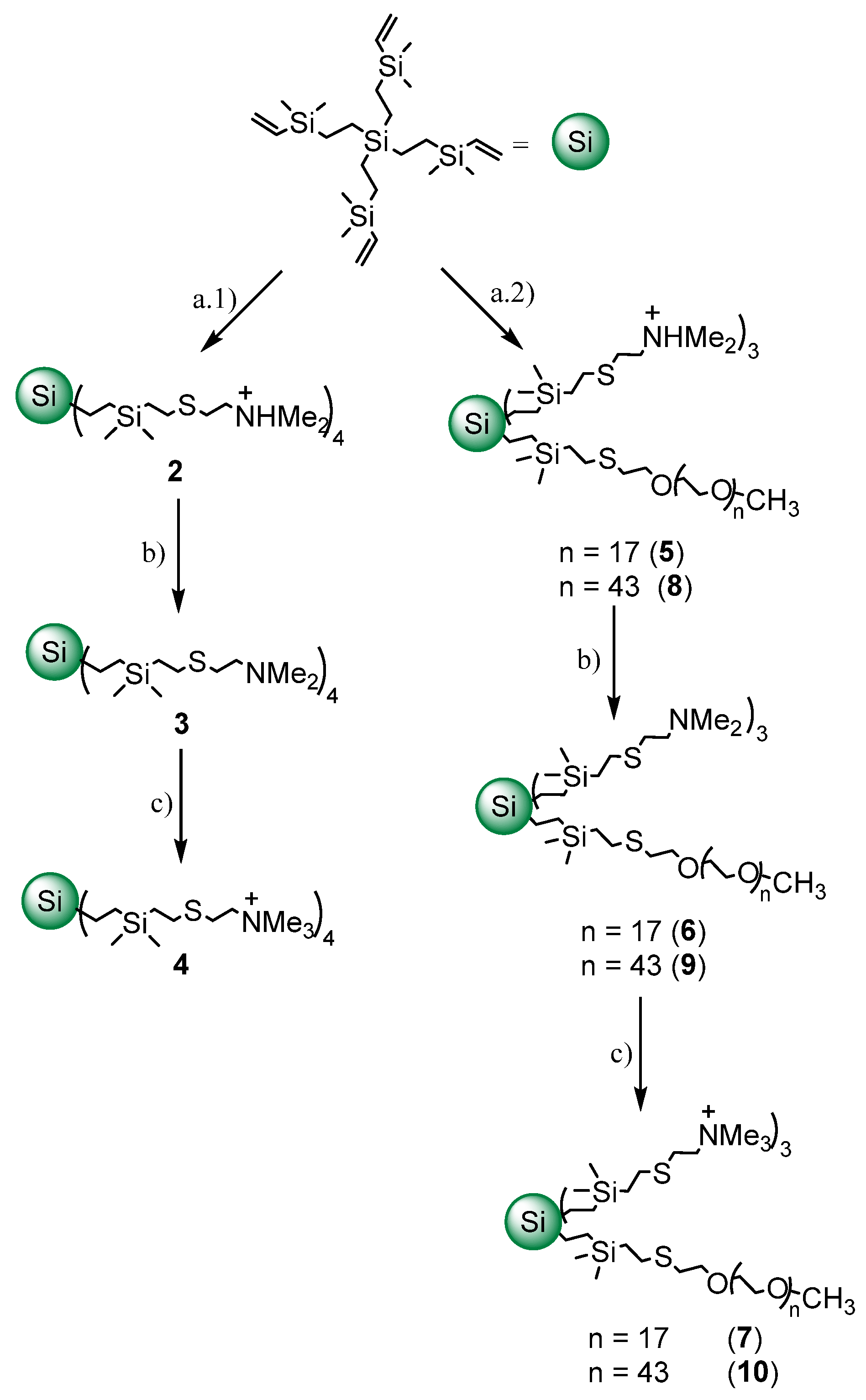
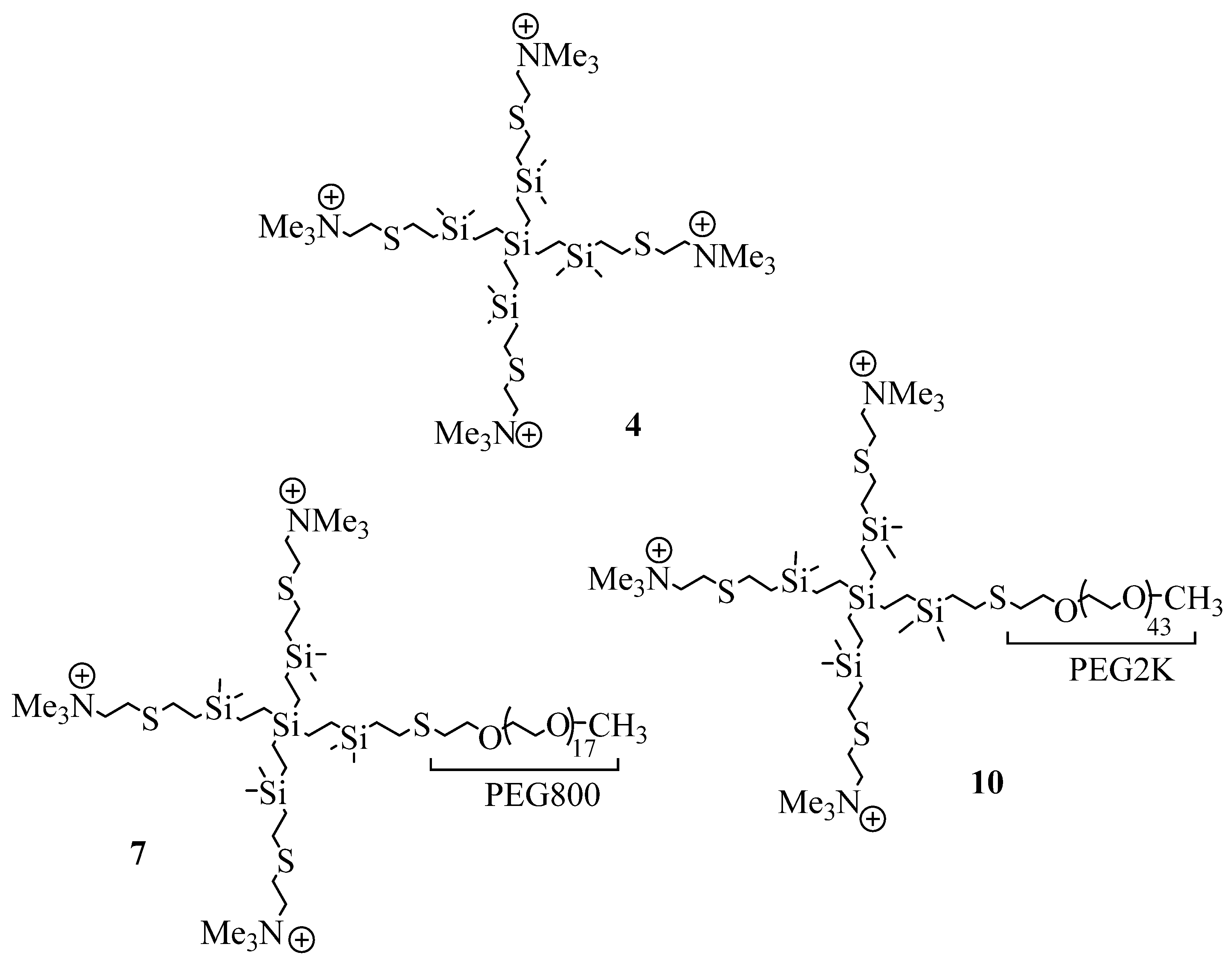
2.1. Synthesis and Characterization of Cationic CBS Dendrimers Homo- and Heterofunctionalized with Ammonium Groups and Polyethylene Glycol
2.2. Antibacterial Activity of Dendrimers against P. aeruginosa
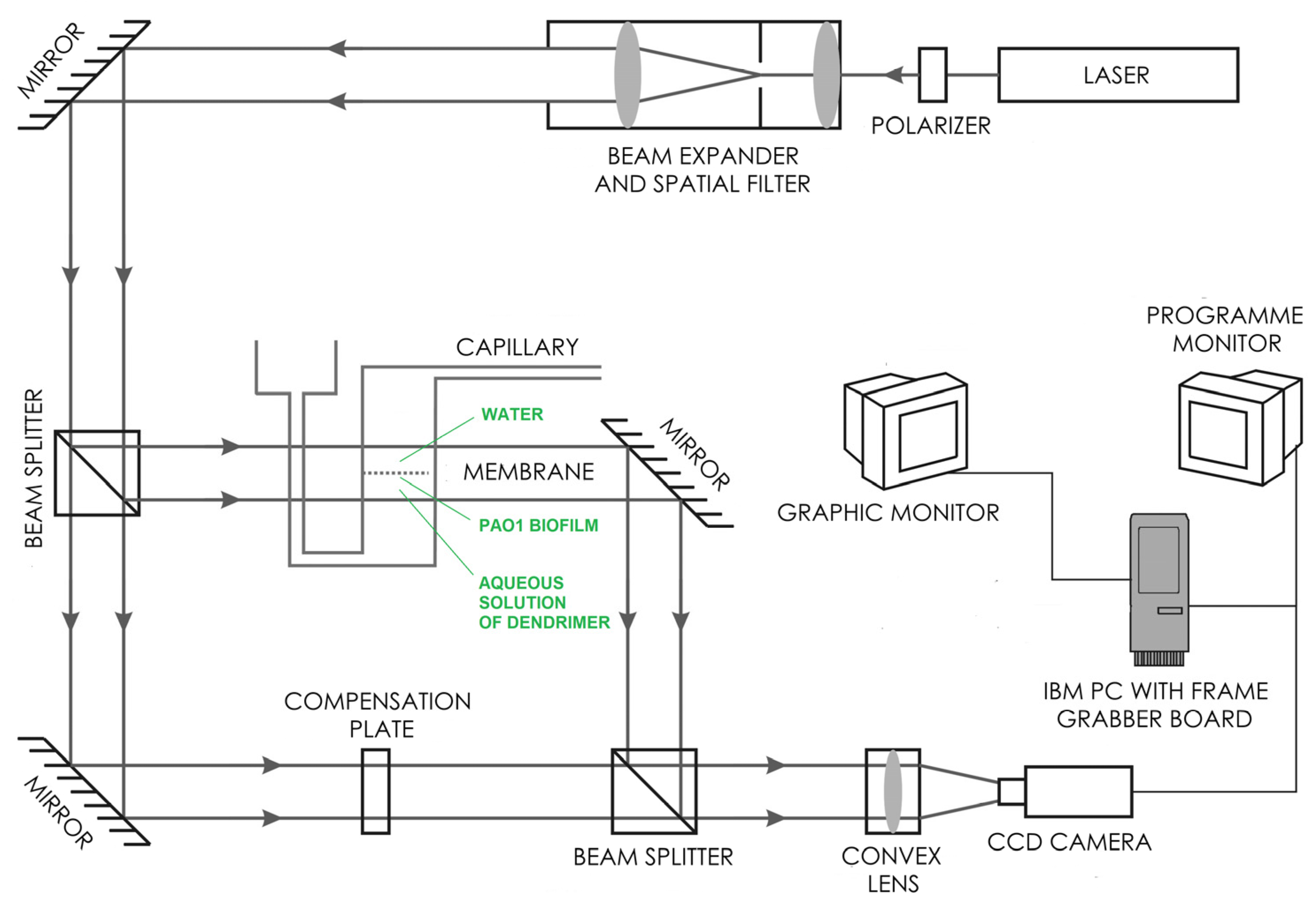
2.3. Diffusion Properties and Antibiofilm Activity of Dendrimers on P. aeruginosa PAO1 Biofilm
2.4. Antibacterial Activity of Dendrimer Combined with Endolysin against P. aeruginosa PAO1
3. Discussion
4. Materials and Methods
4.1. Synthesis of Cationic CBS Dendrimers
4.2. Bacterial Strains
4.3. Analysis of Antibacterial Properties of Dendrimers against Planktonic Cells
4.4. Analysis of Antibacterial Properties of Dendrimers against Biofilm
4.4.1. Biofilm Formation and Quantification Assay
4.4.2. Inhibition of P. aeruginosa Biofilm Formation
4.4.3. Antibiofilm Activity Testing
4.4.4. Resazurin Assay and Drop Plate Method
4.5. Scanning Electron Microscopy
4.6. Laser Interferometry
4.7. Fluorescence Microscopy
4.8. Antibacterial Activity of Endolysin Combined with Dendrimers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savoia, D. New perspectives in the management of Pseudomonas aeruginosa infections. Future Microbiol. 2014, 9, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Shneider, M.M.; Latka, A.; Maciejewska, B.; Browning, C.; Sycheva, L.V.; Cornelissen, A.; Danis-Wlodarczyk, K.; Senchenkova, S.N.; Shashkov, A.S.; et al. The O-specific polysaccharide lyase from the phage LKA1 tailspike reduces Pseudomonas virulence. Sci. Rep. 2017, 7, 16302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Mcdonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Clifton, L.A.; Skoda, M.W.A.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [Green Version]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Aurelia Chis, A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-Dendritic. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-62696-0. [Google Scholar]
- Malkoch, M.; García Gallego, S. Dendrimer Chemistry: Synthetic Approaches towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 1–20. ISBN 978-1-78801-132-7. [Google Scholar]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef] [Green Version]
- Ortega, P.; Sánchez-Nieves, J.; Cano, J.; Gómez, R.; de la Mata, F.J. CHAPTER 5 Poly(carbosilane) Dendrimers and Other Silicon-containing Dendrimers. In Dendrimer Chemistry: Synthetic Approaches towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 114–145. ISBN 978-1-78801-132-7. [Google Scholar]
- Chen, A.; Karanastasis, A.; Casey, K.R.; Necelis, M.; Carone, B.R.; Caputo, G.A.; Palermo, E.F. Cationic Molecular Umbrellas as Antibacterial Agents with Remarkable Cell-Type Selectivity. ACS Appl. Mater. Interfaces 2020, 12, 21270–21282. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From nanobiotechnology, positively charged biomimetic dendrimers as novel antibacterial agents: A review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Panarin, E.F.; Solovskii, M.V.; Zaikina, N.A.; Afinogenov, G.E. Biological activity of cationic polyelectrolytes. Die Makromol. Chemie 1985, 9, 25–33. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Sánchez-Nieves, J.; Hernández-Ros, J.M.; Fernández-Ezequiel, A.; Soliveri, J.; Copa-Patiño, J.L.; Gómez, R.; Javier De La Mata, F. Structure-activity relationship study of cationic carbosilane dendritic systems as antibacterial agents. RSC Adv. 2016, 6, 7022–7033. [Google Scholar] [CrossRef]
- Fuentes-Paniagua, E.; Hernández-Ros, J.M.; Sánchez-Milla, M.; Camero, M.A.; Maly, M.; Pérez-Serrano, J.; Copa-Patiño, J.L.; Sánchez-Nieves, J.; Soliveri, J.; Gómez, R.; et al. Carbosilane cationic dendrimers synthesized by thiol-ene click chemistry and their use as antibacterial agents. RSC Adv. 2014, 4, 1256–1265. [Google Scholar] [CrossRef]
- Ciepluch, K.; Skrzyniarz, K.; Barrios-Gumiel, A.; Quintana, S.; Sánchez-Nieves, J.; de la Mata, F.J.; Maciejewska, B.; Drulis-Kawa, Z.; Arabski, M. Dendronized Silver Nanoparticles as Bacterial Membrane Permeabilizers and Their Interactions with P. aeruginosa Lipopolysaccharides, Lysozymes, and Phage-Derived Endolysins. Front. Microbiol. 2019, 10, 2771. [Google Scholar] [CrossRef] [Green Version]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of important biotech molecules: Delivery aspects. Expert Opin. Drug Deliv. 2008, 5, 371–383. [Google Scholar] [CrossRef]
- Thakur, S.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Impact of pegylation on biopharmaceutical properties of dendrimers. Polymer 2015, 59, 67–92. [Google Scholar] [CrossRef]
- Bahamondez-Canas, T.F.; Zhang, H.; Tewes, F.; Leal, J.; Smyth, H.D.C. PEGylation of Tobramycin Improves Mucus Penetration and Antimicrobial Activity against Pseudomonas aeruginosa Biofilms in Vitro. Mol. Pharm. 2018, 15, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Maciejewska, B.; Gałczyńska, K.; Kuc-Ciepluch, D.; Bryszewska, M.; Appelhans, D.; Drulis-Kawa, Z.; Arabski, M. The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells. Bioorg. Chem. 2019, 91, 103121. [Google Scholar] [CrossRef]
- Love, M.J.; Bhandari, D.; Dobson, R.C.J.; Billington, C. Potential for bacteriophage endolysins to supplement or replace antibiotics in food production and clinical care. Antibiotics 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Ciepluch, K.; Skrzyniarz, K.; Zdańska, J.; Barrios-Gumiel, A.; Sánchez-Nieves, J.; de la Mata, F.J.; Maciejewska, B.; Drulis-Kawa, Z.; Bryszewska, M.; Arabski, M. PEGylation of dendronized silver nanoparticles increases the binding affinity of antimicrobial proteins. J. Mol. Liq. 2020, 319, 114339. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Smith, B.D. High-Generation Polycationic Dendrimers Are Unusually Effective at Disrupting Anionic Vesicles: Membrane Bending Model. Bioconjug. Chem. 2000, 11, 805–814. [Google Scholar] [CrossRef]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol. Biosyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef]
- Barrios-Gumiel, A.; Sanchez-Nieves, J.; Pérez-Serrano, J.; Gómez, R.; de la Mata, F.J. PEGylated AgNP covered with cationic carbosilane dendrons to enhance antibacterial and inhibition of biofilm properties. Int. J. Pharm. 2019, 569, 118591. [Google Scholar] [CrossRef]
- Crabbé, A.; Leroy, B.; Wattiez, R.; Aertsen, A.; Leys, N.; Cornelis, P.; Van Houdt, R. Differential proteomics and physiology of Pseudomonas putida KT2440 under filament-inducing conditions. BMC Microbiol. 2012, 12, 282. [Google Scholar] [CrossRef] [Green Version]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- Plotka, M.; Sancho-Vaello, E.; Dorawa, S.; Kaczorowska, A.K.; Kozlowski, L.P.; Kaczorowski, T.; Zeth, K. Structure and function of the Ts2631 endolysin of Thermus scotoductus phage vB_Tsc2631 with unique N-terminal extension used for peptidoglycan binding. Sci. Rep. 2019, 9, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thandar, M.; Lood, R.; Winer, B.Y.; Deutsch, D.R.; Euler, C.W.; Fischetti, V.A. Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 2971–2979. [Google Scholar] [CrossRef] [Green Version]
- Plotka, M.; Szadkowska, M.; Håkansson, M.; Kovačič, R.; Al-Karadaghi, S.; Walse, B.; Werbowy, O.; Kaczorowska, A.K.; Kaczorowski, T. Molecular characterization of a novel lytic enzyme lysc from clostridium intestinale urnw and its antibacterial activity mediated by positively charged n-terminal extension. Int. J. Mol. Sci. 2020, 21, 4894. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, S.; Percival, S.L. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Thummeepak, R.; Kitti, T.; Kunthalert, D.; Sitthisak, S. Enhanced antibacterial activity of Acinetobacter baumannii bacteriophage �ABP-01 endolysin (LysABP-01) in combination with colistin. Front. Microbiol. 2016, 7, 1402. [Google Scholar] [CrossRef] [Green Version]
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-Lactamase Inhibitor Boronic Acid Derivative SM23 as a New Anti-Pseudomonas aeruginosa Biofilm. Front. Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Llamazares, C.; Del Olmo, N.S.; Ortega, P.; Gómez, R.; Soliveri, J.; Javier De La Mata, F.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial effect of carbosilane metallodendrimers in planktonic cells of gram-positive and gram-negative bacteria and staphylococcus aureus biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Heredero-Bermejo, I.; Gómez-Casanova, N.; Quintana, S.; Soliveri, J.; de la Mata, F.J.; Pérez-Serrano, J.; Sánchez-Nieves, J.; Copa-Patiño, J.L. In vitro activity of carbosilane cationic dendritic molecules on prevention and treatment of candida albicans biofilms. Pharmaceutics 2020, 12, 918. [Google Scholar] [CrossRef]
- Fernandez, J.; Martin-Serrano, Á.; Gómez-Casanova, N.; Falanga, A.; Galdiero, S.; de la Mata, F.J.; Heredero-Bermejo, I.; Ortega, P. Effect of the combination of levofloxacin with cationic carbosilane dendron and peptide in the prevention and treatment of staphylococcus aureus biofilms. Polymers 2021, 13, 2127. [Google Scholar] [CrossRef] [PubMed]
- Danis-Wlodarczyk, K.; Vandenheuvel, D.; Jang, H.B.; Briers, Y.; Olszak, T.; Arabski, M.; Wasik, S.; Drabik, M.; Higgins, G.; Tyrrell, J.; et al. A proposed integrated approach for the preclinical evaluation of phage therapy in Pseudomonas infections. Sci. Rep. 2016, 6, 28115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosztołowicz, T.; Metzler, R.; Wąsik, S.; Arabski, M. Model of ciprofloxacin subdiffusion in Pseudomonas aeruginosa biofilm formed in artificial sputum medium. PLoS ONE 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Olszak, T.; Danis-Wlodarczyk, K.; Arabski, M.; Gula, G.; Maciejewska, B.; Wasik, S.; Lood, C.; Higgins, G.; Harvey, B.J.; Lavigne, R.; et al. Pseudomonas aeruginosa PA5oct jumbo phage impacts planktonic and biofilm population and reduces its host virulence. Viruses 2018, 11, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Dendrimer (Compound No.) | Planktonic Cells | Biofilm | ||||
|---|---|---|---|---|---|---|
| Preventing Biofilm Formation | Removing Biofilm | |||||
| MIC | MBC * | MBIC | MBBC * | MBDC | MBEC * | |
| 4 | 32 | 32 | 64 | 64–128 | 128 | 512 |
| 7 | 256 | 256 | 512 | 512–1024 | 512–1024 | 1024–>1024 |
| 10 | 256 | 256 | >1024 | >1024 | >1024 | >1024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana-Sanchez, S.; Gómez-Casanova, N.; Sánchez-Nieves, J.; Gómez, R.; Rachuna, J.; Wąsik, S.; Semaniak, J.; Maciejewska, B.; Drulis-Kawa, Z.; Ciepluch, K.; et al. The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin. Int. J. Mol. Sci. 2022, 23, 1873. https://doi.org/10.3390/ijms23031873
Quintana-Sanchez S, Gómez-Casanova N, Sánchez-Nieves J, Gómez R, Rachuna J, Wąsik S, Semaniak J, Maciejewska B, Drulis-Kawa Z, Ciepluch K, et al. The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin. International Journal of Molecular Sciences. 2022; 23(3):1873. https://doi.org/10.3390/ijms23031873
Chicago/Turabian StyleQuintana-Sanchez, Sara, Natalia Gómez-Casanova, Javier Sánchez-Nieves, Rafael Gómez, Jarosław Rachuna, Sławomir Wąsik, Jacek Semaniak, Barbara Maciejewska, Zuzanna Drulis-Kawa, Karol Ciepluch, and et al. 2022. "The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin" International Journal of Molecular Sciences 23, no. 3: 1873. https://doi.org/10.3390/ijms23031873
APA StyleQuintana-Sanchez, S., Gómez-Casanova, N., Sánchez-Nieves, J., Gómez, R., Rachuna, J., Wąsik, S., Semaniak, J., Maciejewska, B., Drulis-Kawa, Z., Ciepluch, K., Mata, F. J. d. l., & Arabski, M. (2022). The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin. International Journal of Molecular Sciences, 23(3), 1873. https://doi.org/10.3390/ijms23031873








